Abstract
When present in the extracellular environment, the nucleoside adenosine protects cells and tissues from excessive inflammation and immune-mediated damage while promoting healing processes. This role has been highlighted experimentally using distinct disease models, including those of colitis, diabetes, asthma, sepsis, and ischemic injury. Adenosine also suppresses immune responses, as in the tumor microenvironment, assisting immune evasion while promoting angiogenesis. The mechanisms involved in adenosine signaling are addressed elsewhere in this issue. Here, the authors specifically address the generation of adenosine from extracellular nucleotides. This process is catalyzed by a series of plasma membrane ectonucleotidases, with the focus in this article on members of the CD39, CD73, and CD38 families and on their role in inflammatory and neoplastic hematological diseases. Pharmacological modulation of adenosine generation by drugs that either have or modulate ectonucleotidase function might be exploited to treat these diverse conditions.
Keywords: Adenosine, Cancer, Colitis, Ectonucleotidase, Inflammation, Ischemic injury
Introduction
Adenosine is a purine nucleoside comprising a molecule of adenine attached to a ribose sugar molecule moiety via glycosidic bonds. When present in the extracellular environment, this mediator protects cells and tissues from excessive immune-mediated damage. This role has been highlighted recently in several disease models, including colitis [1], diabetes [2], asthma [3], sepsis [4], and ischemic injury [5]. Adenosine also suppresses immune responses, and as such, may mediate in part, the immune-suppression often seen within the tumor microenvironment thus facilitating tumor immune evasion [6]. Therefore, mechanisms involved in both the generation of adenosine as well as its downstream signaling pathways are of considerable therapeutic interest [7].
Purinergic signaling
Under normal physiologic conditions, the levels of adenosine in the tissue microenvironment are relatively low and certainly below the sensitivity threshold of immune cells. However, during hypoxia, ischemia, inflammation, infection, metabolic stress, and tumor transformation tissue, adenosine levels rapidly increase [8]. These elevated levels of extracellular adenosine in response to tissue-disturbing signals has the dual function of signaling tissue injury in an autocrine and paracrine manner and eliciting a range of tissue responses that can be generally viewed as organ protective.
Growing evidence indicates that in addition to the nucleoside adenosine, extracellular nucleotides, such as adenosine triphosphate (ATP) or nicotinamide adenine dinucleotide (NAD), participate in the regulation of host immune responses [9].
Extracellular ATP binds multiple type-2 purinergic/pyrimidinergic (P2Y and P2X) receptors, and this interaction influences cellular metabolism, migration, proliferation, and apoptosis (reviewed in [10, 11]). This nucleotide may also serve as substrates for ectonucleotidases, surface enzymes having their catalytic domain located in the extracellular compartment.
The most well-characterized ectonucleotidase pathway proceeds through the sequential action of CD39 (ectonucleoside triphosphate diphosphohydrolase-1 (ENTPD-1)), the prototype of the ENTPDase enzyme family, which converts extracellular ATP (or ADP) to AMP. CD39 is located on the cell surface and contains one extracellular facing catalytic domain and two transmembrane domains, important for maintaining catalytic activity and substrate specificity [12]. The ectonucleotidase cascade initiated by CD39 is terminated by CD73 (5′-nucleotidase) that converts AMP to adenosine [13]. CD73 is a dimeric extracellular glycoprotein tethered to the cell membrane by a glycosyl phosphatidyl inositol anchor. Alternatively, adenosine can be generated from NAD through to the coordinated action of CD38 (NAD glycohydrolase), which generates ADP ribose, and PC-1 (ectonucleotide pyrophosphatase/phosphodiesterase family member 1), which generates AMP. The removal of the final phosphate is operative via CD73, which consequently acts as a bottleneck enzyme in the setting of both cascades.
Adenosine elicits its physiological responses by binding to and activating one or more of the four transmembrane adenosine receptors, denoted A1, A2A, A2B, and A3. Each of these four adenosine receptor subtypes is coupled to a G protein, which can stimulate (Gs protein) or inhibit (Gi protein) the production of intracellular cAMP. Changes in the levels of cAMP influence the activity of intracellular protein kinases that phosphorylate intracellular proteins or transmembrane ion channels during physiological responses [14]. Adenosine at physiological levels (below 1 μM) can activate A1, A2A, and A3 adenosine receptors, whereas much higher concentrations of this nucleoside (i.e., generated under pathophysiological conditions) are required to stimulate the A2B adenosine receptor.
Transcriptional regulation of ecto-enzyme expression
The expression of the CD39 and CD73 ecto-enzymes can be regulated by hypoxia or by exposure to cytokines present in the extracellular milieu.
A study from Synnestvedt et al. demonstrated that hypoxia was able to induce CD39 and CD73 expression and function [15]. In this same study, analysis of the CD73 gene promoter region revealed one binding site for hypoxia-inducible factor 1 (HIF-1) [15] suggesting that hypoxia may regulate CD73 expression through HIF-1.
