Abstract
Diets rich in omega-3s have been thought to prevent both obesity and osteoporosis. However, conflicting findings are reported, probably as a result of gene by nutritional interactions. Peroxisome proliferator-activated receptor-gamma (PPARγ), is a nuclear receptor that improves insulin sensitivity but causes weight gain and bone loss. Fish oil is a natural agonist for PPARγ and thus may exert its actions through PPARγ pathway. We examined the role of PPARγ in body composition changes induced by a fish or safflower oil diet using two strains of C57BL6J (B6); i.e. B6.C3H-6T (6T) congenic mice created by backcrossing a small locus on Chr 6 from C3H carrying ‘gain of function’ polymorphisms in the Pparγ gene onto a B6 background, and C57BL6J mice. After 9 months of feeding both diets to female mice, body weight, percent fat and leptin levels were less in mice fed the fish oil vs those fed safflower oil, independent of genotype. At the skeletal level, fish oil preserved vertebral bone mineral density (BMD) and microstructure in B6 but not in 6T mice. Moreover, fish oil consumption was associated with an increase in bone marrow adiposity and a decrease in BMD, cortical thickness, ultimate force and plastic energy in femur of the 6T but not B6 mice. These effects paralleled an increase in adipogenic inflammatory and resorption markers in 6T but not B6. Thus, compared to safflower oil, fish oil (high ratio omega-3/-6) prevents weight gain, bone loss, and changes in trabecular microarchitecture in the spine with age. These beneficial effects are absent in mice with polymorphisms in the Pparγ gene (6T), supporting the tenet that the actions of n-3 fatty acids on bone microstructure are likely to be genotype dependent. Thus caution must be used in interpreting dietary intervention trials with skeletal endpoints in mice and in humans.
Keywords: aging, Omega-3s, Bone-fat interactions, Genetic animal models, bone microCT
Introduction
The loss of bone mineral density (BMD) and the increase in bone marrow adiposity are common hallmarks of the aging process (1). As the average life expectancy continues to increase, the need to develop new strategies to prevent osteoporosis and fragility fractures is rising. Dietary factors have long been known to influence bone remodelling and fragility (2). Several epidemiological studies have particularly focused on Mediterranean diets (3,4) and have suggested an association between fish oil consumption and lower rate of bone loss in older adults (5–7). However, conflicting results are reported in clinical and pre-clinical studies. For example, in epidemiological studies, BMD was positively associated to n-3 polyunsaturated fatty acids (PUFA) (8) or fish (9,10) consumption. Cross sectional studies also reported a lower risk of osteoporosis fracture among women with a higher intake of sea food (11). In contrast, others found no significant association between PUFA consumption, bone formation markers, BMD or risk fracture (12,13,14). Moreover, other studies highlight opposite findings by showing a higher fracture risk in women taking higher polyunsaturated fat (15). Although those discrepancies may arise due to various confounding factors (age, dose, time of treatment…), one of the main explanations might relate to the interaction between dietary intake and the genome, since genetic factors are thought to contribute between 55% to 85% to the variance of bone loss with age (16).
B6.C3H-6T congenic (6T) mice were developed to gain insights into the genetics and biology underlying phenotypes of the C3H/HeJ (C3H, High BMD and IGF-1) and the C57BL/6J (B6, low BMD and IGF-1) mice. 6T mice were generated by intercrossing a region of Chromosome 6 from C3H onto a B6 background and then backcrossing 10 generations onto B6. The Chromosome 6 quantitative trait locus (QTL) encompasses close to 20cM on mid-distal mouse chromosome 6; there are more than 500 genes in this region; however, finer mapping revealed a list of 21 candidate genes; of those there are hundreds of SNPs between C3H and B6; however there are only 3 coding non synonymous SNPs, none of which differed between C3H and B6 (17). Based on previous studies, 2 SNPs located in the distal UTR of PPARγ were identified to be the most important contributor to the 6T phenotype and both these were associated with a gain of function of PPARγ. In previous work, low bone mass and reduced IGF-1 (systemic and locally) were the major phenotypic characteristics of the 6T mouse; both these were worsened by feeding a high fat diet due to the increased activity of PPARγ (18). This supported the possibility that PPARγ itself was one of the determinants of Igf-1 gene expression and protein level (19). Nocturnin, a circadian deadenylase was found to be markedly enhanced when PPARG was activated, and this in turn was thought to cause the low skeletal and circulating IGF-1 levels (20). Association between bone and PPARγ was verified in a human cohort, showing different skeletal responses to dietary environment depending on the genetic variation of Pparγ (18, 21).
Other genetic mouse models support the notion that enhanced PPARγ signalling worsens bone health with age. PPARγ regulates osteoclastogenesis directly through C-fos expression (22) and indirectly by decreasing the OPG/RANKL ratio in osteoblast/osteocytes (23, 24). As such PPARγ activation may have several effects on bone, including increased bone marrow adiposity, decreased osteoblastogenesis and increased osteoclastogenesis (20, 22, 23).
In vitro, fish oil has been shown to directly up-regulate PPARγ in different cell lines such as C2C12 myocyte or HEK293T kidney cells (25, 26, 27); however in vivo fish oil decreased the absolute amount of PPARγ by decreasing peripheral fat. In rodents, fish oil supplementation has been shown to reduce fat mass and to improve BMD in aging mice after 4 and 6 months of supplementation respectively in Balb/c and C57BL6J mice (28, 29). However, our groups and others reported also in C57BL6J mice either a modest or no structural and mechanical effect on bone of the n-3 fatty acids (FA), respectively after 5 and 14 months of supplementation, mainly found in fish oil (30).
We therefore postulated that the conflicting results of fish oils on bone health with aging may result from a gene×diet interaction between fish oil intake and Pparγ genotype. In this study we used the 6T congenic mice to investigate independent and interactive effects of fish oil with Pparγ genotypes on bone mass, structure and strength in female mice aged from 3 to 12 months old.
Materials and methods
Animals
Forty 10 weeks-old female B6.C3H-6T (6T, n=20) and C57BL/6J (B6J, n=20) mice were obtained from the Jackson laboratory (Bar Harbor, ME, USA). The B6.C3H-6T congenic strain was made as previously described (17, 18). Weight-matched mice were housed in a laboratory animal care facility in cages (5 mice/cage) and fed a standard pellet diet for 2 weeks. At 12 weeks of age, B6.C3H-6T and C57BL/6J mice were divided into two dietary groups (n = 10 per group) and fed 22% safflower oil or 22% fish oil diets (Harlan Teklad TD.08324 and TD08323, supplemental table S1–S2). Isocaloric diets were ensured by Harlan. The two diets were constituted by weight of pellet 21.2% of protein, 42.3% of carbohydrate and 22.2% of fat. Animals were pair feed. Food consumption was recorded weekly in 6T safflower oil, 6T fish oil, B6 safflower oil or B6 fish oil groups and the smallest amount of food intake observed was then fed to all the 4 groups over the following week. Supplements were freshly prepared every three months, stored in aliquots at 4°C and distributed every 2 or 3 days. Mice were maintained on a 12-h light/dark cycle at an ambient temperature of 22–25°C. All mice were sacrificed at the age of 12 months consider to be old mice. Animal procedures were approved by the University Of Geneva School Of Medicine Ethical Committee and the State of Geneva Veterinarian Office.
In vivo measurement of bone mineral density and body composition
Total body mass, lean body mass, fat mass, femoral and spinal bone mineral density (BMD, g/cm2) were measured in vivo at baseline and just before euthanasia by dual-energy X-ray absorptiometry (PIXImus2, GE lunar, Madison WI) (31).
