Abstract
The Oncotype DX® Recurrence Score® (RS) is a validated genomic predictor of outcome and response to adjuvant chemotherapy in ER-positive breast cancer. Adjuvant! was developed using SEER registry data and results from the Early Breast Cancer Clinical Trialists’ overview analyses to estimate outcome and benefit from adjuvant hormonal therapy and chemotherapy. In this report we compare the prognostic and predictive utility of these two tools in node-negative, ER-positive breast cancer. RS and Adjuvant! results were available from 668 tamoxifen-treated NSABP B-14 patients: 227 tamoxifen-treated NSABP B-20 patients, and 424 chemotherapy-plus-tamoxifen-treated B-20 patients. Adjuvant! results were also available from 1952 B-20 patients. The primary endpoint was distant recurrence-free interval (DRFI). Cox proportional hazards models were used to compare the prognostic and predictive utility of RS and Adjuvant!. Both RS (p<0.001) and Adjuvant! (p=0.002) provided strong independent prognostic information in tamoxifen-treated patients. Combining RS and individual clinicopathologic characteristics provided greater prognostic discrimination than combining RS and the composite Adjuvant!. In the B-20 cohort with RS results (n=651), RS was significantly predictive of chemotherapy benefit (interaction p=0.031 for DRFI, p=0.011 for overall survival [OS], p=0.082 for disease-free survival [DFS]), but Adjuvant! was not (interaction p=0.99, p=0.311 and p=0.357, respectively). However, in the larger B-20 sub-cohort (n=1952), Adjuvant! was significantly predictive of chemotherapy benefit for OS (interaction p=0.009) but not for DRFI (p=0.219) or DFS (p=0.099). Prognostic estimates can be optimized by combining RS and clinicopathologic information instead of simply combining RS and Adjuvant!. RS should be used for estimating relative chemotherapy benefit.
Keywords: Breast cancer, Oncotype DX, Recurrence Score, Adjuvant!, Node Negative, ER Positive
Introduction
During the past decade, gene expression profiling has become a powerful tool for predicting risk of breast cancer recurrence and mortality. Several gene expression profiles have been found to predict risk of recurrence in untreated breast cancer patients and in those treated with hormonal therapy and/or chemotherapy [1–6]. Oncotype DX is a commercially available, validated 21-gene assay that quantifies risk of distant recurrence in node-negative (N0), estrogen-receptor-positive (ER+), tamoxifen-treated breast cancer patients [5,7]. Oncotype DX has also been shown to significantly predict benefit from adjuvant non-anthracycline-based and anthracycline-based chemotherapy in N0 and node-positive, ER+ patients [8,9]. Clinical practice guidelines have included the 21-gene Recurrence Score (RS) in the management of N0, ER+ breast cancer [10,11].
Adjuvant! is a popular tool that uses patient and tumor characteristics to predict 10-year breast cancer outcome based on SEER registry data [12,13]. It also utilizes results from the Early Breast Cancer Clinical Trialists’ Collaborative Group overview analyses to assign benefit from adjuvant therapies, and it assumes the same proportional reduction in recurrence and mortality across different prognostic categories [14]. Adjuvant! has been validated as a prognostic tool and is widely used by patients and oncologists to determine prognosis and to choose appropriate systemic therapy [15].
It is clinically important to evaluate and compare the prognostic and predictive utility of RS and Adjuvant! in order to determine if they could be integrated in a manner that would enhance their clinical usefulness. Two previously reported studies have compared the prognostic utility of Adjuvant! and RS in N0 and node-positive patients treated with hormonal therapy with or without adjuvant chemotherapy and have demonstrated their independent prognostic contributions [16,17]. However, no study to date has compared genomic classifiers with Adjuvant! in terms of their abilities to predict chemotherapy benefit.
In the present study, we performed a comprehensive comparison of both the prognostic and predictive utilities of Oncotype DX and Adjuvant! at 10 years of follow-up using data from patients with N0, ER+ breast cancer who participated in two NSABP clinical trials (B-14 and B-20).
Methods
Patient population
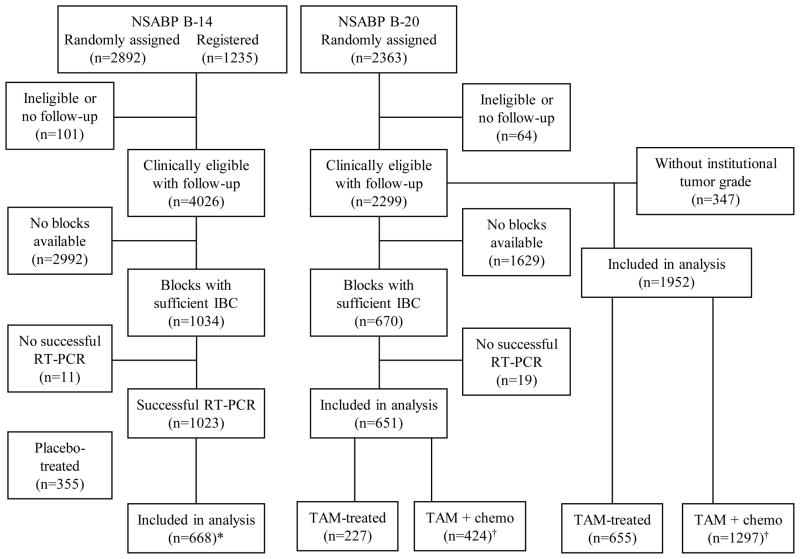
In NSABP B-14, 2892 N0 and ER+ breast cancer patients were randomized between 1/82 and 1/88 to 5 years of placebo or 5 years of tamoxifen, and an additional 1235 patients were registered between 1/88 and 10/88 to receive 5 years of tamoxifen [18,19]. Of the 2615 patients who were assigned to tamoxifen, the Oncotype DX assay was successfully performed in 668 clinically eligible patients with follow-up (CONSORT Fig. 1).
