Abstract
Nigeria has a high burden of vector borne diseases such as malaria and lymphatic filariasis (LF). This study aimed to determine the species composition of mosquitoes in Ibadan, Southwest Nigeria as well as determine their role in malaria and LF transmission. Adult mosquitoes were collected by Pyrethrum Spray Catch (PSC) and identified and graded according to their abdominal conditions. The mosquitoes were dissected to determine the parity status and to check for microfilariae of Wuchereria bancrofti. The presence of circumsporozoite protein of Plasmodium falciparum was examined using ELISA. A total of 1600 mosquitoes were collected of which 31 (1.9%) were Anopheles gambiae s.l. while 1756 (98%) were Culex sp. None of the mosquitoes examined was positive for Plasmodium falciparum and Wuchereria bancrofti. The lack of adequate sanitary conditions in the area could be responsible for the large number of mosquitoes collected. Health education could help in sensitizing the inhabitants.
Keywords: Mosquitoes, Lymphatic filariasis, Malaria, Nigeria, Ibadan
INTRODUCTION
Malaria and lymphatic filariasis are vector-borne diseases that account for the largest global burdens of mortality and morbidity in the world’s poorest countries[1, 2]. More than half of the world’s population is at risk of at least one of these diseases. Malaria is caused by Plasmodium and transmitted by Anopheles mosquitoes. It kills about 881,000 people every year, 90% of whom are in Africa and 85% of whom are children under five[3]. It is highly endemic in Nigeria with about 97% of people in Nigeria at risk of the disease[4].
Lymphatic filariasis (LF) which is one of the most debilitating neglected tropical diseases (NTD) in the world is caused by the parasitic worms Wuchereria bancrofti, Brugia malayi and B. timori and is transmitted by Anopheles, Culex, Aedes, Ochlerotatus and Mansoni mosquitoes[5]. The disease is endemic in 81 countries with an estimated 120 million people infected and 40 million people with clinical manifestations including lymphoedema (elephantiasis) of the limbs and urogenital disorders, especially hydrocele in men[5]. Nigeria bears the highest burden of LF in Africa, with an estimated 80 to 120 million people at risk[6].
It is common to find malaria and LF in the same human population and sharing the same mosquito vectors[7]. It is therefore common to find co infections of malaria and LF in a single mosquito vector in these areas. Malaria and LF are both transmitted by Anopheles mosquitoes in Nigeria[8]. The diseases had been observed to coexist in some parts of Nigeria, such as New Bussa, Niger State[9]. Therefore, any control method geared towards the vector has the capability of controlling both diseases.
However, there is paucity of information on the transmission of LF and malaria in south west, Nigeria. Therefore, this research was carried out in an urban area in Ibadan metropolis to determine the species composition and temporal distribution of mosquitoes in Ibadan, South west Nigeria as well as to determine their role in the transmission of malaria and lymphatic filariasis in the area.
2. MATERIALS AND METHODS
2.1 Study Area
This study was conducted in randomly selected houses in Beere (07°38’0”N, 03°90’0”E), Ibadan North/East Local Government Area, Oyo State. Nigeria. The study area is within the core residential areas in Ibadan which has limited basic infrastructure services. The area has an inadequate waste disposal system and lacks comprehensive water and sewage systems.
2.2 Mosquito Collection
Adult mosquitoes were collected by Pyrethrum Spray Catch (PSC)[10] between the hours of 06:00 and 08:00 fortnightly between December, 2012 and July, 2013.
2.3 Mosquito identification and determination of other entomological indices
Mosquitoes collected were identified morphologically using taxonomic keys[11]. Mosquitoes were graded according to their abdominal conditions, namely; blood fed (BF), unfed (UF), gravid (G), and half gravid (HG).
A subset of the mosquitoes collected was dissected to determine the parity status and to check for microfilariae of Wuchereria bancrofti[10].
For molecular identification, DNA extraction was done using boil preparation method, 2–3 legs from each mosquito were employed for the extraction. The legs were crushed in 50 µl of distilled water and boiled at 95°C for 10 minutes. The supernatant was used as template for species identification using Polymerase Chain Reaction (PCR)[12].
The head and thorax of female anopheles were used to test for the presence of circumsporozoite protein (CSP) of Plasmodium falciparum using the Enzyme-Linked Immunosorbent Assay (ELISA)[13].