In vivo measurement of morphology and microarchitecture
A high-resolution in vivo microcomputed tomography system (microCT Skyscan 1076, Skyscan, Aartselaar, Belgium) was used to scan the left tibia and the caudal vertebrae at 3 and 12 months of age. The in vivo microCT system consists of an X-ray source and detector rotating around the animal bed. The microCT machine is equipped with a 100 Kv X-ray source with a spot size of 5 µm. A scan lasted approximately 20 min, resulting in shadow projections with a pixel size of 12 µm. A modified Feldkamp algorithm, using undersampling to reduce noise, was applied to the scan data, resulting in reconstructed 3D data sets with a voxel size of 20 µm (32). A detailed description and validation of the algorithm is published elsewhere (33). Cortical and trabecular bones were separated manually with “CT analyzer” software. Outcomes measure for the trabecular and cortical structure are described by Bouxsein et al. (34). To evaluate bone marrow adiposity tibias, we processed ex-vivo protocol with osmium staining as described (35). After labeling of lipids by osmium tetroxide, the bones were imaged using energy of 45 keV (UCT40, Scanco Medical AG, Basserdorf Switzerland).
Histomorphometry
Femur and lumbar spine L5 were embedded in methyl-methacrylate (Merck, Darmstadt, Germany) as previously described (36). Five-8µm thick sagital sections were cut with a Leica Corp. Polycut E microtome (Leica Corp. Microsystems AG, Glattburg, Switzerland) and stained with modified Goldner’s trichrome, and histomorphometric measurements were performed on the secondary spongiosa of the distal femur metaphysis and vertebral body of L5, using a Leica Corp. Q image analyser at 40X magnification. Two sections for each stained sample were quantified per animal. TRAP was detected by using hexazotized pararosanilin (Sigma, St Louis, MO) and naphtol ASTR phosphate (Sigma, St Louis, MO) to reveal osteoclasts, ; non-osteoclastic acid phosphatase was inhibited by adding 100mMol/L L(+)-tartric acid (Sigma, St Louis, MO) to the substrate solution. The following parameters were recorded: the number of TRAP+ osteoclasts in contact with trabeculae (N.Oc/TBPm ; expressed in cells per millimeter of trabecular bone surface) ; the resorption surface (OcS/BS ; expressed in %) ; the average length of the zone of contact per osteoclast (Oc.Pm/N.Oc ; given in microns).
Testing of mechanical resistance
The night before mechanical testing, bones were thawed slowly at 7°C and then maintained at room temperature. Femur was placed on the material testing machine on two supports separated by a distance of 9.9 mm and load was applied to the midpoint of the shaft, thus creating a three-point bending test. The mechanical resistance to failure was tested using a servo-controlled electromechanical system (Instron 1114, Instron corp., High Wycombe, UK) with actuator displaced at 2mm/minute. Outcomes measures were ultimate force, stiffness, and energy as described by Turner and Burr (37).
RNA extraction and Quantitative PCR. Bone
The whole tibia was excised and immediately pulverized to a fine powder in peqGold Trifast (peQLab Biotechnologie GmbH) using FastPrep System tube and appartus (QBiogene, Illkirch, France) in order to achieve quantitative RNA extraction. We used a lysis time and speed respectively of 14sec and 5 m/s, as recommended by the maker. Total RNA extraction and quantitative PCR were performed as previously described (36). The following pre-designed TaqMan® gene expression assays were used for the quantitative RT-PCR (References, Gapdh: Mm00437762_m1, Igf-1: Mm00439559_m1, Il-6: Mm00446190_m1, Rankl: Mm00441908_m1, Opg: Mm00435452_m1, Fabp4: Mm00445878_m1, AdipoQ: Mm01343606_m1, Adipsin: Mm00439559_m1, Mcsf: Mm00432689_m1, Il1-ra: Mm00446185, Tnfα: Mm99999068_m1, Trap: Mm00475698_m1, Fsp27: Mm00617672_m1, Lpl: Mm00434764_m1, Pepck: Mm00440635_m1, Pparγ: Mm01184322_m1 Applied Biosystems, Rotkreuz, Switzerland) consisting of two unlabeled primers and a FAM™ dye-labeled TaqMan® MGB probe, and the correspondent buffer TaqMan® Universal PCR Master Mix (Applied Biosystems, Rotkreuz, Switzerland). Relative quantities (RQ) were calculated with the formula RQ=E-Ct using an efficiency (E) of 2 by default. The mean quantity was calculated from triplicates for each sample and this quantity was normalized to the similarly measured mean quantity of the GAPDH normalization gene. Finally, normalized quantities were averaged for 3 to 4 animals and represented as mean ± SEM.
White adipose tissue (WAT)
Total RNA from peri-ovarian WAT was extracted using the Trizol reagent (Life technologies, Zug, Switzerland) to the manufacturer’s protocol. Three micrograms total RNA were reverse-transcribed using 400 units of Moloney Murine Leukemia Virus (MMLV) Reverse Transcriptase (Invitrogen, Basel, Switzerland), in the presence of 1 unit/µl RNAsin (Promega Corp, Madison, WI, USA), 0.2 µg random primers (oligo(dN)6) (Promega Corp, Madison, WI, USA), 2 mM dNTP and 20 µM DTT (Invitrogen, Basel, Switzerland). The expression of the cDNAs was determined by quantitative real-time PCR using an ABI step one plus sequence detection System (Applera Europe, Rotkreuz, Switzerland) and were normalized using the housekeeping genes Ribosomal Protein S29. PCR products were quantified using the Power Master SYBR Green mix (Applera Europe, Rotkreuz, Switzerland) and results are expressed in arbitrary units (A.U) relative to the control group mean value. Primers sets were designed using the Primer Express software (Applera Europe, Rotkreuz, Switzerland) and were chosen when possible on both sides of an intron to avoid amplification of possible contaminating genomic DNA. The annealing temperature (60°C) and amplicon size (50–150 bp) were automatically determined by the software. Oligos were used at 217nM each (Microsynth, Switzerland). The sequence of the primers used is provided in supplemental table S3.
Collection of serum
Blood from all mice was obtained by retro-orbital collection at baseline and after 6 and 14 months of diets. After centrifugation, serum was removed and stored at −80°C until analysis. Serum TRACP5b (tartrate-resistant alkaline phosphatise form 5b) and osteocalcin were measured according to manufacturer’s instructions (SB-TR103 SBA Sciences, Turku, Finland; and CN50-1300 Biomedical Technologies Inc., Stoughton, MA, USA, respectively). IGF-I (AC-18F1 Immunodiagnostic Systems, Paris, France), myostatin (9875-11 US biological life science, Salem, MA01970) and leptin (90030 Crystal Chem Inc, Downers Grove, IL 60515) were measured in serum by immunoenzymometric assay (IEMA) with a kit from IDS following the manufacturer’s instructions.
Data analysis
We tested the significance of diet effects by a two way ANOVA repeat measurements with the type of diet and genotype as factors. As appropriate, post hoc testing was performed using Fisher’s protected Least Squares Difference (PLSD). The p of interaction between the diet and genotype was only mentioned when significant. Differences were considered significant at p < 0.05. Data are presented as mean ± SEM.