Fig. 1.
CONSORT diagram for patients from the National Surgical Adjuvant Breast and Bowel Project B-14 and B-20 trials.
Abbreviations: IBC, invasive breast cancer; RT-PCR, Reverse transcription polymerase chain reaction; TAM, tamxifen; chemo, chemotherapy.
*Includes 290 randomized and 378 registered patients.
†Tamoxifen plus methotrexate and fluorouracil (MFT): 203 patients; tamoxifen plus. cyclophosphamide, methotrexate, and fluorouracil (CMFT): 221 patients.
In NSABP B-20, 2363 N0, ER+ patients were randomized between 10/88 and 3/93 to tamoxifen alone or to tamoxifen plus cyclophosphamide, methotrexate, and fluorouracil (CMFT) or tamoxifen plus methotrexate and fluorouracil (MFT) [20]. Of those, 2299 were clinically eligible with follow-up. Blocks containing sufficient invasive breast cancer tissue were available for 670 patients. The Oncotype DX assay was successfully performed in 651 patients (227 tamoxifen-treated and 424 chemotherapy-plus-tamoxifen-treated). In order to provide additional information on the predictive utility of Adjuvant!, a total 1952 patients from B-20 with known institutional tumor grade assessment were also evaluated in a separate analysis.
Study design and endpoints
The objective of this study was to compare the prognostic and predictive utility of Oncotype DX and Adjuvant! to determine if the two methodologies supply independent prognostic information and, if so, to develop an algorithm to integrate both into a single prognostic algorithm.
The primary pre-specified endpoint was distant recurrence-free interval (DRFI), defined as time from study entry to the first distant recurrence. Contralateral breast cancer, non-breast second primary cancers, and deaths before distant recurrence were considered censoring events. Loco-regional recurrences were not considered either as events or as censoring events. DRFI was used as the primary endpoint because distant recurrence is the endpoint of greatest direct clinical consequence to the patient with early-stage breast cancer. Secondary endpoints included overall survival (OS), disease-free survival (DFS) and breast cancer-specific mortality (BCSM).
Patient eligibility criteria have been presented in previous publications [5]. ER-positivity was determined by the ligand-binding assay. Tumor grade for all patients with Oncotype DX assessment was determined by a board-certified pathologist from Stanford University using the Elston modification of the Bloom-Richardson grading criteria [21].
This study was approved by the Essex IRB (NJ), the Allegheny General Hospital IRB (PA), and the University of Pittsburgh IRB (PA).
Sample preparation, RS algorithm, and Adjuvant! Risk Index (RI) algorithm
Gene expression in fixed paraffin-embedded tumor tissue was performed by Genomic Health, Inc, using the Oncotype DX assay [5]. The assay results provide a Recurrence Score (RS) for each patient, scaled from 0 to 100, that is derived from the reference-normalized expression measurements for the cancer-related genes. The pre-specified cutoff points classified patients into low-risk (RS<18), intermediate-risk (18–30), and high-risk (RS≥31) groups [5]. The development of the computing algorithm and the cutoffs for RS have been previously described in detail [5]. An emulator of Adjuvant! Breast version 9.0 was kindly provided by Peter Ravdin, MD, and was used to develop an Adjuvant! risk index (RI) for breast cancer mortality based on clinicopathologic information. Pathologic tumor size was available from 603 (90%) of the 668 eligible B-14 patients and from 638 (98%) of the 651 eligible B-20 patients. For patients without known pathologic tumor size, clinical tumor size was used instead. We could not assess the co-morbidity for individual patients from the study case report forms and the default assumption of minor health problems was used for all patients. For a patient of known age who had ER+ with N0 breast cancer, and whose tumor size, tumor grade were known but who had not undergone any adjuvant treatment, the Adjuvant! estimate of breast cancer mortality without competing risks at 10 years of follow-up was regarded as the baseline risk, which is shown as “10 Year Risk” in Adjuvant! Breast and does not depend on the co-morbidity. Accounting for the 32% consensus risk reduction from tamoxifen, 10-year breast cancer mortality (with tamoxifen) was derived and was used as the risk integrator of those clinicopathologic factors. One hundred times this 10-year breast cancer mortality estimate was defined as the Adjuvant! risk index (RI) for breast cancer recurrence in this comparative study. This RI was also adopted by Goldstein and co-authors [16]. Two cutoff points in RI were chosen so that the RI risk group had a distribution similar to that of the RS group
Statistical analysis
Kaplan-Meier estimates were used to estimate 10-year event rates for RS and RI risk groups. As the basis for an appropriately based comparison of Adjuvant! Breast and Oncotype DX, the continuous percentiles of RS and RI were used for further assessment in regression analyses. These percentiles were denoted as RS-PCT and RI-PCT, respectively. Because RS-PCT and RI-PCT had a range from 0 to 100, they were divided by 50 and re-scaled to a range from 0 to 2 in regression analyses so that the associated hazard ratios (HRs) were comparable to those hazard ratios associated with clinicopathologic factors. The original choice to present the HR for the continuous RS for a 50 unit increment was based on a desire to achieve comparability with categorization of tumor size and age into two groups and the categorization of grade into three groups. The HR of 3.12 a 50-point increment in RS is clinically relevant, as it was comparable to the greater than four-fold difference in distant recurrence risk at 10 years observed between the low RS and high RS groups (6.8% vs. 30.5%).[5] Cox proportional hazards models were used to examine the association of either RS-PCT or RI-PCT with risk of distant recurrence. Multivariate Cox proportional hazards models were also used to examine whether RS and RI provide independent information, how RS and traditional clinicopathologic factors could be integrated into an enhanced predictor of patient prognosis, and whether the RI predicts benefits from chemotherapy. A P value <0.05 for the likelihood ratio test was used to determine the statistical significance of findings.
