Abstract
IMPORTANCE
Intellectual lifestyle enrichment throughout life is increasingly viewed as a protective strategy against commonly observed cognitive decline in the elderly.
OBJECTIVE
To investigate the association of lifetime intellectual enrichment with baseline cognitive performance and rate of cognitive decline in a non-demented elderly population and to estimate difference (in years) associated with lifetime intellectual enrichment to the onset of cognitive impairment.
DESIGN, SETTING, PARTICIPANTS
Prospective analysis of subjects enrolled in the Mayo Clinic Study of Aging (MCSA), a longitudinal population-based study of cognitive aging in Olmsted County, Minnesota. We studied 1995 non-demented (1718 cognitively normal, 277 MCI) participants in MCSA who completed intellectual lifestyle measures at baseline and underwent at least one follow-up visit.
MAIN OUTCOMES AND MEASURES
We studied the effect of lifetime intellectual enrichment by separating the variables into two non-overlapping principal components: education/occupation-score and mid/late-life cognitive activity measure based on self-report questionnaires. A global cognitive Z-score served as our summary cognition measure. We used linear mixed-effects models to investigate the associations of demographic and intellectual enrichment measures with global cognitive Z-score trajectories.
RESULTS
Baseline cognitive performance was lower in older subjects and in those with lower education/occupation, lower mid/late-life cognitive activity, apolipoprotein E4 (APOE) genotype, and in men. The interaction between the two intellectual enrichment measures was significant such that the beneficial effect of mid/late-life cognitive activity on baseline cognitive performance was reduced with increasing education/occupation. Only baseline age, mid/late-life cognitive activity, and APOE4 genotype were significantly associated with longitudinal change in cognitive performance from baseline. For APOE4 carriers with high lifetime intellectual enrichment (75th percentile of both education/occupation and mid/late-life cognitive activity), the onset of cognitive impairment was about 8.7 years later compared with low lifetime intellectual enrichment (25th percentile of both education/occupation and mid/late-life cognitive activity) in an 80 year old subject.
CONCLUSIONS AND RELEVANCE
Higher levels of education/occupation were associated with higher levels of cognition. Higher levels of mid/late-life leisure activity were also associated with higher levels of cognition, but the slope of this relationship slightly increased over time. Lifetime intellectual enrichment might delay the onset of cognitive impairment and be used as a successful preventive intervention to reduce the impending dementia epidemic.
INTRODUCTION
The elderly population in the United States is expected to more than double from 35 million in 2000 to 72 million in 2030.1 Commonly observed cognitive decline in the elderly due to the pathological aging of the brain will have a significant impact on public health. Intellectual lifestyle enrichment throughout life is increasingly viewed as a protective strategy against cognitive decline in the elderly. Numerous studies demonstrate that all components of intellectual enrichment – higher lifetime non-leisure learning components such as education and primary occupation2–5,6 as well as cognitively stimulating activities7–10 are protective against cognitive decline and AD dementia. Intellectual enrichment may succeed as a preventive intervention if we: 1) understand the relative influence of each of these intellectual enrichment factors on baseline cognitive performance and rate of decline and 2) estimate the years of protection provided against cognitive impairment by each of the factors.
Lifetime intellectual enrichment can be grouped into two major components – the first comes from early and mid-life non-cognitive activities - education and the major occupation/job; and the second component comes from mid/late-life cognitive activities. In this manuscript, we separated these two components and closely examined the effect of each on the baseline cognition and the subsequent rate of cognitive decline in a population based sample of non-demented elderly individuals. Then, we estimated numbers of years of protection provided by each component for subsequent onset of cognitive impairment.
METHODS
Selection of Participants
Study subjects were participants in the Mayo Clinic Study of Aging (MCSA), an epidemiological study of the prevalence, incidence, and risk factors for Mild Cognitive Impairment (MCI) and dementia among Olmsted County residents ages 70–89. The subjects consisted of the original sampled cohort from October 1, 2004 and replenishment sampling cohorts that occurred in years 2008 and 2009. We included all 1995 baseline non-demented (1718 cognitively normal, 277 MCI) subjects with APOE genotype, intellectual enrichment variables (see below), complete neuropsychological assessments, and at least one additional clinical follow-up with complete neuropsychological assessments. The MCSA uses the Rochester Epidemiology project records – linkage system infrastructure11,12 and complete details of the MCSA design have been published elsewhere.13–15
Standard protocol approvals, registrations, and patient consents
These studies were approved by the Mayo institutional review board and informed consent was obtained from all participants or their surrogates.
