Abstract
Although the negative selection of self-reactive B cells in the bone marrow of mammals has been clearly demonstrated, it remains unclear in models of gut-associated B cell lymphopoiesis, such as that of the chicken (Gallus gallus). We have generated chicken surface IgM–related receptors in which the diversity region of the lamprey variable lymphocyte receptor (VLR) has been fused to the C region of chicken surface IgM (Tμ). Expression of a VLR:Tμ receptor with specificity for PE supported normal development of B cells, whereas a VLR:Tμ receptor specific to hen egg lysozyme (a self-antigen with respect to chicken B cells) induced, in vivo, complete deletion of VLRHELTμ-expressing B cells. In ovo i.v. injection of PE resulted in deletion of VLRPETμ-expressing Β cells in the embryo spleen, demonstrating that negative selection was independent of the bursal microenvironment. Although chickens transduced with a murine CD8α:chicken Igα fusion protein contained B cells expressing mCD8α:chIgα, cotransfection of the mCD8α:chIgα construct, together with thymus leukemia Ag (a natural ligand for mCD8α), resulted in reduced levels of mCD8α:chIgα-expressing B cells in inverse proportion to the levels of thymus leukemia Ag–expressing cells. Deletion of mCD8a: chIga-expressing cells was specific for B cells and required active signaling downstream of the mCD8α:chIgα receptor. Ag-mediated negative selection of developing chicken B cells can therefore occur independently of the bursal microenvironment and is dependent on signaling downstream of the BCR.
Blymphopoiesis is a regulated process that occurs in the bone marrow of humans and rodents and GALT of other mammals and birds. To generate protection against pathogens, the humoral immune system requires a diverse pool of BCRs that can recognize a broad range of foreign Ags. The diversification of variable Ig regions, however, runs the risk of generating self-reactive specificities that must be eliminated from the competent pool of B cells.
B cell development in mammals such as mice and humans occurs in the bone marrow throughout life. B lymphopoiesis in the bone marrow purges self-reactive specificities, using mechanisms such as receptor editing and clonal deletion (1–3). Approximately 50% of autoreactive B cells, however, escape these central tolerance mechanisms (4), but they peripheralize in a state of anergy, and in the presence of specific Ag and competition with other B cells remain quiescent, with a substantially reduced lifespan. Anergic B cells show compromised signaling downstream of BCR cross-linking (5).
It has been shown in murine models that the form of the self-antigen defines the fate of self-reactive B cells. Whereas a membrane-bound form of neo–self-antigen leads to deletion of specific transgenic B cells, a soluble form of the self-antigen leads to specific B cell anergy, despite binding to the B cell with the same intrinsic affinity. The capacity of membrane-bound Ag to induce deletion, therefore, likely reflects its valence and greater potential to cluster large numbers of Ag receptors on developing B cells (6–9).
In contrast to bone marrow models of B cell development, mechanisms involved in the selection of avian B cells remain unclear. Gut-associated B cell lymphopoiesis in avian species and some mammals differs from human and rodent models of B cell development with respect to anatomical location and mechanisms by which BCR diversity is generated. Furthermore, in contrast to mice and humans, in which rearrangement of Ig light and heavy chains continues throughout life in the bone marrow, B cell commitment and subsequent Ig gene rearrangement in chickens is restricted to a single wave of precursors in embryonic life (10–12). In chickens, B cells rearrange unique variable H and L chain gene segments, generating limited diversity. The limited diversity generated by Ig rearrangement in chickens raises the question of whether germline specificities induce selection of B cells or have been conserved evolutionarily to avoid self-reactivity.
The initial colonization of the bursal follicles requires B cell surface Ig (sIg) expression, and this leads to the induction of Ig diversification. Avian B cells generate extensive Ig diversity upon passing this Ig selection checkpoint by a process of gene conversion in the developing bursa. Therefore, it is likely that avian B cell lymphopoiesis is prone to the development of as much self-reactivity as is seen in mammals. Embryonic B cells are eliminated following surface BCR ligation using anti-allotype Ab in chickens (13–15). These experiments showed that injection of anti-allotype Ab to day 13 allotype heterozygote chickens (IgM-1a/b) eliminated B cells with the relevant BCR. These experiments, however, could not distinguish between the elimination of allotype-expressing B cells as a consequence of receptor ligation leading to the induction of tolerance and simple opsonization of anti-allotype–coated B cells.
To address the issue of tolerance in immature chicken B cells, we have generated defined BCR/ligand combinations and expressed these in the developing chicken embryo. We show in this article that whereas a sIg-related receptor with specificity for a foreign Ag supported B cell development, a sIg-related receptor with spec-ificity for a self-antigen resulted in B cell deletion. We further show that expression of a cell surface neo–self-antigen results in B cell deletion and that this deletion requires signaling downstream of the BCR. We conclude, therefore, that the developing chicken B cell repertoire is subject to tolerance induction and that the induction of tolerance can occur prior to the migration of B cell precursors to the bursa.
Materials and Methods
Generation of replication-competent avian leukosis virus retroviral constructs
To generate BCRs with defined specificity, the Ag binding portion of the VLR molecule, called the diversity region, was fused to the chicken truncated m-chain. The diversity region of hen egg lysozyme (HEL)– or PE-specific VLRs (16) was PCR amplified using Platinum Pfx Polymerase (Invitrogen, San Diego, CA) and the primer combination VLR5’ (5’-ATTATTTGGCCACGGCATGTCCCTCGCAGT-3’) and VLR3’ (5’-ATTATTCGGCCGGGCATTTCGAGGGGCTAGTG-3’) from the VLR-containing vectors and cloned into Zero Blunt (Invitrogen). Cloned VLRs were digested from this shuttle vector, using EaeI and EagI restriction enzyme sites embedded within the primers. VLR sequences of ~ 600 bp were gel purified and cloned into the EagI site of RCAS(BP)B-Tμ (replication-competent avian leukosis virus with splice acceptor Bryan's polymerase [RCAS(BP)]), between the chicken H chain leader sequence and the truncated μ (Tμ) (17).
The nonclassical murine MHC class I molecule, thymus leukemia Ag (TL), and mouse β2-microglobulin (β2m) were cloned from RMA-S TL (18) cells generously provided by Dr. James Carlyle (Sunnybrook Health Sciences Centre, Toronto, ON, Canada). Briefly, TL Ag was amplified from extracted mRNA, using the TL5’ (5’-ATTATTATCGATATCTCCCTAACATGAGGATG-3’) and TL3’ (5’-ATTATTTTCGAATCAGGAGACCAATGGTGGGGC-3’) primers, and the resulting PCR product was gel purified and digested for ligation. Murine β2m was cloned from mRNA of RMA-S TL cells using the β2m5’ (5’-ATTATTATCGATATGGCTCGCTCGGTGACC-3’) and β2m3’ (5’-ATTATTTTCGAATCACATGTCTCGATCCCAGTAG-3’) primers, gel purified and digested for ligation. The IRES was amplified using IRES5’ (5’-ATTATTATCGATGGATCCGTGCCC-3’) and IRES3’ (5’-ATTATTTTCGAAATTATCATCGTGTTTTTCAAAGGAA-3’) primers from the IRES containing plasmid pRetroX-IRES-ZsGreen1 (Clontech Laboratories, Mountain View, CA), gel purified and digested for ligation. The TL-IRES-β2m construct was cloned by sequential ligation of β2m, IRES, and TL, respectively, into RCAS(BP)B using ClaI and BstBI sites, which allowed destruction of 3’ restriction sites, retaining the ClaI cloning site upstream of each insert.
The RCAS(BP)A–mCD8α:chIgα chimeric receptor was previously generated by Pike et al. (19) and was used in double transfections in vitro and inoculations in vivo. The RCAS(BP)A–mCD8α:chIgαF1F2F3 mutant was generated by site-directed mutagenesis to convert each of the three tyrosine residues in the cytoplasmic domain of Iga to phenylalanine.
