Abstract
Objective
This systematic and quantitative review evaluates the literature on associations between depressed mood and flow-mediated dilation (FMD), a measure of endothelial function, in adults.
Methods
Published English-language articles (through December 2010) were identified from literature searches, assessed for data extraction, and evaluated for quality.
Results
The literature includes cross-sectional (n = 9) and retrospective examinations (n = 3) of how FMD correlates with clinical or subclinical depression in healthy adults and cardiovascular patients (total N across 12 studies = 1491). FMD was assessed using a variety of methodologies. Samples were predominately older white and Asian subjects with higher socioeconomic status. In eight of the 12 articles selected for this review, at least one significant inverse association was noted between depressed mood and FMD, with primarily moderate effect sizes. The overall meta-analysis (random-effects model) revealed a combined effect size of correlation coefficient r = 0.19 (95% confidence interval = 0.08–0.29, p = .001). Significant combined effects were found for subgroups of studies that a) received better quality ratings (r = 0.29), b) examined patients with cardiovascular disease or with cardiovascular disease risk factors/comorbidity (r = 0.29), c) used maximum vasodilation to quantify FMD (r = 0.27), and d) assessed samples that had a mean age of 55 years and older (r = 0.15).
Conclusions
Diverse studies support the inverse correlation between depressed mood and endothelial function, as measured by FMD. This literature would be strengthened by prospective studies, increased methodological consistency in FMD testing, and broader sampling (e.g., African Americans, younger age, lower socioeconomic status).
Keywords: review, meta-analysis, depression, mood, flow-mediated dilation, vasodilation
INTRODUCTION
Depressed mood is a prevalent condition that has been implicated as an independent risk factor in the pathogenesis of cardiovascular disease (CVD) and a predictor of impending cardiac events and postcardiac outcomes (1–4). A consistent threshold has not been found for the level at which depressive symptoms become cardiotoxic. Clinical depression (e.g., major depressive disorder [MDD]) and milder subclinical levels of depressive symptoms have been linked to adverse cardiovascular outcomes among initially healthy samples and CVD patients (3–6). Despite considerable work studying depression for links to blood pressure (BP) and autonomic nervous system physiology (6–10), the mechanisms linking depression to CVD remain uncertain.
Endothelial dysfunction is observed in atherosclerosis and cardiovascular events (11,12). Endothelial function can be evaluated by examining flow-mediated dilation (FMD). In FMD, endothelium-dependent vasodilation is elicited by a reactive hyperemia-induced rise in endothelial shear stress (13). In response to increased shear stress, the endothelium increases the release of nitric oxide. Under normal functioning, this leads to the relaxation of vascular smooth muscle and increased artery diameter (11–14). FMD is quantified as the increase in artery diameter relative to resting baseline (15). This noninvasive measure correlates with more invasive measures of endothelial functioning (12,16–18). FMD is predictive of adverse cardiovascular outcomes and is inversely related to cardiovascular risk (11,14,19–25).
Endothelial function has been of interest as a possible mechanism linking depression to CVD. This article evaluates the literature on depressed mood and endothelial function, as measured by FMD (10,26–36).
METHODS
Search Strategy
Based on recommendations for similar reviews (37), literature searches were conducted in MEDLINE and PsycINFO databases for all relevant articles on human studies published in English-language journals through December 2010. Searches involved a combination of search terms (“flow-mediated dilation” OR “dilation” OR “vasodilation” OR “vasodilatation” OR “endothelial” OR “endothelium” OR “reactive hyperemia”) AND (“depression” OR “depressive” OR “depressed” OR “mood” OR “negative affect”). We also reviewed the reference lists of retrieved articles to identify additional studies.
Study Selection Process
Our searches led to 12 articles that met the inclusion criteria (Fig. 1). This review included only published peer-reviewed articles on studies of clinical depression or depressive symptoms that provided data on associations with FMD, as measured by ultrasound imaging of the brachial artery response to reactive hyperemia. We excluded studies that a) measured FMD and depression without analyzing their association, b) sampled only patients with MDD (no comparison group) to test how FMD correlated with other measures, or c) examined depression relative to other types of tests for endothelial function (e.g., coronary artery acetylcholine response) (38–41).
Figure 1.
Flow diagram of process used in selection of studies for review.
Data Extraction
Details from the articles were logged into a standard form to characterize each study. Of interest were the methodological details of FMD and depression assessments, sample characteristics, study designs, study exclusions, the significance of associations, covariate adjustments, blinding of the FMD experimenter, and efforts to limit exposure to acute confounders of FMD (e.g., smoking, caffeine) before testing.
Statistical Analyses
An effect size quantified as correlation coefficient “r” was computed for the reported associations between depressed mood and FMD. (For reference of effect sizes, r = 0.10: small; r = 0.30: moderate; r = 0.50: large) (42,43). Some r’s were taken directly from articles that provided product moment correlations (e.g., Pearson r, Spearman ρ), whereas r’s for other studies were converted from translatable statistics (e.g., mean [standard deviation], F values). We took a conservative approach (42) by assigning r = 0.00 to articles with no translatable data (e.g., “results were nonsignificant”).
To have independent effects, each study reporting multiple analyses was represented in the meta-analytic model by a single effect derived from that study’s strongest methodology. For example, an effect from retrospective data was chosen if the study provided both retrospective and cross-sectional data. The effect derived using a continuous depressive symptom variable was selected if a study analyzed both continuous and dichotomized (weaker statistical power) scores. If a study analyzed a whole sample and subsamples, the effect for the larger whole sample was chosen.
Meta-analytic procedures, as described by Field and Gillett (43), were conducted using SPSS 18 (SPSS Inc, Chicago, IL) and R software (R Foundation for Statistical Computing, Vienna, Austria) (44). On preliminary examination of the Q statistic in a fixed-effect model and in finding significant (p < .001) heterogeneity, we determined that a random-effects model would be most appropriate. Whereas fixed-effects models assume that between-study differences are due to sampling error across homogenous studies measuring the same underlying effects in the same population, random-effects models have greater generalizability by considering such differences to be due to underlying effects among heterogeneous populations (45,46).
