Abstract
Background
BRAF mutations occur in 5–10% of metastatic colorectal cancers and are biomarkers associated with a poor prognosis. However, the outcomes with standard chemotherapy over sequential lines of therapy in a large cohort of patients with BRAF-mutant tumors have not been described.
Methods
We searched the MD Anderson Cancer Center databases for patients with colorectal cancer patients and identified BRAF mutations between December 2003 and May 2012. Patients were analyzed for clinical characteristics, progression-free survival (PFS), overall survival (OS), and chemotherapeutic agents used. Survival was estimated according to the Kaplan-Meier method.
Results
Among the 1567 patients tested for BRAF mutations at our institution, 127 (8.1%) had tumors with BRAF mutations. The 71 patients who presented with metastatic disease received a median of 2 lines of chemotherapy. For the first three lines of chemotherapy, median progression-free survivals were 6.3 months (n=69 patients, 95% confidence interval (CI) of 4.9–7.7 months), 2.5 months (n=58, 95% CI of 1.8–3.0 months), and 2.6 months (n=31, 95% CI of 1.0–4.2 months), respectively. Median PFS was not affected by the backbone chemotherapeutic agent in the first-line setting, whether oxaliplatin-based or irinotecan-based (6.4 months vs. 5.4 months, respectively, p-value = 0.99).
Conclusions
Progression-free survival is expectedly poor for patients with BRAF-mutated metastatic colorectal cancer. Despite the ascertainment bias present (with testing preferentially performed in patients suitable for clinical trials in refractory disease), these data provide historic controls suitable for future study design and support the idea that novel therapeutic options are essential in this population.
Introduction
Colorectal cancer is the second-leading cause of cancer-related mortality in the United States, with more than 50,000 deaths estimated in 2012.1 Even so, survival for patients with metastatic colorectal cancer has improved owing to the advent of improved cytotoxic chemotherapeutic agents,2–4 anti–vascular endothelial growth factor antibodies,5,6 and the more frequent use of curative hepatic metastasectomies.7–9 Additionally, greater understanding of the molecular pathways driving survival and proliferation of colorectal tumor cells has led to the approval of novel targeted therapies—namely, cetuximab and panitumumab—specifically tailored to inhibit epidermal growth factor receptor (EGFR) pathways and thereby thwart tumor growth and improve survival in patients with a wild-type KRAS oncogene.10,11
Despite such advances, patients with colorectal cancer harboring mutations in the BRAF oncogene (present in 5%–10% of all colorectal tumors12,13) have traditionally poor survival outcomes and low response rates when treated with the aforementioned therapies14–16. BRAF mutations, most commonly a valine to glutamic acid substitution of the 600th amino acid (V600E)12, generate a conformational change of the RAF kinase, leading to constitutive activation of the BRAF kinase and the downstream MAPK pathway, which are implicated in tumor cell proliferation and anti-apoptotic behavior17,18. In a phase I trial of the mutated BRAF inhibitor vemurafenib, patients with BRAF-mutated metastatic colorectal cancers demonstrated a response rate of approximately 5% when treated with this drug as a single agent19, much lower than the approximately 50% of patients with metastatic melanoma who responded to the same therapy in the seminal phase III trial20,21
To our knowledge, no prior studies have described progression-free survival (PFS) across sequential lines of chemotherapy among patients with BRAF-mutated metastatic colorectal cancer. To that end, we retrospectively evaluated the clinicopathologic features and survival of 127 patients with BRAF-mutated colorectal cancer treated at The University of Texas MD Anderson Cancer Center.
Methods
Identification of Patients with BRAF-Mutated Colorectal Cancer
The MD Anderson institutional computerized database were searched to identify patients with a diagnosis of colorectal cancer who were evaluated and assessed for treatment at our institution between December 2003 and May 2012. Tumors were classified and staged according to clinical and pathologic information (American Joint Committee on Cancer [AJCC] Cancer Staging Manual, 7th edition)22.
Patients with colorectal cancer were included for analysis in this series only if their pathology reports revealed a BRAF mutation in either the primary tumor or a metastasis, (depending on the tissue available). To determine whether a BRAF mutation was present, DNA was extracted from sections of microdissected paraffin-embedded blocks and analyzed by both polymerase chain reaction and pyrosequencing from codons 595 to 600 of the BRAF oncogene. This assay has the sensitivity to detect approximately 1 in 10 mutation-bearing cells in the microdissected area.
Microsatellite Testing
Microsatellite stability or instability was determined by one of two methods: (1) DNA was extracted from paraffin-embedded sections of microdissected tumor and adjacent sections of non-neoplastic colorectal tissue surrounding the tumor and analyzed by polymerase chain reaction followed by capillary electrophoretic detection of microsatellite repeats. Here, a panel of seven microsatellite markers (BAT25, BAT26, BAT40, D2S123, D5S346, D17S250, and TGFB2) was evaluated to detect changes in the numbers of microsatellite repeats in tumor tissue compared with the adjacent normal tissue from the same patient. Tumors bearing five or more markers with higher numbers of microsatellite repeats relative to the normal tissue controls were deemed to exhibit microsatellite instability; or, (2) tumor samples were tested with immunohistochemical stains using antibodies against the proteins MLH1, MSH2, MSH6, and PMS2. Microsatellite instability was defined as the loss of one or more of these proteins in the tumor tissue compared with the adjacent normal tissue.