Results
Patient demographics
In the B-14 trial, the distributions of demographic, clinical, and treatment characteristics were similar between the 668 evaluable tamoxifen-treated patients with RS information and the remaining 1947 clinically eligible patients with follow-up (Supplemental Table S1A). In B-20, the distributions of demographic, clinical, and tumor characteristics were also similar between the 651 evaluable patients with RS information and the remaining 1648 clinically eligible patients with follow-up (Supplemental Table S1B). Median follow-up time for DRFI was 14.3 years for the 668 B-14 patients and 10.6 years for the 651 B-20 patients.
Comparison of the prognostic utility between RS and RI in tamoxifen- treated patients
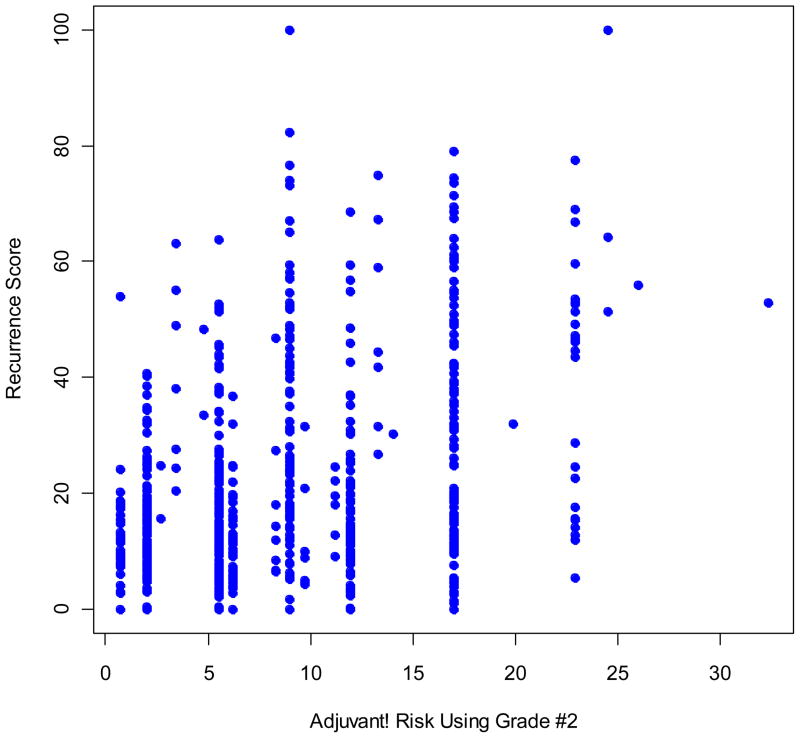
Among the 668 evaluable tamoxifen-treated patients in B-14 with RS information, 338 (50.6%) were in the low RS group, 149 (22.3%) in the intermediate RS group, and 181 (27.1%) in the high RS group. The Adjuvant! RI values ranged from 0.7 to 32.3. Two cutoff points in RI were chosen so that the RI risk group had a distribution similar to that of the RS group: RI low (RI≤5.5), RI intermediate (5.5<RI≤11.9) and RI high (RI>11.9). The corresponding distribution was 52.7%, 18.6%, and 28.7% for the three RI groups, respectively. The concordance between the RS risk group and the RI risk group was 0.49, and the correlation between RS and RI was modest, with a Spearman correlation coefficient of 0.38 (Fig. 2). RS was continuous, with values from 0 to 100, and RI had a discrete nature, with 19 levels for the 668 patients.
Fig. 2.
Scatter plot of RS and the Adjuvant! RI.
The Kaplan-Meier curves of DRFI across different RS and RI groups showed that patients in high-risk groups had the highest risk of distant recurrence, and those in low risk groups had the lowest risk of distant recurrence over time (Supplemental figures S1 and S2). Among the RI low patients, the proportions of distant recurrence at 10 years for the low, intermediate, and high RS groups increased from 5.6% to 10% and 18.2%, respectively. In the RI intermediate group, the proportions of distant recurrence at 10-year for the low, intermediate, and high RS groups increased from 13.4% to 13.9% and 43.2%, respectively. In the RI high group, the proportions of distant recurrence at 10 years for the low, intermediate, and high RS groups increased from 5% to 23.4% and 31.5%, respectively (Fig. 3). On the other hand, among intermediate and high RS groups, the risk of distant recurrence at 10 years clearly increased with the RI risk, although such a relationship was not obvious in the low RS group (Fig. 3 and Supplemental figure S3).
