Abstract
Background
Depressive symptoms and fatigue frequently overlap in clinical samples and the general population. The link of depressive symptoms and fatigue with increased risk of cardiovascular disease has been partly explained by shared biological mechanisms including sympathetic overactivity. Prolonged sympathetic overactivity downregulates the responsiveness of the β-adrenergic receptor (β-AR), a receptor that mediates several end-organ sympathetic responses.
Purpose
The authors studied whether depression and fatigue are related to reduced β-AR responsiveness within the human body (in vivo) in an ethnically diverse sample of African and Caucasian Americans.
Methods
The chronotropic25 dose (CD25) was used to determine in vivo β-AR responsiveness in 93 healthy participants. Psychometric measures included the Center of Epidemiological Studies-Depression Scale and the Multidimensional Fatigue Symptom Inventory.
Results
Hierarchical regression analyses (adjusted for age, gender, body mass index, blood pressure, smoking, and ethnicity) revealed that mental fatigue was significantly related to reduced β-AR responsiveness (i.e., higher CD25 values) in the whole sample. Moderation analyses indicated significant ethnicity × depression/fatigue interactions. Depressive symptoms, total fatigue, emotional fatigue, mental fatigue, and physical fatigue were related to reduced β-AR responsiveness in Caucasian American but not in African Americans.
Conclusions
Our findings suggest that symptoms of depression and fatigue are related to decreased in vivo β-AR responsiveness in Caucasian Americans. The lack of this association in African Americans highlights the importance for considering ethnicity as a potential moderator in research focusing on associations between psychological variables and cardiovascular function.
Keywords: β-adrenergic receptor, African Americans, Caucasian Americans, Depression, Fatigue
Introduction
Depressive symptoms and fatigue frequently overlap in clinical samples, primary care patients, and the general population [1-5]. Although severe manifestations of these symptoms lead to clinical diagnoses (e.g., major depression and chronic fatigue syndrome), they are everyday phenomena and continuously distributed within the population [6-8]. The substantial overlap in these symptoms has been partly explained by common precipitants (e.g., prolonged psychological distress or illness). In addition, shared interacting biological mechanisms have been suggested to underlie depressive symptoms and fatigue, such as the cytokine-induced sickness response, neuroendocrine alterations, and autonomic nervous system dysregulation characterized by sympathetic overactivity and decreased parasympathetic tone [3,9-14].
Symptoms of depression and fatigue may have predictive value for cardiovascular disease (CVD) morbidity and mortality. On the other side, these symptoms increase in patients with cardiovascular events [15-23]. This relationship may be partly based on sympathetic nervous system (SNS) overactivity. SNS overactivity is considered a risk factor for CVD [24-26] and both depression and fatigue have been related to indices of sympathetic predominance, such as increased heart rate, reduced heart rate variability, or higher levels of norepinephrine in both clinical and nonclinical samples [13,27-33]. Curiously, a wide range of research indicates ethnic differences in depression/fatigue, cardiovascular functioning, and relationships between these variables [34-40].
Several studies on the associations between psychological features and SNS functioning have used more global or indirect indicators of SNS functioning inferred from hemodynamic/electrophysiological assessment or analyzed catecholamines in blood or urine samples. A promising approach to study SNS functioning involves measuring responsiveness of the β-adrenergic receptor (β-AR) because this receptor mediates a wide range of catecholamine-induced end-organ sympathetic responses, such as vasodilatation, heart rate increase, and immune functions [41]. As sympathetic stimulation downregulates β-AR responsiveness, reduced β-AR responsiveness is considered an indicator of prolonged SNS overactivity and a physiological reflection of chronic stress [41,42].
β-AR responsiveness can be measured in vitro by assessing isoproterenol-induced cyclic adenosine monophosphate production in human peripheral blood mononuclear cells (PBMC) or in vivo by assessing the chronotropic25 dose (CD25). CD25 refers to the dose of isoproterenol that is necessary to increase heart rate by 25 beats per minute (bpm). High CD25 values indicate low receptor responsiveness [41,42]. Although both measures are interrelated [43], CD25 may be more valid than in vitro assessment because the neurohormonal environment of a PBMC cell receptor may differ from a postsynaptic cell receptor within an organ like the heart [41]. Studies using the CD25 method have found relationships of β-AR function with several stress-related states, such as type A behavior pattern, subjective social status, anxiety, and hostility [36,44-49]. Moreover, CD25 values have been reported to be higher in African Americans than in Caucasian Americans [36].
Although associations of depression and fatigue with sympathetic functioning have frequently been studied, less research has been done on the relationship of these symptoms with β-AR function. We examined whether symptoms of depression and fatigue are related to in vivo β-AR responsiveness in African Americans and Caucasian Americans. Considering the relevance of ethnicity to depressive symptoms, fatigue and cardiovascular functioning, we also focused on potential differences in depression/fatigue-CD25 relationships between African Americans and Caucasian Americans. It was hypothesized that symptoms of depression and fatigue may be related to reduced β-AR responsiveness (i.e., higher CD25 values).
Methods
Participants
Participants were unmedicated volunteers recruited from the local San Diego, California area. Recruitment occurred through local papers, online advertisements, community flyers, and word of mouth. As part of a larger study on the health of African Americans and Caucasian Americans, the study group for isoproterenol testing was roughly evenly divided between Caucasian Americans (20 women and 30 men) and African Americans (19 women and 24 men). Age ranged between 19 and 51 years (see Table 1 for sample characteristics). The study was approved by the University of California, San Diego Institutional Review Board, and all subjects gave both written and verbal consent. Exclusionary criteria were: current diagnoses of a clinical illness other than hypertension, history of psychosis or sleep disorder, current alcohol or drug abuse, moderate or heavy smoking (>10 cigarettes/day), increased caffeine intake (>600 mg/day), hormonal medication (including the contraceptive pill or hormone replacement therapy), blood pressure≥170/105 mmHg, severe obesity (class II obesity, body mass index (BMI)≥35), and any medication use. Two subjects with antihypertensive medication were accepted for participation and enrolled after a 3-week drug washout period supervised by the study physician.