Statistical Analyses
Once those patients with BRAF mutations had been identified, their medical records were retrospectively reviewed to obtain demographic, clinicopathologic, treatment, and outcome data according to an institutional review board–approved protocol. Descriptive statistics were used to characterize the patient population. OS was defined as the time between the date of diagnosis and date of death or date of last follow-up. PFS was defined as the time between the date of treatment initiation and either the date of radiographic disease progression (as determined by the interpreting radiologist at our institution) or the date of death. Survival curves were generated using the Kaplan-Meier method, and the differences between curves were calculated with the log-rank test. The effects of patient demographics, disease, and treatment characteristics on survival outcomes were analyzed using the methods of Kaplan and Meier with a two-sided p-value of less than 0.05 considered significant. Hazard ratios were estimated with univariate Cox proportion hazard models.
Results
Patient Demographics
Among the 1567 patients with colorectal cancer tested for activating BRAF mutations, 127 patients (8.1%) were found to have BRAF–mutant tumors. Table 1 summarizes the demographic characteristics of these 127 patients and the clinicopathologic features of their disease. The median age at diagnosis was 60.0 years (range, 27–73 years). Most tumors (59.8%) were located in the right colon and were of moderate or poor histologic grade (2–3). 94% of the patients had tumors which carried a V600E mutation in the BRAF oncogene. Six tumors had D594G mutations, and one had a G496R mutation.
TABLE 1.
PATIENT DEMOGRAPHICS AND DISEASE CHARACTERISTICS
| Characteristic | Stage I–III (n=56) | Stage IV (n=71) |
|---|---|---|
| Median Age at Diagnosis (years) | 61 | 59 |
| Sex (%) | ||
| Male | 26 (46.4%) | 39 (54.9%) |
| Female | 30 (53.6%) | 32 (45.1%) |
| Race/Ethnicity (%) | ||
| Caucasian | 49 (87.5%) | 60 (84.5%) |
| African American | 5 (8.9%) | 2 (2.8%) |
| Hispanic | 1 (1.8%) | 2 (2.8%) |
| Asian | 1 (1/8%) | 7 (9.9%) |
| Stage at Diagnosis (%) | ||
| I | 8 (14.3%) | -- |
| II | 15 (26.8%) | -- |
| III | 33 (58.9%) | -- |
| IV | -- | 71 |
| T Stage (%) | ||
| T1 | 0 | 0 |
| T2 | 11 (14.3%) | 4 (5.6%) |
| T3 | 35 (62.5%) | 20 (28.2%) |
| T4 | 10 (17.9%) | 25 (35.2%) |
| TX | 0 | 22 (31.0%) |
| N Stage (%) | ||
| N0 | 23 (41.1%) | 3 (4.2%) |