Fig. 3.
Point estimates and 95% confidence intervals for the percentage of B-14 patients with distant recurrence at 10 years by RS and RI risk groups.
Based on Cox models with either continuous RS-PCT or RI-PCT as the sole predictor, the HRs associated with a 50-unit increment in RS-PCT or RI-PCT were 3.61 (95% CI=2.49, 5.24; p<0.001) for RS-PCT and 2.87 (95% CI=1.95, 4.23; p<0.001) for RI-PCT (Table 1).
Table 1.
Results from Multivariate Cox Models Assessing the Relative Associations of RI-PCT/50 and RS-PCT/50 in 668 B-14 Tamoxifen-treated Patients with DRFI as the Endpoint
| Models | Variables | Hazard ratio (95% CI) | P | −2log(L)a |
|---|---|---|---|---|
| 1 | Age(>50 vs ≤50) | 0.74 (0.5, 1.08) | 0.121 | 1299.8 |
| Tumor size (cm) | 1.19 (1.08, 1.32) | <0.001 | ||
| Grade (moderate vs well) | 1.84 (1.04, 3.25) | <0.001 | ||
| Grade (poor vs well) | 4.86 (2.79, 8.46) | |||
| 2 | RI-PCT/50b | 2.87 (1.95, 4.23) | <0.001 | 1326.6 |
| 3 | RS-PCT/50b | 3.51 (2.49, 5.24) | <0.001 | 1306.5 |
| 4 | RI-PCT/50 | 1.93 (1.27, 2.91) | 0.002 | 1296.4 |
| RS-PCT/50 | 2.83 (1.91, 4.18) | <0.001 | ||
| 5 | Age (>50 vs ≤50) | 0.79 (0.54, 1.17) | 0.247 | 1280.9 |
| Tumor size (cm) | 1.21 (1.05, 1.39) | 0.008 | ||
| Grade (moderate vs well) | 1.51 (0.75, 3.05) | 0.003 | ||
| Grade (poor vs well) | 3.18 (1.42, 7.15) | |||
| RI-PCT/50 | 0.86 (0.45, 1.62) | 0.636 | ||
| RS-PCT/50 | 2.37 (1.58, 3.55) | <0.001 | ||
| 6 | Age (>50 vs ≤50) | 0.8 (0.54, 1.18) | 0.257 | 1281.1 |
| Tumor size (cm) | 1.18 (1.06,1.32) | 0.002 | ||
| Grade (moderate vs well) | 1.38 (0.77, 2.48) | 0.001 | ||
| Grade (poor vs well) | 2.8 (1.52, 5.17) | |||
| RS-PCT/50 | 2.34 (1.56, 3.5) | <0.001 | ||
| 7 | Age (>50 vs ≤50) | 0.74 (0.5, 1.09) | 0.122 | 1299.8 |
| Tumor size (cm) | 1.19 (1.04, 1.36) | 0.012 | ||
| Grade (moderate vs well) | 1.81 (0.91, 3.6) | <0.001 | ||
| Grade (poor vs well) | 4.74 (2.19, 10.28) | |||
| RI-PCT/50 | 1.03 (0.55, 1.92) | 0.931 |
Abbreviations: DRFI, distant recurrence-free interval.
L represents the likelihood function for a model.
The ranges for the RI and RS are different. RI-PCT and RS-PCT were used instead to allow direct comparison of these HRs
Integration of RS and RI in tamoxifen-treated patients
The multivariate Cox model with continuous RS-PCT and RI-PCT as predictors showed that both provided independent prognostic information beyond the other variable in tamoxifen-treated patients (Model 4, Table 1).
Results from multivariate Cox models, which included RS-PCT, RI-PCT, age, tumor size, and tumor grade as predictors, showed that while the introduction of RS-PCT and RI-PCT diminished the prognostic power of the traditional clinicopathologic factors, tumor size and grade remained significant predictors in the model, along with RS-PCT, while RI-PCT did not (Model 5, Table 1). This was also true when RS-PCT individually was added to the traditional clinicopathologic factors (Model 6, Table 1). However, as would be expected, the individual addition of RI-PCT to the traditional clinicopathologic factors had minimal effect on their prognostic impact, and RI-PCT was not a significant predictor in that model (Model 7, Table 1). Similar results were observed in multivariate analyses in which RS and RI risk groups were used instead (Supplemental Table S2).