Results
Effect of n-3/n-6 FAs ratio on body composition and gene expression of WAT in B6 and 6T
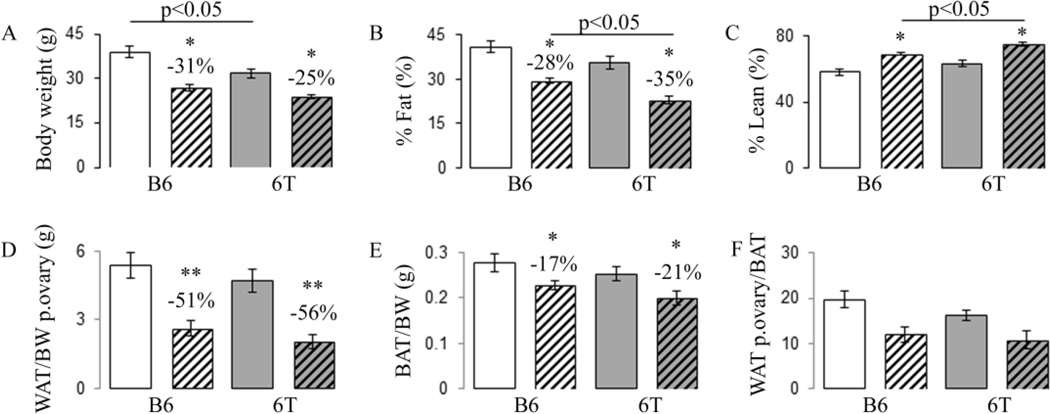
Overall, no significant differences in food intake were observed between groups (Fig. S1). At baseline, 6T tend to have a lower body weight, % fat mass and a higher % lean than B6. There are no significant differences in appendicular lean mass (Table S4). After 9 months of supplementation, the fish oil diet resulted in decreased body weight and % fat mass whereas it led to an increase in the %lean vs the safflower oil. These effects were similar in 6T and B6 (Fig. 1A–C). Interestingly, appendicular lean mass gain was lower in 6T than in B6 (+0.068±0.04% vs +0.162±0.05%, p<0.05) but was not influenced by fish oil. At sacrifice, WAT (peri-ovarian and peri-kidney) and BAT mass per kg of body weight significantly decreased with fish oil vs safflower oil diet in both 6T and B6 (Fig. 1D–E). Interestingly, the fish oil diet led to a more drastic reduction in WAT (around 54%) than BAT (around 20%) in both 6T and B6 (Fig. 1D–F).
Fig 1.
Effects of 9 months of fish oil diet on body composition, WAT and BAT mass in 6T and B6. (A) Total body weight; (B) %fat and (C) %lean evaluated by piximus; (D) WAT ovary mass measured at sacrifice per body weight (BW); (E) BAT/BW; (F) WATovary/BAT. * p<0.05,** p<0.01 significant difference vs safflower mice. Grey bars,6T; open bars, B6; hatched bars, fish oil diet; non-hatched bars, safflower diet. Bars show means (± sem).
In B6 mice, gene expression analysis of peri-ovarian WAT indicated that fish oil significantly decreased the markers of adipogenesis (leptin, adiponectin, Fas) and increased Pparβ (+158% vs safflower oil, p<0.05) (Table 1). In contrast, fish oil did not have any significant effect on leptin, adiponectin and Pparβ expression in 6T; rather it increased the expression of inflammatory markers Fas and Tnfα (respectively, +87% and +51% vs safflower, p<0.05).
Table 1.
Effects of fish oil on WAT gene expression after 9 months of supplementation in 6T and B6.
| B6 | 6T | P Genotype | P Diet | P Int | |||
|---|---|---|---|---|---|---|---|
| Gene | Safflower | Fish | Safflower | Fish | |||
| Leptin | 1.00±0.13 | 0.58±0.08** | 0.69±0.13 | 0.59±0.12 | NS | 0.03 | NS |
| AdipoQ | 1.00±0.10 | 0.58±0.09** | 0.54±0.10 | 0.51±0.19 | 0.05 | 0.08 | NS |
| Pparβ | 1.00±0.19 | 2.58±0.73* | 1.91±0.67 | 1.92±0.45 | NS | NS | NS |
| Tnfα | 1.00±0.23 | 1.59±0.27 | 2.68±0.67 | 4.04±0.67$ | 0.006 | 0.01 | NS |
| Pparγ | 1.00±0.44 | 0.16±0.05 | 0.55±0.28 | 0.84±0.74 | NS | NS | NS |
| Fas | 1.00±0.10 | 0.63±0.17 | 0.90±0.17 | 1.68±0.39* | 0.05 | NS | 0.02 |
| Lpl | 1.00±0.14 | 0.96±0.11 | 1.91±0.59 | 1.29±0.30 | 0.05 | NS | NS |
| Srebp | 1.00±0.14 | 1.94±0.75 | 1.63±0.65 | 2.58±1.07 | NS | NS | NS |
Gene expression was evaluated by qRTPCR (n=6–8 per group).
p<0.05 significant difference vs safflower in each genotype;
p<0.05 significant difference vs B6 in each diet by post-hoc fisher’s PLSD (2F-ANOVA, genotype and diet). Interaction, Int. Means ± SEM.
Effect of n-3/n-6 FAs ratio on BMD and trabecular microarchitecture in B6 and 6T
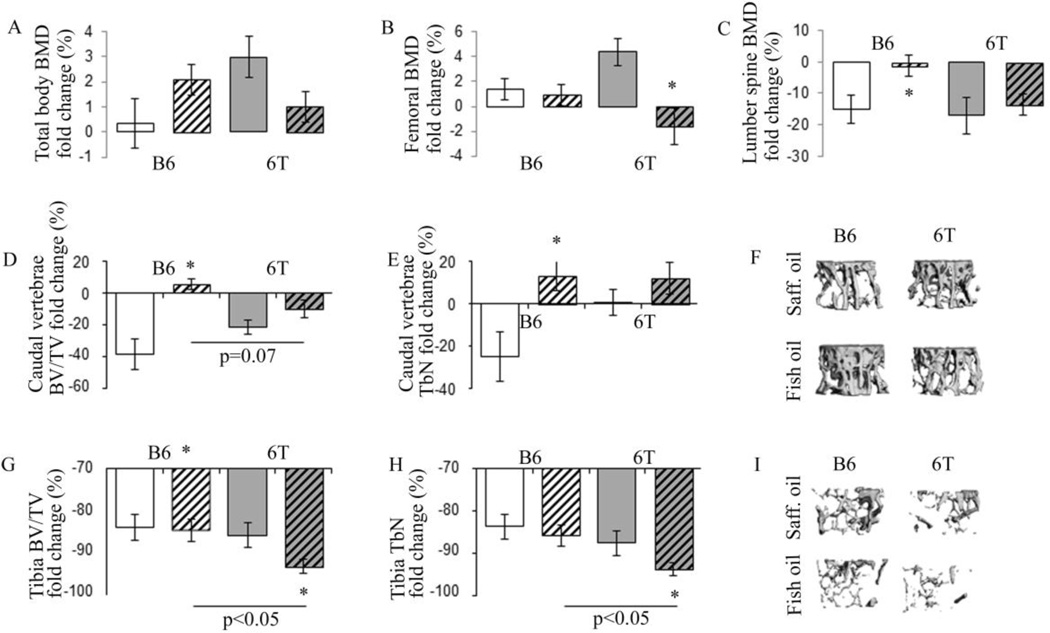
At baseline, 6T mice exhibited lower total body BMD and trabecular bone volume at the lumbar spine compared to B6 mice (Table S4). In B6 mice, fish oil significantly increased lumbar spine BMD vs safflower oil whereas it did not have any impact on total body and femoral BMD. In contrast, in 6T mice, fish oil did not have significant effect on lumbar spine BMD but significantly decreased the total body and femoral BMD vs safflower oil (Fig. 2 A–C). Age-related changes in trabecular microarchitecture followed a similar pattern in the proximal tibia and caudal vertebrae; although in the tibia the loss of BV/TV and TbN was more dramatic (Fig. 2 D–I). At the vertebrae, fish oil significantly prevented the decrease of BV/TV and Tb.N with age in B6 mice. The same trend was observed in 6T mice but the difference was not significant (Fig. 2 D–F). At the proximal tibia, fish oil did not have any significant effect in B6, whereas in 6T mice, it significantly accentuated the loss of bone volume fraction and trabecular number with age (Fig. 2 G–I). In addition, 6T mice on a fish oil diet had increased bone marrow adiposity vs B6 mice on a long term fish oil diet (MicroCT AdV/TV, 0.12±0.05 vs 0.02±0.005, respectively, p<0.05).