In the context of colitis, induction of CD39 and CD73 by hypoxia resulted in a shift from pro-inflammatory ATP to immunosuppressive adenosine [16]. A study from Cummins et al. examined the effect of dimethyl oxalyl glycine (DMOG), a hydroxylase inhibitor, on Caco-2 intestinal cells and reported that DMOG was able to induce HIF-1 and protect mice from dextran sodium solfate (DSS)-induced colitis [17]. In another study, Robinson and et al. evaluated the effect of FG-4497, another hydroxylase inhibitor, on murine colitis induced by trinitrobenzene sulfonic acid (TNBS). FG-4497 induced HIF-1 and attenuated clinical symptoms of colitis [18]. Both investigations suggest that HIF-1 activators could be beneficial to hypoxia-induced tissue inflammation through the generation of the immune-suppressive adenosine.
Regulation of ecto-enzymes expression has been also linked to the cytokine milieu that cells are exposed to. Thus in a study from Regateiro et al., expression of CD73 on murine CD4+ T cells resulted in the hydrolysis of AMP and the subsequent generation of adenosine that in turn could suppress the proliferation of effector CD4 T cells in vitro. In the same study, the authors reported that TGF-β also induced CD73 on CD8+ T cells, macrophages and dendritic cells, thereby enabling adenosine to be generated in different tissue microenvironments [19].
In the tumor setting, Sekar et al. reported that apoptotic tumor cells induce IL-27 secretion by dendritic cells which activates CD39+CD69+ regulatory T cells. They further postulate that the expression of CD69 on such cells facilitates the interaction of CD39+ regulatory T cells with CD73+ CD8 cells resulting in the generation of adenosine, therefore suppressing CD8 T cell cytotoxicity [20].
Ectonucleotidases, adenosine generation, and immunosuppression
In mouse systems, circulating CD4+/CD25high regulatory T lymphocytes have been shown to express high levels of functional CD39 and CD73 thus having the capacity to generate adenosine. Furthermore, adenosine production is an integral component of the suppressive machinery of regulatory T cells. Under conditions of chronic inflammation, considerable amounts of ATP are released in the extracellular environment, while at the same time activated effector T cells express the A2A receptor. Adenosine production is thus believed to be a late mechanism for controlling effector T cell proliferation mainly in chronically activated or inflamed tissues.
The situation in humans is more complicated, with the circulating component of regulatory T cells constitutively expressing CD39 but not CD73. Therefore, such regulatory T cells are unable to produce adenosine. CD73 expression is however rapidly turned on under activation and as such, regulatory T cells infiltrating solid tumors, for example, appear to be CD39+/CD73+, and therefore would be expected to be able to generate adenosine locally in the tumor microenvironment.
Cancer
Extracellular adenosine is elevated in neoplastic tissues as consequence of an increased expression and activity of the ecto-enzymes that produce it. Several types of solid tumors [21, 22] and certain types of leukemia [23, 24] express the enzymatic machinery necessary to convert ATP into adenosine on their cell surface. Accumulation of adenosine within the tumor tissue has complex and diverse effects. On the one side, this nucleoside has been linked to cytoprotection and growth promotion of tumor cells. On the other side, adenosine has a profound impact on the environment surrounding the tumor. Indeed, this nucleoside may increase angiogenesis, inhibit Th1 cytokine production, promote adhesion of immune cells to endothelial cells and contribute to suppression of effector T cells (reviewed in [25]).
Based on this knowledge, an intriguing possibility is that tumor cells themselves may exploit the CD39/CD73 enzymatic cascade to actively produce adenosine and favorably modify its local environment.
We have recently examined these possibilities in mouse models of metastatic disease and melanoma [26] and in human chronic lymphocytic leukemia (CLL). In a comparable manner, recently Salvestrini and et al. [27] have highlighted the significance of purinergic signaling in the inhibition of cell proliferation, homing and engraftment of tumor cells in a murine model of acute myeloblastic leukemia [27].
We will next address the clinical relevance of the role of purinergic signaling in CLL and other hematological malignancies, such as follicular lymphoma.
Chronic lymphocytic leukemia
This chronic leukemia, characterized by the expansion of a mature population of CD5+/CD23+ B lymphocytes, displays markedly different behaviors whether in the blood or in the LN, with proliferation occurring almost exclusively in the latter [28–30]. Circulating CLL cells are characterized by an invariably high expression of CD39 and a variable CD73 expression. Approximately 30 % of CLL patients express high levels of CD73 on their leukemic cells, and this is associated with such cellular markers of poor prognosis as CD38 and ZAP-70. Immunohistochemical analysis of lymph node tissues indicates that the highest intensity of CD73 expression is near the CLL proliferation centers. Furthermore, CD73+ lymphocytes are generally Ki-67+ proliferating cells, which are in close contact with CD2+ T lymphocytes. These arrangements appear essential for the expansion of the pathogenic CLL clones, which in turn localize adjacent to perivascular areas [24].
Together, these observations indicate that CD73 expression on neoplastic CLL cells is associated with more aggressive clinical behavior, higher cellular turnover, and intense recirculation to and from lymphoid organs.