Retroviral gene transfer vector production and in vivo gene transfer
The RCAS retroviral gene transfer system was used to introduce receptor genes of interest into chicken embryo fibroblasts (CEFs) and chicken embryos, as described elsewhere (20). Line 0 CEFs (Regional Poultry Research Laboratories, East Lansing, MI), cultured in IMDM (Life Technologies) supplemented as described earlier, were transfected with RCAS-based plasmids by calcium chloride precipitation, as described elsewhere (20). Within 7 d of transfection, essentially all CEFs expressed the retrovirally transduced chimeric receptors.
DT40 infection was performed as described previously (21). Light chain mutant DT40 chicken B lymphoma cells were infected with the RCAS viruses by coculturing serial dilutions of DT40 (starting at 5 × 104 cells) with semiconfluent transfected CEFs in 6 wells of a 12-well plate, for 48 h in IMDM with 2% chicken serum. Nonadherent DT40 cells were harvested, expanded, and FACS sorted on a FACSAria (BD Biosciences, Mississauga, ON, Canada).
To generate chickens transduced with the receptor of interest, day 3 incubated B line (ISA North America A Hendrix Genetics Company, Cambridge, ON, Canada) chick embryos were inoculated with 1 × 106 CEFs that were > 98% transfected in 100 μl IMDM with 1% chicken serum. After disinfection of the top of the egg (air sac end) with 70% ethanol, a 1-mm hole was cut, and transfected CEF cell suspensions were injected through the top of the egg, using a 1-inch 18-gauge needle, followed by sealing of the hole with melted paraffin wax.
Abs and flow cytometry
Surface expression of VLRHELTμ or VLRPETμ receptors on CEFs was detected using R-PE (Columbia Biosciences, Columbia, MD), FITC-conjugated anti-chicken μ (HY18), and biotinylated HEL (Sunnybrook Health Sciences Centre Ab Facility) followed by Streptavidin-APC (BD Biosciences). Ex vivo B cells were stained with anti-ChB6 pan-B cell marker (Bu1-APC), HY18-FITC, or anti-chicken γ L chain Ab. Ag binding was detected by R-PE or HEL–biotin. TL expression was detected using TL18/20 or biotinylated HD168 (22); murine β2m was detected with anti-mβ2m LYM11 (provided by Dr. David Williams, Department of Biochemistry, Faculty of Medicine, University of Toronto) followed by Streptavidin-PerCP (BD Biosciences). Murine CD8α was detected with mAb 53-6.72 (provided by P. Hugo, MetrioGene Biosciences, Montreal, QC, Canada).
Specificity and affinity of the VLRHELTμ receptor were estimated by inhibition assay. Cells were stained with conjugated HEL in the presence of serial dilutions of nonconjugated HEL. The inhibition curve was calculated by plotting mean fluorescence intensity of binding against inhibitor concentration (GraphPad Prism 4).
TL binding to the mCD8α:chIgα-transfected CEFs was assessed by staining with PE-conjugated TL tetramer. Samples were analyzed on a FACSCalibur or LSRII (BD Biosciences, Mississauga, ON, Canada).
Ag injection in ovo
Incubated embryos were candled to determine location of blood vessels in the chorioallantoic membrane. A 1 cm × 0.5 cm window was cut in the shell and the underlying membrane rendered translucent with paraffin oil. Then 0.1 ml Ag in PBS was injected i.v., using a 30-gauge needle, and the window was sealed with melted paraffin wax.
PCR amplification of integrated VLR sequences
Integrated VLR sequences were amplified from transduced bursa and spleen, using nested PCR. Approximately 1 million cells were solubilized in PCR lysis buffer for 4 h. A total of 104 cell equivalents were amplified with RCAS 5’ and 3’ primers and also with chicken genomic RAG2 gene primers as a control. A total of 1 μl of the first-round reaction was used for the nested PCR, using VLR and RAG2 primers.
Results
Generation of Ag-specific BCR transduced chicken B cells
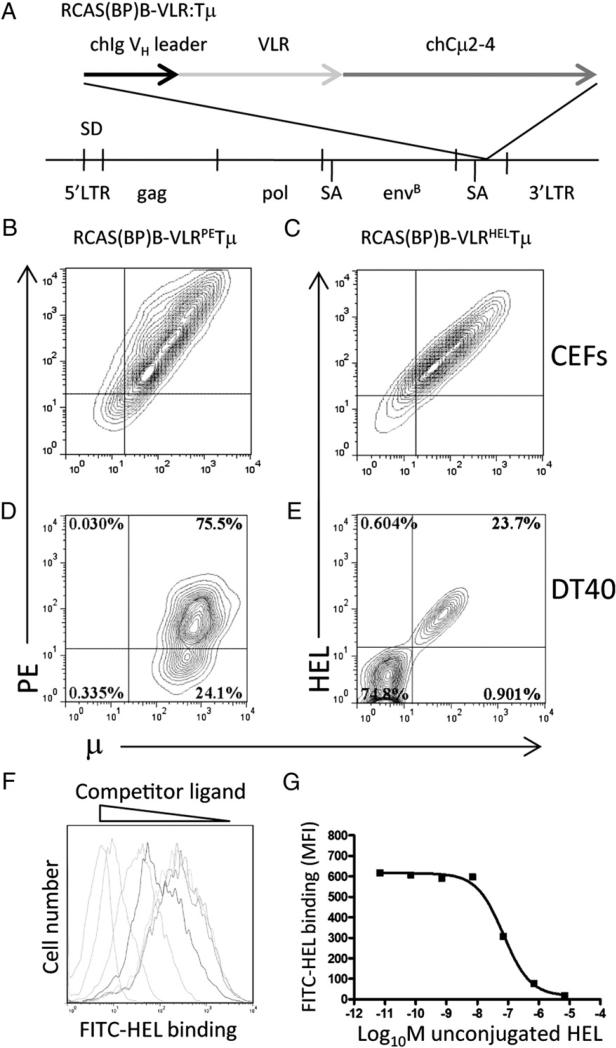
To generate sIg-related BCRs of defined specificity, we made use of Ag-specific lamprey VLRs with specificity for PE and HEL isolated from a yeast display library (16). The diversity region sequences of the lamprey VLRs were fused to the chicken truncated μ (Tμ) sequence, using the chicken VH leader sequence. The resulting constructs were cloned into the RCAS(BP)B productive retroviral vector (Fig. 1A) and transfected into CEFs (Fig. 1B, 1C). We have previously observed that surface expression of chicken Tμ does not require coexpression of Igα and Igβ on CEFs, and under these circumstances does not signal when cross-linked (21). CEFs transfected with the RCAS(BP)B–VLR:Tμ also expressed these constructs at similar levels on the CEF surface (Fig. 1B, 1C). Staining with HEL or PE demonstrated that the RCAS(BP)B–VLRHELTμ and RCAS(BP)B–VLRPETμ constructs maintained the specificity of the original selected VLR.
FIGURE 1.
VLRPETμ and VLRHELTμ receptor expression in chicken cells. (A) Schematic structure of RCAS(BP)B–VLRHEL/PETμ. The VLR diversity region was fused between the chicken VH leader sequence and the truncated chicken (CH2–4) transmembrane m-chain. CEFs were transfected with RCAS(BP)B–VLRPETμ (B) or RCAS(BP)B–VLRHELTμ (C), and surface expression and specificity of the chimeric receptors were assessed. Contour plots are gated on forward scatter and side scatter and are representative of 10,000 cells stained with anti-μ and specific Ag. sIg− DT40 chicken B cells were infected with RCAS(BP)B–VLRPETμ (D) or RCAS(BP)B– VLRHELTμ (E); VLR expression and Ag binding were assessed by anti-μ and specific Ag staining. Contour plots are gated on forward scatter and side scatter and are representative of 10,000 events. Binding affinity of the VLRHELTμ expressed on CEFs (F) was assessed by inhibition of binding by nonfluorescent HEL. Histograms are gated on forward scatter and side scatter and are representative of 10,000 events. (G) Affinity of binding was calculated by plotting the mean fluorescent intensity of bound fluorescent Ag versus concentration of competing nonconjugated HEL Ag.
