Abstract
Cockayne Syndrome (CS) is a rare autosomal recessive segmental progeria characterized by growth failure, lipodystrophy, neurological abnormalities and photosensitivity but without skin cancer predisposition. CS life expectancy ranges from 5 to 16 years for the two most severe forms (Types II and I, respectively). Mouse models of CS have thus far been of limited value due either to very mild phenotypes, or premature death during postnatal development prior to weaning. The cause of death in severe CS models is unknown but has been attributed to extremely rapid aging. Here, we found that providing mutant pups with soft food from as late as postnatal day 14 allowed survival past weaning with high penetrance independent of dietary macronutrient balance in a novel CS model (Csa-/- ∣ Xpa-/-). Survival past weaning revealed a number of CS-like symptoms including small size, progressive loss of adiposity and neurological symptoms, with a maximum lifespan of 19 weeks. Our results caution against interpretation of death before weaning as premature aging, and at the same time provide a valuable new tool for understanding mechanisms of progressive CS-related progeroid symptoms including lipodystrophy and neurodysfunction.
Keywords: DNA repair, Cockayne syndrome, CSA, XPA, segmental progeria, lifespan
Proteins altered in CS, including CSA and CSB, share a common function in transcription-coupled nucleotide excision DNA repair (TC-NER) in the nucleus and base excision repair (BER) of mitochondrial DNA (Sykora et al. 2012). TC-NER specifically removes lesions in DNA that obstruct RNA polymerases and block transcription, and is distinguished from Global Genome NER (GG-NER), which operates throughout the genome to eliminate helix-distorting DNA damage (Hoeijmakers 2009). CSA and CSB are required for removal of RNA polymerase stalled at the site of a lesion in order for the repair reaction to occur (Fousteri & Mullenders 2008). CS proteins are also localized to mitochondria and are thought to recruit and stabilize BER proteins to repair complexes in the inner mitochondrial membrane. Human CS cells exhibit an altered redox balance with increased intracellular ROS and mitochondrial dysfunction (Scheibye-Knudsen et al. 2012). Whether this reflects indirect effects of unrepaired DNA damage, or a more direct role of CS proteins in redox balance remains unknown. NER proteins also participate in a number of transcription related processes including initiation and elongation (Brooks 2013). Mutations in other NER-related genes (XPD, XPG, XPB) can also cause CS in combination with symptoms of Xeroderma Pigmentosum (XP), most notably pigmentation abnormalities and elevated skin cancer in UV-exposed skin, resulting in the combined disorder XPCS (Cleaver et al. 2009). CS includes a spectrum of severities with life expectancies ranging from 5 years for the most severe form (early onset Type II including Cerebro-Oculo-Facio-Skeletal Syndrome, COFS) (Suzumura & Arisaka 2010), 16.1 for moderate (classical Type I) and 30.3 for atypical (mild, late onset Type III) (Natale 2011).
Numerous mouse models of CS (Csb-/-, Csa-/-) and XPCS (XpdXPCS/XPCS) have been established, however with a generally milder phenotype than CS (van de Ven et al. 2007). Further inhibition of NER by crossing these models into backgrounds lacking the GG-NER-specific protein XPC, or XPA which is common to both sub-pathways, results in a more severe disorder, NER progeria, that more closely resembles the small size, lipodystrophy and neurological complications of CS (van de Ven et al. 2007). A hallmark of severe NER progeria in Csb-/- ∣ Xpa-/- (Murai et al. 2001; van der Pluijm et al. 2006), XpdXPCS/XPCS ∣ Xpa-/- (Andressoo et al. 2006), Csb-/- ∣ Xpc-/- (Laposa et al. 2007) and Xpg-/- (Harada et al. 1999) mutant models is death before weaning at approximately 3 weeks of age. Attempts to extend lifespan past the weaning period by such interventions as reducing litter size, moistening the food (van der Pluijm et al. 2006), providing surrogate mothers (de Boer et al. 2002) or extending the nursing period (Andressoo et al. 2006) have so far proven unsuccessful.
