Abstract
Objectives
To evaluate the relationship between myocardial fibrosis identified by cardiac magnetic resonance (CMR) and ventricular performance and arrhythmias in patients late after the Fontan operation.
Background
Patients who have undergone the Fontan palliation may develop ventricular dysfunction and arrhythmias, but the mechanisms and risk factors are poorly defined.
Methods
All patients who have had a Fontan operation and a CMR study with the myocardial delayed enhancement technique from January 2002 to November 2008 were retrospectively identified.
Results
Of 90 patients (mean age at study 23.1 ± 10.9 years), 25 (28%) had positive late gadolinium enhancement (LGE) in the ventricular myocardium. Patients with positive LGE had lower mean ejection fraction (EF) (45% v. 56%, P<0.001), increased median end-diastolic volume (EDVi) (100 mL/BSA1.3 v. 82 mL/BSA1.3, P=0.004), increased median ventricular massi (63 g/BSA1.3 v. 45 g/BSA1.3, P<0.001), higher frequency of regional wall motion abnormalities (52% v. 28%, P=0.05), and higher frequency of non-sustained ventricular tachycardia (NSVT) (36% v. 11%, P=0.01). Multivariate regression analysis demonstrated that more extensive positive LGE, expressed as percent LGE of total myocardial mass, was associated with lower EF (P=0.002), increased EDVi (P<0.001), increased massi (P<0.001), and a higher frequency of NSVT (OR 1.2, 95% CI 1.1 to 1.4, P=0.006).
Conclusions
In this cohort of late Fontan survivors, myocardial fibrosis was common and associated with adverse ventricular mechanics and higher prevalence of NSVT. Further studies are warranted to examine the utility of LGE for risk stratification and treatment of ventricular arrhythmia and dysfunction in Fontan patients.
Keywords: Fontan procedure, magnetic resonance imaging, congenital heart disease, myocardial delayed enhancement, myocardial fibrosis
The Fontan procedure is the most common surgical palliation in patients with a functional single ventricle (FSV). Although advances in medical and surgical management of these patients have dramatically improved the prognosis in young children and adolescents (1), adverse outcomes become increasingly frequent with age and are common in adulthood (2,3). Myocardial fibrosis has been implicated as a potential contributor, but evidence has been scant, in part due to lack of precise quantitative in-vivo methods by which to detect it.
Myocardial delayed enhancement (MDE) is a cardiac magnetic resonance (CMR) technique that detects myocardial fibrosis and infarction. The technique has been validated by studies that correlated the finding of late gadolinium enhancement (LGE) with the presence and extent of myocardial fibrosis detected by histology in animal models and in humans (4-6). The majority of the investigations on LGE have been in the context of acquired adult heart disease. In the congenital heart disease population, positive LGE has been described in patients after tetralogy of Fallot repair and after atrial redirection surgery for transposition of the great arteries (7,8). In these cohorts, positive LGE has been associated with adverse ventricular mechanics, exercise intolerance, and arrhythmias. The impact of myocardial fibrosis on cardiac performance and clinical outcomes in patients with FSV late after the Fontan operation is not known. The purpose of this study was to characterize the frequency, location, and patterns of LGE and to evaluate the relationship between LGE and ventricular performance and arrhythmias in patients late after the Fontan operation.
Methods
Patients
A database search identified all patients who had undergone a Fontan operation and had a CMR study between January 2002 and November 2008. Among these, patients were included in the study if MDE acquisitions were technically successful. If a patient had multiple CMR studies using the MDE technique, only the most recent study was used for analysis. The study was approved by the Scientific Review Committee of the Department of Cardiology and by the Children’s Hospital Boston Committee on Clinical Investigation.
Clinical Variables
Demographic and clinical variables were abstracted from the medical records. Ventricular morphologies were classified as left ventricular (LV), right ventricular (RV), or mixed (e.g., unbalanced atrioventricular canal). Ventricular type was classified as mixed if both ventricles had an end-diastolic volume larger than a z score of −4. Published normative data from Alfakih et al. was used for ventricular z score calculations (9). Type of surgical palliation was classified into 1 of 4 Fontan types: lateral tunnel, right atrium-to-pulmonary artery anastomosis, right atrium-to-right ventricle connection, or extracardiac conduit. Collected surgical variables included age at Fontan, time since Fontan, history of Fontan revision, number of palliations prior to CMR (divided into those with and without cardiopulmonary bypass (CPB)), and age at volume unloading surgery (e.g., bidirectional Glenn or similar procedure).
