Abstract
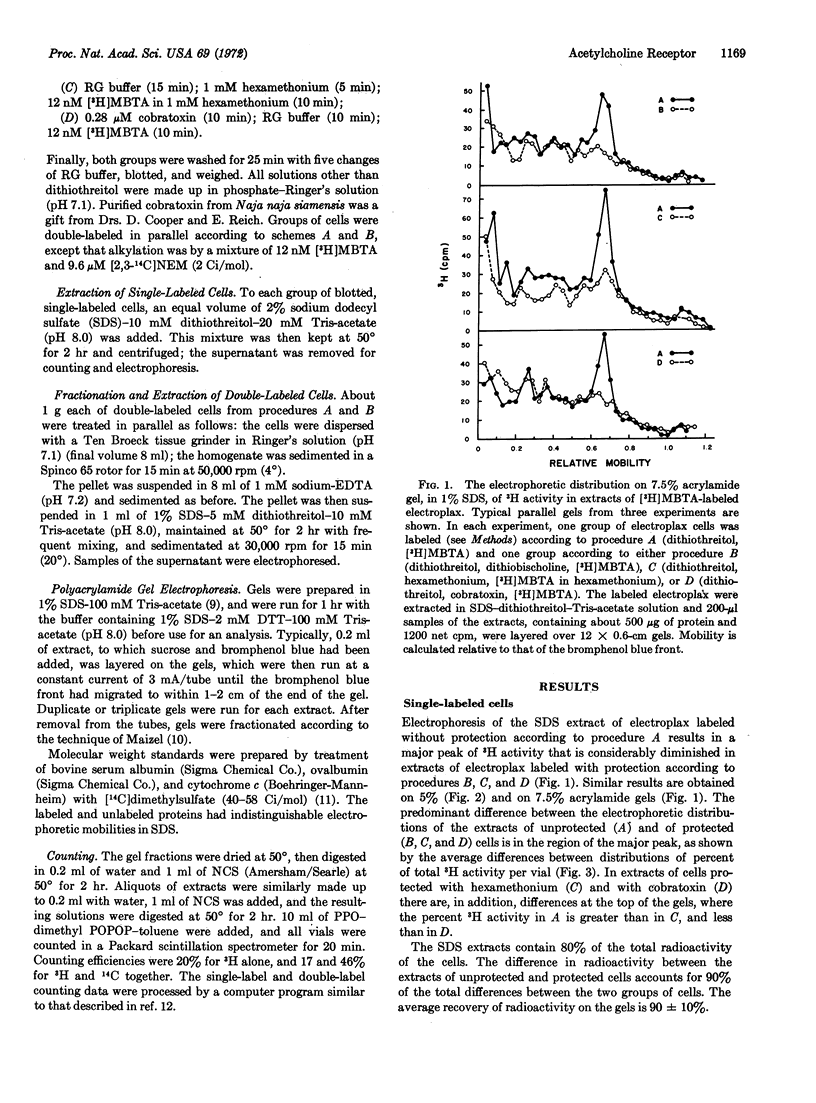
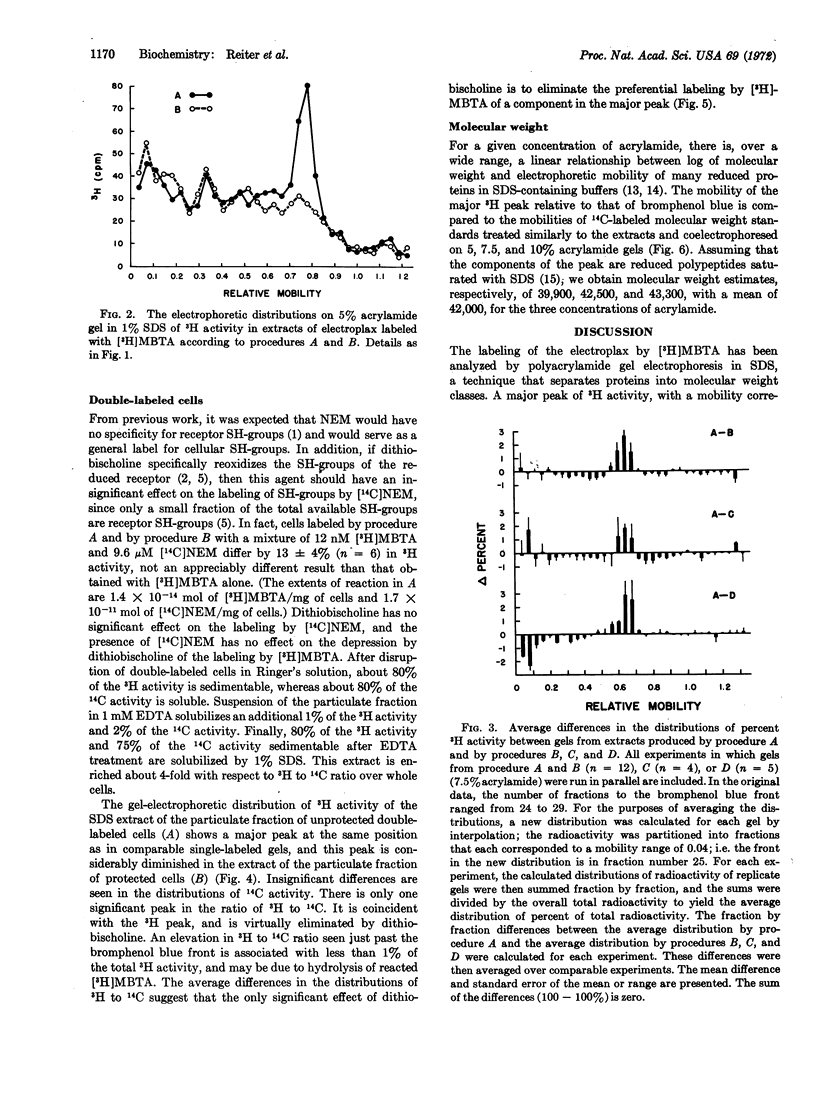
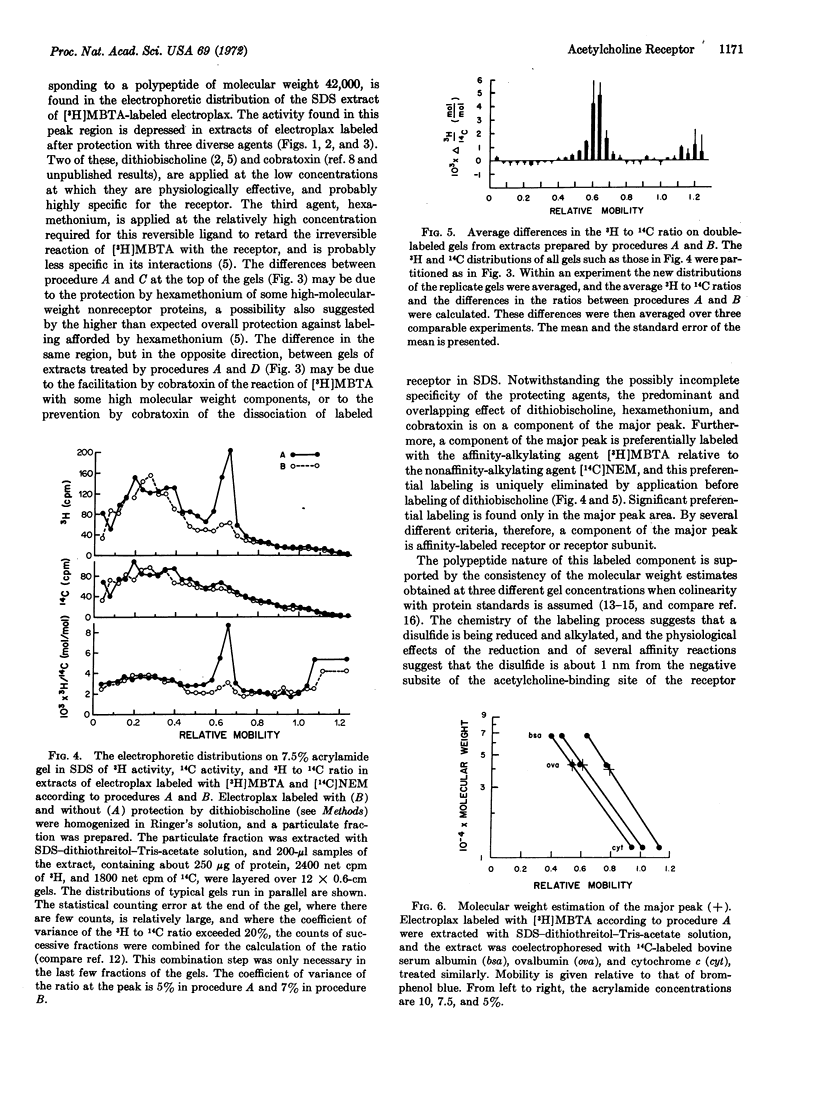
Electroplax, single cells dissected from electric tissue of Electrophorus, are labeled in a two-step procedure: reduction by dithiothreitol followed by alkylation by the affinity label 4-(N-maleimido)-α-benzyltri-[methyl-3H]methylammonium iodide, either alone or in combination with [2,3-14C]N-ethylmaleimide. Electrophoresis in sodium dodecyl sulfate on polyacrylamide gel of an extract, prepared with this detergent, of single-labeled or of double-labeled cells results in a major peak of 3H activity, with a mobility corresponding to a polypeptide of molecular weight 42,000. In addition, in the double-labeled samples, there is a unique peak in the ratio of 3H to 14C that is coincident with the 3H peak. The electrophoretic patterns of extracts of cells in which affinity alkylation of the reduced receptor has been suppressed by dithiobischoline, an affinity oxidizing agent, by cobratoxin, an irreversible ligand, or by hexamethonium, a reversible ligand, show a considerably diminished peak of 3H activity in the region of molecular weight 42,000. This is the predominant difference between the electrophoretic patterns of extracts of unprotected and of protected cells. Furthermore, extracts of cells protected with dithiobischoline before labeling with both tritiated affinity label and [14C]N-ethylmaleimide do not show the peak in the 3H to 14C ratio seen in the absence of protection. Thus, by several diverse criteria, the peak of 3H activity corresponding to a molecular weight of 42,000 contains affinity-labeled acetylcholine receptor or receptor subunit.