Random-effect models (47) were conducted to obtain combined effect sizes for the set of 12 studies and for several study subgroups. The subgroup meta-analyses aggregated the studies by cardiovascular status, depression assessment, age group, FMD quantification, and quality score. The Q statistic of the random-effects model did not indicate significant heterogeneity (p > .10) of effect sizes in the overall meta-analysis, so we did not test study characteristics as potential moderators of the depression-FMD link. Potential publication bias was examined with a funnel plot (43) and Begg and Mazumdar’s adjusted rank correlation test (48), as well as Rosenthal’s (49) “fail-safe n,” which estimates how many unpublished studies with nonsignificant findings (sometimes called the “file-drawer problem”) it would take to render the combined effect size nonsignificant.
Quality Assessment of Reported Studies
Two coauthors (D.C.C. and L.M.T.) evaluated articles for strength of evidence and reporting quality using a rating system adapted from other reviews (3,50), which assigned a priori values to parameters as detailed in Appendix 1. Each article was evaluated for study design/methodology and whether enough information was provided to facilitate study replication and comparison across samples. Whereas strength of evidence total quality scores could range from 5 to 15, total reporting quality scores could range from 0 to 4. Any discrepant scoring was resolved by consensus between the two raters.
RESULTS
Characteristics and Quality of Studies on Depression and FMD
Sample Characteristics
The 12 published studies included a total of 1491 adult subjects drawn from clinical and community populations in the United States, Europe, and Asia (Table 1A, Table 1B). The five studies focusing on MDD (current or remitted) included between 22 and 68 subjects, whereas the seven investigations of depressive symptoms included from 46 to 415 subjects. Studies examined younger (26,27,31) and older (29,32,33–35) healthy adults, as well as younger-to-older patients with documented CVD or elevated risk factors for CVD, including diabetes (10,28,30,36). Data on socioeconomic status (SES) were reported in only five articles (29,30,32,34,35), which indicated primarily well-educated, higher SES samples. Based on the seven articles reporting race/ethnicity data, the samples were predominately white (i.e., 80%–96%) (28–30,35) and Asian (33,34), with African Americans well represented (48%) in the sample of only one study (31).
TABLE 1.
A. Major Depression (Current and Prior History) and FMD
| Reference | Cohort | Confounders Excluded (E), Controlled (C), and Avoided in Pre-FMD Test Preparation (P) |
Depression and FMD (%Δ in Diameter) Association Tested |
Significant: Yes/No; Effect Size (r); SOE; RQ; and Comments |
|---|---|---|---|---|
| Broadley et al. (26) |
N = 22 (12 patients with treated MDD [6m:6f] versus 10 age-/sex-matched controls [8m:2f] [United Kingdom]); mean age = 37 y |
E: Smoke, HTN, DM, obese, menopause, dyslipidemia, cardioactive meds |
Remitted MDD posttreatment (per DSM-IV criteria, no interview specified) and FMD: maximum %Δ |
Yes: MDD group showed ↓ FMD than control group, p < .01; r = 0.57 SOE: 11; RQ: 2 10 of 12 patients were on antidepressants |
| C: Age, sex | ||||
| P: Fast, no caffeine (12 h), alcohol (24 h) | ||||
| Rajagopalan et al. (27) |
N = 30 (15 MDD patients with MDD [4m:11f]) versus 15 age-/sex-matched controls, [4m:11f] [United States]); mean age = 29 y |
E: Smoke, HTN, DM, obese, family hx CAD, dyslipidemia, oral contraceptives |
Current MDD (per DSM-IV criteria, interview not specified) and FMD: %↓ |
Yes: MDD correlated with ↓ FMD, p < .05; r = 0.36 SOE: 9; RQ: 4 No exclusion/control of meds (except oral contraceptives) |
| C: Age, sex, diet, fitness, menstrual phase | ||||
| P: None reported | ||||
| Taylor et al. (28) |
N = 68 (48 MDD patients at ↑ CAD risk [16m:32f] versus 20 age- and CAD risk–matched controls [12m:8f] [United States]); mean age = 62 y |
E: Smoke, HPA/ANS active meds; women had to be postmenopausal/with stable estrogen |
Current MDD (per Depression Interview Structured Hamilton) and FMD: %Δ |
No: FMD similar in MDD and control groups; p > .05; r = 0.15 SOE: 11; RQ: 1 Little information on FMD method/statistics |
| C: Age, CAD risk | ||||
| P: Fast 12 h + no meds | ||||
| Wagner et al. (29) |
N = 39 postmenopausal women assessed for history of MDD (19: hx+/20: hx−) (United States); Mean age = 61 y |
E: Smoke, DM, CVD, current depression |
History of depressive episodes (per SCID) and FMD: %Δ |
Yes: ↑ no. MDD episodes and ↓ FMD, p < .05; r = 0.34 SOE: 11; RQ: 4 |
| C:Metabolic syndrome, race, HRT, CES-D | ||||
| P: Fast 12 h; 24 h avoid lipid/BP meds, exercise, caffeine, high fat, aspirin, vitamins | ||||
| Wagner et al. (30) |
N = 44 postmenopausal women with diabetes tested for MDD/minor depression/dysthymia history (28: hx+/16: hx−) (United States); mean age = 62 y |
E: Smoke, CVD, insulin, current psychological disorder |
History of depressive episodes (per SCID) and FMD: %Δ |
Yes: ↑ recurrence of MDD and FMD ↓, p < .05; r = 0.30 SOE: 11; RQ: 4 |
| C: HTN, DM duration, HbA1c, CES-D | ||||
| P: Fast 12 h; 24 h avoid exercise, high fat, aspirin, caffeine, lipid/BP meds, vitamins |
| B. Depressive Symptoms and FMD | ||||
|---|---|---|---|---|
| Reference | Cohort | Confounders Excluded (E), Controlled (C), and Avoided in Pre-FMD Test Preparation (P) |
Depression and FMD (%Δ in Diameter) Association Tested |
Significant: Yes/No; Effect Size (r) SOE; RQ; and Comments |
| Cooper et al. (31) | 70 normotensive and mild unmedicated HTN (otherwise healthy) adults. (37m:33f) (United States); mean age = 36 y |
E: Smoker (heavy), meds, CVD, DM, major medical or psychiatric illness, obese, postmenopausal |
POMS-Depression subscale (continuous) and FMD: maximum %Δ |