| N1 | 15 (26.8%) | 14 (19.7%) |
| N2 | 18 (32.1%) | 31 (43.7%) |
| NX | 0 | 23 (32.4%) |
| Tumor Grade (%) | ||
| 1 (well differentiated) | 1 (1.8%) | 0 |
| 2 (moderately differentiated) | 35 (62.5%) | 36 (50.7%) |
| 3 (poorly differentiated) | 20 (35.7%) | 35 (49.3%) |
| Site of Primary Tumor (%) | ||
| Right | 35 (62.5%) | 41 (57.8%) |
| Left | 12 (21.4%) | 15 (21.1%) |
| Rectal | 9 (16.1%) | 15 (21.1%) |
| BRAF Mutation Type (%) | ||
| V600E | 53 (94.6%) | 67 (94.4%) |
| D594G | 2 (3.6%) | 4 (5.6%) |
| G496R | 1 (1.8%) | 0 |
Characteristics of Patients with Stages I–III Disease at Diagnosis
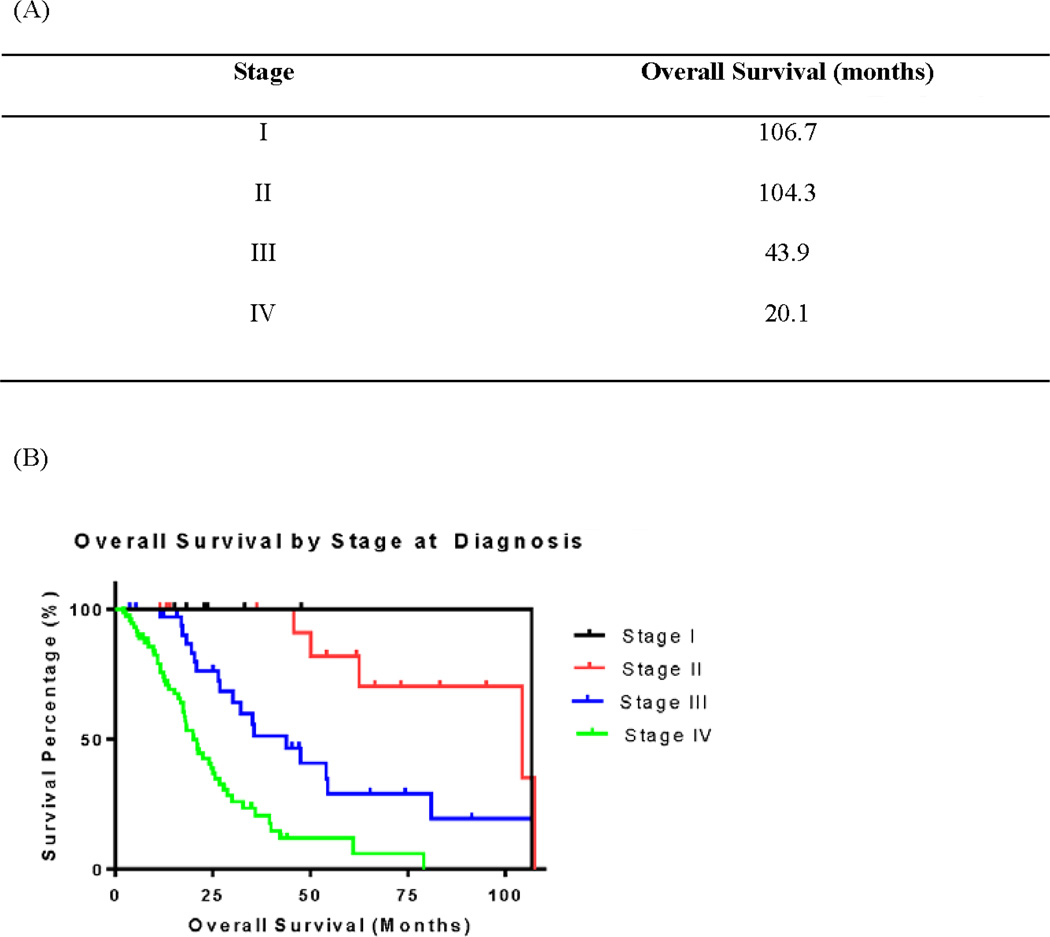
All fifty-six patients with stage I-III disease at diagnosis underwent surgical resection of their primary tumors. The median OS for this group was 62.6 months, and was strongly associated with stage (Figure 1, p<0.001). Higher T stages and higher N stages were associated with shorter median OS (Table 2; p=0.04 and p=0.0006, respectively). Microsatellite stability testing was performed in 36 of these patients. Right-sided primary tumors were more likely to demonstrate microsatellite instability when compared to tumors arising from the left colon/rectum (OR 85.7, p=0.004) (Table 3). In fact, all patients with microsatellite-high (MSI-H) tumors had primary tumors located in the right colon.
Figure 1.
Overall Survival According to Stage at Diagnosis
TABLE 2.
SURVIVAL CHARACTERISTICS OF PATIENTS WITH STAGES I-III DISEASE AT DIAGNOSIS
| Characteristic | N | Events | Median Overall Survival (months) |
Two-Year Overall Survival Rate (%) |
p-value |
|---|---|---|---|---|---|
| Overall Survival | 56 | 22 | 62.6 | 86.8 | |
| Age at Diagnosis | |||||
| Age < 50 years | 8 | 3 | 104.3 | 83.3 | 0.87 |
| Age ≥ 50 years | 48 | 21 | 54.5 | 85.4 | |
| Sex | |||||
| Male | 26 | 12 | 62.6 | 78.5 | 0.66 |
| Female | 30 | 12 | 54.5 | 95.7 | |
| T Stage | |||||
| T1 | -- | -- | -- | -- | 0.04 |
| T2 | 11 | 1 | 106.7 | 100.0 | |
| T3 | 35 | 17 | 54.1 | 86.7 | |
| T4 | 10 | 5 | 45.8 | 64.3 | |
| N Stage | |||||
| N0 | 23 | 6 | 104.3 | 100.0 | <0.01 |
| N1 | 15 | 6 | 54.5 | 83.3 | |
| N2 | 18 | 11 | 32.2 | 67.2 | |
| Site of Primary Tumor | |||||
| Right | 35 | 11 | 54.5 | 88.7 | 0.78 |
| Left | 12 | 8 | 45.8 | 64.1 | |
| Rectal | 9 | 5 | 62.6 | 100.0 | |
| BRAF Mutation Type | |||||
| V600E | 53 | 20 | 54.5 | 86.2 | 0.76 |
| D594G/N | 2 | 1 | 62.6 | 100.0 | |
| G496R | 1 | 1 | 45.8 | 100.0 |
TABLE 3.