We generated double homozygous mutants by intercrossing Csa-/- ∣ Xpa+/- or Csa+/- ∣ Xpa-/- mice. Csa-/- ∣ Xpa-/- (CX) mice were born at Mendelian ratios and were indistinguishable at birth from controls. By postnatal day 5, CX mice were visually smaller in size compared to WT or single KO littermates. By postnatal day 12, clenching of the hind limbs, indicative of neurological dysfunction, was evident. As with other progeroid NER mice, CX mice reached a peak weight at approximately two weeks of age, followed by a decline in fitness, failure to thrive, and premature death with nearly 100% penetrance by postnatal day 28. Control heterozygous and single homozygous mutant CSA (Csa-/-) and XPA (Xpa-/-) mice developed normally as reported previously (de Vries et al. 1995; van der Horst et al. 2002).
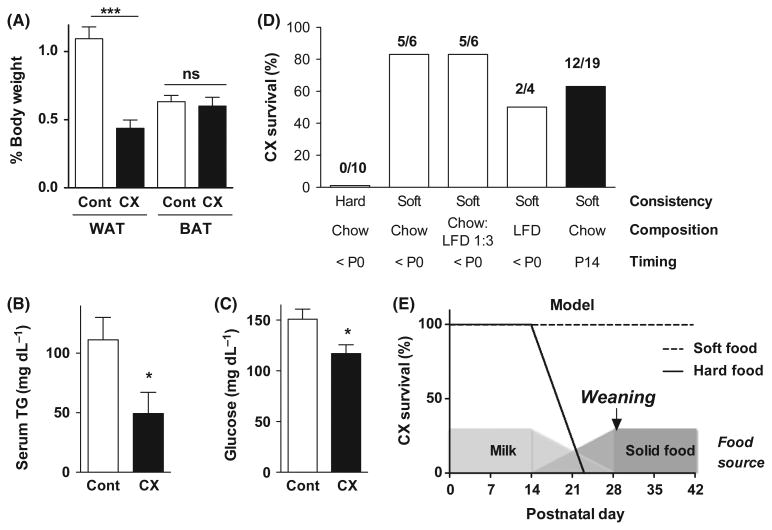
Despite the fact that CX mutants nursed and had milk in their stomachs observable upon sacrifice between days 14 and 21, white adipose but not brown adipose tissue weights were reduced relative to body weight on postnatal day 10 (Fig 1A). Serum triglycerides and blood glucose were also reduced (Fig 1B,C). We hypothesized that a nutritional deficiency might contribute to death at weaning, and reasoned that increasing the energy density of the milk by supplementing lactating dams with a high fat diet may ameliorate this mutant phenotype. Feeding pregnant dams a high fat diet (HFD, 60% calories from fat) vs. standard chow (22% calories from fat) increased triglycerides in their milk without significantly altering protein content (Fig S1) and thus increasing total calories. Consistent with our hypothesis, HFD feeding of lactating dams rescued premature death in CX mice with high penetrance (Fig 1D). However, counter to our hypothesis, a control low fat diet (LFD) with only 10% calories from fat also rescued premature death (Fig 1D) without significantly increasing triglycerides or total calories in the milk (Fig S1).
Figure 1. Death before weaning as a result of starvation.
(A) White and brown adipose tissue (WAT, BAT) expressed as a percent of total body weight in 10-day-old control (Cont, n = 4) and CX (n = 5) animals as indicated. Asterisks indicate significance by Student's t-test between the indicated groups; *** P < 0.0001. (B) Triglycerides (TG) measured in serum of control (n = 6) and CX (n = 4) mice at postnatal day 14; *P < 0.05 by Student's t-test. (C) Blood glucose measured in control (n = 12) and CX (n = 12) mice between P7 and P14; *P < 0.05 by Student's t-test. (D) Survival of CX mice past weaning at P28. Diets of the indicated consistency (hard pellets vs. pulverized pellets in soft agar form) and composition (standard chow, refined LFD, or a 1:3 mixture of the two) were present from before birth (<P0) or from P14 as indicated. The number of surviving/total CX mice in each diet group is indicated above the bar. (E) Model for a bottleneck in CX mouse development comprising an inability to consume hard food during the transition from milk to solids beginning around P14. Presentation of soft food at this time can prevent malnutrition and death. Beyond this bottleneck, neither consistency nor macronutrient balance affects longevity.