Yes: FMD ↓ as POMS-Depression ↑, p < .05; r = 0.26 SOE: 10; RQ: 4 |
| C: Age, sex, BMI, BP, response bias (smokinga) | ||||
| P:12 h: no smoking, exercise, caffeine, alcohol, high fat/nitrates | ||||
| Harris et al. (32) | 193 postmenopausal women (United States). Mixed retrospective and cross-sectional study; mean age = 61 y |
E: (at entry) HRT, diastolic BP >100 mm Hg, insulin, meds: CVD, psychiatric |
BDI (continuous) and FMD: %Δ over time (test intervals as within subjects factor) |
No: Retrospective, p > .05; r = 0.00 Yes: Cross-sectional: FMD ↓ and BDI ↑ in HRT users only, p < .01; r = 0.17 SOE: 10/9 (retro/cross-sect); RQ: 2 |
| C: BMI, baseline diameter | ||||
| P: None reported | ||||
| Lin et al. (33) | 89 healthy adults (57m:32 f) (Taiwan); mean age = 59 y |
E: Self-reported CVD, HTN, DM, metabolic syndrome, psychiatric illness, cardioactive meds |
SCL-90: Depression (Chinese version) (continuous) and FMD: %Δ |
No: p > .05; r = 0.04 SOE: 8; RQ: 4 Focus was hostility, and FMD, but depression, was included as a covariateb |
| C: Smoke, sex, BP, glucose, lipids, SCL-90b | ||||
| P: None reported | ||||
| Narita et al. (34) | 46 healthy older adults (27 men:19 postmenopausal women) (Japan); mean age = 61 y |
E: Smoke, CVD/HTN, DM, high cholesterol level, obese, meds, psychiatric or neurological illness, alcohol |
CES-D/Zung Self-Rating Depression (continuous) and FMD: maximum %Δ |
No: p > .05; r = 0.00 SOE: 7; RQ: 3 Anxiety was the primary focus but included Zung Self-Rating and CES-D |
| C: None reported | ||||
| P: None reported | ||||
| Pizzi et al. (10) | 415 adults (212m:203f), ≥2 CHD risk factors: HTN, smoke, male, age ≥60 y, family hx CHD age <55 y, dyslipidemia (Italy); mean age = 57 y |
E: CHD, DM, antidepressants, kidney/liver failure, systemic inflammatory disease, neoplasm |
BDI ≥10 (n = 96) versus BDI <10 (n = 319) (also BDI continuous) and FMD: maximum %Δ |
Yes: BDI ↑ and FMD ↓, p < .001; r = 0.33 SOE: 12/11 (BDI cut/ continuous); RQ: 3 Did not report avoidance of smoking or exercise before FMD testing |
| C: Age, sex, smoke, HTN | ||||
| P: 12+ h fast, taper/avoid CHD meds (48 h) | ||||
| Schott et al. (35) | 332 healthy older adults (168men:164 postmenopausal women) (United States); mean age = 61 y |
E: CVD, HTN, DM, major illness, HTN/lipid meds |
BDI (continuous) and FMD: %Δ |
No: p > .05; r = 0.00 SOE: 9; RQ: 3 Not significant in men or women |
| C: Age, smoke BP, BMI, HRT (women) | ||||
| P: None reported | ||||
| Sherwood et al. (36) | 143 patients with CHD (99m:44f) (United States); mean age = 63 y |
E: BP ≥ 200/120 mm Hg, heart failure, valvular heart disease, severe arrhythmia, cardiomyopathy |
BDI ≥10 (n = 47) versus BDI <10 (n = 96) and FMD: maximum %Δ |
Yes: BDI ≥10 and ↓ FMD, p < .001; r = 0.21 SOE: 12; RQ: 3 Did not report avoidance of smoking exposure before FMD testing |
| C: Age, sex, baseline, cardioactive/depression meds (smokinga) | ||||
| P: Fast overnight, 48 h taper anti-ischemic meds | ||||
ANS = autonomic nervous system; BP = blood pressure; CAD = coronary artery disease; CES-D = Center for Epidemiological Studies–Depression; CVD = cardiovascular disease; DM = diabetes mellitus; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; FMD = flow-mediated dilation; HbA1c = hemoglobin A1c; HPA = hypothalamic-pituitary-adrenal axis; HRT = hormone replacement therapy; HTN = hypertension; hx = history; MDD = major depressive disorder; meds = medications; m/f = male/female; RQ = reporting quality; SCID = Structured Clinical Interview for DSM-IV; SOE = strength of evidence.
Depression and/or FMD did not vary by smoking status.
The Depression and Anxiety subscales of the Revised 90 Symptom Checklist (SCL-90) were analyzed as covariates.
BDI = Beck Depression Inventory; BMI = body mass index; BP = blood pressure; CES-D = Center for Epidemiological Studies–Depression; CHD = coronary heart disease; CVD = cardiovascular disease; DM = diabetes mellitus; FMD = flow-mediated dilation; HTN = hypertension; HRT=hormone replacement therapy; meds = medications; m/f = male/female; POMS = Profile of Mood States; RQ = reporting quality; retro/cross-sect = retrospective/cross-sectional (mixed study); SOE = strength of evidence.
Study Design Characteristics
The literature on FMD and depressed mood is predominately cross-sectional, with some retrospective work (29,30,32) but no prospective studies. The studies examined whether FMD differed when patients with current MDD (27,28) or remitted MDD (26) were compared with controls or if it varied according to histories of clinical depression (29,30). The remaining studies (10,31–36) on FMD used well-established self-report measures of depressive symptoms (e.g., Beck Depression Inventory [BDI]). All studies addressed potential confounders through exclusion criteria and most studies included statistical adjustments, with the exception of four smaller studies (26–28,34). Most articles reported limiting subjects’ exposure to acute confounders of FMD in the hours before testing.
FMD Assessment Characteristics
The protocol variations in testing FMD are shown in Table 2. The cuff pressures applied for occlusion ranged from 10 mm Hg higher than the participants’ systolic BP up to a standard systolic BP of 300 mm Hg. Approaches differed with regard to how long the brachial artery was occluded to induce reactive hyperemia and where the cuff used for occlusion was positioned. The number of postocclusion ultrasound images collected varied because of differences in measurement intervals and testing durations. Eight of the articles (10,26–29,33,34,36) reported that FMD testing was followed by administration of nitroglycerin (or similar agents) to quantify endothelial-independent dilation. Only one study found a significant relationship between nitroglycerin-induced dilation and depressed mood (10).