Analysis of the enzymatic activities on purified CLL cells indicates that this enzymatic cascade is highly functional. Indeed, CD39+/CD73+ cells can actively convert the substrate ADP to AMP and to the final product adenosine. Because circulating CLL lymphocytes constitutively express significantly higher levels of the A2A adenosine receptor as compared with age- and sex-matched controls, adenosine binding to these receptors (or activation through a selective A2A agonist) leads to increases in intracellular cAMP concentrations, which can modulate different signaling pathways [24].
These data indicate the existence of an autocrine adenosinergic loop in a selected subset of CLL patients, where adenosine is produced and then consumed by leukemic cells. The activation of this axis modulates chemotactic responses of CLL cells, for example inhibiting CXCL12 chemokine induced migration, and protects such cells from spontaneous or drug-induced apoptosis. Exposure of CLL cells to a commercially available CD73 inhibitor as well as to selective adenosine receptor agonists or antagonists confirm that these effects are directly mediated by CD73-generated extracellular adenosine and by the A2A receptor [24].
These observations suggest that in CLL cells, CD73 might be a major modulatory ecto-enzyme in the activation of the adenosinergic axis. Furthermore, adenosine production is restricted by CD73 expression, hence mostly confined in sites of higher cellular turnover, like the lymph node proliferation centers. In the CLL model, CD73 expression, increased levels of extracellular nucleotides and adenosine generation may cooperate together to create local microenvironmental conditions that favor the survival of the leukemic clone and partially protect the clone from the action of chemotherapy. Therefore, targeting the adenosinergic axis might have important therapeutic impact in the control of CLL progression and in increasing the efficacy of chemotherapy.
Lymphoma
Hilchey and co-workers [23], have demonstrated that human follicular lymphoma (FL) infiltrating T lymphocytes are anergic, due in part to regulatory T cell suppression mediated through an adenosine-dependent pathway. Indeed, extracellular adenosine, by activating purinergic A2A and A2B receptors expressed by FL infiltrating effector T cells, in part, mediates FL infiltrating T cell hypo-responsiveness in a subset of patient samples. In this system, inhibition of the ATP-ectonucleotidase-adenosine axis with putative CD39 inhibitors, e.g., ARL67156 or with specific A2A and A2B receptor antagonists, partially overcomes the T cell hypo-responsiveness to stimulation seen with untreated single cell suspensions derived from FL lymph nodes. Specifically, a significant increase in INF-gamma and IL-2 production is observed in stimulated FL lymph node mononuclear cells, compared with that seen in stimulated normal lymph node mononuclear cells, when either CD39 or A2A/A2B receptor signaling is inhibited. Consistent with these observations, the proportion of CD4+ T cells that express CD39 is higher in lymph node biopsies from FL patients as compared with that of normal or reactive lymph nodes or to peripheral blood from normal donors. Furthermore, a proportion of these CD39+ T cells express the classical phenotype of T regulatory cells (CD4+/CD25high/FOXP3+).
These results indicate that adenosine may play an important role in FL-mediated immunosuppression and that the ATP-ectonucleotidase-adenosine axis could be a relevant pharmacological target for cancer immunotherapy [23, 31].
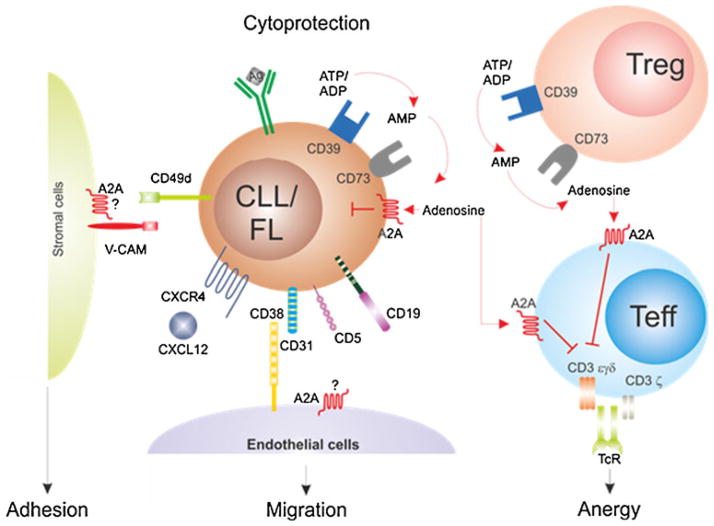
Figure 1 illustrates the role of the ecto-enzyme network in cancer models.
Fig. 1.