Intellectual Enrichment Variables
The primary intellectual activity variables of interest, assessed at baseline, included: 1) education, job-level score based on the primary occupation throughout life; and 2) current weekly cognitive activity over the last 12 months and mid-life weekly cognitive activity (ages 50–65).16 These intellectual enrichment data were recorded for all subjects at the enrollment visit into the MCSA. Education is self-reported and is based on the number of years of school completion. The job level score is based on the subjects’ primary occupation during most of their adult life. All of the occupations were then assigned to one of six groupings based on similar attributes and complexity of their jobs. Details about the cognitive questionnaires used for recording are attached as Appendix to this manuscript. The same form was used to record their cognitive activities over the past 12 months (late-life) and cognitive activities between the ages 50–65 (mid-life). Each component score is weighted based on the amount of activity participation. The first 10 components are added together to determine the cognitive activity scores. Television is the eleventh component which is captured, but is not included in the final cognitive activity score.17
Education/Occupation-score and Mid/Late-life Cognitive activity
Using principal components applied to these four measures (i.e. education, job-level score, current cognitive activity, and mid-life cognitive activity), we separated the uncorrelated components of early life non-leisure activity and mid/late-life cognitive activity. The first two principal components explained 84 % of the variance, and after a varimax rotation the data consolidated into two distinct composite measures of intellectual enrichment: 1) Education/Occupation-score (i.e. lifelong non-leisure intellectual learning) assessed from years of education and job-score (weighted contribution for education was 0.688 and for job-score was 0.725); and 2) mid/late-life cognitive activity from a self-report of cognitive activities in the previous 12 months and at mid-life (50–60 years) (weighted contribution for mid-life was 0.708 and for previous 12 months cognitive activities was 0.7).
Global Cognition Measure
The neuropsychological battery was constructed as previously described.13–15 Four cognitive domains were assessed from nine tests: Executive (TMT: Part B, WAIS-R Digit Symbol); Language (BNT, category fluency); Memory (WMS-R Logical Memory-II (delayed recall), WMS-R Visual Reproduction-II (delayed recall), AVLT delayed recall); and Visuospatial performance (WAIS-R Picture Completion, WAIS-R Block Design). Individual test scores were first converted to z-scores using the mean and standard deviation from the MCSA 2004 enrollment cohort that consisted of non-demented subjects (n=1969). A global cognitive summary score was estimated from the z-transformation of the average of the four domain z-scores and was used to assess cognitive impairment in our subjects. The baseline global z-score, and rate of decline, were the primary outcomes of interest. Out of the 1995 subjects in the study, 1675 were test naive at the time of enrollment into this study and 320 subjects had previously completed the battery as part of an earlier study. We controlled for the number of times the subject had the battery prior to enrollment into the MCSA using a variable named “baseline visit number” because practice effects influence on the measured outcome variable – global cognition over time 18,19. A total of 1675 patients had a baseline visit number of 1 i.e. the first time they took the test was at baseline of the study, 34 patients had a baseline visit number of 2 i.e. tested once before baseline, 39 patients had a baseline visit number of 3 i.e. tested twice before baseline, and 247 patients had a baseline visit number of 4 or more.
Statistical Analysis
We examined the intellectual enrichment measures and demographic variables as predictors of longitudinal global cognitive z-scores using linear mixed models fit by maximum likelihood. In these models, the coefficients that are not associated with time from baseline or any interactions including time from baseline estimate shifts in global z-scores which are consistent over time from baseline. The coefficients associated with time from baseline and any interactions including time from baseline estimate deviations in the rates of global z-score change. A significant interaction indicates that shifts in global z-scores vary with time rather than remaining constant. The initial model included baseline age (years), sex, APOE, time from baseline (years), the intellectual enrichment variables, baseline visit number, all 2-way interactions of these variables, and all 3-way interactions containing time. The models were fit with random subject-specific intercepts and slopes. We tested for the statistical significance of these random terms using likelihood ratio (LR) tests. We also used LR tests to compare independence (where the within-subject errors are independent) and continuous first-order autoregression. Both random terms were significant (p<0.0001). The final models incorporated continuous first-order autoregressive AR(1) correlation structures (estimated correlation for values 1 year apart=0.41, p<0.0001).
There were no significant 3-way interactions, so we removed them from further consideration. We then used a backwards elimination procedure, respecting the need to retain nested terms, to remove predictors and to form the most parsimonious model. The final model contained baseline age (years), sex, APOE4 carrier status, time from baseline (years), baseline visit number, education/occupation-score and mid/late-life cognitive activity. The model also included six two-way interactions: baseline age with time from baseline, mid/late life cognitive score with time from baseline, APOE4 with time from baseline, baseline visit number with time from baseline, baseline visit number with education/occupation-score, and an interaction of the two intellectual enrichment variables. Both random intercepts (p<0.0001) and random slopes (p<0.0001) were deemed necessary.
RESULTS
The demographics, clinical, and intellectual enrichment variables of the non-demented subjects included in this analysis are shown in Table 1. The results of the linear mixed effects models are presented in Table 2. Baseline global z-scores were lower in men, older subjects, APOE4 carriers, those with lower education/occupation and lower mid/late-life cognitive activity. Subjects who had previous exposure to the neuropsychological battery before performed better [practice effects discussed earlier] and this effect diminished as time progressed.