TABLE 2.
Heterogeneity of Methods in Studies of Depression and Flow-Mediated Dilation
| Study Characteristics | No. Studies |
|---|---|
| Depression measures (some studies used multiple measures) |
|
|
2 |
| 3 | |
| 5 | |
| 1 | |
| 1 | |
| 1 | |
| 1 | |
| FMD values used in statistical analyses | |
|
6 |
| 1 | |
| 5 | |
| Timing and no. postdeflation images during FMD testing | |
|
1 |
| 2 | |
| 1 | |
| 1 | |
| 3 | |
| 1 | |
| 2 | |
| 1 | |
| Cuff pressure during FMD testing | |
|
2 |
| 3 | |
| 1 | |
| 3 | |
| 1 | |
| 2 | |
| Cuff inflation time | |
|
1 |
| 2 | |
| 1 | |
| 7 | |
| 1 | |
| Cuff position | |
|
7 |
| 3 | |
| 1 | |
| 1 | |
| Nitroglycerin test reported | |
|
8 |
| 4 |
BP = blood pressure; FMD = flow-mediated dilation.
Quality Assessment
Table 1A and Table 1B include quality scores for strength of evidence and quality of reporting. With a possible range of 5 to 15, the strength of evidence quality scores ranged from 7 to 12, with a mean of 9.9 (standard deviation = 1.5). (Of the 12 articles, two studies received two quality scores due to multiple designs/measures (10,32), but only the lowest score for each was used to calculate the overall mean strength of evidence score). We considered the studies with strength of evidence scores of 11 or more to have the strongest methodological rigor and to be “better-quality” studies (n = 6). Of those six studies, significant correlations between FMD and depression status were shown in five studies (10,26,29,30,36), whereas one study (28) reported no association (Fig. 2). With a possible range from 0 to 4, the reporting quality scores ranged from 1 to 4. The details most often lacking in articles were regarding how baseline was assessed and quantified for use in the calculation of FMD and whether the experimenter testing FMD was blinded to subjects’ depression status.
Figure 2.
Percent change (or maximum percent change) in diameter and effect sizes of better-quality studies. Of these six studies, only Taylor et al. (28) showed a nonsignificant (ns) association between FMD and depression. max = maximum; %Δ = percent change.
Study Results and Meta-Analyses
Study Results
Table 1A and Table 1B summarize the 12 reviewed studies, including effect sizes (r). Eight studies found at least one significant inverse association between depression and FMD (10,26,27,29–32,36). Of the five studies examining correlations between MDD and FMD (26–30), four found significance with moderate to large effects (26,27,29,30). The largest effect (r = 0.57) found was for a study (26) showing less vasodilation among healthy adults with recently treated MDD (n = 12) compared with age-/sex-matched controls (n = 10). Of the seven studies examining a link between FMD and depressive symptoms, three studies found small-to-moderate effects for inverse associations between vasodilation and scores on the BDI (10,36) and on the Profile of Mood States–Depression subscale (31). Of these three studies, the largest effect (r = 0.33) was found (10) for FMD and BDI among adults with two or more CVD risk factors (n = 415).
Meta-Analysis: Overall
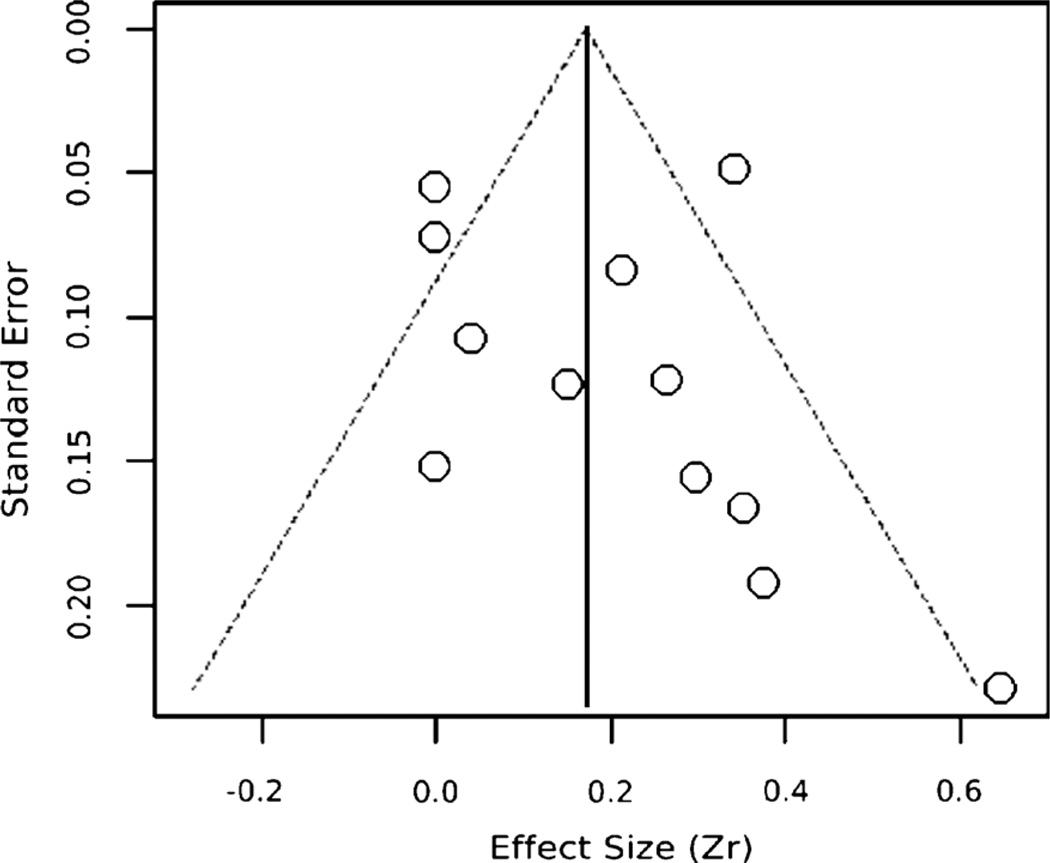
As shown in Table 3, the 12 studies yielded a combined effect size of r = 0.19, 95% confidence interval = 0.08–0.29, p = .001, for the association between depressed mood and FMD. While the Begg and Mazumdar’s test (48) indicates some publication bias (p < .05), the funnel plot’s (Fig. 3) asymmetry is not striking, given our conservative use of r = 0.00 for articles reporting results as “nonsignificant.” Moreover, publication bias due to unpublished studies was not indicated by the fail-safe n of 169, which exceeds Rosenthal’s (49) critical value of 5k + 10 (i.e., 70 in this meta-analysis). Thus, neither the funnel plot nor the fail-safe n shows substantial publication bias, which suggests that these results are reasonably robust. The nonsignificant Q statistic indicated that effect sizes were not heterogeneous in this random-effects model, so we did not conduct moderator analyses. However, we conducted meta-analyses on subgroups.