Role of the ecto-enzyme network in cancer models. Lymphoma/leukemia cells express the enzymatic machinery necessary to convert ATP/ADP into adenosine. Increased extracellular adenosine levels contribute to creating a favorable microenvironment by (1) inducing cytoprotection in an autocrine fashion, by (2) switching off T cell responses, and (3) promoting production and secretion of soluble mediators of inflammation. Alternatively, adenosine may be generated by regulatory Tcells, contributing to immunosuppression and effector T cell anergy
Asthma, chronic obstructive pulmonary disorder, and acute lung injury
A number of studies have supported the evidence that adenosine signaling is involved in the pathophysiology of asthma and chronic obstructive pulmonary disorder (COPD). As an example, in an allergic mouse model of asthma, challenge with adenosine enhanced airway inflammation and led to an increase in lymphocytes, eosinophils, neutrophils and activated macrophages in the bronchoalveolar lavage [3]. Administration of theophylline, a nonspecific adenosine receptor antagonist, prevented enhancement of the inflammatory infiltrate induced by adenosine [3]. Moreover, a wealth of studies over the years clearly demonstrated that adenosine can induce bronchoconstriction in patients suffering from asthma and COPD but not in healthy individuals. The A2B adenosine receptor was found to be one of the main receptors involved in mediating the stimulatory effect of adenosine on mast cells [32]; based on these observations, the use of A2B receptor antagonists has been proposed to treat asthma and COPD. A study from Hua et al., however, reported the opposite results as they showed that the enhanced mast cells activation seen in mice was the result of A2B adenosine receptor loss. Loss of this receptor resulted in decreased cyclic AMP basal levels and the influx of extracellular calcium through store-operated calcium channels after activation with antigen [33]. Moreover, A2B−/− mice displayed increased anaphylaxis, suggesting that A2B adenosine receptor is a negative regulator of mast cell function [33].
Extracellular adenosine has also been implicated as an immunomodulatory molecule during acute lung injury. Previous studies have shown the presence of acute lung injury, particularly edema, inflammation, and diminished gas exchange in mutant mice with CD39 deletion [34, 35]. Further work has demonstrated that deletion of the A2B adenosine receptor in mice with ventilation-induced lung injury (VILI) resulted in reduced survival time and that treatment with the A2B adenosine receptor agonist—BAY 60-6583—was able to attenuate VILI-induced damage [36].
Ischemia and reperfusion
Several lines of evidence indicate that extracellular adenosine can control tissue damage from ischemia and reperfusion injury in several organs, including the heart, liver, kidney, and intestine. A study from Eckle et al. demonstrated the importance of adenosine generation for cardioprotection by ischemic preconditioning [37]. Results from the same investigations showed that ischemic preconditioning led to cardioprotection in A1−/−, A2A−/−, and A3−/− but not in A2B−/− mice and that treatment with an A2B adenosine receptor antagonist reduced the size of infarction induced by ischemia [37].
In the renal setting, increases in adenosine by ischemic preconditioning were found to be attenuated in CD39−/− mice while administration of apyrase (a plasma membrane-bound enzyme catalyzing the hydrolysis of ATP) was capable of restoring renal protection [38]. Similarly, no increase in adenosine generation from ischemia preconditioning was found in CD73−/− mice; administration of 5′-ectonucleotidase resulted in complete restoration of renal protection [39]. In a study from Crikis et al., overexpression of CD39, known to induce heightened levels of adenosine generation, conferred protection in a model of warm renal ischemia–reperfusion [40]. Mice displayed reduced histological lesions, lower levels of apoptosis and preserved serum creatinine and urea levels. Treatment with A2A adenosine receptor antagonist attenuated the protective effect of CD39 overexpression [40]. In the hepatic setting increases in the production of adenosine were absent in CD73−/− mice and were restored after administration of soluble 5′-ectonucleotidase [41]. In the same context, CD39 null mice were protected from acute vascular injury after single-lobe warm ischemia–reperfusion injury and, compared with wild-type control mice, displayed lower aminotransferase levels and less marked histological changes. The same investigation highlighted that it was CD39 deletion on hepatic NK cells that provides protection against ischemia–reperfusion injury [42]. A subsequent study from Pommey et al. demonstrated that liver grafts from CD39 overexpressing mice were protected from ischemia–reperfusion injury and that this protection was mediated by the absence of CD4 T cells [43].
In a model of intestinal ischemia–reperfusion, CD73−/− mice displayed lower adenosine levels that did not increase following ischemia–reperfusion injury [44]. Administration of soluble 5′-ectonucleotidase was accompanied by decreased injury [44]. In another model of intestinal ischemia–reperfusion injury, CD39 null mice showed an increased mortality rate when compared with wild-type control mice. Apyrase supplementation, while able to fully protect wild-type mice from death, did not fully protect CD39 null mice [45].
Sepsis
Extracellular levels of adenosine are high in sepsis. Studies on adenosine signaling have shown that genetic deficiency of the A2B receptor resulted in the increased mortality of such mice after cecal ligation and puncture-induced sepsis [4]. High levels of pro-inflammatory cytokines were similarly associated with the death of such mice [4].
A recent study in humans in whom experimental endo-toxemia was induced by intravenous injection of LPS showed modifications in adenosine metabolism and signaling that were associated with an increase in adenosine levels and a dose dependent decrease in adenosine deaminase and adenosine kinase [46].