2.4 Clinical report of malaria
Malaria report cases were collected from the Primary Health Center in the area (Alafara Aderogba PHC) for the period of the study in order to compare malaria cases in the area with the mosquito density. Malaria diagnosis was based on the use of Rapid Diagnostic Tests.
2.5 Meteorological data
Mean and maximum temperature, relative humidity and annual rainfall data for the study period were obtained from a local weather station in Geography Department, University of Ibadan.
2.6 Data Analysis
The entomological parameters for each vector species were calculated as follows:
Man biting rates = Number of mosquito collected / (Number of collectors × Number of captures)
Indoor resting density (IRD) = Number of mosquitoes/ Number of rooms sprayed
- The Man Biting Rate (MBR) was indirectly calculated as
- MBR = F/W
Sporozoite rate = number positive/ number processed
Entomological inoculation rate (EIR) = man biting rate × sporozoite rate
Parity = (number parous)/ (number parous + number nulliparous)
Chi square test was used to compare the parous rate between species.
2.7 Ethical clearance
Ethical approval for this study was obtained from the University of Ibadan and the University College Hospital, Ibadan (UI/UCH) Ethics Review Committee with approval number UI/UCH/EC/12/0246.
3. RESULTS
3.1 Entomological data
A total of 1600 mosquitoes were collected of which 31 (1.9%) were Anopheles gambiae s.s.. while 1756 (98%) were Culex sp (Table 1). All Anopheles sp were identified molecularly as An. gambiae s.s. The highest number of Anopheles sp and Culex sp were collected in December and January respectively. No Anopheles species was collected in the months of February and March. The lowest number of Culex species collected was in June.
Table 1.
The total number of Anopheles gambiae s.s and Culex sp collected during the study period showing to their abdominal condition
| Month* | Anopheles gambiae s.s | Culex sp | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BF | UF | G | HG | Total | BF | UF | G | HG | Total | |
| December 2012 | 3 | 19 | 0 | 0 | 22 | 86 | 398 | 0 | 0 | 484 |
| January 2013 | 0 | 1 | 0 | 0 | 1 | 98 | 354 | 19 | 75 | 546 |
| February 2013 | 0 | 0 | 0 | 0 | 0 | 63 | 66 | 6 | 38 | 173 |
| March 2013 | 0 | 0 | 0 | 0 | 0 | 8 | 31 | 5 | 27 | 71 |
| April 2013 | 0 | 5 | 0 | 0 | 5 | 0 | 28 | 5 | 24 | 57 |
| May 2013 | 0 | 0 | 0 | 1 | 1 | 15 | 28 | 5 | 10 | 58 |
| June 2013 | 0 | 1 | 0 | 0 | 1 | 3 | 29 | 6 | 18 | 56 |
| July 2013 | 0 | 0 | 0 | 1 | 1 | 56 | 35 | 4 | 29 | 124 |
| Total | 3 | 26 | 0 | 2 | 31 | 329 | 969 | 50 | 221 | 1569 |
UF: unfed; BF: blood-fed; G: gravid; HG: half-gravid
The classification of the female mosquitoes based on their abdominal condition is presented in Table 1. The numbers of unfed mosquitoes were highest in both species in the month of December.
More than fifty percent (57.1%) of An. gambiae s.s. and 64.9% of Culex sp. were parous (Table 2). The parity rate of An. gambiae s.s. and Culex sp. was not significantly different (λ2 = 0.1780, p=0.1780). All the 31 An. gambiae s.s tested negative to Plasmodium falciparum circumsporozoite antigen. None of the mosquitoes dissected was infective with microfilaria worm of Wuchereria bancrofti (Table 2).
Table 2.
Summary of the mosquitoes dissected the parity status and parasite infectivity rate
| Mosquito Species | Number Dissected |
Parous (%) | Number examined for P. falciparum (%) |
Number infective with W. bancrofti (%) |
|---|---|---|---|---|
| An. gambiae s.s | 7 | 4 (57.1) | 31 (0) | 0 (0) |
| Culex sp | 242 | 157 (64.9) | - | 0 (0) |
| Total | 249 | 161 | 31 (0) | 0 (0) |
The proportion of the indoor resting populations of An. gambiae and the Culex species respectively comprised of 26 (84%) and 969 (62%) of unfed females (Table 1). The total indoor resting densities of An. gambiae s.s. and Culex sp were 0.25 and 12.65 respectively for the study period (Table 3). The total man biting rate of An. gambiae and Culex sp were 0.01 and 0.99 respectively for the study period (Table 3).