CMR Technique
Studies were performed with commercially available 1.5 Tesla scanners (GE Medical Systems, Milwaukee, Wisconsin and Philips Healthcare, Best, The Netherlands). The details of the CMR protocols and MDE acquisitions used in our laboratory for assessment of patients with congenital heart disease have been published (10-12). The MDE technique has evolved during the study period as technologic advances became available. A representative protocol comprised an inversion-recovery prepared, phase sensitive, ECG-triggered, breath-hold segmented fast gradient echo pulse sequence in the short-axis planes acquired 15-20 minutes after injection of 0.2 mmol/kg gadopentetate dimeglumine (Magnevist, Berlex Laboratories, Wayne, New Jersey). Image data was acquired in diastole in every other beat. Imaging parameters were: repetition time: 5.9 ms; echo time: 3.5 ms; flip angle: 20 degrees; field-of-view: 260 by 260 mm; slice thickness: 7-8 mm; receiver bandwidth: 345.5 Hz; matrix: 144 × 144; spatial resolution: 1.8 × 1.8 × 7-8 mm, reconstructed to 1.0 × 1.0 × 7-8 mm. Inversion times were selected using an inversion-recovery fast multi-shot echo-planar imaging (Look-Locker sequence) to optimally null the signal of normal myocardium. Post-acquisition analysis of LGE and ventricular size and function were done using commercially available software packages (QMass version 7.0, Medis Medical Imaging Systems, Leiden, The Netherlands).
LGE Analysis
On the basis of visual assessment, patients were divided into 2 groups according to the presence or absence of LGE in the ventricular myocardium. LGE was further characterized by spatial location, pattern, and LGE quantification. Spatial location of LGE in the ventricular myocardium was categorized into one or more of the following locations: free wall, septum, apex, papillary muscles, trabeculations, septal insertion, and surgical sites. Patterns of LGE were categorized as transmural, subendocardial, subepicardial, and speckled. A transmural pattern of LGE required involvement of 100% of the ventricular wall thickness. Subendocardial patterns had LGE in the inner 50% of the ventricular wall or had LGE lesions contiguous with the endocardium. Patients with circumferential subendocardial patterns of LGE were further classified as having endocardial fibroelastosis (EFE) (13). Subepicardial patterns had LGE predominantly in the outer 50% of the ventricular wall or had LGE lesions contiguous with the epicardium. Patients were categorized as speckled if the LGE pattern was characterized by multiple small foci of enhancement as described by Babu-Narayan et al. (7). For categorization of LGE locations and patterns, each distinct LGE lesion was counted. LGE was quantified by the percent LGE of ventricular mass. Percent LGE was calculated by a single observer (RHR) using the previously reported and histologically verified full-width at half-maximum (FWHM) technique (4). Contours were manually adjusted to avoid false identification of artifacts as LGE. The identified LGE was then quantified as a mass and expressed as a percentage of the total ventricular mass. The total ventricular mass was calculated using Simpson’s method based on the contours from the LGE acquisition images. Patients with EFE and LGE in the septal insertion and surgical sites were re-contoured to allow for the calculation of percent LGE without EFE, percent LGE without septal insertion, and percent LGE without surgical sites. In 12 randomly selected patients with positive LGE, the percent LGE measurements were repeated by a second blinded observer (AP) using the FWHM technique to assess interobserver variability.
Ventricular Size and Function
Ventricular volumes and function were analyzed by manual tracing of endocardial and epicardial borders on each short-axis steady-state free precession cine slice as previously described (10). Simpson’s method was applied to calculate end-diastolic volume (EDV), end-systolic volume (ESV), ejection fraction (EF), stroke volume (SV), ventricular mass, and mass-to-volume ratio. In patients whose ventricular type was categorized as mixed, ventricular volumes and mass were summed to allow for calculation of total EDV, ESV, EF, SV, and mass-to-volume ratio. If the ventricular type was categorized as LV or RV, only the dominant ventricle was used for data analysis. In order to account for variations related to body size, SV was indexed to BSA, and EDV, ESV, and ventricular mass were indexed to BSA raised to the 1.3 power (14). To allow comparison with other studies, the univariate results for ventricular volumes and mass were also reported as indexed to BSA alone.
The cine steady-state free precession acquisitions in long- and short-axis views were visually reviewed to identify regional wall motion abnormalities (RWMA). RWMA were classified as hypokinesis, akinesis, and dyskinesis. The corresponding cine and MDE images were compared to determine whether RWMA spatially corresponded to regions of positive LGE.
Arrhythmia History
Patients’ arrhythmia history was compiled by review of Holters, electrocardiograms, electrophysiology catheterizations, and clinic notes. Episodes of atrial ectopy, atrial fibrillation, atrial flutter, supraventricular tachycardia, ventricular ectopy, non-sustained ventricular tachycardia (NSVT), sustained ventricular tachycardia, arrhythmia-related cardiac arrest, and electrophysiology studies were recorded. Pacemaker or defibrillator placement, heart transplantation, or death occurring after the CMR study was also documented.