MICROSATELLITE TESTING RESULTS ACCORDING TO SITE OF PRIMARY TUMOR
| MSS | MSI-H | |
|---|---|---|
| Stage I–III: No Recurrent Disease | ||
| Left Colon/Rectum | 0 | 0 |
| Right Colon | 1 | 16 |
| Stage I–III: Recurrent Disease | ||
| Left Colon/Rectum | 11 | 0 |
| Right Colon | 4 | 4 |
| Stage IV at Presentation | ||
| Left Colon/Rectum | 16 | 0 |
| Right Colon | 17 | 3 |
Among the stage I–III patients, 39 (69.6%) developed recurrent disease after undergoing initial surgical resection (Table 4). Those with recurrent disease had a median OS of 27.1 months and were less likely to be alive at the time of data analysis (p<0.001) than those without disease recurrence, all of whom were still alive at the same time of data analysis (median follow-up of 24.8 months, range 5.3–110.8 months). The primary tumor site was not associated with survival in patients with recurrent disease. Recurrent disease was observed in 94% of patients with microsatellite-stable (MSS) tumors and was more strongly associated with MSS-tumors when compared to the population with MSI-H tumors (OR 60.0, p< 0.001).
TABLE 4.
CHARACTERISTICS OF PATIENTS WITH RECURRENCE AFTER SURGICAL RESECTION
| Characteristic | No Recurrence (Stage I- III) |
p-value1 | Recurrence (Stage I- III) |
p-value2 | Stage IV |
|---|---|---|---|---|---|
| Number of Patients | 17 | 39 | 71 | ||
| Sex | |||||
| Male | 5 | 0.36 (0.10) | 21 | 0.96 (0.91) | 39 |
| Female | 12 | 18 | 32 | ||
| Primary Tumor Site | |||||
| Right | 17 | 40.7 (0.01) | 18 | 0.63 (0.24) | 41 |
| Left | 0 | 12 | 15 | ||
| Rectum | 0 | 9 | 15 | ||
| Site of Recurrence/Metastasis | |||||
| Lung | -- | 5 | 0.66 (0.46) | 13 | |
| Liver | -- | 7 | 0.08 (<0.01) | 43 | |
| Peritoneum | -- | 21 | 2.98 (<0.01) | 20 | |
| Lymph Nodes | -- | 10 | 0.53 (0.15) | 28 | |
| Rectum | -- | 3 | -- | -- | |
| Survival (months) | |||||
| Overall | Not reached | 27.1 | 0.18 | 21.0 | |
| Right | 23.4 | ||||
| Left | 17.0 | ||||
| Rectum | 37.5 | ||||
| Vital Status at Time of Analysis | |||||
| Alive | 17 | 68.7 (<0.01) | 13 | 1.10 (0.83) | 21 |
| Deceased | 0 | 26 | 50 | ||
| Stage at Diagnosis | |||||
| I | 5 | 2 | -- | ||
| II | 7 | 8 | -- | ||
| III | 5 | 29 | -- | ||
| IV | -- | -- | 71 | ||
| Microsatellite Status3 | |||||
| Stable | 1 | 0.02 (<0.01) | 15 | ||
| Unstable | 16 | 4 |
Compares the recurrence and no recurrence groups
Compares the recurrence and stage IV groups
Only 36 of the patients with initial non-metastatic disease had tissue available for microsatellite testing.
Characteristics of Patients with Stages IV Disease at Diagnosis
Seventy-one patients had metastatic disease at diagnosis (Table 5). The liver was the most common site of metastasis at diagnosis, with metastases detected in 43 (60.6%) patients (Table 4). Of the 43 patients with liver metastases, eight (18.6%) were able to undergo hepatic metastasectomy. After a median follow up of 14.3 months, 63% of patients had disease recurrence, with a median recurrence-free survival of 9.1 months following hepatic resection. Other sites of metastases detected at diagnosis were the lungs (13 patients), distant/non-regional lymph nodes (28), and the peritoneum (20).
TABLE 5.
CHARACTERISTICS OF PATIENTS WITH STAGE IV DISEASE AT DIAGNOSIS
| Characteristic | N | Events | Median Overall Survival Time (months) |
Two–Year Overall Survival Rate (%) |
p-value |
|---|---|---|---|---|---|
| Overall Survival | 71 | 50 | 20.1 | 42.5 | |
| Age at Diagnosis | |||||
| Age < 50 | 19 | 15 | 17.4 | 28.9 | 0.38 |
| Age ≥ 50 | 52 | 35 | 24.1 | 47.3 | |
| Sex | |||||
| Male | 39 | 27 | 21.2 | 42.7 | 0.23 |
| Female | 32 | 23 | 21.0 | 45.5 | |
| T Stage | |||||
| T1 | 0 | -- | -- | -- | 0.17 |
| T2 | 4 | 4 | 12.5 | 25.0 | |
| T3 | 20 | 15 | 12.4 | 34.1 | |
| T4 | 25 | 18 | 22.5 | 43.7 | |
| N Stage | |||||
| N0 | 3 | 3 | 12.4 | 0 | 0.34 |
| N1 | 14 | 10 | 34.5 | 71.4 | |
| N2 | 31 | 23 | 17.4 | 25.6 | |
| Site of Primary Tumor | |||||
| Right | 41 | 28 | 21.0 | 41.0 | 0.88 |
| Left | 15 | 11 | 24.6 | 53.6 | |
| Rectal | 15 | 11 | 17.8 | 37.5 | |
| BRAF Mutation | |||||
| Type | |||||
| V600E | 67 | 47 | 20.0 | 40.0 | 0.04 |
| D594G | 4 | 3 | 47.2 | 100.0 |
Characteristics of patients initially diagnosed with stage I–III disease whose tumors recurred were compared with those of patients who had stage IV disease at the time of diagnosis. Patients with recurrent tumors were most likely to develop oligometastatic disease in the peritoneum and/or at the site of their initial surgical resection (24 of 39 patients who developed recurrent disease); peritoneal disease was more common in patients with recurrent tumors than in those with metastatic disease detected at diagnosis (21 of 39 patients vs. 20 of 71 patients, respectively; p<.01).