Table 1.
Descriptive statistics of sample and study variables
| Total (n =93) | African Americans (n =43) | Caucasian Americans (n =50) | |
|---|---|---|---|
| Age (years)*** | 35.1 (9.7) | 39.0 (7.8) | 31.8 (10.0) |
| Females (N (%)) | 39 (41.9) | 19 (20.4) | 20 (21.5) |
| Body mass index (kg/m2)*** | 26.3 (3.6) | 27.9 (2.8) | 24.9 (3.6) |
| Mean arterial blood pressure (mmHg) | 91.7 (12.4) | 94.1 (11.8) | 89.5 (12.7) |
| Current smoker (N, (%))* | 10 (10.8) | 8 (8.6) | 2 (2.2) |
| β-adrenergic receptor responsiveness (CD25) | 1.4 (0.8) | 1.5 (0.8) | 1.3 (0.9) |
| Depression (CES-D) | 10.7 (9.3) | 10.3 (8.7) | 11.0 (9.9) |
| Total fatigue (MFSI total score) | 2.5 (17.6) | 1.2 (18.2) | 3.6 (17.1) |
| MFSI general fatigue | 5.6 (5.4) | 5.1 (5.9) | 6.0 (4.9) |
| MFSI physical fatigue | 2.6 (3.4) | 2.7 (3.7) | 2.5 (3.1) |
| MFSI emotional fatigue | 4.2 (4.5) | 4.5 (5.1) | 4.0 (4.1) |
| MFSI mental fatigue | 4.6 (4.7) | 3.9 (3.9) | 5.2 (5.2) |
| MFSI vigor | 14.3 (4.7) | 14.4 (5.3) | 14.3 (4.3) |
Values shown as mean (SD) unless otherwise noted. Differences between ethnic groups were calculated using t tests with 1,000 bootstrap samples and Chi-square tests
CES-D Center for Epidemiologic Studies Depression Scale, CD25 chronotropic25 dose, MFSI Multidimensional Fatigue Inventory
p <0.05 (two tailed);
p <0.01;
p <0.001
In Vivo Testing of β-Adrenergic Receptor Responsiveness
The CD25 isoproterenol stimulation test was used to assess in vivo β-AR function. Low CD25 values indicate high β-AR sensitivity [41,42]. Heart rates were assessed by electrocardiogram. After a 30-min rest and assessment of basal blood pressure and heart rate, an intravenous low-dose bolus (0.1 μg) of isoproterenol was administered to ensure that no adverse reactions to the drug occurred. Following the 0.1-μg bolus, participants were infused with incremental bolus doses (0.25, 0.5, 1.0, 2.0, and 4.0 μg) until an increase of heart rate by 25 bpm above basal heart rate was observed or until the 4.0-μg bolus was completed. The maximum heart rate after each bolus was calculated as the mean of the three shortest R–R intervals [42]. There was a 5-min time interval between bolus injections. Standardized calculation of CD25 values was performed as described previously [42].
Depression, Fatigue, and Covariate Measures
Participants completed psychological questionnaires the following day. The severity of depressive symptoms was assessed with the 20-item Center of Epidemiological Studies—Depression (CES-D) scale [50]. The CES-D primarily addresses cognitive/affective features of depression. The range of scores is 0–60 with higher scores indicating more severe depressive symptoms. The mean score for patients with a diagnosis of clinical depression is 39, whereas scores higher than 15 suggested an increased risk of depression. High internal consistency as measured by Cronbach’s alpha has been reported for the general population, clinical samples and across several ethnicities [50,51].
Fatigue was assessed with the 30-item short form of the Multidimensional Fatigue Symptom Inventory (MFSI), which was designed to assess multidimensional aspects of fatigue. The scale has six subscales (total fatigue, general fatigue, physical fatigue, emotional fatigue, mental fatigue, and vigor). Although originally designed for cancer patients, the MFSI has also been shown to be valid and highly reliable in both clinical samples and the general population [52,53]. The range of scores is 0–24 for each subscale and −24 to 96 for the total fatigue score, which is calculated by subtracting the vigor score from the sum of the four fatigue scales. In a population-based sample of African Americans, mean scores (standard deviations) were 4.02 (4.44) for physical fatigue, 4.02 (4.44) for physical fatigue, 4.23 (4.12) for mental fatigue, 4.02 (4.58) for emotional fatigue, 7.28 (5.90) for general fatigue, and 13.75 (5.55) for vigor [53]. Higher scores indicate more fatigue.
Demographic and health-related variables, such as age, gender, BMI, smoking status (smoker/nonsmoker), and blood pressure may be related to β-AR function [36,41,44] and may also be confounded with symptoms of depression and fatigue [5,54-56]. Thus, these variables were considered as control variables in adjusted regression models.