TABLE 3.
Meta-analyses of Associations Between Depression and FMD in Complete Set of Studies and Subgroups
| Studies | n | Z (p) | Mean r Effect | 95% CI | Heterogeneity Q: χ2 (df), p |
Fail-Safe n |
|---|---|---|---|---|---|---|
| Complete set of depression and FMD studies | 12 | 3.43 (.001) | 0.19 | 0.08 to 0.29 | 9.49 (11), .58 | 169 |
| Studies by quality status | ||||||
|
6 | 6.92 (<.001) | 0.29 | 0.21 to 0.37 | 5.01 (5), .42 | 109 |
| 6 | 1.25 (.212) | 0.06 | −0.04 to 0.16 | 5.60 (5), .35 | 2 | |
| Studies by cardiovascular status | ||||||
|
4 | 6.82 (<.001) | 0.29 | 0.20 to 0.36 | 2.88 (3), .41 | 55 |
| 8 | 2.18 (.029) | 0.14 | 0.01 to 0.26 | 8.93 (7), .26 | 25 | |
| Studies by depression status | ||||||
|
5 | 4.21 (<.001) | 0.30 | 0.16 to 0.42 | 3.99 (4), .41 | 33 |
| 7 | 1.87 (.062) | 0.13 | −0.01 to 0.26 | 4.11 (6), .66 | 46 | |
| Studies by age group | ||||||
|
9 | 2.44 (.015) | 0.15 | 0.03 to 0.26 | 5.58 (8), .69 | 76 |
| 3 | 3.63 (<.001) | 0.35 | 0.17 to 0.51 | 2.00 (2), .37 | 15 | |
| Studies by FMD change score useda | ||||||
|
5 | 4.04 (<.001) | 0.27 | 0.14 to 0.38 | 5.04 (4), .28 | 73 |
| 6 | 2.17 (.030) | 0.15 | 0.02 to 0.28 | 4.64 (5), .46 | 16 |
In this subgroup meta-analysis, one study was omitted because its FMD measure differed from those in both of the two subgroups.
CI = confidence interval; CVD = cardiovascular disease; FMD = flow-mediated dilation; MDD = major depressive disorder.
Figure 3.
Funnel plot of the overall effect estimates (r) by their standard errors to assess publication bias. The vertical line reflects the summary effect estimate, and the dashed lines show pseudo 95% confidence limits for the summary effect estimate.
Meta-Analysis by Subgroups
Five subgroups showed significant (or marginal) and reasonably robust (based on fail-safe n) combined effect sizes (Table 3). The subgroups included studies that a) rated as better quality (r = 0.29), b) sampled patients with CVD or elevated CVD risk factors/comorbidity (r = 0.29), c) used maximum vasodilation to measure FMD (r = 0.27), d) examined samples with a mean age of 55 years or older (r = 0.15), and e) assessed depressive symptoms (r = 0.13). Several subgroups had significant combined effect sizes that were vulnerable to publication bias (per fail-safe n), including studies that measured FMD as percent change in diameter or focused on healthy adults, patients with current/remitted MDD, or samples with mean age of older than 55 years. The only nonsignificant effect in these meta-analyses was found for the subgroup of studies that were not rated as better quality.
DISCUSSION
To the best of our knowledge, this is the first review of the literature on the relationship between depressed mood and FMD. The 12 studies meeting our inclusion criteria were generally of good quality but varied considerably methodologically. Still, most of these studies found associations for FMD with depressive mood, whether it was assessed as current MDD, remitted MDD, or depressive symptoms.
Quantitative Findings
The overall meta-analysis combined effect size of r = 0.19 for the association between depressed mood and FMD may be an underestimation, given our fairly conservative approach in determining study effect sizes (e.g., r = 0.00 for results reported as not significant). To put this overall effect size for depression and FMD (r = 0.19) in perspective, another meta-analysis found combined effect sizes of r = 0.19 and r = 0.13 for the relationship of obesity to systolic BP and diastolic BP, respectively (51).
A larger effect (r = 0.29) was found when meta-analyses were limited to subgroups of either better-quality studies or studies examining patients with CVD/CVD risk factors (all of which were rated better quality). The subgroup of studies that did not receive better-quality status may have had a nonsignificant combined effect partly because it included several studies with null findings that had examined depressive symptoms only as a secondary variable. Studies specifically designed to assess depressive symptoms and FMD in larger samples of healthy adults would strengthen this literature.
Meta-analyses of the FMD measurement subgroups revealed that the combined effects for both subgroups reached statistical significance, but only the effect (r = 0.27) for the group quantifying FMD as maximum percentage increase seemed to be robust against publication bias. It has not yet been established whether the prognostic value of FMD in predicting CVD varies according to how FMD is quantified. It may be useful to examine whether depression actually has a stronger relationship with the maximum vasodilation response compared with other measures of FMD.
Although both subgroups focusing on the mean age of samples showed significant combined effects for depression and FMD, only the studies with a mean age of 55 years and older produced an effect (r = 0.15) that fail-safe n indicated was relatively robust. The publication bias suggested in the subgroup of study samples with mean ages of 54 and younger may be partly due to this subgroup only including three studies. More research on younger samples is needed to confirm that a depression-FMD link can be demonstrated among adults in whom age-associated cardiovascular risk factors are less prevalent.
Although the subgroup of FMD studies that examined links to depressive symptoms had a marginally significant and relatively robust combined effect (r = 0.13), the subgroup focusing on MDD produced a larger effect (r = 0.30) that fell slightly short of Rosenthal’s suggested fail-safe n (49). The marginal significance for depressive symptoms and slight publication bias for MDD studies might be due to the small number of studies in each subgroup. Expanding the number of FMD studies with both types of depression measures could strengthen the evidence provided by this literature.