Autoimmunity
Inflammatory bowel disease
Given the immunosuppressive properties of adenosine, modulation of its signaling has been exploited in the context of inflammatory bowel disease (IBD) and other autoimmune conditions to curb inflammation. IBD results from massive lymphocyte infiltration of the gut lamina propria, with T helper type 1 (Th1) [47–49] and 17 (Th17) cells [50–54] likely playing the major role in the perpetration of damage against the luminal flora.
Alterations in the generation of adenosine, such as those associated with defects in CD39 or CD73 expression, led to a more severe course of the colitis. Thus, mice null for CD39 were highly susceptible to DSS-induced colitis, displaying dense wall thickening and profound lymphocytic infiltration of the colonic mucosa compared with that of wild-type mice [55].
In humans, a polymorphism adjacent to the CD39 promoter region has been associated with low levels of CD39 mRNA and with susceptibility to Crohn’s disease [55]. Decreases in CD39 expression levels and consequently adenosine generation are likely to be linked to the impairment of CD4+CD25high regulatory T cells [56, 57]. Such a defect might lead to overwhelming T cell autoreactivity because of the lack of immune-modulatory adenosine.
That adenosine is key in controlling inflammation in IBD, was previously shown by a study from Cronstein et al. who reported that sulfasalazine, commonly used to treat IBD, can inhibit lymphocyte accumulation through an adenosine-dependent mechanism [58]. Similarly, methotrexate, another drug used in IBD treatment, controls inflammation by promoting adenosine release [59]. A subsequent investigation demonstrated that the oral administration of ATL313, an agonist of the A2A adenosine receptor can attenuate colitis in mice injected with CD45RBhigh cells, by suppressing production of pro-inflammatory cytokines (IL-2, IFNγ, and TNFα) while sparing that of anti-inflammatory (IL-10 and TGFβ) cytokines [1]. Later on, a study from Frick et al. reported that activation of the A2B adenosine receptor is involved in the modulation of the acute phase of DSS colitis in mice through the induction of IL-10 by intestinal epithelial cells [60].
Type 1 diabetes
There is evidence that adenosine signaling regulates glucose homeostasis through the modulation of both insulin and glucagon release [61]. Given the inverse correlation between levels of adenosine in the islets and extracellular glucose concentration [62], modulation of adenosine signaling by treatment with adenosine receptor agonists has been attempted. These investigations showed that treatment with the non-selective adenosine receptor agonist 5′ N-ethylcarboxamidoadenosine (NECA) prevented the development of diabetes in multiple-low-dose-streptozotocin-challenged mice and in NOD mice injected with cyclophosphamide [2].
The central role of adenosine in preventing the development of diabetes was further highlighted by a series of subsequent studies in which A2A adenosine receptor knock-out mice were found to be highly susceptible to MSDL-induced diabetes that was attenuated following NECA administration [61].
A recent study from Johnston-Cox et al. in the context of type 2 diabetes reported that the A2B adenosine receptor is protective against high fat diet-induced insulin resistance. A2B adenosine receptor null mice exposed to high fat diet show impaired glucose and insulin homeostasis. Moreover, mice injected with the A2B adenosine receptor-specific agonist—BAY 60-6583—displayed lower glucose plasma and insulin levels [63].
The A2B adenosine receptor is also linked to regulation of hyperlipidemia and atherosclerosis. Compared with apolipoprotein E (ApoE) and A2B adenosine receptor knock-out mice, ApoE and A2B adenosine receptor double knock-out mice showed a significant increase in lipid plaques. In vivo administration of BAY 60-6583 in control mice on a high fat diet reduced the lipid profile as well as atherosclerosis [64].
Multiple sclerosis
Extracellular adenosine was found to modulate disease progression in mice with experimental autoimmune encephalomyelitis (EAE), the mouse model of multiple sclerosis. Mice lacking the A2A adenosine receptor developed more severe EAE that was characterized by a greater degree of lymphocyte infiltration in the central nervous system compared with wild-type mice [65].
That adenosine plays a role in curbing disease activity has been indirectly supported by data in patients with multiple sclerosis in whom numerical and functional impairment of regulatory T cells expressing CD39 has been reported [66, 67].
Conclusions
This review has summarized the role of adenosine in different disease settings such as those of hematological malignancies, autoimmunity, chronic and acute respiratory disorders, ischemia–reperfusion, and sepsis and has suggested how pharmacological modulation of adenosine generation and signaling could be exploited to treat these diverse conditions. The review has highlighted how targeting the adenosinergic axis could: (1) control tumor progression and partially overcome the T cell hypo-responsiveness seen in the tumor setting; (2) modulate the extent of the inflammatory infiltrate in asthma and COPD (via adenosine receptor antagonists) and in acute lung injury (via adenosine receptor agonists); (3) attenuate damage during ischemia–reperfusion injury (by increasing breakdown of ATP into adenosine); and (4) control inflammation in autoimmunity (via adenosine receptor agonists).