Statistical Analyses
Statistical analyses were conducted with SPSS version 17.0 for Windows (Chicago, SPSS, Inc.). CD25 values above 3 standard deviations were considered to be outliers and treated as missing values. Screening for multivariate outliers was performed by calculating studentized deleted residuals and centered leverage values [57]. The rate of missing values for CD25 was 3 %. With respect to psychosocial measures and covariates, the rate of missing values was <7 % for each variable. Correlational analyses were conducted for the total sample and separately for African and Caucasian Americans to examine associations between study variables. Hierarchical regression analyses were conducted to examine if symptoms of depression and fatigue explained variance in CD25 (after taking into account aforementioned covariates) and whether ethnicity moderated this relationship between depression/fatigue and CD25. Hierarchical regression analyses were separately conducted for each psychometric scale. Covariates were entered on step 1, ethnicity on step 2, centered depression/fatigue scores on step 3, and centered depression/fatigue score by ethnicity interaction terms on step 4. Interactive effects were further examined by a subsequent series of ethnicity stratified regressions. These separate regressions consisted of the covariates entered on step 1 and depression/fatigue scores entered on the final step. To increase robustness of results and because not all psychological measures were normally distributed (tested by Kolmogorov–Smirnov tests), t tests, correlational analyses, and regression analyses were performed by bootstrapping with 1,000 bootstrap samples and bias-corrected and accelerated (BCa) confidence intervals.
Results
Table 1 shows sample characteristics as well as differences between African and Caucasian Americans. Independent-sample t tests indicated that in vivo β-AR sensitivity (CD25), depressive symptoms, and fatigue did not differ by ethnic groups. However, African Americans were significantly older (p <0.001), had higher BMI values (p <0.001) and were more likely to smoke (p <0.05).
Table 2 shows bivariate correlations of study variables with CD25 for the whole sample and for each ethnic group. Correlation analyses indicated that individuals with more physical and mental fatigue exhibited higher CD25 values (i.e., reduced β-AR sensitivity; p <0.05). Ethnicity stratified analyses indicated differences in depression/fatigue-CD25 relationships between African and Caucasian Americans. While β-AR sensitivity was significantly related to depression (p <0.05), total fatigue (p <0.01), physical fatigue (p <0.001), emotional fatigue (p <0.01), and mental fatigue (p <0.01) in Caucasian Americans, no significant relationship between psychometric measures and CD25 was observed for African Americans (p>0.1). Between-group comparison of correlation coefficients, which were calculated with Cohen and Cohen’s formula 2.8.5 [58] after Fisher’s r-to-z transformation, indicated that correlation coefficients for CD25 and all psychopathological measures differed between ethnic groups (p >0.5).
Table 2.
Pearson correlations (metric variables) and point-biserial correlations (dichotomous variables) of study variables with in vivo β-adrenergic receptor sensitivity (chronotropic25 dose)
| Study variables | Chronotropic25 dose
|
|||
|---|---|---|---|---|
| Total | African Americans | Caucasian Americans | Significance (r) z | |
| Depression (CES-D) | .13 (−.11, .32) | −.23 (−.54, .05) | .32 (.01, .59)* | −2.63** |
| Total fatigue (MFSI total score) | .18 (−.08, .38) | −.18 (−.43, .07) | .47 (.13, .66)** | −3.22** |
| MFSI general fatigue | .04 (−.18, .25) | −.18 (−.44, .09) | .25 (−.10, .49) | −2.03* |
| MFSI physical fatigue | .24 (.01, .45)* | −.11 (−.40, .14) | .51 (.13, .76)*** | −3.13*** |
| MFSI emotional fatigue | .14 (−.11, .35) | −.19 (−.47, .09) | .42 (.06, .62)** | −2.98** |
| MFSI mental fatigue | .25 (.003, .44)* | −.02 (−.31, .22) | .42 (.14, .63)** | −2.17* |
| MFSI vigor | −.06 (−.26, .17) | .19 (−.12, .53) | −.26 (−.49, .04) | 2.13* |
| Age | .25 (.02, .43)* | −.13 (−.43, .19) | .34 (−.003, .58)* | −2.25* |
| Gender | −.26 (−.45, −.01)* | −.22 (−.61, −.05) | −.21 (−.47, .11) | −0.05 |
| Ethnicity | −.15 (−.38, .05) | – | – | – |
| Body mass index (kg/m2) | .17 (−.08, .37) | −.10 (−.43, .25) | .25 (−.15, .52) | −1.65 |
| Smoking status | −.06 (−.22, .08) | −.29 (−.53, −.06) | .11 (−.01, .35) | −1.90 |
| Mean arterial blood pressure (mmHg) | −.07 (−.28, .12) | −.18 (−.49, .15) | −.06 (−.39, .16) | −0.57 |
Correlation analyses based on 1,000 bootstrap samples; 95 % bias corrected and accelerated confidence intervals for correlational coefficients are reported in parentheses. The right column illustrates significant differences between ethnic groups in correlation coefficients calculated with formula 2.8.5 from Cohen and Cohen [58] after Fisher’s r-to-z transformation
CES-D Center for Epidemiologic Studies Depression Scale, MFSI Multidimensional Fatigue Inventory
p<0.05 (two tailed);
p<0.01;
p <0.001; p <0.1 (values set in italics)
Table 3 illustrates results of adjusted hierarchical regression analyses for the whole sample. After adjusting for covariates (step 1) and ethnicity (step 2), a significant main effect was shown for mental fatigue (p <0.05). Table 3 shows that depression, total fatigue, physical fatigue and emotional fatigue interacted with ethnicity (step 4) to predict CD25 (p <0.05). Figure 1 displays these moderating effects.
Table 3.