Table 1.
Patient Characteristics*
| All subjects n=1995 |
CN n=1718 |
MCI n=277 |
|
|---|---|---|---|
| No. of Women (%) | 960 (48.1) | 846 (49.2) | 114 (41.2) |
| No. of ε4 carriers (%) | 539 (27.0) | 438 (25.5) | 101 (36.5) |
| Age at visit (years) | 78.9 (74.3, 82.8) | 78.4 (74.1, 82.5) | 81.5 (76.7, 84.2) |
| Education (years) | 13 (12, 16) | 13 (12, 16) | 12 (12, 15) |
| Job level score | 4 (3, 6) | 4 (3, 6) | 3 (3, 5) |
| Education/Occupation-score | −0.27 (−0.76, 0.73) | −0.25 (−0.76, 0.97) | −0.51 (−1.02, 0.24) |
| Cognitive mid-life | 20 (14, 28) | 20.5 (14, 28.5) | 18.5 (13, 25.5) |
| Cognitive late-life | 21.5 (15.5, 28.5) | 22.5 (16, 29.0) | 18 (14, 25) |
| Mid/late life cognitive activity | −0.10 (−0.76, 0.67) | −0.04 (−0.73, 0.72) | −0.42 (−0.97, 0.39) |
| Short Test of mental status | 34 (32, 36) | 35 (33, 36) | 30 (28, 32) |
| z-global | 0.24 (−0.44, 0.85) | 0.38 (−0.15, 0.95) | −1.07 (−1.73, −0.53) |
| z-memory | 0.17 (−0.56, 0.85) | 0.35 (−0.28, 0.96) | −1.24 (−1.66, −0.66) |
| z-language | 0.22 (−0.44, 0.78) | 0.34 (−0.25, 0.87) | −0.76 (−1.52, −0.11) |
| z-attention | 0.29 (−0.35, 0.82) | 0.40 (−0.19, 0.90) | −0.77 (−1.68, 0.01) |
| z-visualspatial | 0.21 (−0.51, 0.77) | 0.31 (−0.34, 0.85) | −0.65 (−1.28, 0.05) |
| Non-amnestic MCI | 66 (3.3) | ---- | 66 (23.8) |
| Baseline visit number | |||
| 1 | 1932 (96.8) | 1672 (97.3) | 260 (93.9) |
| 2 | 35 (1.8) | 24 (1.4) | 11 (4.0) |
| 3 | 20 (1.0) | 14 (0.8) | 6 (2.2) |
| 4 | 8 (0.4) | 8 (0.5) | 0 |
| Follow-up time (years) | 3.6 (1.6, 5.2) | 3.9 (1.9, 5.2) | 2.6 (1.4, 4.0) |
CN= Cognitively Normal; MCI=Mild Cognitive Impairment
All measures are expressed as median (25th percentile, 75th percentile) or number (percent).
Table 2.
Lifetime intellectual enrichment, baseline cognition and cognitive decline.
| Coefficient | Std.Error | p-value | |
|---|---|---|---|
| (Intercept) | 6.05 | 0.29 | <0.0001 |
| Baseline age (years) | −0.07 | 0.004 | <0.0001 |
| Men | −0.18 | 0.04 | <0.0001 |
| Time (years) | 0.70 | 0.06 | <0.0001 |
| Education/occupation-score | 0.33 | 0.03 | <0.0001 |
| Mid/late-life cognitive activity | 0.17 | 0.02 | <0.0001 |
| APOE4 carrier | −0.20 | 0.04 | <0.0001 |
| Baseline visit number | 0.05 | 0.01 | <0.0001 |
| Baseline age x time | −0.01 | 0.001 | <0.0001 |
| Mid/late-life cognitive activity x time | 0.01 | 0.003 | 0.0445 |
| APOE4 x time | −0.04 | 0.01 | <0.0001 |
| Baseline visit number x time | −0.01 | 0.002 | 0.0157 |
| Baseline visit number x Education/occupation | −0.02 | 0.01 | 0.0411 |
| Education/occupation x Mid/late-life cognitive activity | −0.04 | 0.02 | 0.0296 |
Time here refers to time from baseline. The terms without interaction with time indicate the variables that were significantly associated with baseline global cognition and the terms with interaction with time refer to variables that were significantly associated with annual change in cognition over time.