Survival Outcomes of Patients with Metastatic Disease
The patients with stage IV disease had a median OS of 20.1 months, with a 2-year OS rate of 42.5% (Figure 1). No associations between OS and age, sex, or site of the primary tumor were found in patients with metastatic disease. However, 67 patients (94.3%) with metastatic disease had V600E BRAF mutations. The remaining four patients with D594G mutations were still alive 2 years after their initial diagnosis, with a median OS of 42.2 months, whereas patients with V600E mutations had a median OS of 20.0 months (hazard ratio for death among those with D594G mutations=0.45, p=0.04).
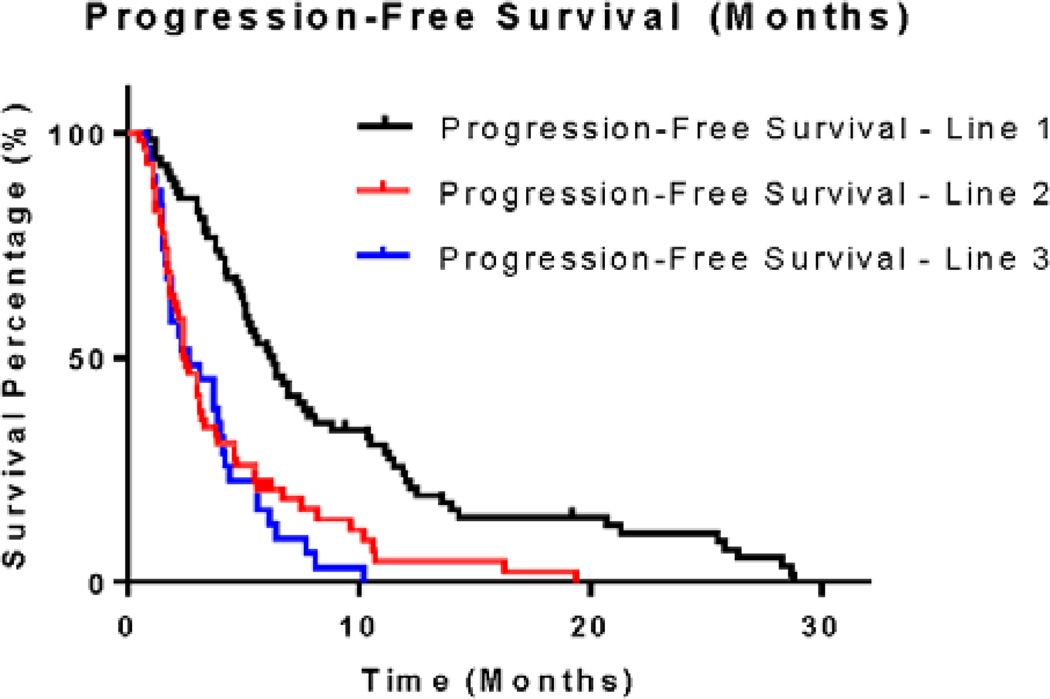
Sixty-nine of the 71 patients with metastatic disease received systemic therapy (Table 6). The performance status of the remaining two patients did not allow treatment initiation. The median number of lines of systemic therapy was two. The type of systemic therapy used (e.g., oxaliplatin-based or irinotecan-based) did not significantly affect PFS in any line of treatment. Most patients (61 of 69) received an oxaliplatin-based regimen as their first-line treatment, and irinotecan-based therapies were most common in the second-line setting (39 of 58). The median progression-free survivals for patients after one, two, and three lines of therapy were 6.3 months, 2.5 months, and 2.6 months, respectively (Figure 2). Although a short duration of benefit was noted with first-line treatment, the median PFS in patients who received two or three lines of therapy corresponded to the time of the first restaging scans. In the second-line setting, 28 of the 39 patients treated with irinotecan concomitantly received an anti-EGFR monoclonal antibody (cetuximab or panitumumab). No difference in PFS was observed for those patients receiving an anti-EGFR monoclonal antibody when compared to those patients who did not.