Acknowledgments
The work summarized in this review article was supported by: Clinician Scientist Fellowship from the Medical Research Council (UK); National Institute of Health; R01 HL094400; P01HL107152, P01 HL087203, and P01 AI045897 and Associazione Italiana Ricerca Cancro (IG #12754); Italian Ministries of Health (Bando Giovani Ricercatori 2008) and Education (Bando FIRB Giovani 2008 and Bando PRIN 2009).
Footnotes
Disclosure The authors declare that they have no conflict of interests.
Contributor Information
Maria Serena Longhi, Email: maria.longhi@kcl.ac.uk, Institute of Liver Studies, King’s College London School of Medicine at King’s College Hospital, Denmark Hill, SE5 9RS, London, UK.
Simon C. Robson, Division of Gastroenterology, and Transplant Institute, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard University, Boston 02215, USA
Steven H. Bernstein, James P. Wilmot Cancer Center, University of Rochester Medical Center, Rochester, NY 14642, USA
Sara Serra, Human Genetics Foundation (HuGeF) and Department of Medical Sciences, University of Turin, Turin, Italy.
Silvia Deaglio, Email: silvia.deaglio@unito.it, Human Genetics Foundation (HuGeF) and Department of Medical Sciences, University of Turin, Turin, Italy.
References
- 1.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 2.Nemeth ZH, Bleich D, Csoka B, Pacher P, Mabley JG, Himer L, Vizi ES, Deitch EA, Szabo C, Cronstein BN, et al. Adenosine receptor activation ameliorates type 1 diabetes. FASEB J. 2007;21:2379–2388. doi: 10.1096/fj.07-8213com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan M, Jamal Mustafa S. Role of adenosine in airway inflammation in an allergic mouse model of asthma. Int Immunopharmacol. 2006;6:36–45. doi: 10.1016/j.intimp.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Csoka B, Nemeth ZH, Rosenberger P, Eltzschig HK, Spolarics Z, Pacher P, Selmeczy Z, Koscso B, Himer L, Vizi ES, et al. A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J Immunol. 2010;185:542–550. doi: 10.4049/jimmunol.0901295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peart JN, Headrick JP. Adenosinergic cardioprotection: multiple receptors, multiple pathways. Pharmacol Ther. 2007;114:208–221. doi: 10.1016/j.pharmthera.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cronstein BN. Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol. 1994;76:5–13. doi: 10.1152/jappl.1994.76.1.5. [DOI] [PubMed] [Google Scholar]
- 9.Di Virgilio F, Boeynaems JM, Robson SC. Extracellular nucleotides as negative modulators of immunity. Curr Opin Pharmacol. 2009;9:507–513. doi: 10.1016/j.coph.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnstock G. Unresolved issues and controversies in purinergic signalling. J Physiol. 2008;586:3307–3312. doi: 10.1113/jphysiol.2008.155903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burnstock G, Verkhratsky A. Evolutionary origins of the purinergic signalling system. Acta Physiol (Oxf) 2009;195:415–447. doi: 10.1111/j.1748-1716.2009.01957.x. [DOI] [PubMed] [Google Scholar]
- 12.Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linden J. Regulation of leukocyte function by adenosine receptors. Adv Pharmacol. 2011;61:95–114. doi: 10.1016/B978-0-12-385526-8.00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colgan SP, Eltzschig HK. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu Rev Physiol. 2012;74:153–175. doi: 10.1146/annurev-physiol-020911-153230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, Taylor CT. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–155. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regateiro FS, Howie D, Nolan KF, Agorogiannis EI, Greaves DR, Cobbold SP, Waldmann H. Generation of anti-inflammatory adenosine by leukocytes is regulated by TGF-beta. Eur J Immunol. 2011;41:2955–2965. doi: 10.1002/eji.201141512. [DOI] [PubMed] [Google Scholar]
- 20.Sekar D, Hahn C, Brune B, Roberts E, Weigert A. Apoptotic tumor cells induce IL-27 release from human DCs to activate Treg cells that express CD69 and attenuate cytotoxicity. Eur J Immunol. 2012;42:1585–1598. doi: 10.1002/eji.201142093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A. 2010;107:1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187:676–683. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 23.Hilchey SP, Kobie JJ, Cochran MR, Secor-Socha S, Wang JC, Hyrien O, Burack WR, Mosmann TR, Quataert SA, Bernstein SH. Human follicular lymphoma CD39+-infiltrating T cells contribute to adenosine-mediated T cell hyporesponsiveness. J Immunol. 2009;183:6157–6166. doi: 10.4049/jimmunol.0900475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serra S, Horenstein AL, Vaisitti T, Brusa D, Rossi D, Laurenti L, D’Arena G, Coscia M, Tripodo C, Inghirami G, et al. CD73-generated extracellular adenosine in chronic lymphocytic leukemia creates local conditions counteracting drug-induced cell death. Blood. 