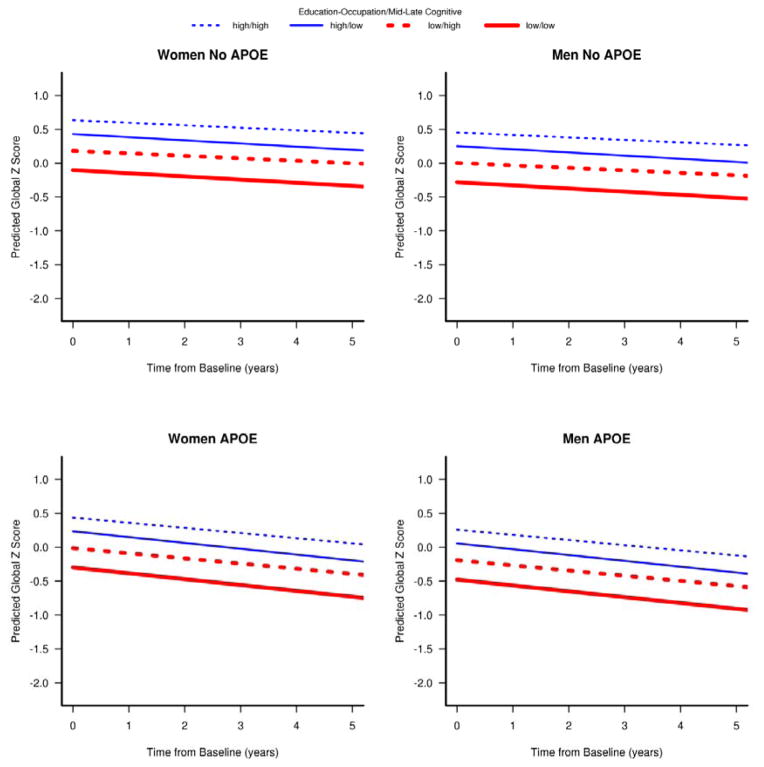
Both better education/occupation and mid/late-life cognitive activity were associated with better cognitive performance. Mid/late life cognitive activity also had a significant interaction with time from baseline (p=0.045) where the slope of this relationship increased over time. Qualitatively, the change in slope of this relationship was small relative to the magnitudes of the shifts in cognition associated with intellectual activity. Additionally, there was a significant interaction between the two intellectual enrichment variables (p = 0.03). Within the observed follow-up period, higher mid/late-life cognitive activity was associated with higher baseline global z-scores but the association was slightly attenuated as the education/occupation increased (see Figure 1). We separated the plots by sex and APOE4 status because the baseline cognitive performance differed between these groups. Low to high mid/late-life cognitive activity was related to better cognitive performance if education/occupation was low thus shifting the low education/occupation curve higher. This shift in the cognitive performance was smaller if education/occupation was high.
Figure 1.
Predicted cognitive global z-scores as a function of time from baseline for different levels of intellectual enrichment measures. The graphs illustrate the interaction between education/occupation-score and mid/late-life cognitive activity. In the figure, low and high intellectual enrichment were defined by 25th and 75th percentiles. The red lines indicate low and blue indicate high education/occupation-scores. The solid lines indicate low mid/late-life leisure activity and dashed lines indicate high mid/late-life cognitive activity.
To estimate the number of additional years remaining cognitively normal associated with high intellectual activity, we used the fitted model to predict when these curves cross a threshold of −0.74 which is the 10th percentile of z-scores in cognitive normal individuals. We chose this cut point because it was used in our previous work for the operationalization of NIA-AA preclinical criteria for AD to indicate cognitive impairment in cognitively normal individuals.20 This cut point is also close to the average global z-scores of −0.8 seen in incident MCIs in the MCSA. Since the predicted times to reach the threshold sometimes exceeded the follow-up in our study, requiring extrapolation, we limited our example of prediction to 80 year old APOE4 carrier subjects. We had 142 subjects with follow-up extending more than 6 years, and 602 subjects with follow-up extending more than 5 years. Predicted times within a few years of these values are likely fairly accurate, whereas times farther away could be subject to increasing inaccuracies from non-linearity and other unmeasurable factors. Predicted times for 80 year old APOE4 non-carrier subjects exceeded 10 years and are therefore not shown.