Exercise Testing
Metabolic exercise testing data were included if the exercise test occurred within one year of the CMR study and if the patient reached maximal aerobic effort. Maximal aerobic effort was defined as a respiratory exchange ratio of ≥1.09 or if the patient reached 95% percent of predicted heart rate. Studies with submaximal aeorobic effort were not included to eliminate bias from factors unrelated to the patient’s Fontan cardiovascular system (e.g., leg pain) (15).
Statistical Analysis
Categorical data were described as number (percent). Nominal data were compared using Pearson’s chi-square test or Fisher’s exact test. Normally distributed continuous variables were described as mean ± standard deviation (SD) and compared using Student’s t test. Non-normally distributed continuous data were described as median [interquartile range (IQR)] and comparisons between subgroups were made using the Mann-Whitney U test.
Multivariate linear and logistic regression analysis with forward stepwise selection was used to investigate associations of LGE patterns, LGE locations, percent LGE, and the variables listed in Table 1 with each dependent outcome variable (EF, EDVi, ESVi, massi, and non-sustained ventricular tachycardia). The analysis included the entire study group.
Table 1. Patient Characteristics.
| Characteristic | All Patients (n = 90) |
LGE(−) (n=65) |
LGE(+) (n=25) |
P Value* |
|---|---|---|---|---|
| Age at CMR (years) | 23.1 ± 10.9 | 22.9 ± 10.2 | 23.2 ± 12.9 | 0.94 |
| Male | 56 (62%) | 39 (60%) | 17 (68%) | 0.63† |
| Age at Fontan (years) | 4.5 [2.0, 11.3] | 3.1 [2.0, 4.7] | 3.1 [1.9, 11.3] | 0.56‡ |
| Time since Fontan (years) | 15.7 ± 6.4 | 16.2 ± 5.9 | 14.3 ± 7.7 | 0.20 |
| Cardiac Diagnosis | 0.29 | |||
| Tricuspid atresia | 24 (27%) | 17 (26%) | 7 (28%) | |
| Double-inlet left ventricle | 18 (20%) | 16 (25%) | 2 (8%) | |
| Hypoplastic left heart syndrome | 17 (19%) | 10 (15%) | 7 (28%) | |
| Double-outlet right ventricle | 14 (15%) | 11 (17%) | 3 (12%) | |
| PA/IVS | 6 (7%) | 4 (6%) | 2 (8%) | |
| Atrioventricular canal defect | 6 (7%) | 5 (8%) | 1 (4%) | |
| Hypoplastic TV/RV | 5 (5%) | 2 (3%) | 3 (12%) | |
| Ventricular Type | 0.11 | |||
| Left ventricle | 46 (51%) | 34 (52%) | 12 (48%) | |
| Right ventricle | 30 (33%) | 24 (37%) | 6 (24%) | |
| Mixed ventricle | 14 (16%) | 7 (11%) | 7 (28%) | |
| Fontan Type | 0.20 | |||
| Lateral tunnel | 51 (56%) | 36 (55%) | 15 (60%) | |
| RA-Pulmonary artery | 29 (32%) | 24 (37%) | 5 (20%) | |
| RA-RV | 5 (6%) | 3 (5%) | 2 (8%) | |
| Extracardiac | 5 (6%) | 2 (3%) | 3 (12%) | |
| Number of operations (prior to CMR) | 3 [2, 3] | 3 [3, 3] | 3 [3, 4] | 0.67‡ |
| Bypass procedures | 2 [1, 3] | 3 [2, 3] | 3 [2, 3] | 0.20‡ |
| Off-bypass procedures | 1 [0, 1] | 0 [0, 1] | 0 [0, 1] | 0.31‡ |
| Prior volume unloading surgery | 33 (37%) | 22 (34%) | 11 (44%) | 0.47† |
| Age at volume unloading surgery | 0.7 [0.4, 1.4] | 0.7 [0.5, 1.5] | 0.6 [0.4, 1.4] | 0.62‡ |
| Number of catheterizations | 4 [2, 5] | 4 [2, 6] | 4 [2, 6] | 0.38‡ |
| History of Fontan revisions | 11 (12%) | 8 (12%) | 3 (12%) | 1.0† |
| Heart transplant | 2 (2%) | 2 (3%) | 0 (0%) | 1.0 |
| Death | 4 (4%) | 2 (3%) | 2 (8%) | 0.3 |
Values are expressed as mean ± SD, n (%), or median [IQR]
Student’s t test or chi-squared test of independence
Fisher’s exact test
Mann-Whitney U test
Abbreviations: PA/IVS = pulmonary atresia with intact ventricular septum; TV = tricuspid valve; RA = right atrium; RV = right ventricle.