2011;118:6141–6152. doi: 10.1182/blood-2011-08-374728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gessi S, Merighi S, Sacchetto V, Simioni C, Borea PA. Adenosine receptors and cancer. Biochim Biophys Acta. 2011;1808:1400–1412. doi: 10.1016/j.bbamem.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Sun X, Wu Y, Gao W, Enjyoji K, Csizmadia E, Muller CE, Murakami T, Robson SC. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology. 2010;139:1030–1040. doi: 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salvestrini V, Zini R, Rossi L, Gulinelli S, Manfredini R, Bianchi E, Piacibello W, Caione L, Migliardi G, Ricciardi MR, et al. Purinergic signaling inhibits human acute myeloblastic leukemia cell proliferation, migration, and engraftment in immunodeficient mice. Blood. 2012;119:217–226. doi: 10.1182/blood-2011-07-370775. [DOI] [PubMed] [Google Scholar]
- 28.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 29.Dighiero G, Hamblin TJ. Chronic lymphocytic leukaemia. Lancet. 2008;371:1017–1029. doi: 10.1016/S0140-6736(08)60456-0. [DOI] [PubMed] [Google Scholar]
- 30.Caligaris-Cappio F, Ghia P. Novel insights in chronic lymphocytic leukemia: are we getting closer to understanding the pathogenesis of the disease? J Clin Oncol. 2008;26:4497–4503. doi: 10.1200/JCO.2007.15.4393. [DOI] [PubMed] [Google Scholar]
- 31.Yegutkin GG, Marttila-Ichihara F, Karikoski M, Niemela J, Laurila JP, Elima K, Jalkanen S, Salmi M. Altered purinergic signaling in CD73-deficient mice inhibits tumor progression. Eur J Immunol. 2011;41:1231–1241. doi: 10.1002/eji.201041292. [DOI] [PubMed] [Google Scholar]
- 32.Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Dikov MM, Blackburn MR, Biaggioni I, Feoktistov I. Effect of A2B adenosine receptor gene ablation on proinflammatory adenosine signaling in mast cells. J Immunol. 2008;180:7212–7220. doi: 10.4049/jimmunol.180.11.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hua X, Kovarova M, Chason KD, Nguyen M, Koller BH, Tilley SL. Enhanced mast cell activation in mice deficient in the A2b adenosine receptor. J Exp Med. 2007;204:117–128. doi: 10.1084/jem.20061372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reutershan J, Vollmer I, Stark S, Wagner R, Ngamsri KC, Eltzschig HK. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 2009;23:473–482. doi: 10.1096/fj.08-119701. [DOI] [PubMed] [Google Scholar]
- 35.Eckle T, Fullbier L, Wehrmann M, Khoury J, Mittelbronn M, Ibla J, Rosenberger P, Eltzschig HK. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol. 2007;178:8127–8137. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]
- 36.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, et al. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 38.Grenz A, Zhang H, Hermes M, Eckle T, Klingel K, Huang DY, Muller CE, Robson SC, Osswald H, Eltzschig HK. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia–reperfusion injury. FASEB J. 2007;21:2863–2873. doi: 10.1096/fj.06-7947com. [DOI] [PubMed] [Google Scholar]
- 39.Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, Kohle C, Kloor D, Thompson LF, Osswald H, Eltzschig HK. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 2007;18:833–845. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 40.Crikis S, Lu B, Murray-Segal LM, Selan C, Robson SC, D’Apice AJ, Nandurkar HH, Cowan PJ, Dwyer KM. Transgenic overexpression of CD39 protects against renal ischemia–reperfusion and transplant vascular injury. Am J Transplant. 2010;10:2586–2595. doi: 10.1111/j.1600-6143.2010.03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hart ML, Much C, Gorzolla IC, Schittenhelm J, Kloor D, Stahl GL, Eltzschig HK. Extracellular adenosine production by ecto-5′-nucleotidase protects during murine hepatic ischemic preconditioning. Gastroenterology. 2008;135:1739–1750. e1733. doi: 10.1053/j.gastro.2008.07.064. [DOI] [PubMed] [Google Scholar]
- 42.Beldi G, Banz Y, Kroemer A, Sun X, Wu Y, Graubardt N, Rellstab A, Nowak M, Enjyoji K, Li X, et al. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice. Hepatology. 2010;51:1702–1711. doi: 10.1002/hep.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pommey S, Lu B, McRae J, Stagg J, Hill P, Salvaris E, Robson SC, d’Apice AJ, Cowan PJ, Dwyer KM. Liver grafts from CD39-overexpressing mice are protected from ischemia reperfusion injury due to reduced numbers of resident CD4(+) T cells. Hepatology. 2012 doi: 10.1002/hep.25985. [DOI] [PubMed] [Google Scholar]
- 44.Hart ML, Henn M, Kohler D, Kloor D, Mittelbronn M, Gorzolla IC, Stahl GL, Eltzschig HK. Role of extracellular nucleotide phosphohydrolysis in intestinal ischemia–reperfusion injury. FASEB J. 2008;22:2784–2797. doi: 10.1096/fj.07-103911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guckelberger O, Sun XF, Sevigny J, Imai M, Kaczmarek E, Enjyoji K, Kruskal JB, Robson SC. Beneficial effects of CD39/ectonucleoside triphosphate diphosphohydrolase-1 in murine intestinal ischemia–reperfusion injury. Thromb Haemost. 2004;91:576–586. doi: 10.1160/TH03-06-0373. [DOI] [PubMed] [Google Scholar]