Table 3.
Indoor resting densities and estimated man biting rate (MBR) per night of An. gambiae and Culex sp mosquitoes
| Anopheles gambiae s.s | Culex sp | |||
|---|---|---|---|---|
| Month | Indoor resting density (IRD) |
Man biting rate (MBR) |
Indoor resting density (IRD) |
Man biting rate (MBR) |
| December 2012 | 0.92 | 0.06 | 20.17 | 1.59 |
| January 2013 | 0.05 | 0 | 28.73 | 1.96 |
| February 2013 | 0 | 0 | 13.30 | 1.91 |
| March 2013 | 0 | 0 | 5.46 | 0.23 |
| April 2013 | 0.31 | 0 | 3.56 | 0 |
| May 2013 | 0.09 | 0 | 5.27 | 0.48 |
| June 2013 | 0.06 | 0 | 3.50 | 0.06 |
| July 2013 | 0.09 | 0 | 11.27 | 1.65 |
| Total | 0.25 | 0.01 | 12.65 | 0.99 |
3.2 Clinical report
A total of 1,615 malaria cases were recorded in the primary health center in the area. Malaria cases were lowest in February (128 cases) and highest in July (307 cases) (Table 4). More females (962 cases) were infected with malaria compared with the males (653 cases) (Table 4).
Table 4.