The RCAS(BP)B–VLRHELTμ and RCAS(BP)B–VLRPETμ constructs were introduced into the chicken bursal B cell lymphoma DT40 (Fig. 1D, 1E). Expression of RCAS(BP)B–VLRPETμ was stable and cells expressing the construct could be readily sorted to homogeneity. In contrast, RCAS(BP)B–VLRHELTμ expression on DT40 cells was less stable in cultures including normal chicken serum that contains HEL (Fig. 1E). When expressed on the B cell surface, Tμ cross-linking results in signaling indicative of association with Igα/Igβ (20). Similarly, cross-linking of VLRPETμ on DT40 cells induces calcium mobilization (D. Davani, Z. Pancer, and M.J.H. Ratcliffe, submitted for publication), again consistent with expression of VLRPETμ associated with Igα/Igβ. Thus whereas Tμ and VLR-Tμ can be expressed on the cell surface in the absence of Igα/Igβ, Tμ and VLR-Tμ will associate with the Igα/Igβ complex if available.
Specificity and affinity of binding of VLRHELTμ on RCAS(BP)B– VLRHELTμ–transfected CEFs were further assessed by inhibition of VLRHELTμ binding to FITC–HEL conjugates by nonconju-gated HEL. Affinity of binding was estimated to be ~ 100 nM, reflecting the concentration of HEL that reduced the binding of FITC–HEL to 50% (Fig. 1F, 1G).
Self-reactive VLRHELTμ-expressing B cells do not productively colonize the developing chicken bursa
VLRHELTμ and VLRPETμ were introduced into developing hematopoietic precursors by inoculating day 3 chicken embryos with CEFs transfected with RCAS(BP)B–VLRHELTμ and RCAS(BP) B–VLRPETμ, respectively. Under these circumstances, inoculated CEFs themselves do not become chimeric with the recipient embryo, as shown by the expression of chicken pan-B cell marker (ChB6) alleles in previous studies (20).
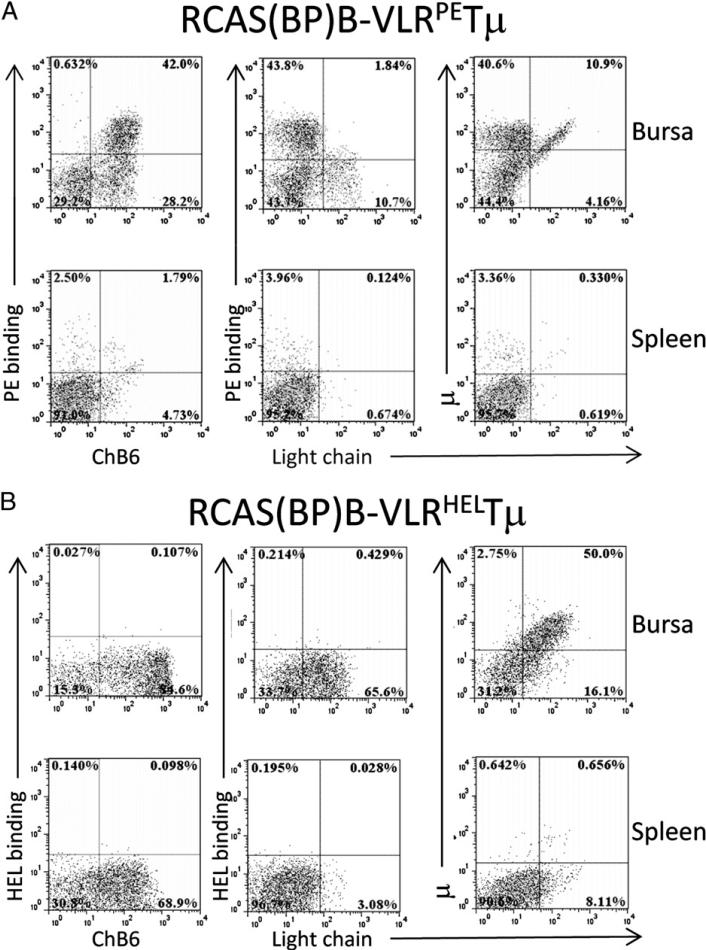
All chicken bursal and splenic B cells express the pan-B cell marker ChB6, and all B cells in the normal bursa are sIgM+ (μ+L+), consistent with the finding that sIg expression is required for productive bursal colonization (19, 20, 23–25). In chicks infected with the RCAS(BP)B–VLRPETμ virus, a significant proportion of neonatal bursal and splenic ChB6+ B cells expressed the chimeric VLRPETμ and lacked endogenous sIgM, as judged by the lack of Ig L chain expression. The proportion of VLRPETμ+/IgM− B cells was 10–40% in the bursae of 30 chickens analyzed (Fig. 2A). In contrast, VLRHELTμ-expressing B cells were not found in the spleen or bursa of chickens infected with the RCAS(BP)B– VLRHELTμ virus (Fig. 2B).
FIGURE 2.
Colonization of bursa and spleen by VLR:Tμ constructs. Bursa and splenic cells were isolated from neonatal chicks infected at day 3 of embryogenesis with RCAS(BP)B–VLRPETμ (A) or RCAS (BP)B–VLRHELTμ (B) transduced CEFs. Presence of VLRPETμ- or VLRHELTμ-expressing cells was assessed by flow cytometry on cells stained for ChB6, μ, Ig L chain, and PE or HEL. Plots are representative of 30 animals from 3 independent experiments. Dot plots are gated on forward scatter and side scatter, and 50,000 cells are represented.
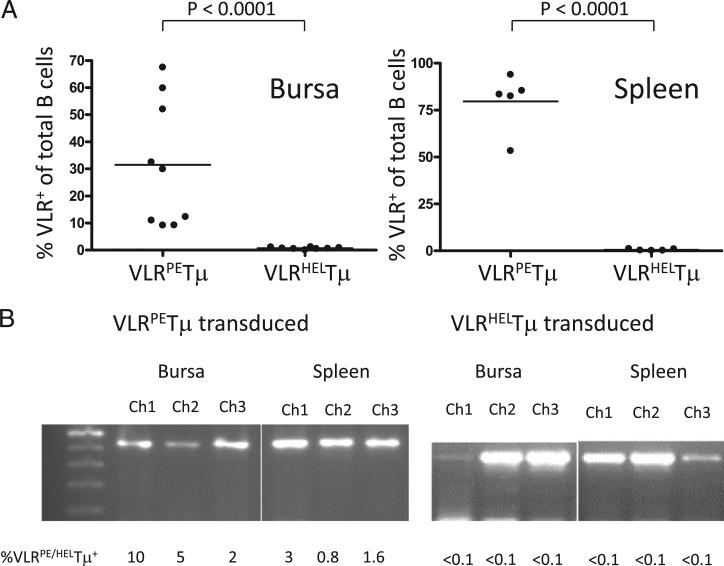
Because the VLRHELTμ receptor was expressed on CEFs at levels equivalent to those of the VLRPETμ receptor, the lack of VLRHELTμ-expressing B cells in vivo could be due to either selective deletion of these cells or a lack of RCAS(BP)B–VLRHELTm viral penetrance in vivo. We therefore confirmed the penetrance of the RCAS(BP)B–VLRHELTμ virus by PCR amplification of the integrated VLRHELTμ sequence from ex vivo spleen and bursal cells, containing both B and non-B cells, because the RCAS virus infects both B and non-B lineage cells (Fig. 3). We conclude, therefore, that the presence of high levels of endogenous HEL resulted in deletion of VLRHELTμ-expressing B cells in vivo (Fig. 3).
FIGURE 3.
Self-reactive VLRHELTμ+ B cells are not detected in RCAS(BP)B– VLRHELTμ transduced chickens. Neonatal bursal and splenic cells of chicks infected at day 3 of embryogenesis by RCAS(BP)B– VLRPETμ or RCAS(BP)B–VLRHELTμ were isolated. (A) The percentage of VLR: Tm-expressing B cells from nine VLRPETμ- and eight VLRHELTμ-infected individual neonatal chickens from three independent experiments was compared (unpaired t test with Welch's correction). (B) Integrated VLRPE and VLRHEL with distinct sizes were amplified by nested-PCR from neonatal bursal and splenic cells; each lane is representative of an individual animal, and the percentage of VLR:Tμ-expressing cells in each sample is indicated below the lane.