Heterogeneity of Studies
Although we feel our meta-analysis results are informative, we acknowledge that their validity could be compromised because of the heterogeneity of the 12 reviewed studies. Random-effects models were used to account for this heterogeneity. The differences in the depression measures, FMD testing methods, sample compositions, and approaches to handling confounders can be viewed as a weakness of this literature. However, the fact that an association between depressed mood and FMD was found with a wide variety of methods and samples may in fact be a strength because it is unlikely that these significant associations are mere artifacts of shared methodologies across studies.
One of the difficulties with the varying approaches used in this literature is that there often are few, if any, studies that can be directly compared with investigations reporting null findings, such as Taylor et al. (28). One study by Rajagopalan and colleagues (27) found a moderate effect for FMD when comparing a group of healthy, young MDD patients to nonde-pressed age-/sex-matched controls using equally sized groups and the same male-to-female ratio in both groups (n = 15; 4:11). Along with the two studies’ (27,28) inconsistencies in sample ages and cardiovascular health, Taylor et al. (28) had a higher proportion of women in their MDD group (n = 48; 16:32) than in their notably smaller control group (n = 20; 12:8), which could have resulted in the patient group having a smaller baseline diameter. Given the influence of baseline diameter on vasodilation changes in diameter (13), that study’s (28) null findings might have been different if its patient and control groups had equal proportions of women or if analyses had been adjusted for baseline diameter (52).
Some reports with null findings for depression-FMD link have no directly comparable studies because of their use of depression questionnaires not found in the existing FMD literature (33,34). However, none of the cross-sectional (33–35) and retrospective (32) studies with null findings for FMD and depressive symptoms reported limiting subjects’ exposure to acute confounders of FMD (e.g., caffeine, high fat) in the hours before FMD testing, unlike the three studies with significant findings for depressive symptoms and FMD (10,31,36). The aforementioned study with null findings for retrospective data (32) included a cross-sectional analysis that found an inverse association between FMD and depressive symptoms in a subsample of women on hormone replacement therapy (32). More research is needed to test whether that finding can be replicated.
Future Research
It is unknown whether variations in FMD methodology affected the degree to which associations were found between depressed mood and FMD. The literature would be strengthened by a more consistent approach to FMD assessment. Given that eight studies of FMD included separate analyses of nitroglycerin-mediated dilation and depression (10,26–29,33,34,36) and only one found significance (10), it may be useful to examine the utility of analyzing these dilation measures in separate models versus the same model (e.g., nitroglycerin response as a covariate).
The reported demographic data suggest that most samples had little racial/ethnic or socioeconomic diversity. The only study with African Americans well represented in its sample did not report any race-/ethnicity-specific data (31). More research is needed on minority populations, particularly African Americans, to further understand this group’s high rates of CVD. Studies of individuals with lower SES could be informative, given their vulnerabilities to CVD and economic stressors that can contribute to depression.
The literature suffers from a lack of prospective research, which is needed to assess whether depression-related endothelial dysfunction is predictive of CVD. It would be helpful to further elucidate the underlying mechanisms linking depression and FMD and the direction of the relationship. Although depression could contribute to endothelial dysfunction by increasing the likelihood of adverse health behaviors (e.g., smoking, lack of exercise, overeating, substance abuse), most studies showing a correlation between depression and FMD reported exclusions or adjustments made for these behaviors with direct and/or indirect measures (e.g., body mass index/ obesity for overeating/lack of exercise).
Thus, direct physiological pathways could be involved. For example, depressed individuals could have an impairment of the l-arginine–nitric oxide pathway (53). Nitric oxide is synthesized from the amino acid l-arginine by a family of enzymes called nitric oxide synthases (53). In addition to the literature linking depression to reduced FMD (a functional measure of nitric oxide–mediated dilation), depression also may be linked to lower nitrogen dioxides (metabolites of nitric oxide) and reduced platelet nitric oxide synthase activity (54). Research investigating such pathways would further enhance our understanding of the association among mood, endothelial dysfunction, and CVD.
Limitations
Several limitations of this review must be considered. The results of our meta-analyses should be viewed cautiously given the small sample sizes, array of methodologies, and variations in the populations sampled. We made a strong effort to identify and evaluate all of the relevant published studies in English-language journals. However, one or more articles may have been inadvertently omitted, and consideration of non–English-language articles could have changed the results. In addition, the use of another quality rating system and/or different raters could have produced a different set of better-quality studies. Finally, FMD is not the only approach that can be used to assess endothelial function, but several studies using other measures of endothelial dysfunction also suggest an association with depressed mood (38–41).
CONCLUSIONS
Literature emerging in the last 10 years suggests that healthy adults and patients with CVD or elevated risk for CVD exhibit an inverse association between depressed mood and endothelial function, as measured by FMD. Prospective studies are needed to advance this research. Increasing the diversity (age, ethnicity, and SES) of samples and the consistency of methodology for FMD testing would further enhance this literature.
Acknowledgments
National Institutes of Health Grants HL36005, RR00827, and P60MD00220 supported this research.
Glossary
- BDI
Beck Depression Inventory
- CVD
cardiovascular disease
- FMD
flow-mediated dilation
- MDD
major depressive disorder
- SES
socioeconomic status
Appendix
APPENDIX 1.
Criteria for Strength of Evidence and Reporting Quality Ratings of Studies
| Parameter | Criteria | Points |
|---|---|---|
| Protocol’s strength of evidence | ||
|
N ≤ 50 | 1 |
| N = 51–100 | 2 | |
| N ≥ 100 | 3 | |
| Cross-sectional | 1 | |
| Retrospective | 2 | |
| Prospective | 3 | |
| No comparison group | 1 | |
| Unmatched, nondepressed group | 2 | |
| Matched (or quasi matched), nondepressed group |
3 | |
| 0 | 1 | |
| 1–2 | 2 | |
| ≥3 | 3 | |
| 0–1 | 1 | |
| 2 | 2 | |
| ≥3 | 3 | |
| Article’s reporting of study protocol | ||
|
No | 0 |
| Yes | 1 | |
| No | 0 | |
| Yes | 1 | |
| No | 0 | |
| Yes | 1 | |
| No or not reported | 0 | |
| Yes | 1 |
CVD = cardiovascular disease; BMI = body mass index; FMD = flow-mediated dilation.
