Abstract
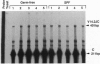
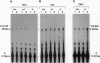
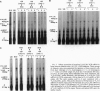
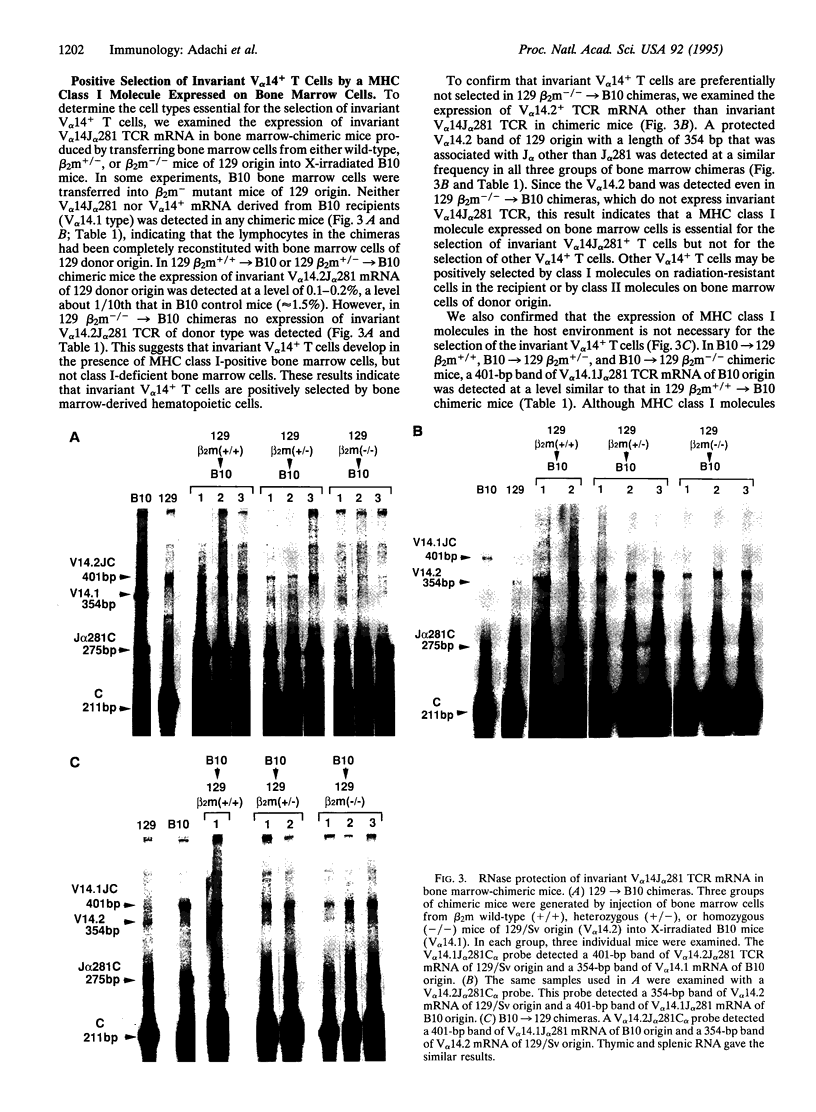
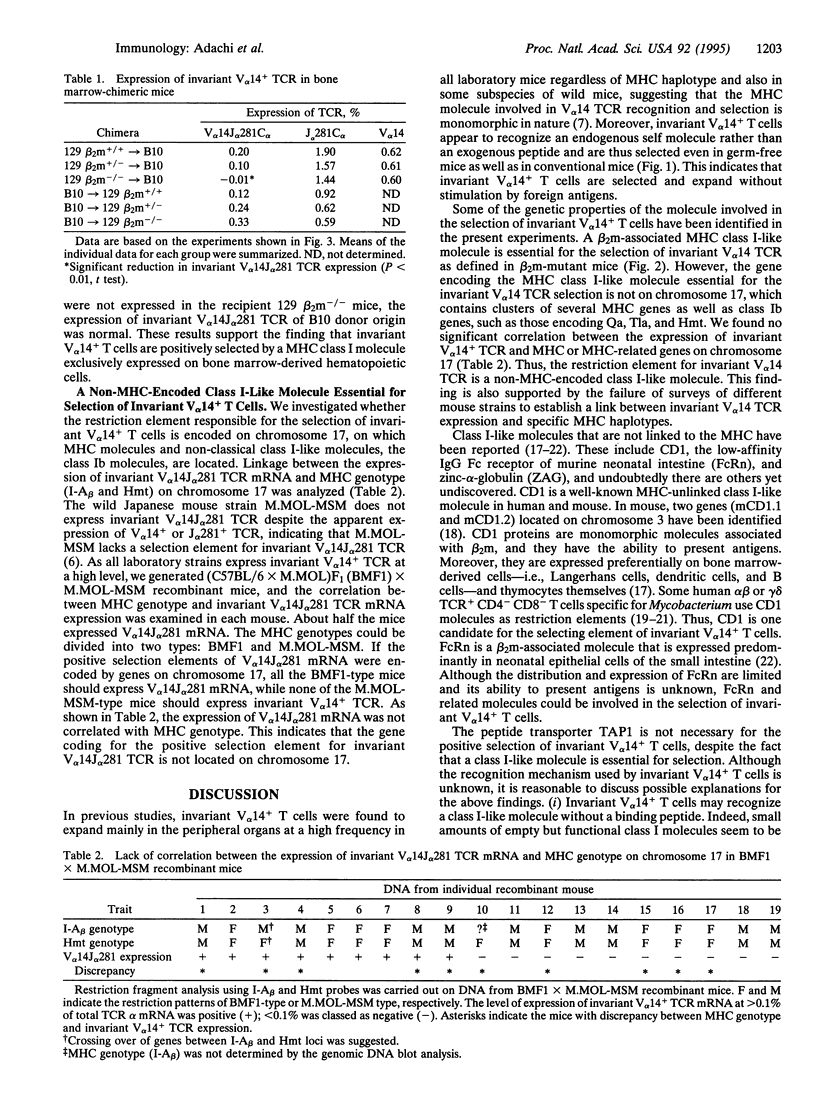
V alpha 14+ T cells are a unique subset expressing an invariant T-cell antigen receptor alpha chain encoded by V alpha 14 and J alpha 281 gene fragments with a 1-nt N region. Most invariant V alpha 14+ T cells develop in extrathymic organs, independent of thymus, and expand at a high frequency in various mouse strains regardless of major histocompatibility complex (MHC) haplotype. In this paper, we show that the positive selection of invariant V alpha 14+ T cells requires a beta 2-microglobulin-associated MHC class I-like molecule not linked to the MHC on chromosome 17. This was determined by linkage analysis on DNA from recombinant mice generated by crossing a C57BL/6 mouse with a wild mouse, Mus musculus molossinus, that is negative for invariant V alpha 14 TCR expression. However, the peptide transporter TAP1 is not necessary for positive selection of invariant V alpha 14+ T cells, indicating the direct recognition of the MHC class I-like molecule without peptide by the invariant V alpha 14 TCR. Further, experiments with bone marrow-chimeric mice show that invariant V alpha 14+ T cells in the periphery are selected by bone marrow cells, suggesting a unique lineage of V alpha 14+ T cells differentiated through a selection process distinct from that of conventional alpha beta TCR+ T cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balk S. P., Bleicher P. A., Terhorst C. Isolation and expression of cDNA encoding the murine homologues of CD1. J Immunol. 1991 Jan 15;146(2):768–774. [PubMed] [Google Scholar]
- Bendelac A., Killeen N., Littman D. R., Schwartz R. H. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 1994 Mar 25;263(5154):1774–1778. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- Bix M., Coles M., Raulet D. Positive selection of V beta 8+ CD4-8- thymocytes by class I molecules expressed by hematopoietic cells. J Exp Med. 1993 Sep 1;178(3):901–908. doi: 10.1084/jem.178.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D., Gray D., Dierich A., Kaufman J., Lemeur M., Benoist C., Mathis D. Mice lacking MHC class II molecules. Cell. 1991 Sep 6;66(5):1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- Elliott J. I. The identity of the cells that positively select thymocytes. Immunol Rev. 1993 Oct;135:215–225. doi: 10.1111/j.1600-065x.1993.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Faure F., Jitsukawa S., Miossec C., Hercend T. CD1c as a target recognition structure for human T lymphocytes: analysis with peripheral blood gamma/delta cells. Eur J Immunol. 1990 Mar;20(3):703–706. doi: 10.1002/eji.1830200336. [DOI] [PubMed] [Google Scholar]