Multivariate logistic regression with forward stepwise selection was used to investigate why patients did or did not have the LGE technique performed and included variables such as age at Fontan, age at CMR, sex, ventricular type, Fontan type, history of volume unloading surgery, and if study occurred during the first or second half of the study period.
All statistical tests were 2-sided and results were considered statistically significant if P < 0.05. All data analysis was performed using SPSS version 15.0 (SPSS Inc, Chicago, IL).
Statement of Responsibility
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Patient Characteristics
A total of 294 CMR studies performed on 205 patients who underwent the Fontan operation were reviewed. Among those studies, MDE imaging was performed and deemed technically adequate in 103 examinations from 90 patients. These patients comprise the study group. Table 1 summarizes patient characteristics and compares between those without and with positive LGE. When comparing patients without and with LGE, there were no significant differences in demographics, anatomic diagnoses, type of Fontan, or other patient characteristics.
There were 115 Fontan patients who had a CMR study without the MDE technique during the study period and, therefore, were not included in the study. When comparing the Fontan patients who had a CMR exam with and without the MDE technique, patients who did not have the MDE technique were younger at the time of their last CMR (17.5 y ± 8.8 y v. 23.1 y ± 10.9 y, P<0.001), closer in time to their Fontan operation (11.6 y ± 5.8 y v. 15.7 y ± 6.4 y, P<0.001), and more likely to have had volume unloading surgery (57% v. 37%, P=0.007). There were no other significant differences in demographics, anatomic diagnoses, type of Fontan, or other patient characteristics between included and excluded patients. Similarly, there were no significant difference in ventricular parameters, including EDVi, ESVi, EF, SVi, massi, and mass-to-volume ratio. Multivariate logistic regression was performed to identify the associations why patients did not the MDE technique performed. In the final model, patients were more likely to have the MDE technique performed if they had their CMR exam during the later half of the study period (OR 11.6, 95% CI 4.9 to 28.0, P<0.001) or if they were older at the time of CMR (OR 1.1, 95% CI 1.1 to 1.2, P<0.001).
LGE Characterization
Among the study group, positive LGE was observed in 25 patients (28%). In patients with positive LGE, median percent LGE was 5.3% [3.3%, 9.8%]. Measurements were repeated by a blinded second observer in 12 of 25 patients with LGE. The interobserver variability between the two observers was good, with a 2.5% mean difference between paired measurements (95% CI - 1.6% to 6.7%, P=0.21).
Counting each discrete lesion separately, LGE was found in the following locations: 64% (n=16) in the free wall of the primary ventricle, 36% (n=9) in the free wall of the secondary ventricle, 16% (n=4) in the ventricular septum, 16% (n=4) at the site of ventricular septal insertion, 12% (n=3) in papillary muscles, 8% (n=2) in the ventricular apex, and 8% (n=2) at surgical sites. Examples of typical locations and patterns of LGE are shown in Figure 1. Counting each discrete LGE lesion separately, 40% (n=10) of patients had transmural LGE patterns, 32% (n=8) demonstrated subendocardial LGE, 20% (n=5) had subepicardial LGE, 16% (n=4) showed EFE, and speckled LGE was seen in 12% (n=3).
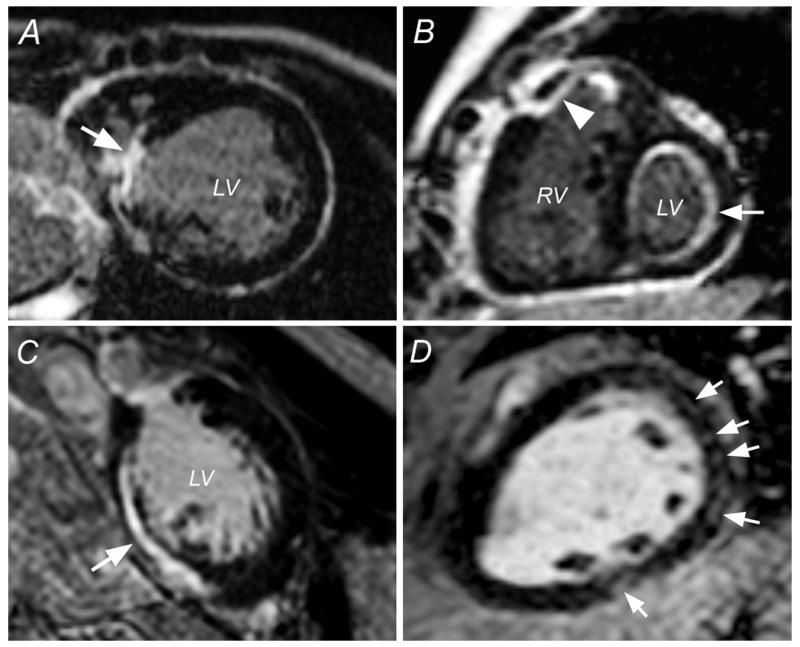
Figure 1.