Summary of adjusted hierarchical regression analyses predicting in vivo β-adrenergic receptor sensitivity (chronotropic25 dose) for the whole sample
| Model | Step | Predictor | Δ R2 | Δ F | b | β |
|---|---|---|---|---|---|---|
| Initial | 1. | Age | 0.20 | 3.96** | 0.2 (0.002, 0.04) | 0.25* |
| Gender | −0.57 (−0.89, −0.25) | −0.34** | ||||
| Body mass index | 0.03 (−0.03, 0.10) | 0.14 | ||||
| Mean arterial blood pressure | −0.11 (−0.03, 0.002) | −0.16 | ||||
| Smoking status | −0.34 (−0.80, 0.13) | 0.21 | ||||
| 2. | Ethnicity | <0.01 | 0.07 | −0.05 (−0.55, 0.41) | −0.03 | |
| Depression (CES-D) | 3. | Depression | 0.01 | 1.06 | 0.10 (−0.01, 0.03) | 0.11 |
| 4. | Ethnicity × depression | 0.05 | 4.41* | 0.04 (0.01, 0.08) | 0.36* | |
| Total fatigue (MFSI total score) | 3. | Total fatigue | 0.01 | 2.89 | 0.01 (−0.01, 0.02) | 0.19 |
| 4. | Ethnicity × total fatigue | 0.06 | 5.54* | 0.03 (0.01, 0.05) | 0.36* | |
| MFSI general fatigue | 3. | General fatigue | 0.04 | 0.32 | 0.01 (−0.02, 0.05) | 0.07 |
| 4. | Ethnicity × general fatigue | 0.02 | 1.68 | 0.26 (0.02, 0.12) | 0.20 | |
| MFSI physical fatigue | 3. | Physical fatigue | 0.04 | 3.22 | 0.05 (−0.02, 0.11) | 0.17 |
| 4. | Ethnicity × physical fatigue | 0.07 | 6.29* | 0.13 (0.01, 0.22) | 0.36* | |
| MFSI emotional fatigue | 3. | Emotional fatigue | 0.01 | 0.78 | 0.02 (−0.03, 0.06) | 0.10 |
| 4. | Ethnicity × emotional fatigue | 0.05 | 4.71* | 0.09 (0.003, 0.19) | 0.31* | |
| MFSI mental fatigue | 3. | Mental fatigue | 0.05 | 4.29* | 0.04 (0.004, 0.09) | 0.22* |
| 4. | Ethnicity × mental fatigue | 0.03 | 3.07 | 0.08 (0.001, 0.17) | 0.30 | |
| MFSI vigor | 3. | Vigor | <0.01 | 0.59 | −0.02 (−0.06, 0.03) | -0.09 |
| 4. | Ethnicity × vigor | 0.01 | 0.65 | −0.02 (−0.12, 0.06) | -0.13 |
Ninety-five percent bias corrected and accelerated confidence intervals for unstandardized beta (b) are reported in parentheses. Confidence intervals based on 1,000 bootstrap samples
Note.
p <0.05 (two tailed);
p <0.01;
p <0.001; p <0.1 (values set in italics)
Fig. 1.
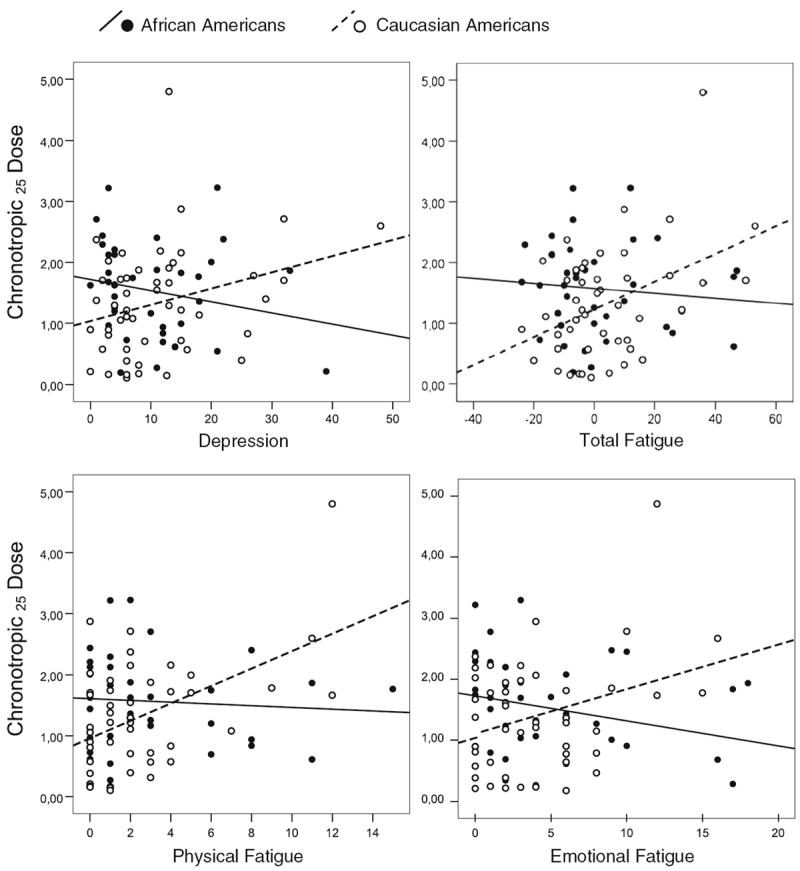
Scatter plots illustrating associations between psychometric measures and in vivo β-adrenergic receptor responsiveness (CD25) in terms of significant depression/fatigue × ethnicity interactions (see Table 3 for regression analyses). Higher values of CD25 indicate reduced sensitivity of β-adrenergic receptors
Follow-up ethnicity stratified regression analyses, adjusted for aforementioned covariates, indicated that CD25 was significantly predicted by total fatigue (ΔR2=0.15, ΔF =7.4, b = 0.03, BCa 95 % CI (0.01, 0.04), β=0.43, p <0.05), physical fatigue (ΔR2=0.17, ΔF =8.75, b =0.12, BCa 95 % CI (0.01, 0.22), β =0.43, p <0.01), emotional fatigue (ΔR2=0.10, ΔF =4.68, b =0.08, BCa 95 % CI (0.001, 0.16), β =0.33, p <0.05), mental fatigue (ΔR2=0.16, ΔF =8.06, b =0.09, BCa 95 % CI (0.03, 0.15), β =0.43, p <0.01), and tended to be predicted by depression in Caucasian Americans (ΔR2= 0.07, ΔF =3.52, b =0.03, BCa 95 % CI (−0.002, 0.05), β = 0.28, p <0.1). No significant relationship was observed in African Americans (p >0.1). The results of adjusted ethnicity stratified regressions have to be interpreted with caution given the reduced statistical power.