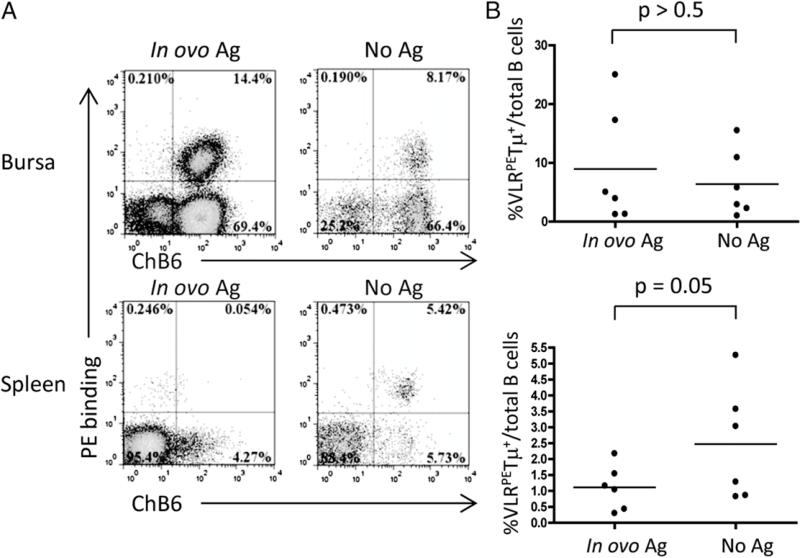
At day 17 of embryonic development, those B cells present in spleen are predominantly prebursal as opposed to postbursal B cells (26). To determine whether negative selection as a consequence of BCR ligation is induced in prebursal cells, we inoculated RCAS(BP)B–VLRPETμ transduced embryos with soluble PE on embryonic day (e) 13. At this time B cell precursors are found in both bursal and splenic tissues. B cells in these tissues were subsequently assessed on day 17e for VLRPETμ expression. Exposure to soluble PE resulted in significant deletion of VLRPETμ-expressing B cells in the spleen (Fig. 4). We can conclude, therefore, that the prebursal stage of chicken B cell development is subject to negative selection following BCR ligation. This conclusion is consistent with the lack of VLRHELTμ-expressing B cells in the neonatal spleen (Fig 3).
FIGURE 4.
In ovo Ag injection reduces Ag-specific B cells in the spleen, but not the bursa. (A) Day 13 RCAS(BP)B–VLRPETμ–infected embryos were injected with 1 mg of PE (Ag treated) or PBS (No Ag) in ovo and assessed on day 17e. VLRPETμ+ B cells were stained with ChB6 and PE and analyzed by flow cytometry. Dot plots are gated on forward scatter and side scatter and are representative of 50,000 cells. (B) The VLRPETμ+/total B cell ratios in the bursa and the spleen of Ag-treated (day 13e) versus control chicks were compared (unpaired t test with Welch's correction).
In contrast, i.v. injection of soluble PE into the day 13 RCAS VLRPETμ-infected embryos failed to induce significant deletion of VLRPETμ-expressing bursal B cells. One possible interpretation of this result is that Ag inoculated into the circulation is poorly accessible to the bursal mesenchyme or to B cells resident in bursal follicles at the time of Ag injection. Under these circumstances, bursal B cells would not be exposed to sufficient Ag to induce BCR ligation and negative selection. Alternatively, bursal B cells may be intrinsically more resistant to negative selection following BCR ligation.
BCR interaction with membrane-bound ligand induces signaling-dependent deletion
The ability of Ag to mediate deletion of Ag-specific B cells in the embryo could either be a consequence of signaling induced downstream of the BCR or, alternatively, be an indirect consequence of Ag binding to the B cell surface. To address this issue, we have taken advantage of an Ig-related chimeric receptor containing the extracellular and transmembrane portion of murine CD8α fused to the cytoplasmic domain of chicken Igα previously generated in the laboratory (19). This mCD8α:chIgα receptor construct is functionally equivalent to intact sIg with respect to its ability to support B cell development past the Ig selection checkpoint. Thus, B cell precursors expressing mCD8α:chIgα colonize bursal follicles and undergo clonal expansion and the induction of gene conversion (25). In contrast, the signaling-defective mutant, mCD8α:chIgαF1F2F3, in which the tyrosine residues of the Igα ITAM motif as well as the non-ITAM tyrosine residue implicated in BLNK recruitment were replaced with phenylalanine failed to support B cell development past the Ig selection checkpoint. Thus, infection of day 3 chicken embryos with the mCD8α:chIgαF1F2F3 construct resulted in B cells coexpressing mCD8α:chIgαF1F2F3 together with endogenous sIgM (21).
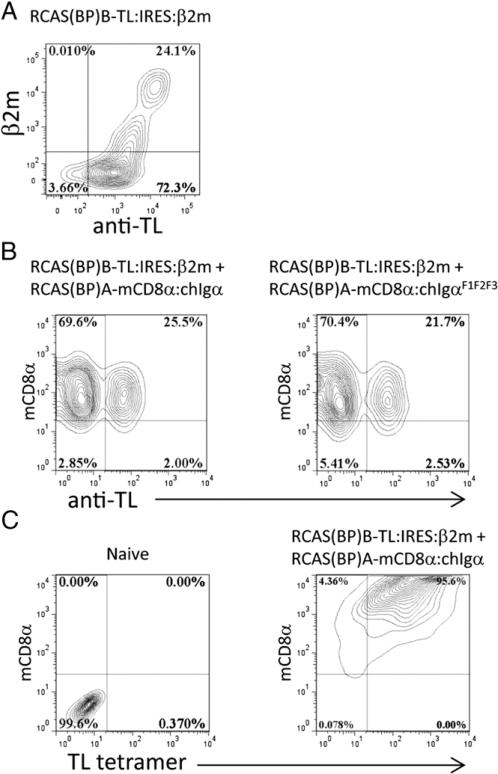
A ligand for the mCD8α homodimer is the TL Ag, a surface nonclassical MHC class I Ag that is expressed as a heterodimer with β2m. We therefore cloned TLa (mouse A strain) from the RMA-S cell line (18) (provided by Dr. James Carlyle, Sunnybrook Research Institute) by RT-PCR and introduced it into the RCAS (BP)B retroviral vector. To provide surface expression of TL in chicken cells, TL was expressed together with murine β2m by cloning TL and β2m bicistronically with an IRES sequence. The RCAS(BP)B–TL:IRES:mβ2m was transfected into CEFs, and TL/mβ2m expression was confirmed by staining with anti-mouse β2m and anti-TL Abs (Fig. 5A). The observation that some cells stained for the surface expression of TL in the absence of staining for mouse β2m is consistent with the surface expression of some TL being supported by the presence of FCS-derived β2m in the tissue culture medium. The RCAS virus includes subgroups that bind to distinct cell surface receptors and allow for double transfection or infection of chicken cells that express both receptors. Thus, individual chicken cells can be doubly infected using A and B subgroup viral strains. The mCD8α:chIgα and mCD8α:chIgαF1F2F3 constructs were cloned into RCAS(BP)A, and the TL:IRES:mβ2m construct was cloned into RCAS(BP)B. Double transfections of CEFs with RCAS(BP)A– mCD8α:chIgα or RCAS(BP)A–mCD8α:chIgαF1F2F3 together with RCAS(BP)B–TL:IRES:mβ2m showed the feasibility of introducing both the CD8α receptor and its ligand into CEFs (Fig. 5B). To confirm the binding of TL to the mCD8α:chIgα used in these experiments, we showed that TL tetramers bound the surface of CD8α:Igα-expressing CEFs (Fig. 5C).
FIGURE 5.
Expression of mCD8α:chIgα and TL/β2m constructs in vitro. (A) TL cell surface expression and association with mβ2m was assessed on RCAS(BP)B–TL/b2m–transfected CEFs by flow cytometry using anti-murine β2m and anti-TL Abs. (B) Cell surface expression of TL, mCD8α:chIgα, and mCD8α-chIgαF1F2F3 was assessed on CEFs transfected with the indicated combinations of RCAS constructs. (C) TL binding capacity of mCD8α:chIgα was demonstrated by TL tetramer staining of mCD8α:chIgα-transfected CEFs. Contour plots are representative of 10,000 cells gated on forward scatter and side scatter.