Keywords: electric eel, synapse, membrane proteins, molecular weight
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartels E., Deal W., Karlin A., Mautner H. G. Affinity oxidation of the reduced acetylcholine receptor. Biochim Biophys Acta. 1970 Jun 2;203(3):568–571. doi: 10.1016/0005-2736(70)90193-8. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Major human erythrocyte glycoprotein spans the cell membrane. Nat New Biol. 1971 Jun 23;231(25):229–232. doi: 10.1038/newbio231229a0. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Kasai M., Lee C. Y. Use of a snake venom toxin to characterize the cholinergic receptor protein. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1241–1247. doi: 10.1073/pnas.67.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrambach A., Rodbard D. Polyacrylamide gel electrophoresis. Science. 1971 Apr 30;172(3982):440–451. doi: 10.1126/science.172.3982.440. [DOI] [PubMed] [Google Scholar]
- Karlin A., Prives J., Deal W., Winnik M. Affinity labeling of the acetylcholine receptor in the electroplax. J Mol Biol. 1971 Oct 14;61(1):175–188. doi: 10.1016/0022-2836(71)90214-2. [DOI] [PubMed] [Google Scholar]
- Karlin A., Prives J., Deal W., Winnik M. Counting acetylcholine receptors in the electroplax. In: Molecular properties of drug receptors. Ciba Found Symp. 1970:247–261. doi: 10.1002/9780470719763.ch12. [DOI] [PubMed] [Google Scholar]