Discussion
Depressive symptoms, total fatigue, emotional fatigue and physical fatigue were associated with reduced in vivo β-AR responsiveness (i.e., higher CD25 values) in Caucasian Americans but not in African Americans. The observed association in Caucasian Americans is consistent with previous studies reporting associations of depression and fatigue with hemodynamic and electrophysiological indicators of sympathetic overactivity or autonomic imbalance in clinical samples and in the general population [13,27-32]. Moreover, reduced in vitro β-AR responsiveness on lymphocytes was found in clinically depressed patients [59]. As both depression and fatigue have been linked to higher levels of norepinephrine [27,32], downregulation of β-AR function may result from chronically increased norepinephrine levels.
The relationship between symptoms of depression/fatigue and reduced β-AR responsiveness may be bi-directional. On one hand, a higher number of depressive symptoms and fatigue may reduce the individuals’ ability to cope with stressors, resulting in exaggerated stress responses, prolonged sympathetic overactivity, and thus reduced β-AR responsiveness [41,60,61]. On the other hand, altered β-AR function may contribute to symptoms of fatigue and depression by causing several maladaptive β-AR-mediated sympathetic responses which affect a wide range of neuroendocrinological and immunological pathways [12,13,41,62].
With respect to the observed ethnicity and depression/fatigue interactions, similar findings have been previously reported in other samples and studies. For example, it was shown that depression scores are significantly related to β-AR-mediated responses to mental stress (i.e., increases in heart rate and stroke volume) among Caucasian Americans but not among African Americans [63,64]. Anger suppression and hostility and psychological constructs that are linked to depressive symptoms [65,66], were more strongly related to systolic blood pressure [37] and higher CD25 values [36] in Caucasian than in African Americans.
Biopsychosocial and genetic ethnic differences may contribute to different association patterns. For example, several genetic and biochemical aspects of β-AR receptors and of pathways that interact with β-AR functioning differ between both ethnic groups [67-69] and there are large ethnic differences in the hypotensive response to β-AR blocking drugs [70]. Moreover, a number of symptom and disease-related ethnic differences have been reported such as higher risk of hypertension and CVD in African Americans [71,72], increased lifetime prevalence for major depression in Caucasian Americans [73] and ethnic differences in attitudes and beliefs toward psychological problems (e.g., higher stigmatization in African Americans) [74].
Given the small number of studies that have used the CD25 method, it is noteworthy that we did not observe a significant difference in β-receptor sensitivity between African and Caucasian Americans. This finding is inconsistent with a previous study that reported increased CD25 values in African Americans [36] but consistent with another study showing no significant effect of race on CD25 [75].
Limitations should be considered when interpreting our results. Given the cross-sectional design, causality in the relation between symptoms of depression/fatigue and CD25 cannot be determined. Moreover, although the observed ethnic differences in smoking status, BMI and age may reflect in part “real-world” differences in the USA, these differences may bias our results although we did control for them in adjusted regression models. Our findings are also limited because we only have observed a population-based sample. Clinical groups (e.g., patients with major depression or cancer) and non-clinical groups may differ in terms of how symptoms of depression and fatigue relate to the β-AR functioning.
To conclude, our findings suggest that symptoms of depression and fatigue are related to decreased in vivo β-AR responsiveness in Caucasian Americans. As decreased in vivo β-AR responsiveness results from prolonged sympathetic overactivity, our findings replicate previous research showing that depressive symptoms and fatigue may be related to increased sympathetic activity. Our findings also extend previous research by showing a direct link between these symptoms and β-AR function within the human body. Considering that altered β-AR sensitivity has been implicated in the pathophysiology of CVD [26] our findings may have clinical implications for understanding the interaction between depression/fatigue and CVD. The lack of this association in African Americans highlights the importance for considering ethnicity as a potential moderator in research focusing on associations between psychological variables and cardiovascular function.
Acknowledgments
This work was supported, in part, by grants HL36005, HL57269, HL44915, and 1UL1RR031980 from the National Institutes of Health (to Joel E. Dimsdale).
Contributor Information
Frank Euteneuer, Email: frank.euteneuer@staff.uni-marburg.de, Department of Psychiatry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0804, USA; Division of Clinical Psychology and Psychotherapy, Philipps Universität Marburg, Gutenbergstrasse 18, 35032 Marburg, Germany.
Michael G. Ziegler, Department of Medicine, University of California, San Diego, 200 West Arbor Drive, San Diego, CA 92103-8341, USA
Paul J. Mills, Department of Psychiatry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0804, USA
Winfried Rief, Division of Clinical Psychology and Psychotherapy, Philipps Universität Marburg, Gutenbergstrasse 18, 35032 Marburg, Germany.