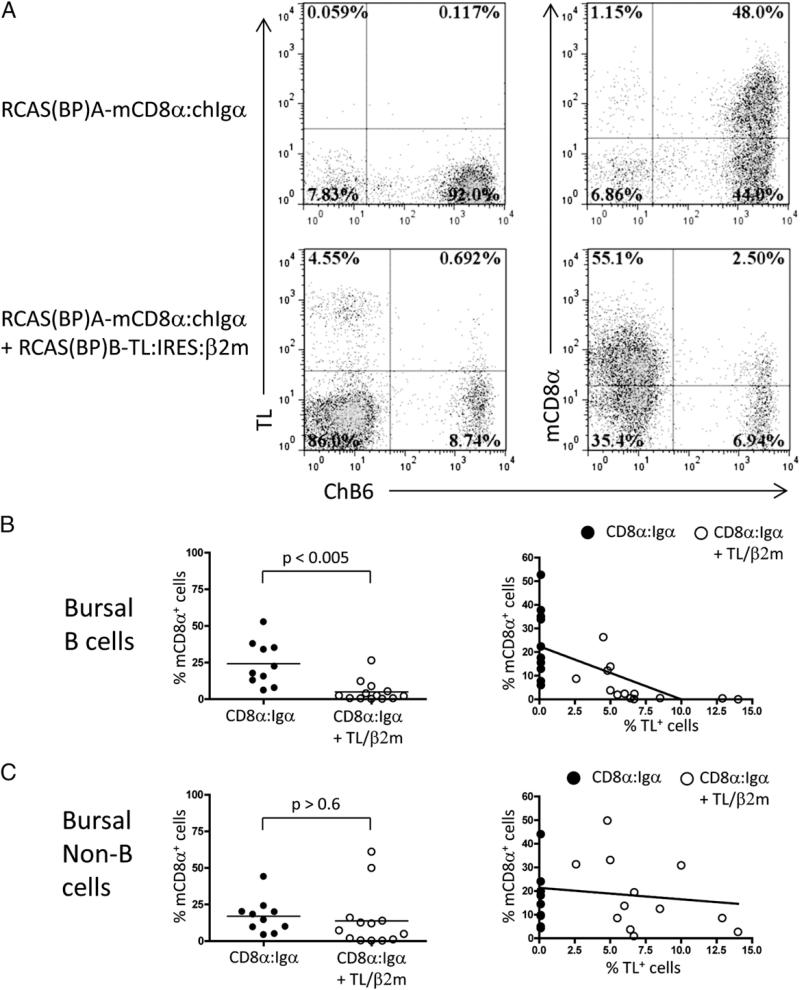
Introduction of RCAS(BP)A–mCD8α:chIgα into day 3 chicken embryos showed colonization of the bursa with cells expressing mCD8α:chIgα. In contrast, neonatal chicks coinfected with RCAS (BP)A–mCD8α:chIgα and RCAS(BP)B–TL:IRES:mβ2m showed reduced levels of mCD8α:chIgα expressing B cells (Fig. 6A, 6B). Strikingly, we observed a clear inverse correlation between the frequency of cells expressing TL/mb2m and the frequency of mCD8α: chIgα expressing B cells. This suggested the possibility that TL/ mβ2m expression was mediating negative selection of mCD8α: chIgα expressing B cells (Fig. 6B).
FIGURE 6.
mCD8α:chIgα-expressing B cells are subject to deletion in the presence of TL/β2m ligand. (A) Presence of ChB6+, mCD8α+ B cells was assessed in RCAS(BP)A–mCD8α:chIgα– or RCAS(BP)A–mCD8α:chIgα + RCAS(BP)B–TL:IRES:mβ2m– infected neonates by flow cytometry. The relationship between the frequency of TL-expressing cells and the percentage of mCD8α+ bursal B cells (B) or non-B cells (C) was determined in single-and double-infected individual day17e or day1 chickens. Plots are representative of 10 single- and 13 double-transduced animals from three independent experiments (unpaired t test with Welch's correction). Dot plots of 50,000 bursal cells gated on FSC and SSC are shown.
Further support for this contention came from the fact that although the frequency of mCD8α:chIgα-expressing non-B cells was also variable in RCAS(BP)A–mCD8α:chIgα transduced chicks, there was no deletion of these cells in RCAS(BP)A–mCD8α:chIgα plus RCAS(BP)B–TL:IRES:mβ2m coinfected chicks (Fig. 6C). Thus negative selection was specific for developing B cells.
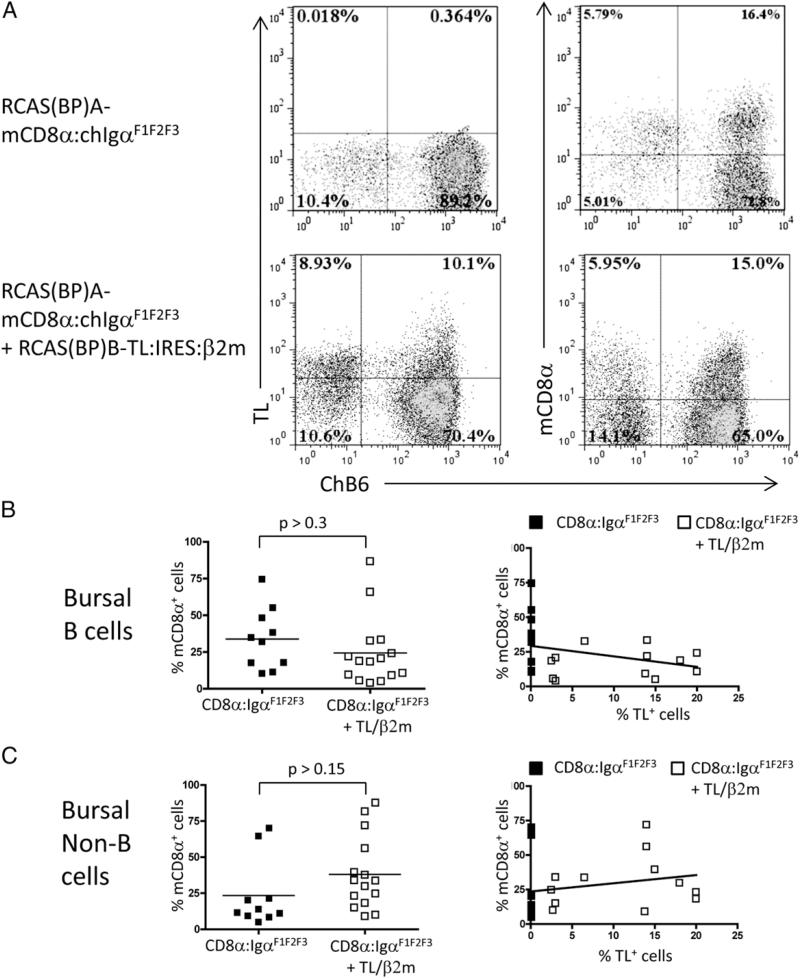
To determine whether deletion of mCD8α:chIgα-expressing cells was due to signaling downstream of the BCR, we took advantage of the mCD8α:chIgαF1F2F3 signaling–defective mutant. In chicks infected with the RCAS(BP)A–mCD8α:chIgαF1F2F3 virus, a substantial population of mCD8α:chIgαF1F2F3 cells were observed (Fig. 7A), although all these cells coexpressed sIgM because the mCD8α:chIgαF1F2F3 receptor does not itself support B cell development (data not shown). Analysis of chicks coinfected with RCAS(BP)B–TL:IRES:mβ2m and RCAS(BP)A–mCD8αchIgαF1F2F3 showed survival of the mCD8α:chIgαF1F2F3-expressing B cells in the presence of the TL/mβ2m ligand with no correlation between the frequency of mCD8α-chIgαF1F2F3–expressing B cells and the frequency of cells expressing TL/mβ2m (Fig. 7B). Not surprisingly, the expression of TL/mβ2m did not significantly affect the levels of mCD8α:chIgαF1F2F3-expressing non-B cells (Fig. 7C). Comparable levels of TL/β2m were expressed in both mCD8α:chIgα and mCD8α-chIgαF1F2F3 coinfected chicks (data not shown). Thus we can conclude the deletion of mCD8α:chIgα-expressing cells in the presence of TL/mβ2m requires signaling downstream of the mCD8α:chIgα receptor.