Locations and Patterns of LGE Late After Fontan Operation
(A) The arrow points to a transmural late gadolinium enhancement (LGE) lesion involving the ventricular septum. (B) The arrowhead points to a primary subendocardial lesion with transmural extension in the free wall of the primary ventricle, whereas the full arrow identifies endocardial fibroelastosis. (C) The arrow points to a subepicardial LGE lesion in the free wall of the primary ventricle. (D) Speckled pattern (arrows) in the free wall of the primary ventricle. LV = left ventricle; RV = right ventricle.
Ventricular Size and Function
Univariate analyses of ventricular size and function are summarized in Table 2. Patients with positive LGE in the ventricular myocardium had higher EDVi and ESVi, lower EF, and larger ventricular massi compared to patients without LGE. Similar univariate analysis showed that LGE in the free wall of the primary ventricle and transmural LGE were both associated with larger ventricular volumes, decreased systolic function, and larger ventricular mass. Univariate linear regression analysis revealed that higher percent LGE correlated with higher EDVi (R2 = 0.27, P<0.001), higher ESVi (R2 = 0.38, P<0.001), lower EF (R2 = 0.32, P<0.001), and higher massi (R2 = 0.16, P<0.001). There were no differences between groups for SVi and mass-to-volume ratios. Similar results were observed when the contribution of EFE, septal insertion sites, and surgical sites were each removed from the total percent LGE.
Table 2. Univariate Analysis.
| All Patients (n = 90) |
LGE(−) (n=65) |
LGE(+) (n=25) |
P Value | |
|---|---|---|---|---|
| EDVi (mL/BSA1.3) | 87 [66, 108] | 82 [63, 98] | 100 [79, 158] | 0.004† |
| EDVi (mL/BSA) | 100 [76, 127] | 95 [73, 115] | 123 [92, 171] | 0.003† |
| ESVi (mL/BSA1.3) | 36 [27, 53] | 34 [26, 44] | 63 [35, 87] | <0.001† |
| ESVi (mL/BSA) | 41 [31, 65] | 39 [29, 52] | 66 [40, 102] | <0.001† |
| SVi (mL/BSA) | 55 ± 18 | 54 ± 17 | 58 ± 19 | 0.36* |
| EF (%) | 53 ± 12 | 56 ± 10 | 45 ± 14 | <0.001* |
| Massi (g/BSA1.3) | 50 [41, 69] | 45 [38, 59] | 63 [49, 89] | <0.001† |
| Massi (g/BSA) | 57 [46, 76] | 52 [42, 72] | 73 [56, 98] | 0.001† |
| Mass / volume ratio (g/mL) | 0.6 [0.5, 0.7] | 0.6 [0.5, 0.8] | 0.6 [0.5, 0.7] | 0.72† |
| RWMA | 31 (34%) | 18 (28%) | 13 (52%) | 0.05‡ |
| Any ventricular arrhythmia | 25 (28%) | 13 (20%) | 12 (48%) | 0.02‡ |
| Ventricular ectopy | 19 (21%) | 9 (14%) | 10 (40%) | 0.01‡ |
| NSVT | 17 (19%) | 7 (11%) | 10 (40%) | 0.005‡ |
| Sustained ventricular tachycardia | 6 (7%) | 3 (5%) | 3 (12%) | 0.3‡ |
| Arrhythmia related cardiac arrest | 3 (3%) | 1 (2%) | 2 (8%) | 0.2‡ |
| Pacemaker | 12 (13%) | 10 (15%) | 2 (8%) | 0.5‡ |
| Defibrillator | 2 (2%) | 2 (3%) | 0 (0%) | 1‡ |
Values are expressed as mean ± SD, median [IQR], or n (%)
Student’s t test
Mann-Whitney U test
Fisher’s exact test
Number of patients with exercise testing data: all patients, 38; without LGE, 24; with LGE, 14.