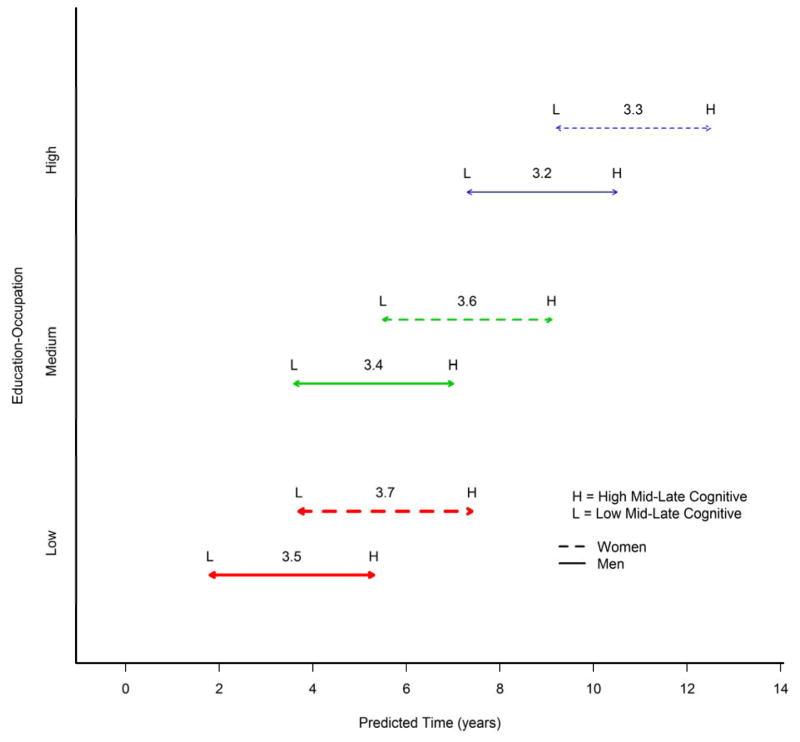
Figure 2 illustrates the differences in the predicted times for an 80 year old APOE4 carrier subject who has never received the battery before baseline to reach a cognitive threshold associated with subtle cognitive impairment depending on sex, education/occupation, and mid/late-life cognitive activity scores. We stratified low, medium and high intellectual enrichment scores by 25th, 50th, and 75th percentiles. A sample interpretation of the data is as follows - in subjects with medium education/occupation, engaging in high mid/late-life activity will have an associated later onset of cognitive impairment of 3.4 years for men and 3.6 years for women APOE4 carriers. Overall going from low lifetime intellectual enrichment (low education/occupation and low mid/late-life cognitive activity) to high lifetime intellectual enrichment (high education/occupation and high mid/late-life cognitive activity) could delay the onset of cognitive impairment by approximately 8.7 years for men and 8.8 years for women APOE4 carriers.
Figure 2.
Differences in the predicted times for an 80 year old APOE4 carrier subject who never received the battery before baseline to reach a cognitive threshold associated with subtle cognitive impairment depending on their sex, , education/occupation and mid/late-life cognitive activity scores. In the figure, low and high intellectual enrichment were defined by 25th and 75th percentiles.
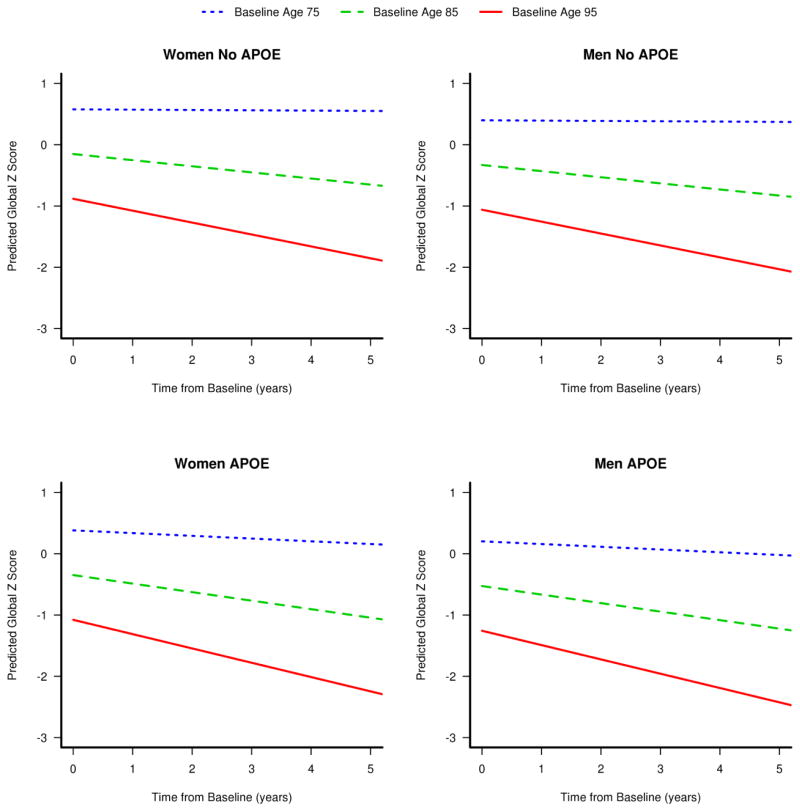
Among all the intellectual enrichment and demographic variables tested, only older age at baseline visit, baseline visit number, mid/late life cognitive activity, and APOE4 genotype had significant interactions with time from baseline. There was also an interaction between baseline visit number and education/occupation indicating that the learning effect that was provided as subjects had more exposures to the test battery was attenuated in subjects with higher education/occupation. The cognitive z-score trajectories for different baseline ages separated by sex and APOE4 status are illustrated in figure 3. Older subjects had lower global z-scores and declined more rapidly after baseline. The steeper decline in cognitive z-scores at older ages would impact the number of years to a cognitive threshold associated with intellectual activity. For example, the constant vertical shift over time in z-scores associated with education/occupation would have a larger horizontal shift (time to threshold) for shallow declines (young ages) than for steep declines (old ages).