Because both diets (HFD, LFD) that rescued death before weaning were composed of refined ingredients, we next hypothesized that a contaminant or toxin present in the standard chow, a grain-based diet made with unrefined ingredients, might interact with the congenital DNA repair deficiency and cause premature death. To address this, we pulverized refined and unrefined pellets, and prepared them in a final 1% semi-solid agar form separately or in a 3:1 ratio. Regardless of the composition of the diet, CX mutant litters receiving agar-based diets survived weaning with high penetrance (Fig 1D). These data are inconsistent with a toxin in unrefined chow or differences in macronutrient content between refined and unrefined diets as causative of premature death in CX mice. Instead, they suggest a defect in the ability to transition from mother's milk to solid food unless that food is soft. Consistent with this, HFD pellets are soft due to their high fat content, and LFD pellets, although initially hard, readily absorbed water (Fig S2) and became soft in the hopper within days, likely due to the absorbance of humidity from the cage. To directly test the potential role of soft food during the transition from milk to solids, we presented litters with standard chow in soft agar form from postnatal day 14, coincident with this transition in C57BL/6 mice (http://jaxmice.jax.org/literature/factsheet/LT0001_Pups.pdf). Twelve out of 19 CX mice survived weaning at 28 days (Fig 1D). We conclude that CX mutants that cannot transition to solid food during this developmental window die of malnutrition (Fig 1E).
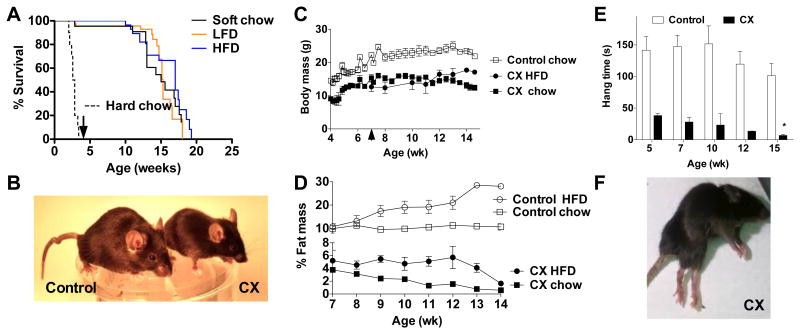
CX mutants weaned at four weeks of age onto chow in soft agar form had an average lifespan of ∼16 weeks (Fig 2A). Five CX mutants weaned onto hard pelleted chow displayed similar longevity (data not shown); the requirement for soft food is thus likely to occur only during an early developmental window. CX mutants weaned onto HFD had slightly extended mean but not maximal lifespan relative to LFD or chow (Fig 2A). CX mutants remained smaller than control littermates throughout their lifespans (Fig 2B, C). Similar to pre-weaned mice, CX mutants weaned onto a chow diet had reduced adiposity, consisting of ∼2% of body weight through most of their adult life. Mutants on a HFD from 7 wks of age achieved higher adiposity of ∼5%, but failed to maintain it towards the end of their lifespan (Fig 2D). Lean % body mass was maintained independent of diet or loss of adiposity; organ weights were also maintained relative to body weight except for a reduction in WAT, while heart, lung and brain were slightly increased (Fig S3).
Figure 2. Progressive lipodystrophy and neurological complications in a new mouse model of Type I/II CS.
A. Kaplan Meier analysis of CX mice born to dams fed hard chow pellets (dotted line, n=10) or soft diets as indicated (LFD, n=47; HFD, n=29; chow agar, n=22). Arrow indicates weaning at 4 weeks of age. B. Representative image of CX and control littermates at 14 wks of age. C. Body weights of CX and control mice weaned onto chow pellets at 4 wks or CX mice switched to HFD at 7 wks (arrowhead); n=3/group. D. Body fat percentage of CX and control mice fed HFD or chow pellets over the indicated time period (n=3/group). E. Grip strength of CX and control mice at the indicated age (n=3/genotype/age) as determined by the length of time hanging on a wire. Asterisks indicate significance according to a one-way ANOVA followed by Dunnett's multiple comparison test vs. the 5 week time point within genotype (* p<0.05); between genotype comparisons were significant (p<0.05) at each timepoint by one-way ANOVA and Tukey's multiple comparison test. F. Representative image of 16 wk old CX mouse displaying hind-limb paralysis, lipodystrophy and kyphosis.