Joel E. Dimsdale, Department of Psychiatry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0804, USA
References
- 1.Martin A, Chalder T, Rief W, Braehler E. The relationship between chronic fatigue and somatization syndrome: a general population survey. J Psychosom Res. 2007;63:147–56. doi: 10.1016/j.jpsychores.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Reuter K, Härter M. The concepts of fatigue and depression in cancer. Eur J Cancer Care. 2004;13:127–34. doi: 10.1111/j.1365-2354.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- 3.Thornton LM, Andersen BL, Blakely WP. The pain, depression, and fatigue symptom cluster in advanced breast cancer: covariation with the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system. Health Psychol Off J Div Health Psychol Am Psychol Assoc. 2010;29:333–7. doi: 10.1037/a0018836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donovan KA, Jacobsen PB. Fatigue, depression, and insomnia: evidence for a symptom cluster in cancer. Semin Oncol Nurs. 2007;23:127–35. doi: 10.1016/j.soncn.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Fuhrer R, Wessely S. The epidemiology of fatigue and depression: a French primary-care study. Psychol Med. 1995;25:895–905. doi: 10.1017/s0033291700037387. [DOI] [PubMed] [Google Scholar]
- 6.Bültmann U, Kant I, Kasl SV, Beurskens AJHM, van den Brandt PA. Fatigue and psychological distress in the working population: psychometrics, prevalence, and correlates. J Psychosom Res. 2002;52:445–52. doi: 10.1016/s0022-3999(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 7.Pawlikowska T, Chalder T, Hirsch SR, Wallace P, Wright DJ, Wessely SC. Population based study of fatigue and psychological distress. BMJ (Clinical Research Ed) 1994;308:763–6. doi: 10.1136/bmj.308.6931.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayuso-Mateos JL, Nuevo R, Verdes E, Naidoo N, Chatterji S. From depressive symptoms to depressive disorders: the relevance of thresholds. Br J Psychiatry J Ment Sci. 2010;196:365–71. doi: 10.1192/bjp.bp.109.071191. [DOI] [PubMed] [Google Scholar]
- 9.Kop WJ. Somatic depressive symptoms, vital exhaustion, and fatigue: divergent validity of overlapping constructs. Psychosom Med. 2012;74:442–5. doi: 10.1097/PSY.0b013e31825f30c7. [DOI] [PubMed] [Google Scholar]
- 10.Rief W, Hennings A, Riemer S, Euteneuer F. Psychobiological differences between depression and somatization. J Psychosom Res. 2010;68:495–502. doi: 10.1016/j.jpsychores.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Dimsdale JE, Dantzer R. A biological substrate for somatoform disorders: importance of pathophysiology. Psychosom Med. 2007;69:850–4. doi: 10.1097/PSY.0b013e31815b00e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverman MN, Heim CM, Nater UM, Marques AH, Sternberg EM. Neuroendocrine and immune contributors to fatigue. PM & R J Inj Funct Rehabil. 2010;2:338–46. doi: 10.1016/j.pmrj.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson G, Maes M, Berk M. Biological underpinnings of the commonalities in depression, somatization, and chronic fatigue syndrome. Med Hypotheses. 2012;78:752–6. doi: 10.1016/j.mehy.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Ekmann A, Osler M, Avlund K. The predictive value of fatigue for nonfatal ischemic heart disease and all-cause mortality. Psychosom Med. 2012;74:464–70. doi: 10.1097/PSY.0b013e318258d294. [DOI] [PubMed] [Google Scholar]
- 16.Carney RM, Freedland KE. Are somatic symptoms of depression better predictors of cardiac events than cognitive symptoms in coronary heart disease? Psychosom Med. 2012;74:33–8. doi: 10.1097/PSY.0b013e3182405ac4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekmann A, Petersen I, Mänty M, Christensen K, Avlund K. Fatigue, general health, and ischemic heart disease in older adults. J Gerontol Ser A Biol Sci Med Sci. 2013 doi: 10.1093/gerona/gls180. in press. [DOI] [PubMed] [Google Scholar]
- 18.Majed B, Arveiler D, Bingham A, Ferrieres J, Ruidavets J-B, Montaye M, et al. Depressive symptoms, a time-dependent risk factor for coronary heart disease and stroke in middle-aged men: the PRIME Study. Stroke J Cereb Circ. 2012;43:1761–7. doi: 10.1161/STROKEAHA.111.645366. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763–74. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 20.Goldston K, Baillie AJ. Depression and coronary heart disease: a review of the epidemiological evidence, explanatory mechanisms and management approaches. Clin Psychol Rev. 2008;28:288–306. doi: 10.1016/j.cpr.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Greco A, Steca P, Pozzi R, Monzani D, D’Addario M, Villani A, et al. Predicting depression from illness severity in cardiovascular disease patients: self-efficacy beliefs, illness perception, and perceived social support as mediators. Int J Behav Med. 2013 doi: 10.1007/s12529-013-9290-5. in press. [DOI] [PubMed] [Google Scholar]
- 22.Low KG, Thoresen CE, Pattillo JR, King AC, Jenkins C. Anxiety, depression, and heart disease in women. Int J Behav Med. 1994;1:305–19. doi: 10.1207/s15327558ijbm0104_2. [DOI] [PubMed] [Google Scholar]
- 23.Profant J, Dimsdale JE. Psychosocial factors and congestive heart failure. Int J Behav Med. 2000;7:236–55. [Google Scholar]
- 24.Leenen FH. Cardiovascular consequences of sympathetic hyperactivity. Can J Cardiol. 1999;15:2A–7A. [PubMed] [Google Scholar]
- 25.Remme JW. The sympathetic nervous system and ischaemic heart disease. Eur Heart J. 1998;19(Suppl F):F62–71. [PubMed] [Google Scholar]