- Karlin A., Winnik M. Reduction and specific alkylation of the receptor for acetylcholine. Proc Natl Acad Sci U S A. 1968 Jun;60(2):668–674. doi: 10.1073/pnas.60.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson E., Eaker D. L., Porath J. Purification of a neurotoxin from the venom of Naja nigricollis. Biochim Biophys Acta. 1966 Oct 31;127(2):505–520. doi: 10.1016/0304-4165(66)90404-1. [DOI] [PubMed] [Google Scholar]
- Kiehn E. D., Holland J. J. Membrane and nonmembrane proteins of mammalian cells. Synthesis, turnover, and size distribution. Biochemistry. 1970 Apr 14;9(8):1716–1728. doi: 10.1021/bi00810a010. [DOI] [PubMed] [Google Scholar]
- Lee C. Y. Elapid neurotoxins and their mode of action. Clin Toxicol. 1970 Sep;3(3):457–472. doi: 10.3109/15563657008990119. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molinoff P., Potter L. T. Isolation of the cholinergic receptor protein of Torpedo electric tissue. Nature. 1971 Feb 19;229(5286):554–557. doi: 10.1038/229554a0. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. The gross conformation of protein-sodium dodecyl sulfate complexes. J Biol Chem. 1970 Oct 10;245(19):5161–5165. [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yund M. A., Yund E. W., Kafatos F. C. A computer method for analysis of radioactivity data from single and double labeled experiments. Biochem Biophys Res Commun. 1971 May 21;43(4):717–722. doi: 10.1016/0006-291x(71)90674-7. [DOI] [PubMed] [Google Scholar]