- 46.Ramakers BP, Wever KE, Kox M, van den Broek PH, Mbuyi F, Rongen G, Masereeuw R, van der Hoeven JG, Smits P, Riksen NP, et al. How systemic inflammation modulates adenosine metabolism and adenosine receptor expression in humans in vivo. Crit Care Med. 2012;40:2609–2616. doi: 10.1097/CCM.0b013e318259205b. [DOI] [PubMed] [Google Scholar]
- 47.Parronchi P, Romagnani P, Annunziato F, Sampognaro S, Becchio A, Giannarini L, Maggi E, Pupilli C, Tonelli F, Romagnani S. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn’s disease. Am J Pathol. 1997;150:823–832. [PMC free article] [PubMed] [Google Scholar]
- 48.Neurath MF, Weigmann B, Finotto S, Glickman J, Nieuwenhuis E, Iijima H, Mizoguchi A, Mizoguchi E, Mudter J, Galle PR, et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J Exp Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in SCID mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 50.Zenewicz LA, Antov A, Flavell RA. CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol Med. 2009;15:199–207. doi: 10.1016/j.molmed.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGovern D, Powrie F. The IL23 axis plays a key role in the pathogenesis of IBD. Gut. 2007;56:1333–1336. doi: 10.1136/gut.2006.115402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nielsen OH, Kirman I, Rudiger N, Hendel J, Vainer B. Upregulation of interleukin-12 and –17 in active inflammatory bowel disease. Scand J Gastroenterol. 2003;38:180–185. doi: 10.1080/00365520310000672. [DOI] [PubMed] [Google Scholar]
- 54.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedman DJ, Kunzli BM, YIAR, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A. 2009;106:16788–16793. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chamouard P, Monneaux F, Richert Z, Voegeli AC, Lavaux T, Gaub MP, Baumann R, Oudet P, Muller S. Diminution of Circulating CD4+CD25 high T cells in naive Crohn’s disease. Dig Dis Sci. 2009;54:2084–2093. doi: 10.1007/s10620-008-0590-6. [DOI] [PubMed] [Google Scholar]
- 57.Ishikawa D, Okazawa A, Corridoni D, Jia LG, Wang XM, Guanzon M, Xin W, Arseneau KO, Pizarro TT, Cominelli F. Tregs are dysfunctional in vivo in a spontaneous murine model of Crohn’s disease. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cronstein BN, Montesinos MC, Weissmann G. Salicylates and sulfasalazine, but not glucocorticoids, inhibit leukocyte accumulation by an adenosine-dependent mechanism that is independent of inhibition of prostaglandin synthesis and p105 of NFkappaB. Proc Natl Acad Sci U S A. 1999;96:6377–6381. doi: 10.1073/pnas.96.11.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morabito L, Montesinos MC, Schreibman DM, Balter L, Thompson LF, Resta R, Carlin G, Huie MA, Cronstein BN. Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5′-nucleotidase-mediated conversion of adenine nucleotides. J Clin Invest. 1998;101:295–300. doi: 10.1172/JCI1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frick JS, MacManus CF, Scully M, Glover LE, Eltzschig HK, Colgan SP. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol. 2009;182:4957–4964. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chia JS, McRae JL, Cowan PJ, Dwyer KM. The CD39-adenosinergic axis in the pathogenesis of immune and nonimmune diabetes. J Biomed Biotechnol. 2012;2012:320495. doi: 10.1155/2012/320495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang GK, Squires PE, Tian F, Kieffer TJ, Kwok YN, Dale N. Glucose decreases extracellular adenosine levels in isolated mouse and rat pancreatic islets. Islets. 2012;4(1):64–70. doi: 10.4161/isl.19037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnston-Cox H, Koupenova M, Yang D, Corkey B, Gokce N, Farb MG, LeBrasseur N, Ravid K. The A2b adenosine receptor modulates glucose homeostasis and obesity. PLoS One. 2012;7:e40584. doi: 10.1371/journal.pone.0040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koupenova M, Johnston-Cox H, Vezeridis A, Gavras H, Yang D, Zannis V, Ravid K. A2b adenosine receptor regulates hyperlipidemia and atherosclerosis. Circulation. 2012;125:354–363. doi: 10.1161/CIRCULATIONAHA.111.057596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mills JH, Kim DG, Krenz A, Chen JF, Bynoe MS. A2A adenosine receptor signaling in lymphocytes and the central nervous system regulates inflammation during experimental autoimmune encephalomyelitis. J Immunol. 2012;188:5713–5722. doi: 10.4049/jimmunol.1200545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell’Acqua ML, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 67.Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farrelly C, Tubridy N, Mills KH. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]