Comparison of the predictive utility of chemotherapy benefit between RS and RI
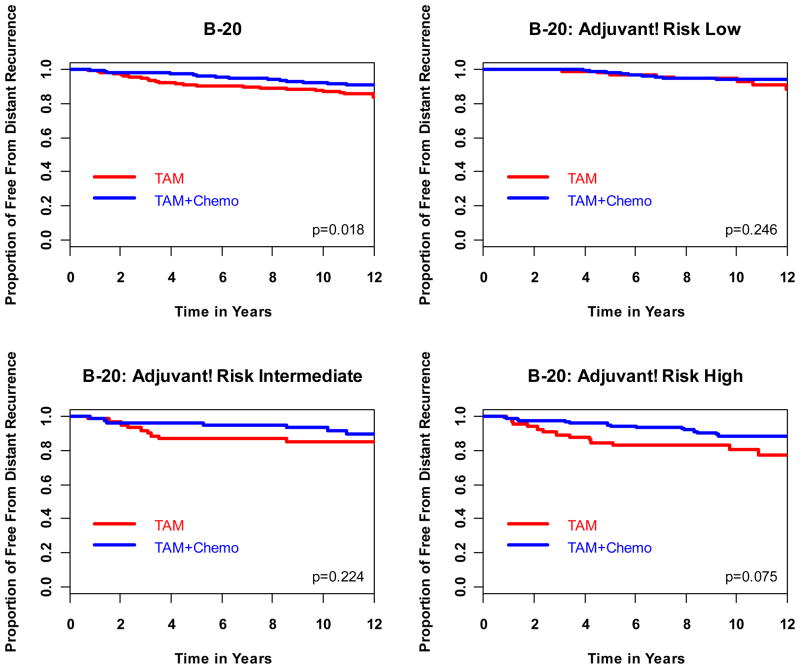
The comparison of the ability of the RS and RI in predicting chemotherapy benefit from MF or CMF was based on the 651 evaluable B-20 patients. Among these patients, 353 (54.2%) were in the low RS group, 134 (20.6%) in the intermediate RS group, and 164 (25.1%) in the high RS group [8]. On the other hand, 294 (45%) were in the low RI group, 145 (22%) in the intermediate RI group, and 212 (33%) in the high RI group. It has been previously shown that RS is a statistically significant predictor of benefit from MF or CMF (p=0.038) [8]. The HRs associated with treatment benefit in DRFI for tamoxifen plus chemotherapy over tamoxifen alone were 1.31 (95% CI=0.46, 3.78), 0.61 (95% CI=0.24, 1.59), and 0.26 (95% CI=0.13, 0.53) for the RS low-, intermediate-, and high-risk groups, respectively (likelihood ratio test for interaction p=0.031) (Table 2, Fig. 3) [8]. However, benefit from chemotherapy in DRFI was fairly uniform within RI categories, with HRs around 0.5–0.6 for the three RI groups (likelihood ratio test for interaction p=0.99) (Table 2, Fig. 5). Similar findings were observed for OS with interaction P-values at 0.011 for RS and 0.311 for RI, respectively, and for DFS with interaction P-values at 0.082 and 0.357 for RS and RI, respectively (Table 2). Because the OS and DFS HRs for chemotherapy benefit according to RI categories trended towards more benefit with increasing RI categories, and in order to provide additional information and to obtain greater power regarding the predictive utility of Adjuvant!, we performed the same analysis for DRFI, OS, and DFS according to RI category in a group of 1952 patients from the original B-20 cohort, for which institutional tumor grade was known. The results indicate that Adjuvant! was predictive of treatment benefit for OS (interaction p=0.009) but not for DRFI (interaction p=0.219 ). For DFS, RI was marginally predictive of chemotherapy benefit (interaction p=0.099) (Table 2).
Table 2.
Association between RS or RI Risk Groups and Benefit from Chemotherapy among 651 B-20 Patients Using DRFI, OS or DFS as the Endpoint
| Endpoint | Cohort | B-20 Patients with RS Assessment (n=651) | All B-20 Patients with Tumor Grade (n=1952) | ||
|---|---|---|---|---|---|
| HR for benefit from MF/CMF (95% CI) | Pa (interaction) | HR for benefit from MF/CMF (95% CI) | Pa (interaction) | ||
| DRFI | Overall | 0.56 (0.34, 0.91) | 0.62 (0.47, 0.81) | ||
| RS low | 1.31 (0.46, 3.78) | 0.031 | N/A | ||
| RS intermediate | 0.61 (0.24, 1.59) | ||||
| RS high | 0.26 (0.13, 0.53) | ||||
| Adjuvant! low | 0.58 (0.23, 1.42) | 0.99 | 0.92 (0.53, 1.62) | 0.219 | |
| Adjuvant! intermediate | 0.54 (0.2, 1.46) | 0.52 (0.29, 0.93) | |||
| Adjuvant! high | 0.53 (0.25, 1.1) | 0.53 (0.36, 0.77) | |||
| OS | Overall | 0.76 (0.49, 1.17) | 0.74 (0.58, 0.95) | ||
| RS low | 1.37 (0.63, 3.01) | 0.011 | N/A | ||
| RS intermediate | 0.94 (0.4, 2.25) | ||||
| RS high | 0.31 (0.16, 0.6) | ||||
| Adjuvant! low | 1.16 (0.55, 2.45) | 0.311 | 1.26 (0.81, 1.95) | 0.009 | |
| Adjuvant! intermediate | 0.7 (0.3, 1.61) | 0.53 (0.31, 0.9) | |||
| Adjuvant! high | 0.53 (0.26, 1.07) | 0.57 (0.4, 0.82) | |||
| DFS | Overall | 0.73 (0.54, 0.99) | 0.75 (0.63, 0.89) | ||
| RS low | 0.91 (0.57, 1.45) | 0.082 | N/A | ||
| RS intermediate | 0.79 (0.43, 1.47) | ||||
| RS high | 0.41 (0.23, 0.71) | ||||
| Adjuvant! low | 0.97 (0.59, 1.61) | 0.357 | 0.91 (0.69, 1.21) | 0.099 | |
| Adjuvant! intermediate | 0.6 (0.33, 1.09) | 0.75 (0.5, 1.13) | |||
| Adjuvant! high | 0.62 (0.36, 1.05) | 0.59 (0.45, 0.78) | |||
Abbreviations: DRFI, distant recurrence-free interval; OS, overall survival; DFS, disease-free survival.