Fig 2.
Effect of fish oil on BMD, tibial and vertebrae trabecular microarchitecture changes with ages in 6T and B6. (A) Total Bone Mineral Density (BMD); (B) femoral BMD; (C) lumbar spine BMD; (D) Caudal vertebrae Trabecular bone volume on tissue volume (BV/TV); E) Caudal vertebrae trabecular number (TbN); (F) illustration of 3D trabecular structure of the vertebrae in mice aged of 12 months; (G) tibial BV/TV; (H) tibial TbN; (I) illustration of 3D trabecular structure of the tibia in mice aged of 12 months. * p<0.05 significant difference vs safflower mice. Grey bars,6T; open bars, B6; hatched bars, fish oil diet; non-hatched bars, safflower diet. Bars show means (± sem).
Effect of n-3/n-6 FAs ratio on cortical architecture and mechanical properties in B6 and 6T
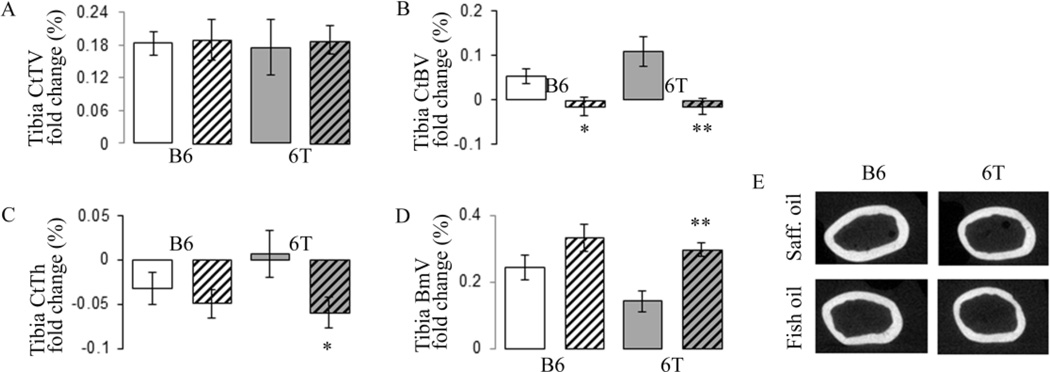
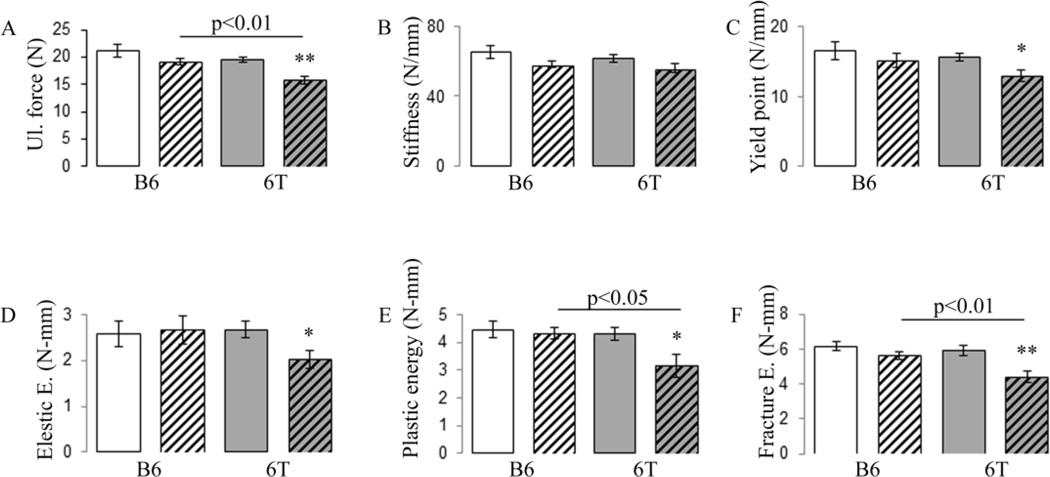
At baseline, 6T mice exhibited lower cortical tissue and bone volume at the mid tibia compared to B6 mice (Table S4). Cortical architecture evolution at the mid-tibia diaphysis is shown in figure 3. Gain in Cortical Bone volume (CtBV) between 3 and 12 months of age is lower in fish oil group’s vs safflower oil either in B6 and 6T mice. However, this effect disappears after adjustment by body weight. Tissue volume (TV) increased equally from 3 to 12 months of age in all groups (around 18%), consistent with a continuous periosteal bone apposition. In B6 mice, bone marrow volume increased with age in a similar manner to fish and safflower oil (around 25%), therefore CtTh decreased equally in the two groups. In 6T, the bone marrow volume increase was higher in fish vs safflower oil; hence fish oil decreased CtTh more than safflower oil (Fig. 3A–E). As a result, in B6 mice fish oil did not significantly change ultimate force, stiffness, elastic, plastic and fracture energy. In contrast, in 6T mice, fish oil significantly decreased ultimate force, yield point, elastic, plastic and fracture energy (Fig. 4A–F). In sum when mice are fed with fish oil, 6T has lower ultimate force, plastic and fracture energy, −17.2%, −27.2% and −21.2% vs B6 respectively, all p<0.05.
Fig 3.
Effect of fish oil diet on tibial cortical microarchitecture changes with ages in 6T and B6. (A) Cortical Tissue Volume (CtTV) at midshaft tibia; (B) Cortical Bone Volume (CtBV); (C) Cortical thickness (CtTh); (D) Bone marrow volume (BmV); (E) 2D reconstruction of cortical femur midshaft illustrating the lower CtTh in 6T mice under fish oil diet also found in femur. * p<0.05, **p<0.01 significant difference vs safflower mice. Grey bars,6T; open bars, B6; hatched bars, fish oil diet; non-hatched bars, safflower diet. Bars show means (± sem).
Fig 4.
Effect of fish oil diet on femur strength in 6T and B6. Mechanical properties have been evaluated by three point bending test. (A) Ultimate (Ul) force; (B) stiffness; (C) yield point; (D) elastic energy; € plastic energy; (F) fracture energy. * p<0.05, **p<0.01 significant difference vs safflower mice. Grey bars,6T; open bars, B6; hatched bars, fish oil diet; nonhatched bars, safflower diet. Bars show means (± sem).