Neurological involvement was observed on several levels, including abnormal gait consistent with cerebellar ataxia, inappropriate hind limb clasping in a tail hang test (Fig S4) and progressive reduction in grip strength (Fig 2E) culminating in uncoordinated hind limb movement, dystonia, and eventual paralysis (Fig 2F, Fig S5 movies). Other symptoms typical of progeroid disorders were also observed, including kyphosis (FigS6). The reasons underlying these progressive progeroid symptoms are unknown, and they could not rescued by feeding refined or high energy-density diets (Fig 2A). Taken together, our data indicate that early postnatal death hampering existing models of severe CS resulted from malnutrition and could be rescued with soft food during the critical developmental transition from milk to solid food (Fig 1E). Surviving animals were smaller in size and had progressive adipose and neurological features reminiscent of CS.
Why were CX mice unable to transition normally to solid food? Prolonged survival on a soft food diet after weaning was previously observed in an inducible mouse model of Hutchinson-Gilford Progeria Syndrome with apparent dental abnormalities (Sagelius et al. 2008). Oral anomalies are frequently noted in CS patients (Schneider 1983; Sorin 1994; Horbelt 2010); however, analysis of bone density in CX teeth revealed no defects in tooth development, enamel, emergence of incisors or molars, or differences in root length during the developmental window of P14 to P19 or later in life (Fig. S7). CS patients typically have problems eating, possibly due to a neuromuscular deficit, such as restricted mandibular range of motion that can hinder chewing (Boraz 1991), or coordination of chewing and swallowing (E. Nielan, personal communication). As a result, many are fed soft food and/or receive nutrition via a gastrojejunostomy feeding tube. Future experiments will be required to address the underlying causes of feeding problems in CS patients, as well as the developmental defect in eating hard chow pellets observed in CX mice, and any potential connection between the two.
Why is rescue of death before weaning in CX mice important? This is the first severe CS model to survive weaning with high penetrance, allowing for the analysis of key progeroid features of CS, including progressive loss of adiposity and neurological dysfunction, and their potential relationship to DNA repair insufficiency and normal outside of the window of early development. It also cautions against the use of lifespan extension beyond weaning as a means to score efficacy of experimental interventions until the cause of the inability to switch to solid food is better understood (van der Pluijm et al. 2006).
Methods
Mice
Mice carrying Xpa and/or Csa knockout alleles in a C57BL/6 background were maintained under standard laboratory conditions (temperature 20–24 °C, relative humidity 50–60%, 12 h light/12 h dark) and allowed free access to water and standard chow pellets (PicoLab 5058, Purina) unless otherwise indicated. All animal experiments were performed with the approval of the appropriate institutional animal care and use committee.
Diets
PicoLab 5058 chow (Purina) consists of unrefined ingredients including wheat, corn, soybean, fish, yeast, and porcine fat, with 23%, 22% and 55% calories from protein, fat and carbohydrates, respectively. High fat (60% calories, D12492) and low fat (10% calories, D12450B) diets (Research Diets, New Brunswick, NJ) consist of refined ingredients (casein, corn starch, maltodextrin 10, sucrose, soybean oil, lard). Agar-based food was prepared by pulverizing pellets of PicoLab 5058 or D12450B and mixing 100g with 100mL of a 2% agar solution. Water absorption of food pellets was determined in a humidified chamber (80% relative humidity) by weighing pellets daily.
Grip strength test
Mice were allowed to hang for 180 seconds on a 55cm wide × 2mm thick wire secured 35cm over soft bedding. The length of time hanging was recorded in three trials with at least 5 minutes rest in between each trial.
Body composition
Fat and lean mass were assessed by non-invasive nuclear magnetic resonance (NMR) in awake animals (EchoMRI, Echo Medical Systems, Houston, TX, USA).