- 26.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure: physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–62. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580–92. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- 28.Gold PW, Wong M-L, Goldstein DS, Gold HK, Ronsaville DS, Esler M, et al. Cardiac implications of increased arterial entry and reversible 24-h central and peripheral norepinephrine levels in melancholia. Proc Natl Acad Sci U S A. 2005;102:8303–8. doi: 10.1073/pnas.0503069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. 2009;12:1–21. doi: 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. 2010;67:1067–74. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka M, Mizuno K, Yamaguti K, Kuratsune H, Fujii A, Baba H, et al. Autonomic nervous alterations associated with daily level of fatigue. Behav Brain Funct BBF. 2011;7:46. doi: 10.1186/1744-9081-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boneva RS, Decker MJ, Maloney EM, Lin J-M, Jones JF, Helgason HG, et al. Higher heart rate and reduced heart rate variability persist during sleep in chronic fatigue syndrome: a population-based study. Auton Neurosci Basic Clin. 2007;137:94–101. doi: 10.1016/j.autneu.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Sheffield D, Krittayaphong R, Cascio WE, Light KC, Golden RN, Finkel JB, et al. Heart rate variability at rest and during mental stress in patients with coronary artery disease: differences in patients with high and low depression scores. Int J Behav Med. 1998;5:31–47. doi: 10.1207/s15327558ijbm0501_3. [DOI] [PubMed] [Google Scholar]
- 34.Anderson NB. Racial differences in stress-induced cardiovascular reactivity and hypertension: current status and substantive issues. Psychol Bull. 1989;105:89–105. doi: 10.1037/0033-2909.105.1.89. [DOI] [PubMed] [Google Scholar]
- 35.Anderson NB, Lane JD, Monou H, Williams RB, Houseworth SJ. Racial differences in cardiovascular reactivity to mental arithmetic. Int J Psychophysiol Off J Int Organ Psychophysiol. 1988;6:161–4. doi: 10.1016/0167-8760(88)90047-5. [DOI] [PubMed] [Google Scholar]
- 36.Jain S, Dimsdale JE, Roesch SC, Mills PJ. Ethnicity, social class and hostility: effects on in vivo [beta]-adrenergic receptor responsiveness. Biol Psychol. 2004;65:89–100. doi: 10.1016/s0301-0511(03)00111-x. [DOI] [PubMed] [Google Scholar]
- 37.Dimsdale JE, Pierce C, Schoenfeld D, Brown A, Zusman R, Graham R. Suppressed anger and blood pressure: the effects of race, sex, social class, obesity, and age. Psychosom Med. 1986;48:430–6. doi: 10.1097/00006842-198607000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Cooper DC, Ziegler MG, Nelesen RA, Dimsdale JE. Racial differences in the impact of social support on nocturnal blood pressure. Psychosom Med. 2009;71:524–31. doi: 10.1097/PSY.0b013e31819e3a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dimsdale JE. Stalked by the past: the influence of ethnicity on health. Psychosom Med. 2000;62:161–70. doi: 10.1097/00006842-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Dinos S, Khoshaba B, Ashby D, White PD, Nazroo J, Wessely S, et al. A systematic review of chronic fatigue, its syndromes and ethnicity: prevalence, severity, co-morbidity and coping. Int J Epidemiol. 2009;38:1554–70. doi: 10.1093/ije/dyp147. [DOI] [PubMed] [Google Scholar]
- 41.Mills PJ, Dimsdale JE. The promise of adrenergic receptor studies in psychophysiologic research II: applications, limitations, and progress. Psychosom Med. 1993;55:448–58. doi: 10.1097/00006842-199309000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Cleaveland CR, Rangno RE, Shand DG. A standardized isoproterenol sensitivity test. The effects of sinus arrhythmia, atropine, and propranolol. Arch Intern Med. 1972;130:47–52. doi: 10.1001/archinte.130.1.47. [DOI] [PubMed] [Google Scholar]
- 43.Brodde OE, Kretsch R, Ikezono K, Zerkowski HR, Reidemeister JC. Human beta-adrenoceptors: relation of myocardial and lymphocyte beta-adrenoceptor density. Science. 1986;231:1584–5. doi: 10.1126/science.3006250. [DOI] [PubMed] [Google Scholar]
- 44.Christou DD, Seals DR. Decreased maximal heart rate with aging is related to reduced β-adrenergic responsiveness but is largely explained by a reduction in intrinsic heart rate. J Appl Physiol. 2008;105:24–9. doi: 10.1152/japplphysiol.90401.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hughes JW. Hostility, social support, and adrenergic receptor responsiveness among African–American and white men and women. Psychosom Med. 2003;65:582–7. doi: 10.1097/01.psy.0000041546.04128.43. [DOI] [PubMed] [Google Scholar]
- 46.Sherwood A, Hughes JW, Kuhn C, Hinderliter AL. Hostility is related to blunted beta-adrenergic receptor responsiveness among middle-aged women. Psychosom Med. 2004;66:507–13. doi: 10.1097/01.psy.0000132876.95620.04. [DOI] [PubMed] [Google Scholar]
- 47.Yu B-H, Kang E-H, Ziegler MG, Mills PJ, Dimsdale JE. Mood states, sympathetic activity, and in vivo β-adrenergic receptor function in a normal population. Depression Anxiety. 2008;25:559–64. doi: 10.1002/da.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Euteneuer F, Mills PJ, Rief W, Ziegler MG, Dimsdale JE. Subjective social status predicts in vivo responsiveness of β-adrenergic receptors. Health Psychol Off J Div Health Psychol Am Psychol Assoc. 2012;31:525–9. doi: 10.1037/a0025990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Mellédo JM, Arthur H, Dalton J, Woo C, Lipton N, Bellavance F, et al. The influence of type A behavior pattern on the response to the panicogenic agent CCK-4. J Psychosom Res. 2001;51:513–20. doi: 10.1016/s0022-3999(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 50.Radloff LS. The CES-D scale. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 51.Naughton MJ, Wiklund I. A critical review of dimension-specific measures of health-related quality of life in cross-cultural research. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 1993;2:397–432. doi: 10.1007/BF00422216. [DOI] [PubMed] [Google Scholar]