- Gastinel L. N., Simister N. E., Bjorkman P. J. Expression and crystallization of a soluble and functional form of an Fc receptor related to class I histocompatibility molecules. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):638–642. doi: 10.1073/pnas.89.2.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo P., Kappler J. W., Marrack P. C. Positive selection of TcR alpha beta thymocytes: is cortical thymic epithelium an obligatory participant in the presentation of major histocompatibility complex protein? Immunol Rev. 1993 Oct;135:133–155. doi: 10.1111/j.1600-065x.1993.tb00647.x. [DOI] [PubMed] [Google Scholar]
- Hugo P., Kappler J. W., McCormack J. E., Marrack P. Fibroblasts can induce thymocyte positive selection in vivo. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10335–10339. doi: 10.1073/pnas.90.21.10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. R., Peterson P. A. Assembly and intracellular transport of MHC class I molecules. Annu Rev Cell Biol. 1993;9:207–235. doi: 10.1146/annurev.cb.09.110193.001231. [DOI] [PubMed] [Google Scholar]
- Koseki H., Asano H., Inaba T., Miyashita N., Moriwaki K., Lindahl K. F., Mizutani Y., Imai K., Taniguchi M. Dominant expression of a distinctive V14+ T-cell antigen receptor alpha chain in mice. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7518–7522. doi: 10.1073/pnas.88.17.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koseki H., Imai K., Nakayama F., Sado T., Moriwaki K., Taniguchi M. Homogenous junctional sequence of the V14+ T-cell antigen receptor alpha chain expanded in unprimed mice. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5248–5252. doi: 10.1073/pnas.87.14.5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino Y., Yamagata N., Sasho T., Adachi Y., Kanno R., Koseki H., Kanno M., Taniguchi M. Extrathymic development of V alpha 14-positive T cells. J Exp Med. 1993 May 1;177(5):1399–1408. doi: 10.1084/jem.177.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski T., Elliott J. D., Loh D. Y., Staerz U. D. Positive selection of T lymphocytes on fibroblasts. Nature. 1993 Aug 12;364(6438):642–645. doi: 10.1038/364642a0. [DOI] [PubMed] [Google Scholar]
- Porcelli S., Brenner M. B., Greenstein J. L., Balk S. P., Terhorst C., Bleicher P. A. Recognition of cluster of differentiation 1 antigens by human CD4-CD8-cytolytic T lymphocytes. Nature. 1989 Oct 5;341(6241):447–450. doi: 10.1038/341447a0. [DOI] [PubMed] [Google Scholar]