TABLE 6.
PROGRESSION-FREE SURVIVAL BY LINE OF TREATMENT AND BY TREATMENT REGIMEN EMPLOYED
| Line of Treatment | N | Progression-Free Survival (months; 95% confidence interval) |
p-value |
|---|---|---|---|
| Line 11 | 69 | 6.3 (4.9–7.7) | |
| Oxaliplatin-based | 61 | 6.4 | 0.63 |
| Irinotecan-based | 6 | 5.4 | |
| 5-FU2-based | 2 | 4.6 | |
| Line 2 | 58 | 2.5 (1.8–3.0) | |
| Oxaliplatin-based | 7 | 3.0 | 0.18 |
| Irinotecan-based | 39 | 3.0 | |
| 5-FU2-based | 4 | 3.1 | |
| Clinical trial | 4 | 3.6 | |
| Other | 4 | 1.7 | |
| Line 3 | 31 | 2.5 (1.0–4.2) | |
| Oxaliplatin-based | 5 | 3.1 | 0.19 |
| Irinotecan-based | 8 | 3.1 | |
| 5-FU2-based | 7 | 6.1 | |
| Clinical trial | 11 | 3.7 | |
| Other | 8 | 1.6 |
Two patients were too ill at presentation to receive systemic therapy.
5-fluorouracil without oxaliplatin or irinotecan.
Figure 2.
Progression-Free Survival by Line of Therapy
Of note, despite the poor prognosis of the majority of patients with BRAF-mutant metastatic tumors, a rare subset of patients had prolonged disease control with systemic chemotherapy and a more indolent course, as represented by an approximate 15% two-year PFS with first-line therapy. A trend toward an improved survival was noted among the eleven patients who underwent metastectomy (8 liver resections, 1 lung resection, 1 lymph node dissection, and 1 brain resection) relative to those who did not proceed to metastectomy (median OS 34.8 versus 17.9 months, respectively; p=0.066). However, the small sample size does not allow better characterization of this subset. With the exception of the majority of patients with D594G BRAF mutations found within this group, no other clinicopathologic parameters (age at presentation, gender, ethnicity, site of primary tumor, distribution of metastases) were unique to this population demonstrating the prolonged survival.
Discussion
To our knowledge, no previous studies have quantified progression-free survival outcomes across multiple lines of therapy specifically for patients with BRAF-mutant metastatic colorectal cancer. With a median PFS of 6.3 months, patients seemed to receive some survival benefit from the first line of systemic therapy upon treatment initiation for metastatic disease. However, the median PFS of patients who underwent second and third lines of treatment were both approximately 2.5 months and correspond to the time of first restaging. That these tumors grew without any apparent radiographic improvement in the second- and third-line settings reinforces the notion that refractory BRAF-mutant metastatic colorectal cancer does not respond well to traditional chemotherapy regimens. Those with metastatic disease tolerated a median of two lines of treatment before having to discontinue therapy permanently. Collectively, these results reinforce the notion that this disease responds very poorly to systemic treatment.
The 71 patients with metastatic disease in this single-institution study had a longer median OS (20.1 months) from the time of diagnosis than those of other cohorts of patients with metastatic, BRAF-mutated colorectal cancer13,23–28. This result may reflect an ascertainment bias present in our cohort representing a group of patients with a robust performance status able to travel for clinical trials. Indeed, we have previously reported median OS of 10.4 months for a retrospective population cohort without the BRAF ascertainment bias23. Nonetheless, a subset analysis of a phase II study of frontline FOLFOXIRI chemotherapy in combination with bevacizumab for patients with metastatic colorectal cancer showed a median PFS of 12.8 months and an OS of 23.8 months for those with BRAF mutations29. Although only ten highly selected patients with BRAF mutations were included in this study, the median OS is similar to what we report and reinforces the notion that patients able to withstand aggressive systemic treatments may demonstrate benefit despite the presence of a BRAF mutation.
In addition, the choice of an oxaliplatin- or an irinotecan-based regimen did not affect PFS. Prior studies of this subset of patients with colorectal cancer have failed to demonstrate significant differences in response between different types of treatments used30,31, a finding reinforcing the idea that the presence of a BRAF mutation is not predictive of response to the currently available therapies.
In our cohort, the four patients with D594G mutations showed a longer OS than those with V600E mutations. Scant information regarding outcomes of patients with metastatic D594G BRAF-mutated colorectal cancer has been reported thus far, with one study citing a lone patient demonstrating an objective response to systemic treatment.32 In vitro work with melanoma cells has shown the D594G BRAF mutation generates a significantly less active BRAF kinase and thereby confers less constitutive activation of the MAPK pathway that is readily triggered by the V600E-mutant BRAF kinase17,33. These clinical results further corroborate the notion that non- V600E mutations may display different clinical phenotypes from those of the more common V600E BRAF-mutated tumors; if these findings are validated prospectively, patients harboring D594 mutations may need to be studied prospectively and independently from patients whose tumors contain BRAF V600E mutations.