FIGURE 7.
mCD8α+ B cell deletion is dependent on BCR signaling. (A) Presence of ChB6+ mCD8α:chIgαF1F2F3-expressing bursal B cells was analyzed by staining of TL/β2m + mCD8α:chIgα transduced day 1 neonates. The relationship between the frequency of mCD8α:chIgαF1F2F3-expressing B cells (B) or non-B cells (C) and the frequency of TL/β2m-expressing cells in TL/β2m + mCD8α:chIgαF1F2F3 transduced day17e or day1 animals was determined. Plots are representative of 10 single- and 15 double-transduced animals from 3 independent experiments (unpaired t test with Welch's correction). Dot plots of 50,000 bursal cells gated on forward scatter and side scatter are shown.
Discussion
Tolerance is a critical step that lymphocytes undergo during their development. The humoral immune system in humans and ro dents generates a diverse pool in excess of 1011 Ag receptors by random assortment of Ig gene segments, the combination of Ig heavy and light chains, and junctional diversity. Therefore, the immune system recruits several mechanisms to keep a tight control over the development of autoreactive B cells at the cost of extensive cell death, as reviewed elsewhere (1, 27).
In chickens, although Ig gene rearrangement does not generate significant diversity, gene conversion is a potent diversifying mechanism for H and L chain V genes. In the same way, therefore, that tolerance is crucial for the normal development of rodent and primate B cells, it might be expected that tolerance would also be important in the development of chicken B cells. Although mechanisms of B cell tolerance have been extensively investigated in mammalian models of bone marrow B cell development, the developmental stages of gut-associated B lymphopoiesis and the stages at which tolerance may occur remain unclear.
We have taken advantage of the specificity-determining regions of the lamprey VLR to generate fusion constructs in which the diversity region of the lamprey VLR is fused to the truncated chicken μ-chain. These constructs are effectively expressed on the surface of CEFs, as is the chicken Tμ construct itself. This finding is a likely consequence of reduced polarity in the chicken transmembrane region of the μ-chain, as discussed elsewhere (24). Importantly, we observed no difference in the efficiency of expression when comparing the VLRPETμ and VLRHELTμ constructs in CEFs.
Although VLRPETμ and VLRHELTμ were expressed on the surface of CEF in vitro, infection of the chicken B cell line, DT40, with VLRHELTμ was stable only in the absence of chicken serum in the media. The DT40 cell line has been used to analyze BCR signaling upon BCR ligation, and DT40 cells undergo apoptosis upon BCR ligation (28, 29). The inability of VLRHELTμ-expressing DT40 cells to grow in the presence of normal chicken serum is consistent with serum-derived HEL ligating the VLRHELTμ construct.
We have used the RCAS(BP) (24, 30, 31) to transduce developing hematopoietic precursors with modified forms of sIg-related receptors in vivo. Because the retroviral transduction is < 100%, transduced BCR–expressing B cells compete with those expressing endogenous sIg for colonization of bursal follicles and subsequent development. In the experiments reported in this article, transduced VLRPETμ penetrance is typically in the order of 10– 50% of B cells. Thus, the transduced VLRPETμ supports the development of B cells as efficiently as the endogenous sIg. This finding is fully consistent with our previous demonstration of effective support of B cell development by Tμ.
In contrast, VLRHELTμ-expressing B cells were not observed in RCAS(BP)B–VLRHELTμ–infected chicks, despite expressing high levels of integrated virus. This lack of observation is based on the inability to detect cells binding the biotinylated HEL. We can rule out the trivial explanation that these cells are, in fact, present and blocked by binding endogenous HEL because we observe no cells expressing IgH in the absence of the L chain, characteristic of cells expressing VLRTμ constructs in the absence of endogenous sIg. We conclude, therefore, that VLRHELTμ-expressing B cells are subject to tolerance induction.
In murine models, in which soluble HEL is used as a neo–self-antigen, B cell anergy rather than deletion is observed. In the experiments reported in this article, we do not observe VLRHELTμ-expressing B cells. This finding may be a consequence of the levels of soluble HEL in the respective systems. In the transgenic soluble HEL model in mice, levels of serum HEL were in the order of 10 ng/ml (32). In contrast, 3–5 mg/ml of HEL is present in the chick embryo or 3 μg/ml in the adult chicken serum, levels that are more than 1000-fold higher than those seen in HEL transgenic mice (33, 34). It is possible, therefore, that deletion of VLRHELTμ-expressing B cells, as opposed to anergy, is a consequence of the extremely high levels of HEL present in the chicken embryo.
Alternatively, anergic murine B cells have a short lifespan in the presence of nonanergic B cells (35). The continued presence of anergic B cells in these models is a consequence of ongoing B cell development and the continual production of new B cells expressing the self-reactive sIg. In chickens, there is a single wave of B cell development, and any anergic B cells with a reduced lifespan would be eliminated without being replaced by newly generated B cells.
A third possibility follows from the absence of IgD in the chicken genome. A characteristic of anergic murine B cells is the downregulation of sIgM while levels of sIgD are maintained (32). Under these circumstances, induction of anergy in developing chicken B cells might induce a downregulation of sIgM in the absence of sIgD. We have previously shown that downregulation of chicken IgM among bursal cells precedes apoptotic cell death (36), presumably because the levels of residual sIgM become too low to support the tonic signaling required for continued B cell development in the absence of sIgD.
Thus, although we can clearly conclude that exposure of VLRHELTμ-expressing B cells to their cognate Ag leads to deletion and tolerance induction, we cannot formally determine whether this is a consequence of direct induction of deletion per se or the induction of anergy leading to deletion.
In chickens, B cell development occurs in a single wave in which B lineage committed precursors colonize the bursa (10–12). This development can be divided temporally into distinct stages. The early stages of chicken B cell development include colonization of bursal follicles, expression of functional sIg, and subsequent cell expansion (23, 31). Chickens have only one functional variable H and L chain gene, and rearrangement of these variable segments generates a prediversified BCR with limited diversity. Expression of that receptor, independent of its specificity, is required and sufficient to support the early stages of B lymphopoiesis. We have shown in this article that peripheral B cells in the embryo are subject to negative selection. These cells include prebursal cells, and so the consequence of ligating the BCR on B cells prior to their migration to the bursa is negative selection. Given the very limited repertoire of prebursal B cells, this clearly places an evolutionary constraint on the specificity of the chicken germline receptor to avoid self-reactivity.
The great majority of newly generated normal B cells in the developing bursa die in situ. This fact cannot be due to the generation of nonproductive gene rearrangements because the formation of a functional sIg receptor is required for colonization of bursal follicles. Similarly, the rate of nonproductive gene conversion is only 2–3% of gene conversion events (37). Although some cell death is likely, owing to the generation of Ig H and L chain combinations that fail to pair appropriately to form a functional sIg molecule (A. Neschadin and M.J.H. Ratcliffe, unpublished observations), the high levels of bursal cell death have not been satisfactorily explained. Those bursal cells that die in situ are those in which levels of sIgM are downregulated (36). The deletion of emerging self-reactive cells likely contributes, therefore, to the levels of cell death seen among developing bursal cells.
Tolerized B cells in the mouse have the potential to undergo receptor editing. This is clearly not possible with our VLRHELTμconstruct, owing to the nature of the specificity-encoding VLR and the random positions at which RCAS(BP)B–VLRHELTμ retroviral integration occurs. In normal chicken bursal cells, however, the potential exists for those B cells that encounter self-antigen to undergo further gene conversion to modify their specificity away from self-antigen recognition.
Introduction of the non–self-reactive VLRPETμ to day 3 chick embryos generated transduced B cells found in both the bursa and the spleen. We therefore investigated whether the presence of PE-specific B cells in VLRPETμ transduced chicks was modified by exposure to PE. In ovo i.v. injection of PE into day 13e VLRPETμ transduced embryos resulted in deletion of splenic VLRPETμ-expressing B cells. Although the deletion we observed was not complete, it was clearly significant and possibly limited by the quantity and persistence of PE that could be administered. Thus, it is plausible that the partial deletion of VLRPE-expressing cells following PE injection may be due to the threshold required for deletion of the autoreactive B cells Nonetheless, at day 17e, the great majority of splenic B cells are prebursal rather than post-bursal cells (26), allowing us to draw the conclusion that the bursal microenvironment is not required for the induction of tolerance.