Clinical Cases of Malaria in the primary health center in the study area from December, 2012 to July, 2013.
| Month/Year | Total Male | Total Female | Grand Total |
|---|---|---|---|
| Dec., 2012 | 102 | 115 | 217 |
| Jan., 2013 | 55 | 100 | 155 |
| Feb., 2013 | 45 | 83 | 128 |
| Mar., 2013 | 54 | 115 | 169 |
| April, 2013 | 54 | 108 | 162 |
| May, 2013 | 107 | 139 | 246 |
| June, 2013 | 109 | 122 | 231 |
| July, 2013 | 127 | 180 | 307 |
| Total | 653 | 962 | 1,615 |
3.3 Meteorological data
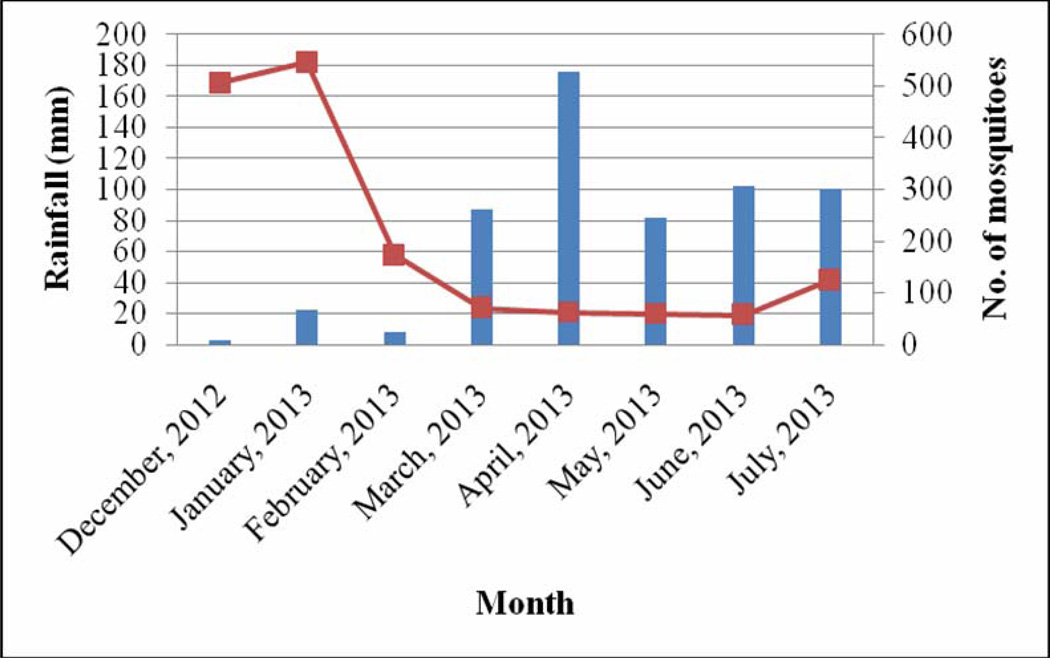
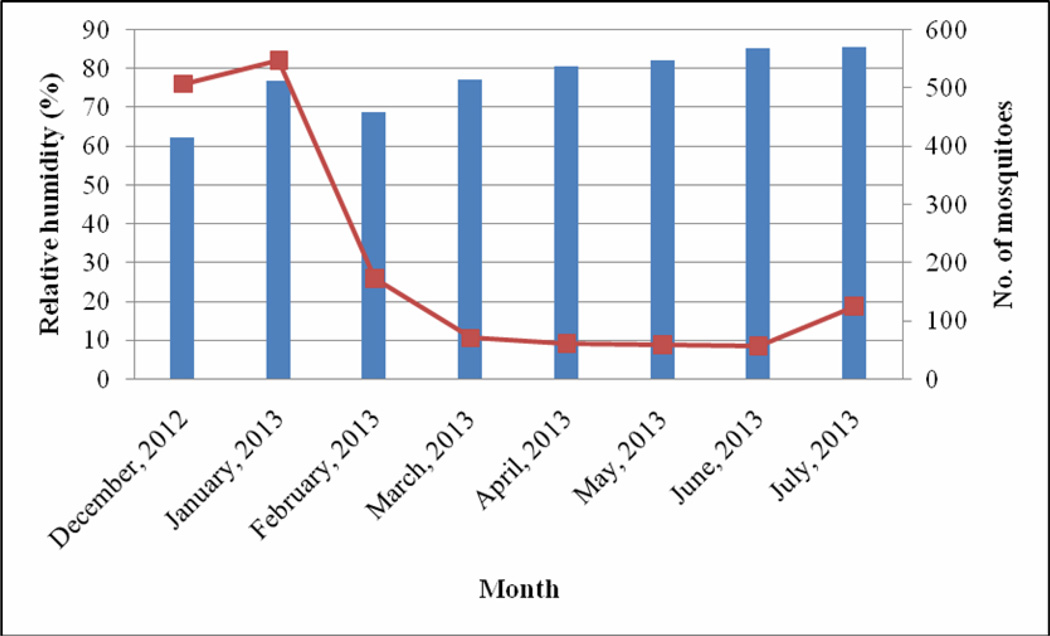
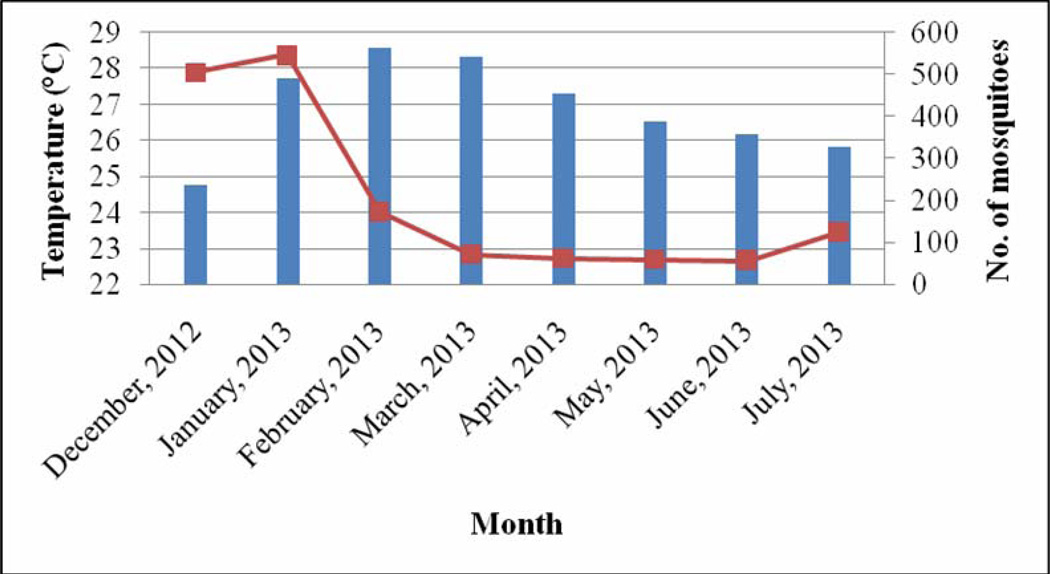
The month of December recorded the lowest rainfall (2.6 mm), temperature (24.7 °C) and relative humidity (62.2%) (Fig 1 – 3). The highest range of rainfall, temperature and relative humidity were recorded in April (175.6 mm), February (28.6 °C) and July (85.7%) respectively. The results showed that mosquito abundance decreased as rainfall increased (Fig 1) while the reverse was the case with temperature (Fig 2) and relative humidity (Fig 3).