Abbreviations: EDV = end-diastolic volume; EF = ejection fraction; ESV = end-systolic volume; NSVT = non-sustained ventricular tachycardia; RWMA = regional wall motion abnormalities; SV = stroke volume
Multivariate linear regression analysis was performed to determine variables associated with EDVi, ESVi, EF, and massi. Table 3 displays the final multivariate models for each of the dependent CMR ventricular parameters. In the final models, higher percent LGE was associated with higher EDVi and ESVi, lower EF, and increased massi. Longer time since Fontan was also associated with lower EDVi and massi, and older age at Fontan was associated with decreased EF.
Table 3. Multivariate Analysis.
| Outcome* | Predictor | Beta | Standard Error |
P Value | R2 |
|---|---|---|---|---|---|
| EDVi (mL/BSA1.3) | 0.45 | ||||
| Percent LGE | 8.6 | 1.9 | <0.001 | ||
| Time since Fontan | −2.9 | 1.1 | 0.02 | ||
| ESVi (mL/BSA1.3) | 0.47 | ||||
| Percent LGE | 8.2 | 1.5 | <0.001 | ||
| EF (%) | 0.50 | ||||
| Percent LGE | −1.3 | 0.4 | 0.002 | ||
| Age at Fontan | −0.8 | 0.3 | 0.008 | ||
| Massi (g/BSA1.3) | 0.43 | ||||
| Percent LGE | 4.2 | 1.0 | <0.001 | ||
| Time since Fontan | −1.6 | 0.6 | 0.01 |
| Outcome† | Predictor | Odds Ratio | Standard Error |
P Value |
|---|---|---|---|---|
| NSVT | ||||
| Percent LGE | 1.2 | 0.06 | 0.006 |
Multivariate linear regression analysis
†Multivariate logistic regression analysis
Abbreviations: EDV = end-diastolic volume; EF = ejection fraction; ESV = end-systolic volume; NSVT = non-sustained ventricular tachycardia
Patients with LGE were more likely to have RWMA (52% v. 28%, P=0.05). Compared to areas of RWMA without LGE, LGE lesions with RWMA were more likely to have dyskinesis (78% v. 22%, P=0.01).
Arrhythmia Results
Univariate results for history of arrhythmias are shown in Table 2. Patients with or without LGE, were similar in terms of frequency of atrial arrhythmias, including non-sinus rhythm, fibrillation, flutter, and supraventricular tachycardia. Compared to patients without LGE, those with LGE were more likely to have ventricular arrhythmias (40% v. 14%, P=0.01) and NSVT (36% v. 11%, P=0.01). With the exception of one patient who had an episode of ventricular tachycardia and cardiac arrest after the CMR study, all other episodes of ventricular arrhythmia occurred before the date of the CMR examination. Multivariate logistic regression analysis demonstrated that higher LGE percent (OR 1.2, 95% CI 1.1 to 1.4, P=0.006) was associated with higher incidence of NSVT.
Exercise Testing Results
Of the 90 study patients, 38 (42%) had a metabolic exercise test reaching maximal aerobic capacity within one year of their CMR study. Patients with or without positive LGE had a similar percent predicted oxygen consumption, oxygen consumption at ventilatory anaerobic threshold, oxygen pulse, and peak workload. Results were similar if patients were analyzed by LGE location or LGE pattern. There was no significant correlation between any of the exercise data parameters and percent LGE.
Discussion
This study is the first to systematically evaluate myocardial fibrosis as identified by the MDE technique in patients late after the Fontan operation. LGE was a common finding and was associated with a more dilated, hypertrophied, and poorly functioning systemic ventricle, and with a higher frequency of non-sustained ventricular tachycardia. Moreover, in multivariate analysis, the percent LGE in the ventricular myocardium was associated with parameters of adverse ventricular size and function, as well as an increased risk for ventricular tachycardia. Both ventricular systolic dysfunction and clinical arrhythmias have been shown to be important predictors of morbidity and mortality (2).
Comparison with Other Studies
LGE has become a well-accepted marker for myocardial fibrosis and has been validated histologically (4,16,17). Babu-Narayan et al. associated clinically important outcomes with LGE in adults after repair of tetralogy of Fallot and in patients with atrial redirection surgery for transposition of the great arteries (7,8). In their studies, LGE was associated with decreased exercise tolerance, higher New York Heart Association class, depressed RV systolic function, and increased frequency of clinical arrhythmias and syncope. Similar findings were recently reported by Wald et al. (12). Our study did not find any associations between LGE and exercise intolerance but had similar results with regard to LGE and depressed ventricular function, RWMA, and arrhythmias.