From likelihood ratio tests.
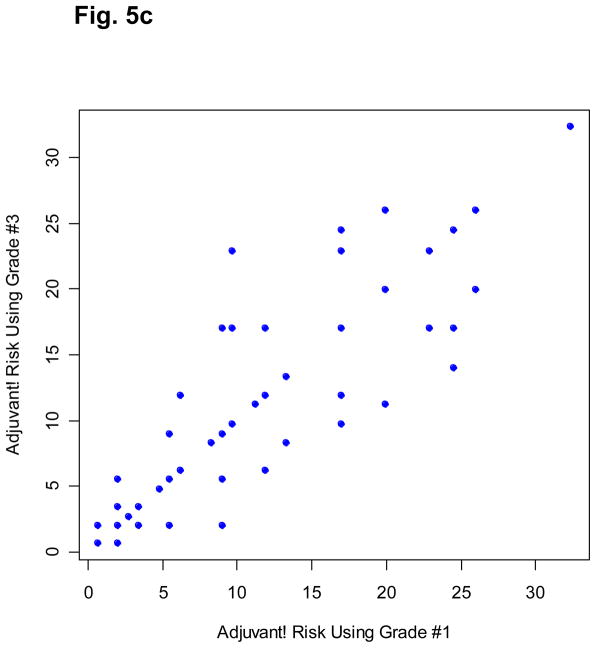
Fig. 5.
Association among the three Adjuvant! RIs based on three independent pathologic gradings (grade #2 was from the pathologist in Standard University). Because of its discrete nature, each point may represent multiple patients.
Discussion
Previous studies of Oncotype DX have demonstrated that the RS provides independent, complementary prognostic information to that provided by traditional clinicopathologic factors in patients with N0 ER+ breast cancer and that the RS is also predictive of chemotherapy benefit in these patients [5,8,17]. Our study demonstrates that the RS from Oncotype DX and RI from Adjuvant! are independent prognostic factors of risk of distant recurrence. As a validated integrator of classical clinicopathologic factors, Adjuvant! might have provided a promising surrogate of these factors to supplement Oncotype DX. However, our study indicates that, as a single composite measure which was derived to act as a standalone measure of prognosis, the individual clinicopathologic factors from which Adjuvant! is comprised provide more information when used to complement the Recurrence Score, and the biology that it already directly captures. Similar findings were observed when other endpoints such as OS, DFS, and BCSM were considered (Supplemental tables S3-S5). Therefore, when the prognosis of tamoxifen-treated N0, ER+ patients is being assessed, one should utilize the individual clinicopathologic factors with RS as opposed to a composite measure such as Adjuvant! with RS.
Although the independent prognostic contribution of Oncotype DX and Adjuvant! has been previously shown by other investigators in N0 and node- positive, ER+ patients treated with hormonal therapy with or without chemotherapy, this is the first study to address the predictive ability of Adjuvant! in patients with N0, ER+ breast cancer. In the B-20 cohort with RS results (n=651), RS was significantly predictive of chemotherapy benefit (interaction p=0.031 for DRFI, p=0.011 for overall survival [OS], p=0.082 for disease-free survival [DFS]), but Adjuvant! was not (interaction p=0.99, p=0.311 and p=0.357, respectively). The comparison of the predictive ability of Adjuvant! with that of RS in the B-20 study may be biased in favor of RS because a cohort of NSABP B-20 tamoxifen-treated patients was used, in part, to develop the gene list and algorithm for RS. However, this is unlikely to have caused appreciable bias, for two reasons. First, the results on the prognostic value of the RS in tamoxifen-treated patients from NSABP-20 were subsequently independently validated in NSABP B-14. Second, consistent with the treatment interaction for RS in NSABP B-20 (i.e. that the relative risk reduction with chemotherapy was greater with high RS than with low RS), such a relationship has been observed either directly or indirectly in other datasets. In SWOG 8814, a significant correlation between RS and benefit from anthracycline-based chemotherapy was demonstrated in node-positive, ER+, postmenopausal patients [9]. In addition, the prognostic relationship between RS and risk of recurrence is consistently strong in groups of patients treated with hormonal therapy alone (as in NSABP B-14, SWOG 8814, and ATAC) [5,9,17], and is consistently much less strong in groups of patients treated with chemotherapy plus hormonal therapy (as in SWOG 8814 and ECOG E2197) [9,16]. The comparison of the predictive utility in B-20 might also be biased in favor of RS because RS was shown to be predictive of treatment benefit with DRFI as the endpoint, and Adjuvant! was developed with breast cancer mortality as the primary endpoint [8,12,15]. However, further analyses based on the 651 B-20 patients showed that the RS was not only predictive of chemotherapy benefit based on DRFI but was also based on BCSM and OS (Table 2 and Supplemental Table S6). Using those endpoints, RI was also shown to be a strong prognostic factor among tamoxifen-treated N0, ER+ breast cancer patients in B-14 (Supplemental Tables S3 and S4).