Blood Measurements
Glucose: Freshly drawn tail vein blood was analyzed with a hand-held blood glucose monitor (One Touch; Johnson & Johnson) according to the manufacturer's instructions. Triglycerides: Serum was analyzed using the Serum Triglyceride Determination Kit (Sigma) according to manufacturer's instructions.
Statistics
Data analyses were performed in GraphPad Prism 5.0 and presented as the mean +/- SEM unless otherwise noted.
Supplementary Material
Acknowledgments
This work was funded in part by NIH training grants (Interdisciplinary Genes and Environment T32ES016645; Radiation Biology T32CA009078), the Glenn Foundation for Medical Research and the Luke O'Brien Foundation. We thank Ediz Calay for technical assistance and Raul Mostoslovsky, Edward Nielan and Jaan-Olle Andressoo for insightful discussions.
Contributor Information
Lear E. Brace, Email: learbrace@fas.harvard.edu.
Sarah C. Vose, Email: Sarah.Vose@state.vt.us.
Dorathy F. Vargas, Email: dvargas@hsph.harvard.edu.
Shuangyun Zhao, Email: shuangyuan_zhao@hsdm.harvard.edu.
Xiu-Ping Wang, Email: xiuping_wang@hsdm.harvard.edu.
James R. Mitchell, Email: jmitchel@hsph.harvard.edu.
References
- Andressoo JO, Mitchell JR, de Wit J, Hoogstraten D, Volker M, Toussaint W, Speksnijder E, Beems RB, van Steeg H, Jans J, de Zeeuw CI, Jaspers NG, Raams A, Lehmann AR, Vermeulen W, Hoeijmakers JH, van der Horst GT. An Xpd mouse model for the combined xeroderma pigmentosum/Cockayne syndrome exhibiting both cancer predisposition and segmental progeria. Cancer Cell. 2006;10:121–132. doi: 10.1016/j.ccr.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Boraz RA. Cockayne's syndrome: literature review and case report. Pediatric dentistry. 1991;13:227–230. [PubMed] [Google Scholar]
- Brooks PJ. Blinded by the UV light: How the focus on transcription-coupled NER has distracted from understanding the mechanisms of Cockayne syndrome neurologic disease. DNA Repair (Amst) 2013 doi: 10.1016/j.dnarep.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver JE, Lam ET, Revet I. Disorders of nucleotide excision repair: the genetic and molecular basis of heterogeneity. Nat Rev Genet. 2009;10:756–768. doi: 10.1038/nrg2663. [DOI] [PubMed] [Google Scholar]
- de Boer J, Andressoo JO, de Wit J, Huijmans J, Beems RB, van Steeg H, Weeda G, van der Horst GT, van Leeuwen W, Themmen AP, Meradji M, Hoeijmakers JH. Premature aging in mice deficient in DNA repair and transcription. Science. 2002;296:1276–1279. doi: 10.1126/science.1070174. [DOI] [PubMed] [Google Scholar]
- de Vries A, van Oostrom CTM, Hofhuis FMA, Dortant PM, Berg RJW, de Gruijl FR, Wester PW, van Kreijl CF, Capel PJA, van Steeg H, Verbeek SJ. Increased susceptibility to ultraviolet-B and carcinogens of mice lacking the DNA excision repair gene. XPA Nature. 1995;377:169–173. doi: 10.1038/377169a0. [DOI] [PubMed] [Google Scholar]
- Fousteri M, Mullenders LH. Transcription-coupled nucleotide excision repair in mammalian cells: molecular mechanisms and biological effects. Cell research. 2008;18:73–84. doi: 10.1038/cr.2008.6. [DOI] [PubMed] [Google Scholar]
- Harada YN, Shiomi N, Koike M, Ikawa M, Okabe M, Hirota S, Kitamura Y, Kitagawa M, Matsunaga T, Nikaido O, Shiomi T. Postnatal growth failure, short life span, and early onset of cellular senescence and subsequent immortalization in mice lacking the xeroderma pigmentosum group G gene. Mol Cell Biol. 1999;19:2366–2372. doi: 10.1128/mcb.19.3.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers JH. DNA damage, aging, and cancer. The New England journal of medicine. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- Horbelt CV. Robinow syndrome, Cockayne syndrome, and Pfeiffer syndrome: an overview of physical, neurological, and oral characteristics. General dentistry. 2010;58:14–17. [PubMed] [Google Scholar]