Effect of n-3/n-6 FAs ratio on osteoclast number and bone marrow adiposity in B6 and 6T
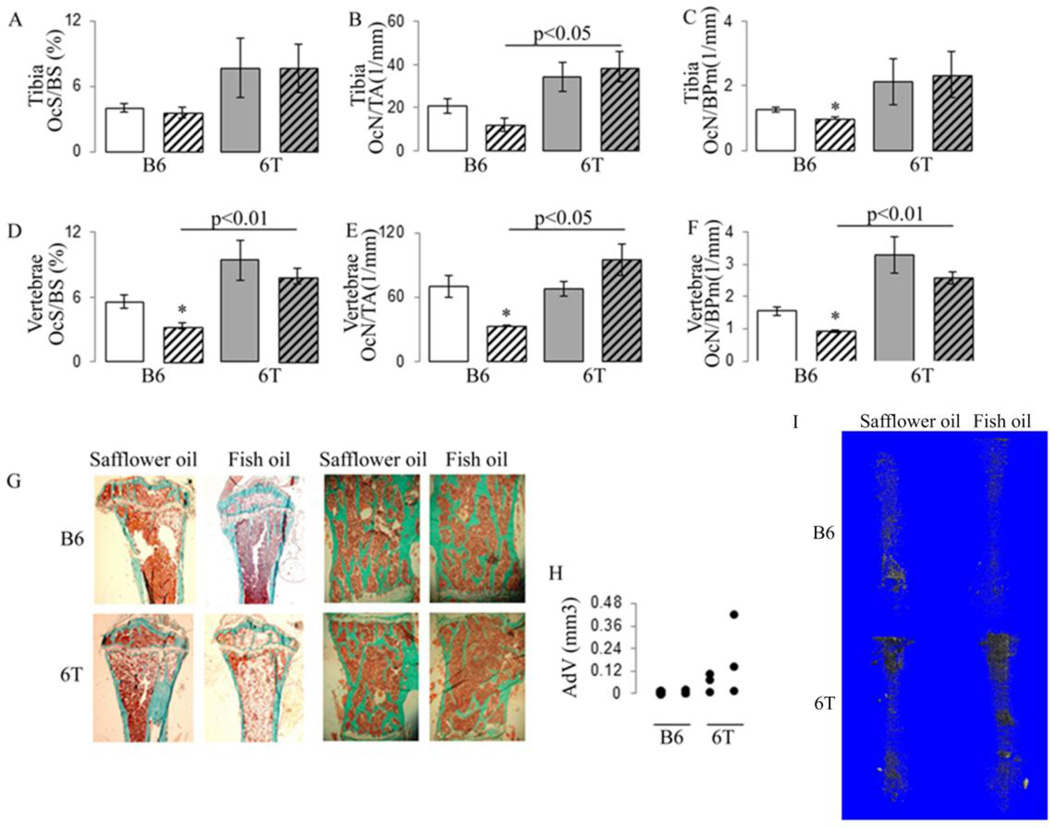
In B6 mice, osteoclast surface and adipocyte volume in femur did not differ between fish and safflower oil (Fig. 5A&H). In lumbar spine, osteoclast surfaces and numbers were significantly decreased with the fish oil diet vs safflower oil and adipocytes were not detectable (Fig. 5D–F). In 6T, fish oil increased osteoclastic and adipocyte number, qualitatively by histmorphometry and quantitatively by microCT with osmium, vs safflower oil in femur whereas it had no significant effect in lumbar spine (Fig. 5G–I). Thus 6T mice fed with fish oil have higher osteoclast surface and numbers, as well as more marrow adiposity compared to B6.
Fig 5.
Effect of fish oil diet on osteoclast and bone marrow adiposity in tibia and vertebrae in 6T and B6. (A) tibial Osteoclast Surface per Bone surface (OcS/BS); (B) tibial Osteoclast Numbers per Tissue Area (OcN/TA); (C) tibial Osteoclast Numbers per Bone Perimeters (OcN/BPm); (D) vertebrae OcS/BS; E) vertebrae OcN/TA; (F) vertebrae OcN/BPm; (G) histological illustration of increased adipocyte infiltration in tibial bone marrow of 6T under fish oil; and absence of adipocyte in bone marrow of vertebrae; (H) Adipocyte volume on tissue volume (Adv/TV) in distal femur quantified by microCT after osmium staining; (I) 3D reconstruction of adipocyte volume present in the marrow space of the all femur.
Effect of n-3/n-6 FAs ratio on bone markers in B6 and 6T
At baseline, 6T mice tend to exhibit lower leptin and IGF-1 levels compared to B6 mice (table S4) similar to what has been reported previously (17). Levels of osteocalcin and TRAP5b were not significantly different between the congenic and B6 mice. In B6, osteocalcin levels decreased less with age in fish oil vs safflower oil diet and TRAP5b remained unchanged between groups. In contrast, in 6T mice, fish oil did not slow the osteocalcin decrease with age and tended to increased TRAP5b (Table S5). In accordance with WAT mass changes, the increase of leptin with age was lower in mice fed the fish oil vs those fed the safflower oil in both 6T and B6 mice (Table S5).
Effect of n-3/n-6 FAs ratio on gene expression of the tibia in B6 and 6T
In order to clarify the molecular mechanisms by which fish oil mediates different skeletal responses to aging between B6 and 6T, we next examined gene expression in the tibia of both strains. In B6 mice, fish oil decreased markers of bone resorption (Mcsf, Trap) and elicited an anti-inflammatory effect as indicated by an increase in Il1-ra and a decrease in Tnfα vs safflower oil (+199% and −18% respectively, p<0.001). Fish oil also decreased Pparγ (−43% vs safflower, p<0.01), i.e. markers of adipogenesis (Table 2).
Table 2.
Effects of fish oil on bone gene expression after 9 months of supplementation in 6T and B6.
| B6 | 6T | P Genotype | P Oil | P Int | |||
|---|---|---|---|---|---|---|---|
| Gene | Safflower | Fish | Safflower | Fish | |||
| Igf-1 | 0.98±0.07 | 1.43±0.11** | 1.50±0.10 | 1.74±0.13 | 0.0004 | 0.002 | NS |
| Il-6 | 1.05±0.10 | 1.03±0.13 | 1.14±0.11 | 1.27±0.11 | NS | NS | NS |
| Opg | 1.18±0.22 | 0.61±0.13* | 1.36±0.29 | 0.71±0.16* | NS | 0.01 | NS |
| Pparγ | 1.08±0.14 | 0.62±0.03** | 1.66±0.08 | 1.31±0.07** | 0.0001 | 0.0001 | NS |
| Rankl | 1.05±0.10 | 0.84±0.10 | 1.05±0.06 | 0.94±0.11 | NS | NS | NS |
| AdipoQ | 1.03±0.08 | 1.19±0.08 | 1.52±0.15 | 2.03±0.18* | 0.0001 | 0.01 | NS |
| Adipsin | 1.15±0.15 | 1.15±0.11 | 5.78±1.4 | 7.07±0.94 | 0.0001 | NS | NS |
| Il-10 | 1.16±0.17 | 0.24±0.03*** | 0.43±0.04 | 0.31±0.04* | 0.002 | 0.0001 | 0.0003 |
| Il1-ra | 1.04±0.14 | 3.11±0.43**** | 1.78±0.43 | 4.33±0.66** | 0.05 | 0.0001 | NS |
| Tnfα | 1.00±0.02 | 0.82±0.04*** | 1.04±0.05 | 1.33±0.08** | 0.0001 | NS | 0.0001 |
| Mcsf | 1.01±0.04 | 0.74±0.06** | 1.00±0.04 | 1.11±0.07 | 0.001 | NS | 0.001 |
| Trap | 1.04±0.09 | 0.74±0.03** | 1.17±0.06 | 1.23±0.02 | 0.0001 | 0.03 | 0.002 |
| Ak2 | 1.11±0.13 | 1.16±0.10 | 1.34±0.24 | 1.30±0.17 | NS | NS | NS |
| Fabp4 | 1.03±0.08 | 1.87±0.05*** | 1.43±0.18 | 2.19±0.19** | 0.01 | 0.0001 | NS |
| Fsp27 | 1.03±0.07 | 1.05±0.08 | 3.34±0.70 | 5.31±0.69* | 0.0001 | 0.05 | 0.05 |
| Lpl | 1.08±0.13 | 1.49±0.15* | 1.25±0.16 | 1.55±0.16 | NS | 0.02 | NS |
| Pepck | 1.15±0.24 | 1.64±0.20 | 2.54±0.61 | 11.64±1.61**** | 0.0001 | 0.0001 | 0.0001 |
Gene expression was evaluated by qRTPCR (n=6–8 per group).
p<0.05 significant difference vs safflower in each genotype;
p<0.05 significant difference vs B6 in each diet by post-hoc fisher’s PLSD (2F-ANOVA, genotype and diet). Interaction, Int. Means ± SEM.