For the 668 B-14 tamoxifen-treated patients included in this study, tumor grade was independently determined by an NSABP pathologist and two other external board-certified pathologists (from Stanford University and University of California, San Francisco) using the Elston modification [21]. The three RIs based on readings from the different pathologists were highly correlated with Spearman correlation coefficients ranging from 0.902 to 0.915. However, the actual values of these RIs can be quite different (Fig. 5). The reproducibility problem of tumor grade in clinical practice can be a major issue that undermines the prognostic power of Adjuvant! Breast.
Although the interaction between chemotherapy benefit and RI risk group was not statistically significant in the 651 patients with OncotypeDX assay from the B-20 trial (Table 2 and Supplemental Table S6), results from 1952 B-20 patients with known institutional tumor grade showed that RI risk group was a significant predictor of chemotherapy benefit for OS and a marginal predictor of chemotherapy benefit for DFS. However, the magnitude of the predictive ability of chemotherapy benefit is much weaker with RI than with RS. Overall in B-20, chemotherapy reduced the risk of mortality by 26%. For RI high-risk patients, chemotherapy reduced the risk of mortality by 43%, whereas for RS high-risk patients, chemotherapy reduced the risk of mortality by 69% (Table 2). Thus, the discriminatory ability of RS to identify patients who would benefit from chemotherapy is stronger than that of Adjuvant! This is further reflected by the consistently significant interaction between RS and chemotherapy benefit observed across all studied endpoints even with the smaller sample size of 651 B-20 patients.
Another potential limitation of our study is that the chosen cutoffs for RI were determined to mimic the distribution of the RS groups among the 668 B-14 patients. Perhaps a dichotomization in RI, high risk versus low risk, would provide a better characterization of RI. However, the multivariate analyses with RS-PCT and RI-PCT, as continuous variables, indicated that selection of cutoffs for either assay had minor impact on the comparison. As can be seen from Fig. 2, no matter which cutoff is chosen for RI, the associated Adjuvant! high-risk group would include a considerable proportion of RS low-risk patients who do not benefit from chemotherapy. This explains why Adjuvant! is a much weaker predictor of chemotherapy benefit when compared with the RS.
Although it is not uncommon for prognostic factors to also be predictive of treatment effect (i.e., HER2 is both prognostic and predictive of trastuzumab benefit), there are several instances where prognostic factors are not predictive. For example, tumor size and nodal status are strongly prognostic but not predictive of chemotherapy benefit. Other factors such as tumor grade and Ki-67 by IHC are consistently prognostic but not consistently predictive of chemotherapy benefit [22–25]. This phenomenon and the inclusion of proliferation genes in RS lend further support to our findings that RS is a stronger predictor of chemotherapy benefit than Adjuvant!
In summary, genomic information from Oncotype DX and clinicopathologic information from Adjuvant! provided independent prognostic contributions in N0, ER+ breast cancer patients. However, only Oncotype DX consistently predicted chemotherapy benefit across various endpoints. Results from the larger parent B-20 cohort showed that Adjuvant! predicted chemotherapy benefit, but only in OS, and the magnitude of prediction was less than that of the RS. Finally, alternative approaches for the integrated use of genomic and clinicopathologic parameters to optimize their prognostic impact should be examined.
Supplementary Material
Fig. S1 Kaplan-Meier curves for distant recurrence across RS risk groups in B-14 tamoxifen-treated patients.
Fig. S2 Kaplan-Meier curves for distant recurrence across RI risk groups in B-14 tamoxifen-treated patients.
Fig. S3 Point estimates and 95% confidence intervals for the percentage of B-14 patients with distant recurrence at 10 years by RS then RI risk groups.
Supplemental Table S1A: Comparison of the Distribution of Demographic, Clinical, and Treatment Characteristics of NSABP B-14 Patients Who Did and Did Not Have RS Assessment
Supplemental Table S1B: Comparison of the Distribution of Demographic, Clinical, and Treatment Characteristics of NSABP B-20 Patients Who Did and Did Not Have RS Assessments
Supplemental Table S2: Results of Multivariate Cox Models Assessing the Relative Associations of RS and RI Risk Groups among 668 B-14 Tamoxifen-treated Patients using DRFI as the Endpoint
Supplemental Table S3: Results from Multivariate Cox Models Assessing the Relative Associations of RI-PCT/50 and RS-PCT/50 among 668 B-14 Tamoxifen-treated Patients Using Breast Cancer-specific Mortality as the Endpoint
Supplemental Table S4: Results from Multivariate Cox Models Assessing the Relative Associations of RI-PCT/50 and RS-PCT/50 among 668 B-14 Tamoxifen-treated Patients Using Overall Survival as the Endpoint
Supplemental Table S6: Association between RS or RI Risk Groups and Benefit from Chemotherapy among 651 B-20 Patients using Breast Cancer-specific Mortality as the Endpoint
Fig. 4.
Kaplan-Meier curves for distant recurrence in 651 B-20 patients.
Acknowledgments
The study is supported by funds from the National Cancer Institute, US Department of Health and Human Services. The funding source did not affect the study design, data collection, analysis, interpretation, writing, or submission of this work. We would like to thank Dr. Drew Watson, Genomic Health, for valuable discussions during this study.
Supported in part by: Public Health Service Grants U10CA-12027, U10CA-69974, U10CA-37377, U10CA-69651, and U24-CA-114732 from the National Cancer Institute, Department of Health and Human Services.