- Laposa RR, Huang EJ, Cleaver JE. Increased apoptosis, p53 up-regulation, and cerebellar neuronal degeneration in repair-deficient Cockayne syndrome mice. Proc Natl Acad Sci U S A. 2007;104:1389–1394. doi: 10.1073/pnas.0610619104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai M, Enokido Y, Inamura N, Yoshino M, Nakatsu Y, van der Horst GT, Hoeijmakers JH, Tanaka K, Hatanaka H. Early postnatal ataxia and abnormal cerebellar development in mice lacking Xeroderma pigmentosum Group A and Cockayne syndrome Group B DNA repair genes. Proc Natl Acad Sci U S A. 2001;98:13379–13384. doi: 10.1073/pnas.231329598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale V. A comprehensive description of the severity groups in Cockayne syndrome. American journal of medical genetics. Part A. 2011;155A:1081–1095. doi: 10.1002/ajmg.a.33933. [DOI] [PubMed] [Google Scholar]
- Sagelius H, Rosengardten Y, Hanif M, Erdos MR, Rozell B, Collins FS, Eriksson M. Targeted transgenic expression of the mutation causing Hutchinson-Gilford progeria syndrome leads to proliferative and degenerative epidermal disease. J Cell Sci. 2008;121:969–978. doi: 10.1242/jcs.022913. [DOI] [PubMed] [Google Scholar]
- Scheibye-Knudsen M, Ramamoorthy M, Sykora P, Maynard S, Lin PC, Minor RK, Wilson DM, Cooper M, Spencer R, de Cabo R, Croteau DL, Bohr VA. Cockayne syndrome group B protein prevents the accumulation of damaged mitochondria by promoting mitochondrial autophagy. J Exp Med. 2012;209:855–869. doi: 10.1084/jem.20111721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider PE. Dental findings in a child with Cockayne's syndrome. ASDC journal of dentistry for children. 1983;50:58–64. [PubMed] [Google Scholar]
- Sorin MS. Cockayne's syndrome: dental findings and management. The Journal of clinical pediatric dentistry. 1994;18:299–302. [PubMed] [Google Scholar]
- Suzumura H, Arisaka O. Cerebro-oculo-facio-skeletal syndrome. Advances in experimental medicine and biology. 2010;685:210–214. doi: 10.1007/978-1-4419-6448-9_19. [DOI] [PubMed] [Google Scholar]
- Sykora P, Wilson DM, 3rd, Bohr VA. Repair of persistent strand breaks in the mitochondrial genome. Mech Ageing Dev. 2012;133:169–175. doi: 10.1016/j.mad.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven M, Andressoo JO, Holcomb VB, Hasty P, Suh Y, van Steeg H, Garinis GA, Hoeijmakers JH, Mitchell JR. Extended longevity mechanisms in short-lived progeroid mice: identification of a preservative stress response associated with successful aging. Mech Ageing Dev. 2007;128:58–63. doi: 10.1016/j.mad.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst GT, Meira L, Gorgels TG, de Wit J, Velasco-Miguel S, Richardson JA, Kamp Y, Vreeswijk MP, Smit B, Bootsma D, Hoeijmakers JH, Friedberg EC. UVB radiation-induced cancer predisposition in Cockayne syndrome group A (Csa) mutant mice. DNA Repair (Amst) 2002;1:143–157. doi: 10.1016/s1568-7864(01)00010-6. [DOI] [PubMed] [Google Scholar]
- van der Pluijm I, Garinis GA, Brandt RM, Gorgels TG, Wijnhoven SW, Diderich KE, de Wit J, Mitchell JR, van Oostrom C, Beems R, Niedernhofer LJ, Velasco S, Friedberg EC, Tanaka K, van Steeg H, Hoeijmakers JH, van der Horst GT. Impaired genome maintenance suppresses the growth hormone--insulin-like growth factor 1 axis in mice with Cockayne syndrome. PLoS Biol. 2006;5:e2. doi: 10.1371/journal.pbio.0050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.