In 6T mice, fish oil decreased Pparγ expression (−21% vs safflower, p<0.01), however this decrease was lower than observed in B6. Interestingly, the fish oil diet in 6T increased others adipogenic markers such as adiponectin +33%, Fsp27 +59% and Pepck +358% vs safflower oil (i.e an important indicator of gain of function in PPARγ), all p<0.05; as well as markers of inflammation (Tnfα: +28% vs safflower, p<0.01). Moreover, markers of bone resorption such as Mcsf and Trap remained unchanged in response to fish oil (Table 2).
Discussion
The mains goal of our study was to elucidate the role of genetic background on the skeletal response to n-3 PUFA. As omega-3 fatty acid is a known ligand for PPARγ (38, 39), we challenged mice with a fish oil diet, utilizing the very modest genotypic differences between the 6T congenic mouse and B6. The 30 cM QTL on mouse Chr 6 that originated from C3H/HeJ and was backcrossed onto B6 includes the Pparγ gene which exhibits a gain of function, due to polymorphisms in the 3’ UTR. Previously these mice were characterized by their high marrow adiposity, low bone mass and reduced circulating IGF-I (40). In the current report, we found that fish oil intake, rich in omega n-3 FA, was associated with an increase in BMD and trabecular microarchitecture at the caudal vertebrae in B6 mice, mainly due to a decrease in osteoclast number through a decrease in inflammatory cytokines. However, fish oil did not significantly improve bone mechanical properties. In 6T, fish oil did not decrease the osteoclast number and surfaces and did not prevent trabecular bone loss with age. This suggests that, when PPARγ activity is initially increased, i.e. as in 6T bone, fish oil was unable to reduce its activity enough to prevent trabecular bone loss with age. Moreover, in 6T, fish oil reduces mechanical properties, i.e. ultimate force and plastic energy, associated with a decrease in cortical thickness and an increase in bone marrow volume. Therefore, these findings offer a potential explanation for the inconsistent effects of omega-3 fatty acid supplementation on the murine skeleton and may provide insight into the heterogeneity in the skeletal response to specific diets in humans.
In accordance with previous experiments, we confirmed that 6T mice had a low vertebral trabecular bone fraction, cortical bone microarchitecture with an increase in bone marrow adiposity and Pparγ expression in bone (41). We also confirmed lower bone marrow adipocytic density in the lumbar spine compared to the femur, similar to what has been reported in humans (42). Moreover we found that 6T did not exhibit more brown or brown like fat, nor more leptin, but did have more osteoclasts, indicating that increased PPARγ activity in bone may directly increase osteoclast numbers (22).
In B6 mice, the fish oil diet significantly reduced the body weight and fat mass gain with age; at sacrifice the fish oil group had lower WAT and BAT mass, with a marked decrease in circulating leptin levels. Interestingly, the decrease of WAT was 3 fold higher than the decrease of BAT, suggesting that fish oil decreased fat mass but preserved a beneficial metabolic profile (43). The pattern of gene expression in white fat indicated a reduction of adipogenesis (leptin, adiponectin, Fas) and an increase of FA oxidation through Pparβ but without changes in Pparγ (44). At the bone tissue levels fish oil significantly decreased Pparγ and increased Igf-1 gene expression while it preserved vertebral BMD and cancellous bone volume supporting the observations that PPARγ regulates IGF-I, probably through indirect mechanisms (19). Surprisingly, fish oil did not affect the other determinants of adipogenesis such as Adiponectin, Adipsin, Fsp27 and Pepck, and as a result bone marrow adiposity was not significantly altered by fish oil in our model. In accordance with Sun et al (28), the fish oil bone effect could be mediated by inflammatory cytokines (TNFα, IL1-Ra). As we previously demonstrated in C57BL6J mice, long-term supplementation of omega-3 FA also may exert these positive effects by decreasing leptin (45). Indeed, intracerebroventricular leptin injection has been shown to induce trabecular bone remodelling in vertebrae by triggering β-adrenergic signalling in the CNS, which in turn increases osteoclast number and activity (46, 47). However, this decrease in osteoclasts had little influence on femoral BMD, cortical thickness or the mechanical properties.
In 6T mice, fish oil diet decreased body weight, fat gain, and circulating leptin in a similar manner to B6 mice. However, markers of adipogenesis that were decreased in B6 in response to fish oil were unchanged in 6T mice. Fish oil can modulate body fat metabolically by increasing lipolysis and fatty acid oxidation (48); or by reducing food intake through the endocannabinoid and mesocorticolimbic dopamine system (49). This second mechanism was not operative in our experiments due to the use of isocaloric diet and pair feeding. So these data suggest that the difference in adipogenesis markers at the gene expression levels were not translated into differences in fat mass gain. At the bone levels, 6T mice exhibited resistance to the positive effect of fish oil on cancellous bone. Moreover, fish oil consumption was associated with a further increase in bone marrow adiposity and a decrease in BMD, cortical thickness and finally femoral mechanical properties. These negative effects paralleled an increase in resorption, adipogenic and inflammatory markers including adiponectin, probably because 6T mice have a gain of function in the Pparγ gene compared to B6 mice. The interaction between omega-3 FA effect and level of PPARγ activation has also been found pharmacologically in skeletal muscle where inhibition of PPARγ, prevented the action of n-3 fatty acid on cell differentiation (50). Pparγ is likely not the only gene that contributes to the skeletal phenotype of the 6T congenic strain since Cidea, Zfp422 and Alox5 genes are within the QTL and could be potential candidates to explain both the bone marrow fat and skeletal response to fish oil diet, although these genes do not have functional polymorphisms between C3H and B6 (51).
There are several limitations to our study. First, the 6T congenic mouse has polymorphic differences in multiple genes that could impact the skeletal response to dietary interventions relative to B6. In particular, this QTL contains other genes that may impact fat mass such as Cidec, a mediator of apoptosis in adipocytes. Notwithstanding, congenic rather than pure inbred mice, are more representative of the heterogeneity in the genetic background of most mammals. Hence our conclusions about diet×gene interactions are not inconsistent with larger cohort studies in humans. Second, Pparγ gene expression does not equate with its nuclear receptor activity. Hence relative differences in Pparγ gene expression by strain may not reflect in vivo activities. Indeed, marrow adiposity was increased in 6T in response to the fish oil diet despite a modest reduction in gene expression. Third, our studies only lasted nine months; hence the long term effects of diet on bone and body composition, particularly in older individuals may be affected by age related changes in several genes including Pparγ. In conclusions, our observations confirm that long-term fish oil diet rich in omega-3 FAs could have a favourable impact on fat mass, skeletal integrity and bone loss with age, i.e bone mass and trabecular bone microarchitecture in vertebrae(52). However, at the skeletal level, the beneficial effects of a fish oil diet may be dependent on the presence of allelic variants in several genes, including Pparγ. Therefore, our findings emphasize the need to consider nutrient by genotype interactions when considering the beneficial or harmful effects of a particular diet.