Of the 56 patients with non-metastatic disease at the time of diagnosis, 39 (69.6%) eventually developed recurrence. Among these patients, the median OS did not significantly differ from that of the patients with metastatic disease at the time of diagnosis (27.1 months vs. 21.0 months, respectively; p=0.18). Prior series have shown that patients with BRAF-mutant colorectal cancer who are initially diagnosed with stage II or III disease have poor survival after recurrence34 and worse OS16 relative to similar patients with wild-type BRAF tumors. Thus, when compared to patients with BRAF-mutated tumors whose disease was metastatic at initial presentation, it is not surprising that the patients in our cohort who recurred displayed similarly poor outcomes and did not respond well to available therapeutic options.
Microsatellite status and recurrence were clearly related. All patients with left colon or rectal primary tumors that were tested for microsatellite instability had microsatellite-stable tumors, and all these patients developed recurrent disease. Likewise, primary tumors arising in the right colon that demonstrated microsatellite instability were less likely to recur. Our findings are consistent with prior findings that patients with BRAF-mutated tumors with microsatellite stability are associated with worse survival outcomes relative to those with microsatellite instability.16,34,35 Large efforts are underway to better define the prognostic role of BRAF mutation in stage II/III colon cancer and the interaction of this prognostic effect with microsatellite instability and tumor location.
We recognize that many of the metastatic patients described here were initially screened for a BRAF mutation because they were being considered for participation in a clinical trial and thus had a performance status suitable for trial eligibility. Therefore, our OS results likely overestimate the survival of all patients with metastatic BRAF-mutated colorectal cancer, as many patients with more aggressive phenotypes would have died before being evaluated for study eligibility. Nonetheless, even with survival outcomes superior to prior reports, this group of patients not only had poor disease response to available systemic chemotherapy and biologic agents but also proved unable to withstand sequential lines of treatment. Treatment of these patients with all available standard-of-care therapies is unlikely to be possible due to declining performance status, and appears to provide little or no improvement in progression-free survival for most patients. Early engagement in the many open or planned studies for this specific population is warranted. In the absence of access to such studies, a regimen of 5-FU, oxaliplatin, and irinotecan may be considered in selected good performance status patients based on the prior report29. Thus, therapeutic options remain poor for patients with BRAF-mutated colorectal cancer, and our findings reinforce the need for a greater understanding of the activated pathways driving these tumors that could be translated into more effective treatment.
Conclusion
Progression-free survivals across sequential lines of systemic therapy are poor in patients with BRAF-mutated metastatic colorectal cancer, and by the second line of treatment, correspond to the time of first restaging scans. Our data provide historical controls for which responses to therapies used in the treatment of trial-eligible BRAF-mutated metastatic colorectal cancer patients can be compared in the future. As patients with this subset of disease do not respond well to currently available traditional treatment options, these results reinforce the notion that additional prospective studies are necessary in order to offer more effective options to patients.
Clinical Practice Points.
-
·
Mutations in the BRAF oncogene are a poor prognostic marker in patients with metastatic colorectal cancer. Responses to standard therapies are poor and are of short duration.
-
·
Enrollment into clinical trials is appropriate for these patients prior to exhausting all available standard-of-care therapies.
-
·
Patients with MSS/BRAF-mutated tumors are more likely to develop disease recurrence than those with MSI-H/BRAF-mutated tumors.
-
·
In the metastatic setting, patients with D594G BRAF mutations may not have as aggressive a disease as patients with V600E BRAF mutations.
Acknowledgments
The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant CA16672.
Funding: The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant CA16672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Dr. Kopetz is supported by CA172670. The remaining authors report no conflicts of interest.