Figure 3.
Predicted cognitive global z-score trajectories for different baseline ages separated by APOE genotype and sex. The average trajectories are shown for subjects with ages 75, 85, and 95 years.
COMMENT
The major conclusions of the study are 1) the protective effect of intellectual enrichment is primarily manifested as relatively consistent higher cognitive performance over time. Mid/late life cognitive activity did have an increasing effect over time, but qualitatively the magnitude of this effect relative to the overall shift in cognitive performance was minor; 2) high lifetime intellectual enrichment (75th percentile) may delay the onset of cognitive impairment by about 8.8 years in men and 8.7 years in women APOE4 carriers compared to a low lifetime intellectual enrichment (25th percentile); 3) the protective effect of mid/late-life cognitive activity on baseline cognitive performance decreases with increasing education/occupation.
Higher levels of education, occupation, and cognitive activity are independently associated with a lower risk of dementia consistent with earlier studies.2–10 The contribution of education/occupation (model coefficient=0.33) was larger than the contribution of mid/late-life cognitive activity (model coefficient=0.17). This result is logical and consistent with our previous finding17 because intellectual development due to education and occupation exerts an impact over the entire adult lifespan, whereas mid/late-life cognitive activities refer to a more limited portion of an individual’s life.
The negative interaction between mid/late-life cognitive activity and education/occupation was intriguing. We found that an individual with low education/occupation benefitted more by engaging in high mid/late-life cognitive activity than an individual with high education/occupation. These findings suggest that the effect of late-life cognitive training programs to delay the onset of AD may be attenuated in those with high education/occupation, and this implies that one may need to account for this interaction while designing preventive trials based on cognitive training. However, it is important to note that a significant protection can be gained from engagement in high mid/late-life cognitive activity irrespective of the subject’s life-long non-leisure activity through education and occupation.
Education/occupation was not associated with the rate of cognitive decline but mid/late-life cognitive activity was slightly associated with the rate of cognitive decline. However the effect of mid/late-life cognitive activity on the rate of cognitive decline (model coefficient=0.01; p=0.0445) was minimal in comparison to its effect on baseline cognitive performance (model coefficient=0.17; p<0.001). The lack of association between education/occupation and rate of cognitive decline is consistent with an earlier longitudinal study that followed over 9000 people semiannually for 15 years.21 The association between mid/late-life cognitive activity and rate of cognitive decline in non-demented elderly is consistent with earlier studies.22,23 However, the weaker association of mid/late-life cognitive activity with rate of decline compared to its larger impact on baseline cognitive performance is important to consider.24 These results support that the protection provided by lifetime intellectual enrichment is largely driven by its effect on baseline cognitive performance and marginally due to its effect on the rate of cognitive decline.
Among the demographic variables [outside of intellectual enrichment variables], being older, being a man, and having APOE4 were predictors of lower baseline global z-scores; being older and having APOE4 were significantly associated with future cognitive decline. The fact that men had lower cognitive performance at baseline is consistent with the literature that men are at higher risk of MCI, particularly at younger ages, due to elevated cardiovascular risk factors.15 Age25 and APOE426,27 are the strongest risk factors for sporadic Alzheimer’s disease. Because the proportion of non-demented individuals progressing to dementia increases with age and in those with APOE4 genotype and because the rate of cognitive decline increases as a person moves closer to dementia diagnosis, it is logical that higher baseline age and APOE genotype may be associated with faster cognitive decline28 as well as worse performance in a non-demented population. It is important to note that the multivariate model enabled us to isolate the significant associations after accounting for all other demographic and intellectual enrichment variables which strengthen our findings of the association of APOE4 and age with rate of cognitive decline. While practice effects were not a focus of this manuscript, finding that greater past exposure to the test resulted in better performance and a reduced effect of education are consistent with the literature.18,19
A recent report by Alzheimer’s Association projected that a treatment breakthrough that can delay the onset of Alzheimer’s disease (AD) by five years means reducing the expected number of AD patients by 2050 by about 43% in the US alone.29 The estimation of the years of protection against cognitive impairment provided by both education/occupation and mid/late-life cognitive activity in a population based sample in this paper (Figure 2) provide guidelines that can be used to understand the public health impact of using intellectual enrichment as a preventive intervention. Education/Occupation – We found that the number of years of protection provided by higher education (keeping cognitive activity constant) is at least five years irrespective of sex and APOE4. The decline in the risk of dementia with increasing education levels in the past century30 support these findings and highlight the importance of intervening early for larger public health impact. Specifically, future reduction in the epidemic of dementia will come from public investments to increase access to education and better jobs for the disadvantaged children and young adults in our populations. Mid/Late-life cognitive activity - Although the effect of education/occupation was larger than mid/late-life cognitive activity, the years of protection provided by high mid/late-life cognitive activity versus low mid/late-life cognitive activity was at least 3.2 years for APOE4 carriers (and 7.3 years for non-carriers – data not shown here). While the optimal intervention time may be intellectual enrichment in early life, there are substantial benefits of employing a public health campaign by providing intellectual enrichment to mid/late-life individuals. In our work, high mid/late-life engagement in cognitively stimulating activities (75th percentile) corresponded to engaging in several cognitively stimulating activities at least 3 times a week during mid/late-life. Examples of these activities include reading books and magazines, playing games and music, artistic activities, crafts, group activities, social activities, and computer activities.