REFERENCES
- 1.Riolo SA, Nguyen TA, Greden JF, King CA. Prevalence of depression by race/ethnicity: findings from the National Health and Nutrition Examination Survey III. Am J Public Health. 2005;95:998–1000. doi: 10.2105/AJPH.2004.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frasure-Smith N, Lespérance F. Recent evidence linking coronary heart disease and depression. Can J Psychiatry. 2006;51:730–737. doi: 10.1177/070674370605101202. [DOI] [PubMed] [Google Scholar]
- 3.Wulsin LR, Vaillant GE, Wells VE. A systematic review of the mortality of depression. Psychosom Med. 1999;61:6–17. doi: 10.1097/00006842-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med. 2003;65:201–210. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- 5.Anda R, Williamson D, Jones D, Macera C, Eaker E, Glassman A, Marks J. Depressed affect, hopelessness, and the risk of ischemic heart disease in a cohort of U.S. adults. Epidemiology. 1993;4:285–294. doi: 10.1097/00001648-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Davidson K, Jonas BS, Dixon KE, Markovitz JH. Do depression symptoms predict early hypertension incidence in young adults in the CARDIA study? Coronary Artery Risk Development in Young Adults. Arch Intern Med. 2000;160:1495–1500. doi: 10.1001/archinte.160.10.1495. [DOI] [PubMed] [Google Scholar]
- 7.Carney RM, Blumenthal JA, Stein PK, Watkins L, Catellier D, Berkman LF, Czajkowski SM, O’Connor C, Stone PH, Freedland KE. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104:2024–2028. doi: 10.1161/hc4201.097834. [DOI] [PubMed] [Google Scholar]
- 8.York KM, Hassan M, Li Q, Li H, Fillingim RB, Sheps DS. Coronary artery disease and depression: patients with more depressive symptoms have lower cardiovascular reactivity during laboratory-induced mental stress. Psychosom Med. 2007;69:521–528. doi: 10.1097/PSY.0b013e3180cc2601. [DOI] [PubMed] [Google Scholar]
- 9.Smith TW, Ruiz JM. Psychosocial influences on the development and course of coronary heart disease: current status and implications for research and practice. J Consult Clin Psychol. 2002;70:548–568. doi: 10.1037//0022-006x.70.3.548. [DOI] [PubMed] [Google Scholar]
- 10.Pizzi C, Manzoli L, Mancini S, Costa GM. Analysis of potential predictors of depression among coronary heart disease risk factors including heart rate variability, markers of inflammation, and endothelial function. Eur Heart J. 2008;29:1110–1117. doi: 10.1093/eurheartj/ehn137. [DOI] [PubMed] [Google Scholar]
- 11.Frick M, Weidinger F. Endothelial function: a surrogate endpoint in cardiovascular studies? Curr Pharm Des. 2007;13:1741–1750. doi: 10.2174/138161207780831211. [DOI] [PubMed] [Google Scholar]
- 12.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 13.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. International Brachial Artery Reactivity Task Force. International Brachial Artery Reactivity Task Force: guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vaso-dilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 14.Moens AL, Goovaerts I, Claeys MJ, Vrints CJ. Flow-mediated vasodilation: a diagnostic instrument, or an experimental tool? Chest. 2005;127:2254–2263. doi: 10.1378/chest.127.6.2254. [DOI] [PubMed] [Google Scholar]
- 15.Korkmaz H, Onalan O. Evaluation of endothelial dysfunction: flow-mediated dilation. Endothelium. 2008;15:157–163. doi: 10.1080/10623320802228872. [DOI] [PubMed] [Google Scholar]
- 16.Fronek A, Allison M. Non-invasive assessment of endothelial activity in patients with coronary heart disease and cardiovascular risk factors. Vasa. 2008;37:137–142. doi: 10.1024/0301-1526.37.2.137. [DOI] [PubMed] [Google Scholar]
- 17.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 18.Takase B, Uehata A, Akima T, Nagai T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol. 1998;82:1535–1539. doi: 10.1016/s0002-9149(98)00702-4. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki T, Hirata K, Elkind MS, Jin Z, Rundek T, Miyake Y, Boden-Albala B, Di Tullio MR, Sacco R, Homma S. Metabolic syndrome, endothelial dysfunction, and risk of cardiovascular events: the Northern Manhattan Study (NOMAS) Am Heart J. 2008;156:405–410. doi: 10.1016/j.ahj.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 21.Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation. 2003;108:2093–2098. doi: 10.1161/01.CIR.0000095273.92468.D9. [DOI] [PubMed] [Google Scholar]
- 22.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 23.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined en-dothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 24.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF, Jr, Gokce N, Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol. 2007;27:2113–2119. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patti G, Pasceri V, Melfi R, Goffredo Chello M, D’Ambrosio A, Montesanti R, DiSciascio G. Impaired flow-mediated dilation and risk of restenosis in patients undergoing coronary stent implantation. Circulation. 2005;111:70–75. doi: 10.1161/01.CIR.0000151308.06673.D2. [DOI] [PubMed] [Google Scholar]
- 26.Broadley AJ, Korszun A, Jones CJ, Frenneaux MP. Arterial endothelial function is impaired in treated depression. Heart. 2002;88:521–523. doi: 10.1136/heart.88.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajagopalan S, Brook R, Rubenfire M, Pitt E, Young E, Pitt B. Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. Am J Cardiol. 2001;88:196–198. doi: 10.1016/s0002-9149(01)01623-x. A7. [DOI] [PubMed] [Google Scholar]
- 28.Taylor CB, Conrad A, Wilhelm FH, Neri E, DeLorenzo A, Kramer MA, Giese-Davis J, Roth WT, Oka R, Cooke JP, Kraemer H, Spiegel D. Psychophysiological and cortisol responses to psychological stress in depressed and nondepressed older men and women with elevated cardiovascular disease risk. Psychosom Med. 2006;68:538–546. doi: 10.1097/01.psy.0000222372.16274.92. [DOI] [PubMed] [Google Scholar]