REFERENCES
- 1.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J. Clin. 2012 Jul-Aug;62(4):220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008 Mar 22;371(9617):1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andre T, Louvet C, Maindrault-Goebel F, et al. CPT-11 (irinotecan) addition to bimonthly, high-dose leucovorin and bolus and continuous-infusion 5-fluorouracil (FOLFIRI) for pretreated metastatic colorectal cancer. GERCOR. Eur. J. Cancer. 1999 Sep;35(9):1343–1347. doi: 10.1016/s0959-8049(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J. Clin. Oncol. 2007 Oct 20;25(30):4779–4786. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 5.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J. Clin. Oncol. 2003 Jan 1;21(1):60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004 Jun 3;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 7.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J. Clin. Oncol. 1997 Mar;15(3):938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 8.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann. Surg. 2002 Jun;235(6):759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J. Clin. Oncol. 2009 Aug 1;27(22):3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 2009 Apr 2;360(14):1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 11.Douillard J-Y, Siena S, Cassidy J, et al. Final results from PRIME: randomized phase III study of panitumumab (pmab) with FOLFOX4 for first-line metastatic colorectal cancer (mCRC) J. Clin. Oncol. 2010;29(suppl):3510. doi: 10.1093/annonc/mdu141. [DOI] [PubMed] [Google Scholar]
- 12.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002 Jun 27;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 13.Tie J, Gibbs P, Lipton L, et al. Optimizing targeted therapeutic development: analysis of a colorectal cancer patient population with the BRAF(V600E) mutation. Int. J. Cancer. 2011 May 1;128(9):2075–2084. doi: 10.1002/ijc.25555. [DOI] [PubMed] [Google Scholar]
- 14.Yokota T, Ura T, Shibata N, et al. BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br. J. Cancer. 2011 Mar 1;104(5):856–862. doi: 10.1038/bjc.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price TJ, Hardingham JE, Lee CK, et al. Impact of KRAS and BRAF Gene Mutation Status on Outcomes From the Phase III AGITG MAX Trial of Capecitabine Alone or in Combination With Bevacizumab and Mitomycin in Advanced Colorectal Cancer. J. Clin. Oncol. 2011 Jul 1;29(19):2675–2682. doi: 10.1200/JCO.2010.34.5520. [DOI] [PubMed] [Google Scholar]
- 16.Ogino S, Shima K, Meyerhardt JA, et al. Predictive and prognostic roles of BRAF mutation in stage III colon cancer: results from intergroup trial CALGB 89803. Clin. Cancer Res. 2012 Feb 1;18(3):890–900. doi: 10.1158/1078-0432.CCR-11-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004 Mar 19;116(6):855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 18.Pritchard C, Carragher L, Aldridge V, et al. Mouse models for BRAF-induced cancers. Biochem. Soc. Trans. 2007 Nov;35(Pt 5):1329–1333. doi: 10.1042/BST0351329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopetz S, Desai J, Chan E, et al. PLX4032 in metastatic colorectal cancer patients with mutant BRAF tumors. J. Clin. Oncol. 2010;28(15 Suppl):a3534. [Google Scholar]
- 20.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011 Jun 30;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N. Engl. J. Med. 2012 Feb 23;366(8):707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enge S, Byrd D, Comptom C, Fritz A, Green F, Trotti A. Cancer Staging Manual. 7th ed. Nwe York, NY: Springer; 2010. [Google Scholar]
- 23.Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011 Oct 15;117(20):4623–4632. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005 Jul 15;65(14):6063–6069. doi: 10.1158/0008-5472.CAN-05-0404. [DOI] [PubMed] [Google Scholar]
- 25.Tol J, Nagtegaal ID, Punt CJ. BRAF mutation in metastatic colorectal cancer. N. Engl. J. Med. 2009 Jul 2;361(1):98–99. doi: 10.1056/NEJMc0904160. [DOI] [PubMed] [Google Scholar]
- 26.Souglakos J, Philips J, Wang R, et al. Prognostic and predictive value of common mutations for treatment response and survival in patients with metastatic colorectal cancer. Br. J. Cancer. 2009 Aug 4;101(3):465–472. doi: 10.1038/sj.bjc.6605164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011 Jun 18;377(9783):2103–2114. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J. Clin. Oncol. 2008;26(35):5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 29.Masi G, Loupakis F, Salvatore L, et al. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trial. Lancet Oncol. 2010 Sep;11(9):845–852. doi: 10.1016/S1470-2045(10)70175-3. [DOI] [PubMed] [Google Scholar]
- 30.Richman SD, Seymour MT, Chambers P, et al. KRAS and BRAF Mutations in Advanced Colorectal Cancer Are Associated With Poor Prognosis but Do Not Preclude Benefit From Oxaliplatin or Irinotecan: Results From the MRC FOCUS Trial. J. Clin. Oncol. 2009 Dec 10;27(35):5931–5937. doi: 10.1200/JCO.2009.22.4295. [DOI] [PubMed] [Google Scholar]
- 31.Hutchins G, Southward K, Handley K, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J. Clin. Oncol. 2011 Apr 1;29(10):1261–1270. doi: 10.1200/JCO.2010.30.1366. [DOI] [PubMed] [Google Scholar]
- 32.De Roock W, Claes B, Bernasconi D, et al. Effects of< i>KRAS, BRAF, NRAS</i>and< i>PIK3CA</i>mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. The lancet oncology. 2010;11(8):753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 33.Smalley KS, Xiao M, Villanueva J, et al. CRAF inhibition induces apoptosis in melanoma cells with non-V600E BRAF mutations. Oncogene. 2009 Jan 8;28(1):85–94. doi: 10.1038/onc.2008.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gavin PG, Colangelo LH, Fumagalli D, et al. Mutation Profiling and Microsatellite Instability in Stage II and III Colon Cancer: An Assessment of Their Prognostic and Oxaliplatin Predictive Value. Clin. Cancer Res. 2012 Dec 1;18(23):6531–6541. doi: 10.1158/1078-0432.CCR-12-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phipps AI, Buchanan DD, Makar KW, et al. BRAF mutation status and survival after colorectal cancer diagnosis according to patient and tumor characteristics. Cancer Epidemiol. Biomarkers Prev. 2012 Oct;21(10):1792–1798. doi: 10.1158/1055-9965.EPI-12-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]