- Porcelli S., Morita C. T., Brenner M. B. CD1b restricts the response of human CD4-8- T lymphocytes to a microbial antigen. Nature. 1992 Dec 10;360(6404):593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- Richards S., Bucan M., Brorson K., Kiefer M. C., Hunt S. W., 3rd, Lehrach H., Lindahl K. F. Genetic and molecular mapping of the Hmt region of mouse. EMBO J. 1989 Dec 1;8(12):3749–3757. doi: 10.1002/j.1460-2075.1989.tb08551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey E., Fowlkes B. J. Selective events in T cell development. Annu Rev Immunol. 1994;12:675–705. doi: 10.1146/annurev.iy.12.040194.003331. [DOI] [PubMed] [Google Scholar]
- Robinson R. R., Germain R. N., McKean D. J., Mescher M., Seidman J. G. Extensive polymorphism surrounding the murine Ia A beta chain gene. J Immunol. 1983 Oct;131(4):2025–2031. [PubMed] [Google Scholar]
- Schild H., Mavaddat N., Litzenberger C., Ehrich E. W., Davis M. M., Bluestone J. A., Matis L., Draper R. K., Chien Y. H. The nature of major histocompatibility complex recognition by gamma delta T cells. Cell. 1994 Jan 14;76(1):29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Schirmbeck R., Reimann J. Peptide transporter-independent, stress protein-mediated endosomal processing of endogenous protein antigens for major histocompatibility complex class I presentation. Eur J Immunol. 1994 Jul;24(7):1478–1486. doi: 10.1002/eji.1830240704. [DOI] [PubMed] [Google Scholar]
- Shawar S. M., Vyas J. M., Rodgers J. R., Rich R. R. Antigen presentation by major histocompatibility complex class I-B molecules. Annu Rev Immunol. 1994;12:839–880. doi: 10.1146/annurev.iy.12.040194.004203. [DOI] [PubMed] [Google Scholar]
- Sherman L. A., Lara A. M. Unrestricted recognition of a nonpeptide antigen by CD8+ cytolytic T lymphocytes. J Immunol. 1989 Dec 1;143(11):3444–3447. [PubMed] [Google Scholar]
- Sumida T., Sado T., Kojima M., Ono K., Kamisaku H., Taniguchi M. I-J as an idiotype of the recognition component of antigen-specific suppressor T-cell factor. Nature. 1985 Aug 22;316(6030):738–741. doi: 10.1038/316738a0. [DOI] [PubMed] [Google Scholar]
- Van Kaer L., Ashton-Rickardt P. G., Ploegh H. L., Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4-8+ T cells. Cell. 1992 Dec 24;71(7):1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- Zijlstra M., Li E., Sajjadi F., Subramani S., Jaenisch R. Germ-line transmission of a disrupted beta 2-microglobulin gene produced by homologous recombination in embryonic stem cells. Nature. 1989 Nov 23;342(6248):435–438. doi: 10.1038/342435a0. [DOI] [PubMed] [Google Scholar]