Fig 1.
Relationship between rainfall and mosquito abundance
Fig 3.
Relationship between relative humidity and mosquito abundance
Fig 2.
Relationship between temperature and mosquito abundance
4. DISCUSSION
This study was carried out to determine the mosquito abundance and distribution as well as their role in the transmission of malaria and lymphatic filariasis transmission in Ibadan, Southwest Nigeria. It revealed that mosquito abundance in the study area is dominated by Culex sp where it accounted for about 98% of the total mosquito collection. The knowledge of vector species is important in understanding the epidemiology of malaria and LF. This study provides the baseline entomological indices of malaria and LF in Beere area of Ibadan, Oyo State, Nigeria.
Mosquitoes were more abundant during the dry months of December, January and February compared to the wet months of March, April, May, June and July. The results showed that mosquito abundance was inversely related with rainfall but not with temperature and relative humidity. Mosquito abundance was reported to have a positive relationship with rainfall and inversely related to temperature in the Imo River Basin of Nigeria[14]. Studies in Abeokuta, South west Nigeria showed that mosquito abundance increased as the season progressed from January with a drastic decline between June and July[15]. Some studies in Nigeria have recorded a preponderance of mosquito species during the rainy season than during the dry season[14, 16, 17]. Since the major breeding site for the mosquitoes in the area was the stream polluted with dung and damaged drainage systems or run-off areas, the heavy rainfall might have flushed larvae and eggs from their breeding sites. Long lasting Insecticide Nets (LLINs) were distributed in the community in April 2013, a decline in mosquito abundance from April to July may be attributed to the use of these LLINs by inhabitants of the community.
The most abundant mosquito in the area was Culex sp. The variation in the abundance of An. gambiae s.s. and Culex sp. can be attributed to the difference in their breeding requirements. Anopheles gambiae s.s. mosquitoes breed in transient habitats such as shallow sun lit fresh water pools or human made habitats, hoof prints and tyre tracks[18]. Culex sp are known to breed in polluted water bodies including open drains, open or cracked septic tanks, flooded pit latrines[14]. The study area had limited basic infrastructure and was characterized by lack of comprehensive water and waste management system that is common to unplanned urbanized areas in Africa. The inhabitants practice open toilet systems and poor sanitary conditions which serve as breeding ground for Culex mosquitoes and could explain the high abundance of this species. Anopheles gambiae s.s. was the only anopheline mosquito found during the study and was more abundant during the dry month of December. The low An. gambiae s.s. reported in this study may be as a result of polluted environment that does not support its breeding.
Anopheles gambiae s.s. mosquito is the vector of malaria and lymphatic filariasis in Nigeria8. However, Culex sp. have been reported to be infective with Wucheraria bancrofti in Nigeria[19, 20]. The possible involvement of Culex species in the transmission of lymphatic filariasis in northern Nigeria has not been substantiated. None of the An. gambiae s.s. mosquitoes analyzed was infected with P. falciparum and W. bancrofti. Conducting the study over a longer period may be necessary to confirm this absence. The non occurrence of the microfilaria worm of lymphatic filariasis may be due to the litte or no transmission in the area. It is interesting to note the high number of clinical cases of malaria reported in the area which does not correlate with the absence of sporozoites in the An. gambiae s.s. mosquitoes. This lack of correlation was probably as a result of the low number of An. gambiae s.s. mosquitoes collected during the study period. The mosquitoes seem to be nuisance pests to the inhabitants of the area. Bed nets and indoor residual spraying would be a worthwhile strategy to reduce the nuisance caused by these mosquitoes as well as reduce the risk of other infections transmitted by them.