The Pediatric Heart Network (PHN) recently published mid-term outcomes in 546 patients after the Fontan operation, of whom 161 patients had a CMR study (1). Compared to our study group, the PHN patients had smaller EDVi (85 mL/BSA1.3 ± 25 mL/BSA1.3 v. 97 mL/BSA1.3 ± 50 mL/BSA1.3, P<0.001) and had higher EF by CMR (57% ± 10% v. 53% ± 12%, P<0.001). There are, however, significant differences between the two patient cohorts. Patients in the PHN study were younger at the time of evaluation (mean 11.9 y ± 3.4 y v. 23.1 y ± 10.9 y, P<0.001), were more likely to have had volume unloading surgery prior to their Fontan (75% v. 38%, P<0.001), and were less likely to have had a RA-PA Fontan (13% v. 32%, P<0.001). These differences suggest that patients with Fontan physiology may have preserved ventricular mechanics in the first and second decades of life but that the risk of adverse ventricular mechanics increases with time from their Fontan operation. This notion is supported by previously published data showing increased morbidity and mortality with age after the Fontan operation (2,3).
Clinical Implications
This study introduces MDE imaging as a potential tool for risk stratification for this patient population. In adult patients, the presence and extent of LGE have been shown to predict adverse outcomes in ischemic heart disease, dilated and hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, and other conditions (18-21). It is therefore reasonable to explore whether LGE and its extent could contribute to risk stratification for heart failure and cardiac death late after the Fontan operation. For example, it would be beneficial to follow the PHN Fontan cohort with serial CMR and MDE evaluations to prospectively explore the temporal relationship between LGE and adverse ventricular mechanics and to study the implications of myocardial fibrosis.
Study Limitations
The single center, retrospective nature of our study limits our ability to make definitive conclusions about the impact of positive LGE on patient outcomes late after the Fontan operation. We have not followed these patients long enough to know if LGE is associated with important clinical outcomes such as cardiac transplantation, ICD implantation, or death. Furthermore, this study was not designed to identify the root causes of myocardial fibrosis seen by MDE imaging. Although CMR has been widely adopted as the preferred technique by which to non-invasively follow these patients (22), our cohort may not be a representative sample of all patients late after the Fontan operation. Further, MDE technique was not applied uniformly to all patients undergoing a CMR examination after the Fontan operation. Logistic regression analysis demonstrated that this reflects both an evolution in clinical practice and that in younger patients with a limited ability to cooperate with the examination peripheral intravenous line placement poses a challenge. It is also worth noting that as with any technological advancement, the MDE technique evolved during the study period, potentially increasing the sensitivity and specificity of the technique for later CMR examinations.
CMR evaluation in patients with pacemakers and defibrillators is currently a strong relative contraindication (23), and these patients are thus not represented in the study cohort. In the cross-sectional PHN Fontan studies, 13% of patients had such a device (1), This selection bias may result in under-identification of ventricular arrhythmia events and arrhythmia related causes of sudden cardiac death in our study. Patients with ventricular dysfunction may also be over-represented in this cohort, as these patients may undergo more frequent CMR surveillance compared to patients who are asymptomatic.
Conclusions
In patients late after the Fontan operation, the presence and extent of myocardial fibrosis as identified by LGE are associated with dilated and hypertrophied systemic ventricles, systolic dysfunction, regional dyskinesis, and non-sustained ventricular tachycardia. Further studies are warranted to examine the utility of LGE for risk stratification and treatment decisions in patients following the Fontan operation.
Acknowledgments
The authors thank Dr. Kimberlee Gauvreau for her suggestions regarding statistical methods.
Funding Sources: This study was supported by the Higgins Family Noninvasive Cardiac Imaging Research Fund and the National Institutes of Health under award number: T32HL007572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations List
- CMR
cardiac magnetic resonance
- CPB
cardiopulmonary bypass
- EDV
end-diastolic volume
- EF
ejection fraction
- EFE
endocardial fibroelastosis
- ESV
endsystolic volume
- FSV
functional single ventricle
- FWHM
full-width at half-maximum
- IQR
interquartile range
- LGE
late gadolinium enhancement
- LV
left ventricular
- MDE
myocardial delayed enhancement
- NSVT
non-sustained ventricular tachycardia
- PHN
Pediatric Heart Network
- RV
right ventricle
- RWMA
regional wall motion abnormalities
- SD
standard deviation
- SV
stroke volume
Footnotes
Disclosures: None.