- 52.Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manag. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cordero ED, Loredo JS, Murray KE, Dimsdale JE. Characterizing fatigue: the effects of ethnicity and acculturation. J Appl Biobehav Res. 2012;17:59–78. doi: 10.1111/j.1751-9861.2012.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mirowsky J, Ross CE. Age and depression. J Health Soc Behav. 1992;33:187–205. discussion 206–12. [PubMed] [Google Scholar]
- 55.Kessler RC, McGonagle KA, Nelson CB, Hughes M, Swartz M, Blazer DG. Sex and depression in the National Comorbidity Survey. II: cohort effects. J Affect Disord. 1994;30:15–26. doi: 10.1016/0165-0327(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 56.Vgontzas AN, Bixler EO, Chrousos GP. Obesity-related sleepiness and fatigue: the role of the stress system and cytokines. Ann N Y Acad Sci. 2006;1083:329–44. doi: 10.1196/annals.1367.023. [DOI] [PubMed] [Google Scholar]
- 57.Fox J. Regression diagnostics: an introduction. Newbury Park: Sage publications; 1991. [Google Scholar]
- 58.Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. Hillsdale: Erlbaum; 1983. [Google Scholar]
- 59.Mazzola-Pomietto P, Azorin J-M, Tramoni V, Jeanningros R. Relation between lymphocyte β-adrenergic responsivity and the severity of depressive disorders. Biol Psychiatry. 1994;35:920–5. doi: 10.1016/0006-3223(94)91238-6. [DOI] [PubMed] [Google Scholar]
- 60.Felton BJ, Revenson TA. Coping with chronic illness: a study of illness controllability and the influence of coping strategies on psychological adjustment. J Consult Clin Psychol. 1984;52:343–53. doi: 10.1037//0022-006x.52.3.343. [DOI] [PubMed] [Google Scholar]
- 61.Hijzen TH, Van Der Gugten J, Bouter L. Active and passive coping under different degrees of stress; effects on urinary and plasma catecholamines and ECG T-wave. Biol Psychol. 1984;18:23–32. doi: 10.1016/0301-0511(84)90023-1. [DOI] [PubMed] [Google Scholar]
- 62.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 63.Delehanty SG, Dimsdale JE, Mills P. Psychosocial correlates of reactivity in black and white men. J Psychosom Res. 1991;35:451–60. doi: 10.1016/0022-3999(91)90040-u. [DOI] [PubMed] [Google Scholar]
- 64.Haeri S, Mills P, Nelesen R, Berry C, Ziegler M, Dillon E, et al. Acute psychologic stress reactivity in blacks versus whites: relationship to psychologic characteristics. Blood Press Monit. 1996;1:27–32. [PubMed] [Google Scholar]
- 65.Cheung RYM, Park IJK. Anger suppression, interdependent self-construal, and depression among Asian American and European American college students. Cult Divers Ethn Minor Psychol. 2010;16:517–25. doi: 10.1037/a0020655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moreno JK, Selby MJ, Fuhriman A, Laver GD. Hostility in depression. Psychol Rep. 1994;75:1391–401. doi: 10.2466/pr0.1994.75.3.1391. [DOI] [PubMed] [Google Scholar]
- 67.Moore JD, Mason DA, Green SA, Hsu J, Liggett SB. Racial differences in the frequencies of cardiac beta(1)-adrenergic receptor polymorphisms: analysis of c145A>G and c1165G>C. Hum Mutat. 1999;14:271. doi: 10.1002/(SICI)1098-1004(1999)14:3<271::AID-HUMU14>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 68.Mills PJ, Dimsdale JE, Ziegler MG, Nelesen RA. Racial differences in epinephrine and 2-adrenergic receptors. Hypertension. 1995;25:88–91. doi: 10.1161/01.hyp.25.1.88. [DOI] [PubMed] [Google Scholar]
- 69.Dimsdale J, Ziegler M, Graham R. The effect of hypertension, sodium, and race on isoproterenol sensitivity. Clin Exp Hypertens Part A Theory Pract. 1988;10:747–56. doi: 10.1080/07300077.1988.11878781. [DOI] [PubMed] [Google Scholar]
- 70.Gupta AK, Poulter NR, Dobson J, Eldridge S, Cappuccio FP, Caulfield M, et al. Ethnic differences in blood pressure response to first and second-line antihypertensive therapies in patients randomized in the ASCOT Trial. Am J Hypertens. 2010;23:1023–30. doi: 10.1038/ajh.2010.105. [DOI] [PubMed] [Google Scholar]
- 71.Long Y, Gracely EJ, Newschaffer CJ, Liu L. Analysis of the prevalence of cardiovascular disease and associated risk factors for European-American and African–American populations in the State of Pennsylvania 2005–2009. Am J Cardiol. 2012;111:72–68. doi: 10.1016/j.amjcard.2012.08.045. [DOI] [PubMed] [Google Scholar]
- 72.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA J Am Med Assoc. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 73.Williams DR, González HM, Neighbors H, Nesse R, Abelson JM, Sweetman J, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean blacks, and non-Hispanic whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305–15. doi: 10.1001/archpsyc.64.3.305. [DOI] [PubMed] [Google Scholar]
- 74.Bailey RK, Patel M, Barker NC, Ali S, Jabeen S. Major depressive disorder in the African American population. J Natl Med Assoc. 2011;103:548–57. doi: 10.1016/s0027-9684(15)30380-1. [DOI] [PubMed] [Google Scholar]
- 75.Hughes JW, Sherwood A, Blumenthal JA, Suarez EC, Hinderliter AL. Hostility, social support, and adrenergic receptor responsiveness among African–American and white men and women. Psychosom Med. 2007;65:582–7. doi: 10.1097/01.psy.0000041546.04128.43. [DOI] [PubMed] [Google Scholar]