PE injection into VLRPETμ transduced embryos did not result in significant deletion of VLRPETμ-expressing cells in the bursa. This finding could again be due to limitations in the amount of Ag delivered to the bursa following i.v. injection, in contrast to the levels of PE in the serum, which would be more readily accessible to peripheral B cells. The levels of HEL in the developing embryo are > 10-fold higher than the levels of injected PE, and the transfer of HEL into bursal follicles may be much more efficient than the transfer of PE, which has a much higher m.w. In this regard, deletion of IgM allotype–expressing B cells in both bursa and spleen has been shown upon injection of anti-allotype Ab in ovo during embryonic life (13, 38). When injected neonatally, anti-allotype Abs deplete allotype-expressing B cells in the spleen more effectively than those in the bursa, although repeated postnatal injection of anti-allotype Abs does deplete allotype-bearing cells in the bursa. This finding supports the contention that there is limited accessibility to the bursal microenvironment of proteins present in serum rather than an intrinsic resistance of bursal cells to tolerance induction and explains the inability of PE inoculated i.v. to induce deletion of VLRPETμ-expressing bursal B cells. This is equivalent to models of self-tolerance in mice in which the affinity and avidity of the BCR for Ag can affect the persistence of tolerant B cells (1, 4). It would be interesting to know whether surviving VLRPETμ bursal B cells in PE-injected embryos are anergic.
We also addressed the effect of membrane-bound self-antigen on B cell selection. To achieve this, we took advantage of two strains of the RCAS vector that can simultaneously infect individual cells. The MHC class I/CD8 interaction is species specific, and comparison of amino acids involved in this interaction between chickens and mice indicates several modifications in the positioning of charged residues (39, 40). Therefore, mouse CD8 does not bind chicken MHC class I (19, 21, 31); in contrast, the murine TL associates with murine CD8aa homodimers with a binding affinity much stronger than that in the interaction of CD8aa with classical murine MHC class I (41). We initially observed that expression of murine TL in the CEFs was dependent on an exogenous source of mammalian β2m and chick embryos transduced with RCAS-TL failed to express the TL Ag on the cell surface. We therefore constructed the RCAS-TL:IRES:mβ2m virus that allowed TL expression in vivo. Chicks doubly transfected with mCD8α: chIgα and TL/mβ2m showed deletion of mCD8α:chIgα-expressing B cells in the bursa and spleen. Deletion was not always complete but was inversely related to the levels of TL expression. The number of B cells colonizing each bursal follicle is low, between 2 and 5. Under circumstances in which the frequency of TL-expressing cells is relatively low, individual bursal follicles may therefore not contain TL-expressing cells and may not provide a tolerizing environment for developing mCD8α:chIgα-expressing B cells. Our conclusion that B cells are being tolerized by exposure to TL/mβ2m is strengthened by the observation that in the same chicks the frequency of mCD8α-expressing non-B cells is not affected by the presence of TL/mβ2m. This finding argues that the mechanism involved in the deletion of mCD8-expressing cells is intrinsic to B cells in contrast to non-B cells.
Negative selection of mCD8α-chIgα–expressing B cells by TL/β2m was shown to involve BCR signaling. This was formally demonstrated by the survival of mCD8α-chIgαF1F2F3–expressing B cells in the presence of TL/β2m. This finding is consistent with studies in murine B cells in which modulation of signaling downstream of sIg affects the capacity of the sIg to mediate negative selection. We have previously shown that whereas B cell expression of the CD8a:Iga construct supported both calcium mobilization, when expressed in DT40 cells, and B cell development in vivo, mutations of tyrosine residues in the Iga sequence compromised both calcium mobilization and B cell development. Although calcium mobilization could be complemented by pairing the mutant mCD8α-Iga with a wild-type mCD8β-Igβ, B cell development was not complemented. In contrast, a mCD8α-Iga construct in which the BLNK binding tyrosine of Iga was mutated when combined with a wild-type mCD8b-Igb supported B cell development while not reconstituting high-level calcium mobilization. We have therefore dissociated the signals required to support B cell development in vivo from the signals required to support high-level calcium mobilization (20). It would be inter esting to determine whether the signals required for negative selection are equivalent to those required to support B cell development or whether additional signals such as those needed for calcium mobilization are necessary.
In conclusion, the results presented in this article demonstrate that developing chicken B cells are subject to self-tolerance by soluble and cell surface Ag, independent of the bursal microenvironment, but dependent on signaling downstream of the BCR.
Acknowledgments
We thank Gisele Knowles and Arian Khandani for expert assistance with flow cytometry and cell sorting, Dr. James Carlyle (Sunnybrook Health Sciences Centre) for providing the RMAS-TL cell line, and Dr. David Williams (University of Toronto) for providing the anti-mβ2m Ab.
This work was supported by the Canadian Institutes for Health Research (to M.J.H.R.) and by a Canadian Institutes for Health Research strategic training grant in regenerative medicine (to D.D.).
Abbreviations used in this article:
- CEF
chicken embryo fibroblast
- e
embryonic
- HEL
hen egg lysozyme
- β2m
β2-microglobulin
- RCAS
replication-competent avian leukosis virus
- RCAS(BP)
replication-competent avian leukosis virus with splice acceptor Bryan's polymerase
- sIg
surface Ig
- TL
thymus leukemia Ag
- VLR
variable lymphocyte receptor
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat. Rev. Immunol. 2007;7:633–643. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat. Immunol. 2004;5:645–650. doi: 10.1038/ni1076. [DOI] [PubMed] [Google Scholar]
- 3.Pelanda R, Torres RM. Receptor editing for better or for worse. Curr. Opin. Immunol. 2006;18:184–190. doi: 10.1016/j.coi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Gauld SB, Benschop RJ, Merrell KT, Cambier JC. Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat. Immunol. 2005;6:1160–1167. doi: 10.1038/ni1256. [DOI] [PubMed] [Google Scholar]
- 5.Healy JI, Dolmetsch RE, Timmerman LA, Cyster JG, Thomas ML, Crabtree GR, Lewis RS, Goodnow CC. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–428. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- 6.Arnold B, Dill O, Küblbeck G, Jatsch L, Simon MM, Tucker J, Hämmerling GJ. Alloreactive immune responses of transgenic mice expressing a foreign transplantation antigen in a soluble form. Proc. Natl. Acad. Sci. USA. 1988;85:2269–2273. doi: 10.1073/pnas.85.7.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 8.Nemazee D, Buerki K. Clonal deletion of autoreactive B lymphocytes in bone marrow chimeras. Proc. Natl. Acad. Sci. USA. 1989;86:8039–8043. doi: 10.1073/pnas.86.20.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemazee D, Russell D, Arnold B, Haemmerling G, Allison J, Miller JF, Morahan G, Buerki K. Clonal deletion of autospecific B lymphocytes. Immunol. Rev. 1991;122:117–132. doi: 10.1111/j.1600-065x.1991.tb00600.x. [DOI] [PubMed] [Google Scholar]