The study has some limitations. First, the results do not preclude the possibility that active lifestyle intervention might prospectively alter the rate of cognitive decline in an active interventional study. However we did not find evidence for this in our observational population-based sample in which subjects self-reported information about their mid/late-life cognitive activities. Second, when estimating delay in disease onset due to higher levels of enrichment we assumed that cognitive decline is linear over time which, while probably true for short intervals (i.e. several years), is likely not true for longer periods of observation. However by limiting estimation of time to only APOE4 carriers we are not extrapolating much more than the follow-up time. Increasing pathological burden with age may cause an acceleration of the decline. Third, the study results are pertinent to non-demented subjects in the population and may be different in demented individuals. Fourth, we did not have measurements for early life cognitive activities and assumed that education and occupation are the major components of the intellectual enrichment in early life.
The study also has major strengths. First, the population based nature of the sample makes the results of the study generalizable and enhances their external validity. Second, the use of principal components aided us in separating two major intellectual enrichments in life – education/occupation and mid/late-life cognitive activities into two uncorrelated variables. Third, the multivariate modeling enabled us to isolate the independent significant associations of the multiple components.
Acknowledgments
Funding and Role of Sponsors:
This work was supported by NIH grants K99/R00 AG37573, R01 AG11378, R01-AG041851, P50 AG16574, U01 AG06786; the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation, U.S.A and Opus building NIH grant C06 RR018898. The funding sources were not involved in the manuscript review or approval.
Footnotes
Disclosure: The authors report no conflicts of interest
Author Contributions:
Dr. Vemuri had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study Design: Vemuri
Statistical Analysis: Lesnick, Przybelski
Interpretation of the data: Vemuri, Lesnick, Przybelski, Jack
Obtained Funding: Vemuri, Petersen, Jack
Data Collection: Machulda, Knopman, Petersen, Jack
Drafting of the manuscript: Vemuri
Critical revision of the manuscript: All authors
Disclosures:
Dr. Vemuri receives research funding from NIH/NIA and Alzheimer’s Association.
Mr. Lesnick reports no disclosures.
Mr. Przybelski reports no disclosures.
Dr. Machulda reports no disclosures.
Dr. Knopman serves as Deputy Editor for Neurology®; serves on a Data Safety Monitoring Board for Lilly Pharmaceuticals; is an investigator in clinical trials sponsored by Janssen Pharmaceuticals, and receives research support from the NIH.
Dr. Mielke has served as a consultant for Eli Lilly and receives funding from NIH/NIA.
Dr. Roberts receives research funding from the NIH
Dr. Geda reports no disclosures.
Dr. Rocca receives research funding from the NIH
Dr. Petersen reports receiving consulting fees from Roche Inc., Merck and Genentech, receiving royalties from Oxford University Press; and serving as chair of data monitoring committees for Pfizer, Inc. and Janssen Alzheimer Immunotherapy; and receives research support from the NIH/NIA.
Dr. Jack serves as a consultant for Siemens and receives research funding from the National Institutes of Health (R01-AG011378, RO1-AG041851, RO1-AG037551, U01-HL096917, U01-AG032438, U01-AG024904), and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation.
References
- 1.Aging Ao. [Accessed 06/14/2013];Projected Future Growth of the Older Population. 2010 http://www.aoa.gov/Aging_Statistics/future_growth/future_growth.aspx.