- 29.Wagner JA, Tennen H, Mansoor GA, Abbott G. History of major depressive disorder and endothelial function in postmenopausal women. Psychosom Med. 2006;68:80–86. doi: 10.1097/01.psy.0000195868.68122.9e. [DOI] [PubMed] [Google Scholar]
- 30.Wagner J, Tennen H, Mansoor G, Abbott G. Endothelial dysfunction and history of recurrent depression in postmenopausal women with type 2 diabetes: a case-control study. J Diabetes Complications. 2009;23:18–24. doi: 10.1016/j.jdiacomp.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Cooper DC, Milic M, Tafur JR, Mills PJ, Bardwell WA, Ziegler MG, Dimsdale JE. Adverse impact of mood on flow-mediated dilation. Psychosom Med. 2009;72:122–127. doi: 10.1097/PSY.0b013e3181cdbfc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris KF, Matthews KA, Sutton-Tyrrell K, Kuller LH. Associations between psychological traits and endothelial function in postmenopausal women. Psychosom Med. 2003;65:402–409. doi: 10.1097/01.psy.0000035720.08842.9f. [DOI] [PubMed] [Google Scholar]
- 33.Lin TK, Weng CY, Wang WC, Chen CC, Lin IM, Lin CL. Hostility trait and vascular dilatory functions in healthy Taiwanese. J Behav Med. 2008;31:517–524. doi: 10.1007/s10865-008-9177-0. [DOI] [PubMed] [Google Scholar]
- 34.Narita K, Murata T, Hamada T, Takahashi T, Omori M, Suganuma N, Yoshida H, Wada Y. Interactions among higher trait anxiety, sympathetic activity, and endothelial function in the elderly. J Psychiatr Res. 2007;41:418–427. doi: 10.1016/j.jpsychires.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Schott LL, Kamarck TW, Matthews KA, Brockwell SE, Sutton-Tyrrell K. Is brachial artery flow-mediated dilation associated with negative affect? Behav Int J Med. 2009;16:241–247. doi: 10.1007/s12529-009-9038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherwood A, Hinderliter AL, Watkins LL, Waugh RA, Blumenthal JA. Impaired endothelial function in coronary heart disease patients with depressive symptomatology. J Am Coll Cardiol. 2005;46:656–659. doi: 10.1016/j.jacc.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 37.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 38.Kim JH, Kim JW, Ko YH, Choi CU, Na JO, Kim EJ, Rha SW, Park CG, Seo HS, Oh DJ. Coronary endothelial dysfunction associated with a depressive mood in patients with atypical angina but angiographically normal coronary artery. Int J Cardiol. 2010;143:154–157. doi: 10.1016/j.ijcard.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Rybakowski JK, Wykretowicz A, Heymann-Szlachcinska A, Wysocki H. Impairment of endothelial function in unipolar and bipolar depression. Biol Psychiatry. 2006;60:889–891. doi: 10.1016/j.biopsych.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 40.Tomfohr LM, Martin TM, Miller GE. Symptoms of depression and impaired endothelial function in healthy adolescent women. J Behav Med. 2008;31:137–143. doi: 10.1007/s10865-007-9141-4. [DOI] [PubMed] [Google Scholar]
- 41.Lavoie KL, Pelletier R, Arsenault A, Dupuis J, Bacon SL. Association between clinical depression and endothelial function measured by forearm hyperemic reactivity. Psychosom Med. 2010;72:20–26. doi: 10.1097/PSY.0b013e3181c2d6b8. [DOI] [PubMed] [Google Scholar]
- 42.Rosenthal R, DiMatteo MR. Meta-analysis: recent developments in quantitative methods for literature reviews. Annu Rev Psychol. 2001;52:59–82. doi: 10.1146/annurev.psych.52.1.59. [DOI] [PubMed] [Google Scholar]
- 43.Field AP, Gillett R. How to do meta-analysis. Br J Math Stat Psychol. 2010;63:665–694. doi: 10.1348/000711010X502733. [DOI] [PubMed] [Google Scholar]
- 44.R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2005. [Google Scholar]
- 45.Field AP. Meta-analysis of correlation coefficients: a Monte Carlo comparison of fixed- and random-effects methods. Psychol Methods. 2001;6:161–180. doi: 10.1037/1082-989x.6.2.161. [DOI] [PubMed] [Google Scholar]
- 46.Tak LM, Meijer A, Manoharan A, de Jonge P, Rosmalen JG. More than the sum of its parts: meta-analysis and its potential to discover sources of heterogeneity in psychosomatic medicine. Psychosom Med. 2010;72:253–265. doi: 10.1097/PSY.0b013e3181d714e1. [DOI] [PubMed] [Google Scholar]
- 47.Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychol Methods. 1998;3:486–504. [Google Scholar]
- 48.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 49.Rosenthal R. Meta-analytic Procedures for Social Research. Beverly Hills, CA: Sage; 1984. [Google Scholar]
- 50.Bland CJ, Meurer LN, Maldonado G. A systematic approach to conducting a nonstatistical meta-analysis of research literature. Acad Med. 1995;70:642–653. doi: 10.1097/00001888-199507000-00014. [DOI] [PubMed] [Google Scholar]
- 51.Oh HS, Seo WS. Inter-relationships between arteriosclerotic risk factors: a meta-analysis. Yonsei Med J. 2000;41:450–458. doi: 10.3349/ymj.2000.41.4.450. [DOI] [PubMed] [Google Scholar]
- 52.Kapuku GK, Treiber FA, Hartley B, Ludwig DA. Gender influences endothelial-dependent arterial dilatation via arterial size in youth. Am J Med Sci. 2004;327:305–309. doi: 10.1097/00000441-200406000-00001. [DOI] [PubMed] [Google Scholar]
- 53.Pinto VL, Brunini TM, Ferraz MR, Okinga A, Mendes-Ribeiro AC. Depression and cardiovascular disease: role of nitric oxide. Cardiovasc Hematol Agents Med Chem. 2008;6:142–149. doi: 10.2174/187152508783955060. [DOI] [PubMed] [Google Scholar]
- 54.Chrapko WE, Jurasz P, Radomski MW, Lara N, Archer SL, Le Melle´do JM. Decreased platelet nitric oxide synthase activity and plasma nitric oxide metabolites in major depressive disorder. Biol Psychiatry. 2004;56:129–134. doi: 10.1016/j.biopsych.2004.03.003. [DOI] [PubMed] [Google Scholar]