Apart from low number of samples (indoor resting densities) and the non occurrence of sporozoite, other malaria transmission risk parameters or indices portrays malaria occurrence. Indoor resting densities (IRD) of both species are strongly linked with the number of rooms sampled. The abundance of mosquitoes predicts the IRD and MBR. Thus, the high IRD and MBR recorded for An. gambiae s.s in December and Culex sp in January could be attributed to high density of mosquitoes obtained at those months.
A remarkable number of the mosquitoes were unfed. This high proportion of the unfed females of An. gambiae s.s. and the Culex sp (84% and 62% respectively) could be due the use of protective clothing during the night. It may also be influenced by some host-seeking factors; these mosquitoes may have been trapped indoors while searching for a blood meal after their emergence from the breeding sites. Also the huge difference between An. gambiae s.s. and Culex sp. in the number of mosquitoes that were unfed and gravid could be due to the high numbers of Culex sp. collected compared to the number of An. gambiae s.s.
There were more parous mosquitoes in the area compared to nulliparous ones. This indicates that the population is an older population and signifies that the high survival rate of the mosquitoes21. Likewise, the high number of nulliparous mosquitoes could be an indication of high productivity of the mosquito breeding sites which continuously supplies the area with young mosquitoes. The monthly variation in the parity rate of both An. gambiae s.s. and Culex mosquitoes is probably associated with the seasonal abundance of mosquito breeding sites. The parity rate is likely influenced by environmental variables such as rainfall and availability of breeding site15. It was observed that inhabitants of the area stay out doors for a long time before going to bed late at night, thus the sleeping habits of the inhabitants may have led to the low number of unfed mosquitoes.
5. CONCLUSION
The lack of good drainage and sewage system in the study area could be one of the factors contributing to the high number of mosquitoes recorded. Health education will go a long way in sensitizing the inhabitants on the importance of environmental and personal hygiene in mosquito control.
Finally, we summarize our findings as follows:
We collected a total of 1600 mosquitoes of which 31 (1.9%) were Anopheles gambiae s.s.. while 1756 (98%) were Culex sp. over the study periods.
The mosquito species were Anopheles gambiae s.s.. and Culex sp.
Majority of the mosquitoes were parous.
None of the mosquitoes examined was positive for Plasmodium falciparum and Wuchereria bancrofti.
Despite the low number of malaria vectors found in the community, malaria cases were high. Lymphatic filariasis transmission was apparently very low/ absent in the study area.
We conclude that the lack of good drainage and sewage system could be contributing to the abundance of mosquitoes in the study area.
ACKNOWLEDGEMENT
This work was supported by a Medical Education Partnership Initiative in Nigeria (R24TW008878) Mentored Research Award from Fogarty International Centre to Patricia N. Okorie. We are grateful to Mr Raifu Kolawole for technical assistance and to Dr. Sammy O. Sam-Wobo for providing the microscope used for dissection. We are grateful to Robert Wirtz for providing the Plasmodium sporozoite ELISA reagents. The cooperation and support of the inhabitants and staff of the primary health center in Beere is highly appreciated.
REFERENCES
- 1.WHO. Progress report 2000–2009 and strategic plan 2010–2020 of the global programme to eliminate lymphatic filariasis: halfway towards eliminating lymphatic filariasis. 2010 WHO/HTM/NTD/PCT/2010.6.
- 2.WHO. World Malaria Report. Geneva, Switzerland: 2013. [Google Scholar]
- 3.National Population Commission (NPC) Nigeria Demographic and Health Survey 2008. Abuja, Nigeria: National Population Commission and ICF Macro; 2009. [Nigeria] and ICF Macro; pp. 1–630. [Google Scholar]
- 4.PMI. President‘s Malaria Initiative Nigeria Malaria Operational Plan FY 2013. 2013:1–60. [Google Scholar]
- 5.WHO. Progress report 2000–2009 and strategic plan 2010–2020 of the global programme to eliminate lymphatic fi lariasis: halfway towards eliminating lymphatic filariasis. 2010 WHO/HTM/NTD/PCT/2010.6.