- 2.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. Jama. 1994 Apr 6;271(13):1004–1010. [PubMed] [Google Scholar]
- 3.Cobb JL, Wolf PA, Au R, White R, D’Agostino RB. The effect of education on the incidence of dementia and Alzheimer’s disease in the Framingham Study. Neurology. 1995 Sep;45(9):1707–1712. doi: 10.1212/wnl.45.9.1707. [DOI] [PubMed] [Google Scholar]
- 4.Ott A, Breteler MM, van Harskamp F, et al. Prevalence of Alzheimer’s disease and vascular dementia: association with education. The Rotterdam study. BMJ. 1995 Apr 15;310(6985):970–973. doi: 10.1136/bmj.310.6985.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu C, Karp A, von Strauss E, Winblad B, Fratiglioni L, Bellander T. Lifetime principal occupation and risk of Alzheimer’s disease in the Kungsholmen project. Am J Ind Med. 2003 Feb;43(2):204–211. doi: 10.1002/ajim.10159. [DOI] [PubMed] [Google Scholar]
- 6.Karp A, Kareholt I, Qiu C, Bellander T, Winblad B, Fratiglioni L. Relation of education and occupation-based socioeconomic status to incident Alzheimer’s disease. Am J Epidemiol. 2004 Jan 15;159(2):175–183. doi: 10.1093/aje/kwh018. [DOI] [PubMed] [Google Scholar]
- 7.Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001 Dec 26;57(12):2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabrigoule C. Do leisure activities protect against Alzheimer’s disease? Lancet neurology. 2002 May;1(1):11. doi: 10.1016/s1474-4422(02)00010-8. [DOI] [PubMed] [Google Scholar]
- 9.Crowe M, Andel R, Pedersen NL, Johansson B, Gatz M. Does participation in leisure activities lead to reduced risk of Alzheimer’s disease? A prospective study of Swedish twins. J Gerontol B Psychol Sci Soc Sci. 2003 Sep;58(5):249–255. doi: 10.1093/geronb/58.5.p249. [DOI] [PubMed] [Google Scholar]
- 10.Sattler C, Toro P, Schonknecht P, Schroder J. Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and Alzheimer’s disease. Psychiatry Res. 2012 Mar 30;196(1):90–95. doi: 10.1016/j.psychres.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012 Dec;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011 May 1;173(9):1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010 Sep 7;75(10):889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts RO, Geda YE, Knopman DS, et al. The incidence of MCI differs by subtype and is higher in men: The Mayo Clinic Study of Aging. Neurology. 2012 Jan 31;78(5):342–351. doi: 10.1212/WNL.0b013e3182452862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geda YE, Topazian HM, Roberts LA, et al. Engaging in cognitive activities, aging, and mild cognitive impairment: a population-based study. J Neuropsychiatry Clin Neurosci. 2011 Spring;23(2):149–154. doi: 10.1176/appi.neuropsych.23.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vemuri P, Lesnick TG, Przybelski SA, et al. Effect of lifestyle activities on alzheimer disease biomarkers and cognition. Ann Neurol. 2012 Nov;72(5):730–738. doi: 10.1002/ana.23665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duff K, Beglinger LJ, Moser DJ, Paulsen JS, Schultz SK, Arndt S. Predicting cognitive change in older adults: the relative contribution of practice effects. Arch Clin Neuropsychol. 2010 Mar;25(2):81–88. doi: 10.1093/arclin/acp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dodge HH, Wang CN, Chang CC, Ganguli M. Terminal decline and practice effects in older adults without dementia: the MoVIES project. Neurology. 2011 Aug 23;77(8):722–730. doi: 10.1212/WNL.0b013e31822b0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jack CR, Jr, Knopman DS, Weigand SD, et al. An operational approach to NIA-AA crtiteria for preclinical Alzheimer’s disease. Ann Neurol. 2012 Apr; doi: 10.1002/ana.22628. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider AL, Sharrett AR, Patel MD, et al. Education and cognitive change over 15 years: the atherosclerosis risk in communities study. J Am Geriatr Soc. 2012 Oct;60(10):1847–1853. doi: 10.1111/j.1532-5415.2012.04164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology. 2003 Sep 23;61(6):812–816. doi: 10.1212/01.wnl.0000083989.44027.05. [DOI] [PubMed] [Google Scholar]
- 23.James BD, Wilson RS, Barnes LL, Bennett DA. Late-life social activity and cognitive decline in old age. J Int Neuropsychol Soc. 2011 Nov;17(6):998–1005. doi: 10.1017/S1355617711000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell MB, Cimino CR, Benitez A, et al. Cognitively Stimulating Activities: Effects on Cognition across Four Studies with up to 21 Years of Longitudinal Data. J Aging Res. 2012;2012:461592. doi: 10.1155/2012/461592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans DA, Funkenstein HH, Albert MS, et al. Prevalence of Alzheimer’s disease in a community population of older persons. Higher than previously reported. Jama. 1989 Nov 10;262(18):2551–2556. [PubMed] [Google Scholar]
- 26.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993 Aug 13;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 27.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993 Aug;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 28.Salmon DP, Ferris SH, Thomas RG, et al. Age and apolipoprotein E genotype influence rate of cognitive decline in nondemented elderly. Neuropsychology. 2013 Jul;27(4):391–401. doi: 10.1037/a0032707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alzheimer’s Association. Changing the Trajectory of Alzheimer’s Disease. 2010 http://www.alz.org/alzheimers_disease_trajectory.asp.
- 30.Rocca WA, Petersen RC, Knopman DS, et al. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011 Jan;7(1):80–93. doi: 10.1016/j.jalz.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]