The authors retain the right to provide a copy of the final manuscript to the NIH upon acceptance for journal publication, for public archiving in PubMed Central as soon as possible but no later than 12 months after publication by the journal.
Footnotes
Clinical Trials registration: NSABP B-14: PDQ: NSABP-B-14; NSABP B-20: PDQ: NSABP-B-20
CONFIDENTIAL
Not to be distributed or submitted without explicit permission of the NSABP Operations Office.
Conflicts of interest
Even though the NSABP Statistical Center has received research funding from Genomic Health, this study was not supported by Genomic Health. SS is a full-time employee and a stockholder in Genomic Health. GT, SJA, and JPC declare no potential conflicts of interest. EPM has been a consultant and on the speaker’s bureau of Genomic Health. There are no other potential conflicts of interest reported.
References
- 1.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. PNAS. 2004;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van De Vijver M, He YD, Van’t Veer LJ, et al. A Gene-Expression Signature as a Predictor of Survival in Breast Cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 3.Van 't Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 4.Foekens JA, Atkins D, Zhang Y, et al. Multicenter Validation of a Gene Expression-Based Prognostic Signature in Lymph Node-Negative Primary Breast Cancer. J Clin Oncol. 2006;24:1665–1671. doi: 10.1200/JCO.2005.03.9115. [DOI] [PubMed] [Google Scholar]
- 5.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 6.Dowsett M, Cuzick J, Wales C, et al. Risk of distant recurrence using oncotype DX in postmenopausal primary breast cancer patients treated with anastrozole or tamoxifen: a TransATAC study. Cancer. 2009;69:75s (abstr 53). [Google Scholar]
- 7.Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006;8:R25. doi: 10.1186/bcr1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 9.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 11. [Accessed 9/18/08];NCCN Clinical Practice Guidelines in OncologyTM Breast Cancer, (Version 2.2008) http://www.nccn.org.
- 12.Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 13.Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 17 Regs Limited Use + Katrina Impacted Louisiana Cases. [Accessed 1-29-10];Surveillance Research Program, National Cancer Institute SEER* Software. Nov Released april 2008, based on the November 2007 submission. at: www.seer.cancer.gov/seerstat [version 6.4.4]
- 14.Early Breast Cancer Trialist’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 15.Olivotto IA, Bajdik CD, Ravdin PM, et al. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol. 2005;23:2716–2725. doi: 10.1200/JCO.2005.06.178. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein LJ, Gray R, Badve S, et al. Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol. 2008;26:4063–4071. doi: 10.1200/JCO.2007.14.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28:1829–1834. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 18.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320:479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 19.Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 2001;93:684–690. doi: 10.1093/jnci/93.9.684. [DOI] [PubMed] [Google Scholar]
- 20.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997;89:1673–1682. doi: 10.1093/jnci/89.22.1673. [DOI] [PubMed] [Google Scholar]
- 21.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 22.Assersohn L, Salter J, Powles TJ, et al. Studies of the potential utility of Ki67 as a predictive molecular marker of clinical response in primary breast cancer. Breast Cancer Res Treat. 2003;82:113–123. doi: 10.1023/B:BREA.0000003968.45511.3f. [DOI] [PubMed] [Google Scholar]
- 23.Burcombe RJ, Makris A, Richman PI, et al. Evaluation of ER, PgR, HER-2 and Ki-67 as predictors of response to neoadjuvant anthracycline chemotherapy for operable breast cancer. Br J Cancer. 2005;92:147–155. doi: 10.1038/sj.bjc.6602256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheang MCU, Chia SK, Voduc D, et al. Ki67 Index, HER2 Status, and Prognosis of Patients With Luminal B Breast Cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penault-Llorca F, Andre F, Sagan C, et al. Ki67 expression and docetaxel efficacy in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2809–2815. doi: 10.1200/JCO.2008.18.2808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Kaplan-Meier curves for distant recurrence across RS risk groups in B-14 tamoxifen-treated patients.
Fig. S2 Kaplan-Meier curves for distant recurrence across RI risk groups in B-14 tamoxifen-treated patients.
Fig. S3 Point estimates and 95% confidence intervals for the percentage of B-14 patients with distant recurrence at 10 years by RS then RI risk groups.
Supplemental Table S1A: Comparison of the Distribution of Demographic, Clinical, and Treatment Characteristics of NSABP B-14 Patients Who Did and Did Not Have RS Assessment
Supplemental Table S1B: Comparison of the Distribution of Demographic, Clinical, and Treatment Characteristics of NSABP B-20 Patients Who Did and Did Not Have RS Assessments
Supplemental Table S2: Results of Multivariate Cox Models Assessing the Relative Associations of RS and RI Risk Groups among 668 B-14 Tamoxifen-treated Patients using DRFI as the Endpoint
Supplemental Table S3: Results from Multivariate Cox Models Assessing the Relative Associations of RI-PCT/50 and RS-PCT/50 among 668 B-14 Tamoxifen-treated Patients Using Breast Cancer-specific Mortality as the Endpoint
Supplemental Table S4: Results from Multivariate Cox Models Assessing the Relative Associations of RI-PCT/50 and RS-PCT/50 among 668 B-14 Tamoxifen-treated Patients Using Overall Survival as the Endpoint
Supplemental Table S6: Association between RS or RI Risk Groups and Benefit from Chemotherapy among 651 B-20 Patients using Breast Cancer-specific Mortality as the Endpoint