Supplementary Material
Highlights.
-
-
Fish oil reduced body weight and fat mass gain with age in B6 mice
-
-
In B6, fish oil preserved vertebral BMD and cancellous bone by a decrease in osteoclast
-
-
6T mice exhibit resistance to fish oil effect on bone due to a gain of function of PPARγ
-
-
In 6T mice, fish oil increase bone marrow adiposity, resorption and inflammatory markers
-
-
Fish oil effects may dependent on allelic variants in several genes, including Pparγ
Acknowledgements
We thank Ms Madeleine Lachize and Juliette Cicchini for her technical assistance. We thank Pr S Ferrari (Geneva University Hospital and Faculty of Medicine, Switzerland) for his intellectual support. Authors’s roles: Study design: NB and CJR. Study conduct: NB and ES. Data analysis: NB. Data interpretation: NB and CJR. Drafting manuscript: NB. Revising manuscript content and approving final version: NB, ES and CJR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nicolas Bonnet, Division of Bone Diseases, Department of Internal Medicine Specialties, Geneva University Hospital & Faculty of Medicine, Geneva 14, Switzerland..
Emmanuel Somm, Service of Endocrinology, Diabetology, and Metabolism, Centre Hospitalier Universitaire Vaudois/Department of Physiology, Lausanne CH-1005, Switzerland. 3 Division of Development and Growth, Department of Paediatrics, University of Geneva School of Medicine, 1211 Geneva 14, Switzerland..
Clifford J Rosen, Maine Medical Center Research Institute, Scarborough, Maine 04074..
References
- 1.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2(1):35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 2.Abellan van Kan G, Gambassi G, de Groot LC, Andrieu S, Cederholm T, André E, et al. Nutrition and aging, the Carla Workshop. J Nutr Health Aging. 2008;12(6):355–364. doi: 10.1007/BF02982667. [DOI] [PubMed] [Google Scholar]
- 3.Ohnell O, Gullberg B, Allander E, Kanis JA. The apparent incidence of hip fracture in Europe: a study of national register sources MEDOS Study Group. Osteoporos Int. 1992;2(6):298–302. doi: 10.1007/BF01623186. [DOI] [PubMed] [Google Scholar]
- 4.Kannus P, Parkkari J, Sievänen H, Heinonen A, Vuori I, Järvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 Suppl):57S–63S. doi: 10.1016/8756-3282(95)00381-9. [DOI] [PubMed] [Google Scholar]
- 5.Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61(6 Suppl):1402S–1406S. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 6.Kontogianni MD, Melistas L, Yannakoulia M, Malagaris I, Panagiotakos DB, Yiannakouris N. Association between dietary patterns and indices of bone mass in a sample of Mediterranean women. Nutrition. 2009;25(2):165–171. doi: 10.1016/j.nut.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Puel C, Coxam V, Davicco MJ. Mediterranean diet and osteoporosis prevention. Med Sci. 2007;23(8–9):756–760. doi: 10.1051/medsci/20072389756. [DOI] [PubMed] [Google Scholar]
- 8.Farina EK, Kiel DP, Roubenoff R, Schaefer EJ, Cupples LA, Tucker KL. Protective effects of fish intake and interactive effects of long-chain polyunsaturated fatty acid intakes on hip bone mineral density in older adults: the Framingham Osteoporosis Study. Am J Clin Nutr. 2011;93(5):1142–1151. doi: 10.3945/ajcn.110.005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Högström M, Nordström P, Nordström A. n-3 Fatty acids are positively associated with peak bone mineral density and bone accrual in healthy men: the NO2 Study. Am J Clin Nutr. 2007;85(3):803–807. doi: 10.1093/ajcn/85.3.803. [DOI] [PubMed] [Google Scholar]
- 10.Nawata K, Yamauchi M, Takaoka S, Yamaguchi T, Sugimoto T. Association of n-3 polyunsaturated fatty acid intake with bone mineral density in postmenopausal women. Calcif Tissue Int. 2013;93(2):147–154. doi: 10.1007/s00223-013-9743-5. [DOI] [PubMed] [Google Scholar]
- 11.Farina EK, Kiel DP, Roubenoff R, Schaefer EJ, Cupples LA, Tucker KL. Dietary intakes of arachidonic acid and alpha-linolenic acid are associated with reduced risk of hip fracture in older adults. J Nutr. 2011;141(6):1146–1153. doi: 10.3945/jn.110.133728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salari Sharif P, Asalforoush M, Ameri F, Larijani B, Abdollahi M. The effect of n-3 fatty acids on bone biomarkers in Iranian postmenopausal osteoporotic women: a randomized clinical trial. Age. 2010;32(2):179–186. doi: 10.1007/s11357-009-9122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brownbill RA, Ilich JZ. Lipid profile and bone paradox: higher serum lipids are associated with higher bone mineral density in postmenopausal women. J Womens Health. 2006;15(3):261–270. doi: 10.1089/jwh.2006.15.261. [DOI] [PubMed] [Google Scholar]
- 14.Virtanen JK, Mozaffarian D, Cauley JA, Mukamal KJ, Robbins J, Siscovick DS. Fish consumption, bone mineral density, and risk of hip fracture among older adults: the cardiovascular health study. J Bone Miner Res. 2010;25(9):1972–1979. doi: 10.1002/jbmr.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orchard TS, Cauley JA, Frank GC, Neuhouser ML, Robinson JG, Snetselaar L, et al. Fatty acid consumption and risk of fracture in the Women's Health Initiative. Am J Clin Nutr. 2010;92(6):1452–1460. doi: 10.3945/ajcn.2010.29955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ralston SH. Genetics of osteoporosis. Proc Nutr Soc. 2007;66(2):158–165. doi: 10.1017/S002966510700540X. [DOI] [PubMed] [Google Scholar]
- 17.Ackert-Bicknell CL, Salisbury JL, Horowitz M, DeMambro VE, Horton LG, Shultz KL, et al. A chromosomal inversion within a quantitative trait locus has a major effect on adipogenesis and osteoblastogenesis. Ann N Y Acad Sci. 2007;1116:291–305. doi: 10.1196/annals.1402.010. [DOI] [PubMed] [Google Scholar]
- 18.Ackert-Bicknell CL, Demissie S, Marín de Evsikova C, Hsu YH, DeMambro VE, Karasik D, et al. PPARG by dietary fat interaction influences bone mass in mice and humans. J Bone Miner Res. 2008;23(9):1398–1408. doi: 10.1359/JBMR.080419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lecka-Czernik B, Ackert-Bicknell C, Adamo ML, Marmolejos V, Churchill GA, Shockley KR, et al. Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) by rosiglitazone suppresses components of the insulin-like growth factor regulatory system in vitro and in vivo. Endocrinology. 2007;148(2):903–911. doi: 10.1210/en.2006-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lecka-Czernik B, Rosen CJ, Kawai M. Skeletal aging and the adipocyte program: New insights from an "old" molecule. Cell Cycle. 2010;9(18):3648–3654. doi: 10.4161/cc.9.18.13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harsløf T, Tofteng CL, Husted LB, Nyegaard M, Børglum A, Carstens M, et al. Polymorphisms of the peroxisome proliferator-activated receptor γ (PPARγ) gene are associated with osteoporosis. Osteoporos Int Oct. 2011;22(10):2655–2666. doi: 10.1007/s00198-010-1491-z. [DOI] [PubMed] [Google Scholar]