- 6.Hotez PJ, Asojo OA, Adesina AM. Nigeria: “Ground Zero” for the high prevalence neglected tropical diseases. PLoS Negl Trop Dis. 2012;6:e1600. doi: 10.1371/journal.pntd.0001600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burkot T, Garner P, Paru R, Dagoro H, Barnes A, McDougall S, et al. Effect of Untreated Bednets on the Transmission of Plasmodium falciparum, Plasmodium vivax, and Wuchereria Bancrofti in Papua New Guinea. Trans R Soc Trop Med Hyg. 1990;84:773–779. doi: 10.1016/0035-9203(90)90073-n. [DOI] [PubMed] [Google Scholar]
- 8.Okorie PN, McKenzie FE, Ademowo OG, Bockarie M, Kelly-Hope L. Nigeria Anopheles vector database: an overview of 100 years’ research. PLoS One. 2011;6:e28347. doi: 10.1371/journal.pone.0028347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Awolola T, Idowu E, Adeneye A, Mafe M, Oduola A, Ogunrinade A, et al. Entomological survey and infection rates of Plasmodium falciparum and Wuchereria bancrofti in mosquito populations in the Kainji Lake Area, Nigeria. Niger J Parasitol. 2006;27:58–61. [Google Scholar]
- 10.WHO. Lymphatic filariasis: a handbook of practical entomology for national lymphatic filariasis elimination programmes. 2013 WHO/HTM/NTD/PCT/2013.10.
- 11.Gillies M, Coetzee M. A supplement to the Anophellinae of Africa south of the sahara Publication of the South African. Institute for Medical Research. 1987:1–138. [Google Scholar]
- 12.Scott J, Brogdou W, Collins F. Identification of single specimens of the Anopheles gambiae. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 13.Wirtz R, Burkot T, Graves P, Andre R. Field evaluation of enzyme-linked immunosorbent assays for Plasmodium falciparum and Plasmodium vivax sporozoites in mosquitoes (Diptera: Culicidae) from Papua New Guinea. J Med Entomol. 1987;24:433–437. doi: 10.1093/jmedent/24.4.433. [DOI] [PubMed] [Google Scholar]
- 14.Uttah EC, Wokem GN, Okonofua C. The Abundance and Biting Patterns of Culex quinquefasciatus Say (Culicidae) in the Coastal Region of Nigeria. ISRN Zool. 2013;2013:1–7. [Google Scholar]
- 15.Adeleke MA, Mafiana CF, Idowu AB, Sam-Wobo SO, Idowu OA. Population dynamics of indoor sampled mosquitoes and their implication in disease transmission in Abeokuta, south-western Nigeria. J Vector Borne Dis. 2010;47:33–38. [PubMed] [Google Scholar]
- 16.Olayemi IK. Survivorship of Anopheles gambiae in relation to malaria transmission in Ilorin, Nigeria. Online J Heal Allied Sci. 2008;7:3–7. [Google Scholar]
- 17.Oduola AO, Olojede JB, Oyewole IO, Otubanjo OA, Awolola TS. Abundance and diversity of Anopheles species (Diptera: Culicidae) associated with malaria transmission in human dwellings in rural and urban communities in Oyo State, Southwestern Nigeria. Parasitol Res. 2013;112:3433–3439. doi: 10.1007/s00436-013-3522-0. [DOI] [PubMed] [Google Scholar]
- 18.Gillies M, De Meillon B. The Anophelinae of Africa, south of the sahara (Ethiopian zoogeographical region) Publication of the South African Institute for Medical Research. 1968:1–343. [Google Scholar]
- 19.Anosike JC, Nwoke BE, Ajayi EG, Onwuliri CO, Okoro OU, Oku EE, et al. Lymphatic filariasis aming the Ezza people of Ebonyi State, Eastern Nigeria. Ann Agric Env Med. 2005;12:181–186. [PubMed] [Google Scholar]
- 20.Udonsi J. Bancroftian filariasis in the Igwun Basin, Nigeria. Acta Trop. 1988;45:171–179. [PubMed] [Google Scholar]
- 21.WHO. Malaria entomology and vector control Trial Edition. 2003 WHO/CDS/CPE/SMT/2002.18 Rev.1 Part I.