References
- 1.Anderson PA, Sleeper LA, Mahony L, et al. Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol. 2008;52:85–98. doi: 10.1016/j.jacc.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khairy P, Fernandes SM, Mayer JE, Jr., et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117:85–92. doi: 10.1161/CIRCULATIONAHA.107.738559. [DOI] [PubMed] [Google Scholar]
- 3.Piran S, Veldtman G, Siu S, Webb GD, Liu PP. Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation. 2002;105:1189–94. doi: 10.1161/hc1002.105182. [DOI] [PubMed] [Google Scholar]
- 4.Amado LC, Gerber BL, Gupta SN, et al. Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol. 2004;44:2383–9. doi: 10.1016/j.jacc.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Kehr E, Sono M, Chugh SS, Jerosch-Herold M. Gadolinium-enhanced magnetic resonance imaging for detection and quantification of fibrosis in human myocardium in vitro. Int J Cardiovasc Imaging. 2008;24:61–8. doi: 10.1007/s10554-007-9223-y. [DOI] [PubMed] [Google Scholar]
- 6.Wagner A, Mahrholdt H, Holly TA, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374–9. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- 7.Babu-Narayan SV, Goktekin O, Moon JC, et al. Late gadolinium enhancement cardiovascular magnetic resonance of the systemic right ventricle in adults with previous atrial redirection surgery for transposition of the great arteries. Circulation. 2005;111:2091–8. doi: 10.1161/01.CIR.0000162463.61626.3B. [DOI] [PubMed] [Google Scholar]
- 8.Babu-Narayan SV, Kilner PJ, Li W, et al. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of fallot and its relationship to adverse markers of clinical outcome. Circulation. 2006;113:405–13. doi: 10.1161/CIRCULATIONAHA.105.548727. [DOI] [PubMed] [Google Scholar]
- 9.Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging. 2003;17:323–9. doi: 10.1002/jmri.10262. [DOI] [PubMed] [Google Scholar]
- 10.Garg R, Powell AJ, Sena L, Marshall AC, Geva T. Effects of metallic implants on magnetic resonance imaging evaluation of Fontan palliation. Am J Cardiol. 2005;95:688–91. doi: 10.1016/j.amjcard.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 11.Geva T, Powell AJ. Pediatric Heart Disease. In: Edelman RR, Hesselink JR, Zlatkin MB, Crues JV, editors. Clinical Magnetic Resonance Imaging. Elsevier Science; Philadelphia, PA: 2006. pp. 1041–1069. [Google Scholar]
- 12.Wald RM, Haber I, Wald R, Valente AM, Powell AJ, Geva T. Effects of Regional Dysfunction and Late Gadolinium Enhancement on Global Right Ventricular Function and Exercise Capacity in Patients With Repaired Tetralogy of Fallot. Circulation. 2009;119:1370–1377. doi: 10.1161/CIRCULATIONAHA.108.816546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tworetzky W, del Nido PJ, Powell AJ, Marshall AC, Lock JE, Geva T. Usefulness of magnetic resonance imaging of left ventricular endocardial fibroelastosis in infants after fetal intervention for aortic valve stenosis. Am J Cardiol. 2005;96:1568–70. doi: 10.1016/j.amjcard.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 14.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–57. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 15.Paridon SM, Mitchell PD, Colan SD, et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol. 2008;52:99–107. doi: 10.1016/j.jacc.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 16.Moon JC, Reed E, Sheppard MN, et al. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;43:2260–4. doi: 10.1016/j.jacc.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 17.Simonetti OP, Kim RJ, Fieno DS, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–23. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 18.Wu KC, Weiss RG, Thiemann DR, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–21. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teraoka K, Hirano M, Ookubo H, et al. Delayed contrast enhancement of MRI in hypertrophic cardiomyopathy. Magn Reson Imaging. 2004;22:155–61. doi: 10.1016/j.mri.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Tandri H, Saranathan M, Rodriguez ER, et al. Noninvasive detection of myocardial fibrosis in arrhythmogenic right ventricular cardiomyopathy using delayed-enhancement magnetic resonance imaging. J Am Coll Cardiol. 2005;45:98–103. doi: 10.1016/j.jacc.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 21.Bondarenko O, Beek AM, Nijveldt R, et al. Functional outcome after revascularization in patients with chronic ischemic heart disease: a quantitative late gadolinium enhancement CMR study evaluating transmural scar extent, wall thickness and periprocedural necrosis. J Cardiovasc Magn Reson. 2007;9:815–21. doi: 10.1080/10976640701547335. [DOI] [PubMed] [Google Scholar]
- 22.Fogel MA. Cardiac magnetic resonance of single ventricles. J Cardiovasc Magn Reson. 2006;8:661–70. doi: 10.1080/10976640600713814. [DOI] [PubMed] [Google Scholar]
- 23.Levine GN, Gomes AS, Arai AE, et al. Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2007;116:2878–91. doi: 10.1161/CIRCULATIONAHA.107.187256. [DOI] [PubMed] [Google Scholar]