- 22.Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med. 2007;13:1496–1503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- 23.Cho SW, Yang JY, Her SJ, Choi HJ, Jung JY, Sun HJ, et al. Osteoblast-targeted overexpression of PPARγ inhibited bone mass gain in male mice and accelerated ovariectomy-induced bone loss in female mice. J Bone Miner Res. 2011;26(8):1939–1952. doi: 10.1002/jbmr.366. [DOI] [PubMed] [Google Scholar]
- 24.Mieczkowska A, Baslé MF, Chappard D, Mabilleau G. Thiazolidinediones Induce Osteocyte Apoptosis by a G Protein-coupled Receptor 40-dependent Mechanism. J Biol Chem. 2012;287(28):23517–23526. doi: 10.1074/jbc.M111.324814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magee P, Pearson S, Whittingham-Dowd J, Allen J. PPARγ as a molecular target of EPA anti-inflammatory activity during TNF-α-impaired skeletal muscle cell differentiation. J Nutr Biochem. 2012;23(11):1440–1448. doi: 10.1016/j.jnutbio.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Flachs P, Rossmeisl M, Bryhn M, Kopecky J. Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin Sci (Lond) 2009;116(1):1–16. doi: 10.1042/CS20070456. [DOI] [PubMed] [Google Scholar]
- 27.Lengqvist J, Mata De Urquiza A, Bergman AC, Willson TM, Sjövall J, Perlmann T, et al. Polyunsaturated fatty acids including docosahexaenoic and arachidonic acid bind to the retinoid X receptor alpha ligand-binding domain. Mol Cell Proteomics. 2004;3(7):692–703. doi: 10.1074/mcp.M400003-MCP200. [DOI] [PubMed] [Google Scholar]
- 28.Sun D, Krishnan A, Zaman K, Lawrence R, Bhattacharya A, Fernandes G. Dietary n-3 fatty acids decrease osteoclastogenesis and loss of bone mass in ovariectomized mice. J Bone Miner Res. 2003;18(7):1206–1216. doi: 10.1359/jbmr.2003.18.7.1206. [DOI] [PubMed] [Google Scholar]
- 29.Halade GV, Rahman MM, Williams PJ, Fernandes G. Combination of conjugated linoleic acid with fish oil prevents age-associated bone marrow adiposity in C57Bl/6J mice. J Nutr Biochem. 2011;22(5):459–469. doi: 10.1016/j.jnutbio.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonnet N, Ferrari SL. Effects of long-term supplementation with omega-3 fatty acids on longitudinal changes in bone mass and microstructure in mice. J Nutr Biochem. 2011;22(7):665–672. doi: 10.1016/j.jnutbio.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Iida-Klein A, Lu SS, Yokoyama K, Dempster DW, Nieves JW, Lindsay R. Precision, accuracy, and reproducibility of dual X-ray absorptiometry measurements in mice in vivo. J Clin Densitom. 2003;6(1):25–33. doi: 10.1385/jcd:6:1:25. [DOI] [PubMed] [Google Scholar]
- 32.Waarsing JH, Day JS, van der Linden JC, Ederveen AG, Spanjers C, De Clerck N, et al. Detecting and tracking local changes in the tibiae of individual rats: a novel method to analyse longitudinal in vivo micro-CT data. Bone. 2004;34:163–169. doi: 10.1016/j.bone.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Waarsing JH, Day JS, Weinans H. An automatic algorithm to segment 3D microCT scans of bone. The IXth congress of the international society of bone morphometry; Edingburgh, UK. 2002. [Google Scholar]
- 34.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 35.Clinthorne JF, Adams DJ, Fenton JI, Ritz BW, Gardner EM. Short-term re-feeding of previously energy-restricted C57BL/6 male mice restores body weight and body fat and attenuates the decline in natural killer cell function after primary influenza infection. J Nutr. 2010;140:1495–1501. doi: 10.3945/jn.110.122408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonnet N, Standley KN, Bianchi EN, Stadelmann V, Foti M, Conway SJ, et al. The matricellular protein Periostin is required for Sclerostin inhibition and the anabolic response to mechanical loading and physical activity. J Biol Chem. 2009;284(51):35939–35950. doi: 10.1074/jbc.M109.060335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14(4):595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 38.Edwards IJ, O'Flaherty JT. Omega-3 Fatty Acids and PPARgamma in Cancer. PPAR Res. 2008:358052. doi: 10.1155/2008/358052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deckelbaum RJ, Worgall TS, Seo T. n-3 fatty acids and gene expression. Am J Clin Nutr. 2006;83(6 Suppl):1520S–1525S. doi: 10.1093/ajcn/83.6.1520S. [DOI] [PubMed] [Google Scholar]
- 40.Rosen CJ, Ackert-Bicknell CL, Adamo ML, Shultz KL, Rubin J, Donahue LR, et al. Congenic mice with low serum IGF-I have increased body fat, reduced bone mineral density, and an altered osteoblast differentiation program. Bone. 2004;35(5):1046–1058. doi: 10.1016/j.bone.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Bouxsein ML, Rosen CJ, Turner CH, Ackert CL, Shultz KL, Donahue LR, et al. Generation of a new congenic mouse strain to test the relationships among serum insulin-like growth factor I, bone mineral density, and skeletal morphology in vivo. J Bone Miner Res. 2002;17(4):570–579. doi: 10.1359/jbmr.2002.17.4.570. [DOI] [PubMed] [Google Scholar]
- 42.Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bonesource. 2004;34(3):376–383. doi: 10.1016/j.bone.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 43.Motyl KJ, Bishop KA, DeMambro VE, Bornstein SA, Le P, Kawai M, et al. Altered thermogenesis and impaired bone remodeling in Misty mice. J Bone Miner Res. 2013;28(9):1885–1897. doi: 10.1002/jbmr.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flachs P, Rossmeisl M, Bryhn M, Kopecky J. Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin Sci. 2009;116(1):1–16. doi: 10.1042/CS20070456. [DOI] [PubMed] [Google Scholar]
- 45.Bonnet N, Rizzoli R, Ferarri SL. Effects of a diet enriched in omega-3 on bone remodeling and bone structure. journal of nutrigenetics and nutrigenomics. 2008;1(FP35):299. [Google Scholar]
- 46.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 47.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 48.Kopecky J, Rossmeisl M, Flachs P, Kuda O, Brauner P, Jilkova Z, et al. n-3 PUFA: bioavailability and modulation of adipose tissue function. Proc Nutr Soc. 2009;68(4):361–369. doi: 10.1017/S0029665109990231. [DOI] [PubMed] [Google Scholar]
- 49.Golub N, Geba D, Mousa SA, Williams G, Block RC. Greasing the wheels of managing overweight and obesity with omega-3 fatty acids. Med Hypotheses. 2011;77(6):1114–1120. doi: 10.1016/j.mehy.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magee P, Pearson S, Whittingham-Dowd J, Allen J. PPARγ as a molecular target of EPA anti-inflammatory activity during TNF-α-impaired skeletal muscle cell differentiation. J Nutr Biochem. 2012;23(11):1440–1448. doi: 10.1016/j.jnutbio.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Le P, Kawai M, Bornstein S, DeMambro VE, Horowitz MC, Rosen CJ. A high-fat diet induces bone loss in mice lacking the Alox5 gene. Endocrinology. 2012;153(1):6–16. doi: 10.1210/en.2011-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelly OJ, Gilman JC, Kim Y, Ilich JZ. Long-chain polyunsaturated fatty acids may mutually benefit both obesity and osteoporosis. Nutr Res. 2013;33(7):521–533. doi: 10.1016/j.nutres.2013.04.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.