- 10.Weber WT, Foglia LM. Evidence for the presence of precursor B cells in normal and in hormonally bursectomized chick embryos. Cell. Immunol. 1980;52:84–94. doi: 10.1016/0008-8749(80)90402-5. [DOI] [PubMed] [Google Scholar]
- 11.Houssaint E, Toraño A, Ivanyi J. Ontogenic restriction of colonization of the bursa of Fabricius. Eur. J. Immunol. 1983;13:590–595. doi: 10.1002/eji.1830130715. [DOI] [PubMed] [Google Scholar]
- 12.Ratcliffe MJH, Lassila O, Pink JR, Vainio O. Avian B cell precursors: surface immunoglobulin expression is an early, possibly bursa-independent event. Eur. J. Immunol. 1986;16:129–133. doi: 10.1002/eji.1830160204. [DOI] [PubMed] [Google Scholar]
- 13.Ratcliffe MJH, Ivanyi J. Allotype suppression in the chicken. I. Generation of chronic suppression in heterozygous but not in homozygous chickens. Eur. J. Immunol. 1979;9:847–852. doi: 10.1002/eji.1830091104. [DOI] [PubMed] [Google Scholar]
- 14.Ratcliffe MJH, Ivanyi J. Allotype suppression in the chicken. II. Suppression in homozygous chickens with antiallotype antibody and allotype-disparate B cells. Eur. J. Immunol. 1981;11:296–300. doi: 10.1002/eji.1830110406. [DOI] [PubMed] [Google Scholar]
- 15.Ratcliffe MJH, Ivanyi J. Allotype suppression in the chicken. IV. Deletion of B cells and lack of suppressor cells during chronic suppression. Eur. J. Immunol. 1981;11:306–310. doi: 10.1002/eji.1830110408. [DOI] [PubMed] [Google Scholar]
- 16.Tasumi S, Velikovsky CA, Xu G, Gai SA, Wittrup KD, Flajnik MF, Mariuzza RA, Pancer Z. High-affinity lamprey VLRA and VLRB monoclonal antibodies. Proc. Natl. Acad. Sci. USA. 2009;106:12891–12896. doi: 10.1073/pnas.0904443106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sayegh CE, Rao MA, Ratcliffe MJH. Avian B cell development: lessons from transgenic models. Vet. Immunol. Immunopathol. 1999;72:31–37. doi: 10.1016/s0165-2427(99)00114-2. [DOI] [PubMed] [Google Scholar]
- 18.Rodgers JR, Mehta V, Cook RG. Surface expression of beta 2-microglobulin-associated thymus-leukemia antigen is independent of TAP2. Eur. J. Immunol. 1995;25:1001–1007. doi: 10.1002/eji.1830250421. [DOI] [PubMed] [Google Scholar]
- 19.Pike KA, Iacampo S, Friedmann JE, Ratcliffe MJH. The cytoplasmic domain of Ig alpha is necessary and sufficient to support efficient early B cell development. J. Immunol. 2004;172:2210–2218. doi: 10.4049/jimmunol.172.4.2210. [DOI] [PubMed] [Google Scholar]
- 20.Sayegh CE, Demaries SL, Iacampo S, Ratcliffe MJH. Development of B cells expressing surface immunoglobulin molecules that lack V(D)J-encoded determinants in the avian embryo bursa of fabricius. Proc. Natl. Acad. Sci. USA. 1999;96:10806–10811. doi: 10.1073/pnas.96.19.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pike KA, Ratcliffe MJH. Dual requirement for the Ig alpha immunoreceptor tyrosine-based activation motif (ITAM) and a conserved non-Ig alpha ITAM tyrosine in supporting Ig alpha beta-mediated B cell development. J. Immunol. 2005;174:2012–2020. doi: 10.4049/jimmunol.174.4.2012. [DOI] [PubMed] [Google Scholar]
- 22.Holcombe HR, Castaño AR, Cheroutre H, Teitell M, Maher JK, Peterson PA, Kronenberg M. Nonclassical behavior of the thymus leukemia antigen: peptide transporter-independent expression of a nonclassical class I molecule. J. Exp. Med. 1995;181:1433–1443. doi: 10.1084/jem.181.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCormack WT, Tjoelker LW, Barth CF, Carlson LM, Petryniak B, Humphries EH, Thompson CB. Selection for B cells with productive IgL gene rearrangements occurs in the bursa of Fabricius during chicken embryonic development. Genes Dev. 1989;3:838–847. doi: 10.1101/gad.3.6.838. [DOI] [PubMed] [Google Scholar]
- 24.Sayegh CE, Demaries SL, Pike KA, Friedman JE, Ratcliffe MJH. The chicken B-cell receptor complex and its role in avian B-cell development. Immunol. Rev. 2000;175:187–200. [PubMed] [Google Scholar]
- 25.Pike KA, Ratcliffe MJH. Ligand-independent signaling during early avian B cell development. Immunol. Res. 2006;35:103–116. doi: 10.1385/IR:35:1:103. [DOI] [PubMed] [Google Scholar]
- 26.Lydyard PM, Grossi CE, Cooper MD. Ontogeny of B cells in the chicken. I. Sequential development of clonal diversity in the bursa. J. Exp. Med. 1976;144:79–97. doi: 10.1084/jem.144.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grimaldi CM, Hicks R, Diamond B. B cell selection and susceptibility to autoimmunity. J. Immunol. 2005;174:1775–1781. doi: 10.4049/jimmunol.174.4.1775. [DOI] [PubMed] [Google Scholar]
- 28.Takata M, Homma Y, Kurosaki T. Requirement of phospholipase C-gamma 2 activation in surface immunoglobulin M-induced B cell apoptosis. J. Exp. Med. 1995;182:907–914. doi: 10.1084/jem.182.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winding P, Berchtold MW. The chicken B cell line DT40: a novel tool for gene disruption experiments. J. Immunol. Methods. 2001;249:1–16. doi: 10.1016/s0022-1759(00)00333-1. [DOI] [PubMed] [Google Scholar]
- 30.Hughes SH, Greenhouse JJ, Petropoulos CJ, Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J. Virol. 1987;61:3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pike KA, Baig E, Ratcliffe MJH. The avian B-cell receptor complex: distinct roles of Igalpha and Igbeta in B-cell development. Immunol. Rev. 2004;197:10–25. doi: 10.1111/j.0105-2896.2004.0111.x. [DOI] [PubMed] [Google Scholar]
- 32.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 33.Kreukniet MB, Nieuwland MG, van der Zijpp AJ. Phagocytic activity of two lines of chickens divergently selected for antibody production. Vet. Immunol. Immunopathol. 1995;44:377–387. doi: 10.1016/0165-2427(94)05304-b. [DOI] [PubMed] [Google Scholar]
- 34.Wilcox FH, Cole RK. The inheritance of differences in the lysozyme level of hens’ egg white. Genetics. 1957;42:264–272. doi: 10.1093/genetics/42.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodnow CC, Cyster JG, Hartley SB, Bell SE, Cooke MP, Healy JI, Akkaraju S, Rathmell JC, Pogue SL, Shokat KP. Self-tolerance checkpoints in B lymphocyte development. Adv. Immunol. 1995;59:279–368. doi: 10.1016/s0065-2776(08)60633-1. [DOI] [PubMed] [Google Scholar]
- 36.Paramithiotis E, Jacobsen KA, Ratcliffe MJH. Loss of surface immunoglobulin expression precedes B cell death by apoptosis in the bursa of Fabricius. J. Exp. Med. 1995;181:105–113. doi: 10.1084/jem.181.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sayegh CE, Drury GL, Ratcliffe MJH. Efficient antibody diversification by gene conversion in vivo in the absence of selection for V(D)J-encoded determinants. EMBO J. 1999;18:6319–6328. doi: 10.1093/emboj/18.22.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ratcliffe MJH, Ivanyi J. Allotype suppression in the chicken. III. Analysis of the recovery form suppression by neonatally injected or maternal antibodies. Eur. J. Immunol. 1981;11:301–306. doi: 10.1002/eji.1830110407. [DOI] [PubMed] [Google Scholar]
- 39.Kaufman J, Salomonsen J, Flajnik M. Evolutionary conservation of MHC class I and class II molecules—different yet the same. Semin. Immunol. 1994;6:411–424. doi: 10.1006/smim.1994.1050. [DOI] [PubMed] [Google Scholar]
- 40.Tregaskes CA, Kong FK, Paramithiotis E, Chen CL, Ratcliffe MJH, Davison TF, Young JR. Identification and analysis of the expression of CD8 alpha beta and CD8 alpha alpha isoforms in chickens reveals a major TCR-gamma delta CD8 alpha beta subset of intestinal intraepithelial lymphocytes. J. Immunol. 1995;154:4485–4494. [PubMed] [Google Scholar]
- 41.Attinger A, Devine L, Wang-Zhu Y, Martin D, Wang JH, Reinherz EL, Kronenberg M, Cheroutre H, Kavathas P. Molecular basis for the high affinity interaction between the thymic leukemia antigen and the CD8alphaalpha molecule. J. Immunol. 2005;174:3501–3507. doi: 10.4049/jimmunol.174.6.3501. [DOI] [PubMed] [Google Scholar]