Abstract
Head direction (HD) cells, found in the rodent Papez circuit, are thought to form the neural circuitry responsible for directional orientation. Because NMDA transmission has been implicated in spatial tasks requiring directional orientation, we sought to determine if the NMDA antagonist dizocilpine (MK-801) would disrupt the directional signal carried by the HD network. Anterior thalamic HD cells were isolated in female Long-Evans rats and initially monitored for baseline directional activity while the animals foraged in a familiar enclosure. The animals were then administered MK-801 at a dose of .05 mg/kg or 0.1 mg/kg, or isotonic saline, and cells were re-examined for changes in directional specificity and landmark control. While the cells showed no changes in directional specificity and landmark control following administration of saline or the lower dose of MK-801, the higher dose of MK-801 caused a dramatic attenuation of the directional signal, characterized by decreases in peak firing rates, signal to noise, and directional information content. While the greatly attenuated directional specificity of cells in the high dose condition usually remained stable relative to the landmarks within the recording enclosure, a few cells in this condition exhibited unstable preferred directions within and between recording sessions. Our results are discussed relative to the possibility that the findings explain the effects of MK-801 on the acquisition and performance of spatial tasks.
Keywords: navigation, spatial orientation, anterior thalamus, place cells, dizocilpine, N-methyl-D-aspartate, rat
Investigations of the neurochemical substrates of navigational behavior have indicated an important role for the N-methyl-D-aspartate (NMDA) subtype of the glutamate receptor. Given the widely held view of the critical role of the hippocampal formation in spatial memory (e.g., Jarrard, 1993; Morris, Garrud, Rawlins, & O'Keefe, 1982; Olton, Walker, & Gage, 1978), and the likelihood that NMDA receptor-mediated plasticity may be a cellular mechanism of memory formation in the hippocampus (e.g., Bliss & Collingridge, 1993), it is perhaps not surprising that administration of NMDA antagonists have been shown to impair behavior in a variety of spatial memory paradigms including the Morris water maze (Ahlander, Misane, Schott, Ogren, 1999; Davis, Butcher, & Morris, 1992; Morris, Anderson, Lynch, and Baudry, 1986; Morris, Steele, Bell, & Martin, 2013; Steele and Morris, 1999), the radial arm maze (Butelman, 1989; Ward, Mason, & Abraham, 1990; Shapiro and O'Connor, 1992), and the active allothetic place avoidance task (Stuchlik, and Vales, 2005).
While the issue of the true mechanism by which NMDA antagonists interfere with spatial behavior has been debated (e.g. Cain, Saucier, Hall, Hargreaves, & Boon, 1996; Keith & Rudy, 1990), the role of the NMDA receptor in spatial memory acquisition continues to be the subject of investigation, some of the more recent studies finding that knockout mice lacking functional NMDA receptors in distinct subregions of the hippocampus show a pattern of spatial deficits dependent on which hippocampal regions are affected (Bannerman et al., 2008; Nakazawa, Sun, Quirk, Rondi-Reig, Wilson, & Tonegawa, 2003; Place et al., 2012; Tsien, Huerta, & Tonegawa, 1996).
Another critical discovery related to our understanding of the neurophysiology of spatial orientation is the identification of several classes of neurons that seem to encode correlates of spatial navigation. Place cells, recorded most often in the CA regions of the hippocampus, become active when the animal is within a particular area of an environment, the ‘place field’ of the recorded cell (O'Keefe & Dostrovsky, 1971). Head direction (HD) cells, found in regions of the thalamus and other limbic structures, become active when the animal orients its head in a particular direction of the horizontal plane, the ‘preferred direction’ of the recorded cell (Sharp, Blair & Cho, 2001; Taube, 1995). Grid cells, found in the dorsal caudal medial entorhinal cortex, discharge at multiple locations in an environment forming a repeating grid-like pattern that could be used to encode movement trajectory (Fyhn, Molden, Witter, Moser, & Moser, 2004; Moser & Moser, 2008). Lastly, border cells of the medial entorhinal cortex become active when the animal approaches the walls or boundaries of the proximal recording environment (Solstad, Boccara, Kropff, Moser, & Moser, 2008). These four cell types respond to many of the same cues that are thought to be important for navigation, such as environmental landmarks and signals of self-movement, and are thought to work together to form the brain network used for navigational behavior (Derdikman & Moser, 2010; McNaughton et al., 1996; O'Keefe & Nadel, 1978).
The present study examines the effect of NMDA blockade on the directional selective activity of anterior thalamic HD cells. In accordance with the view that the HD cell system plays an important role in the brain circuitry that mediates directional orientation, lesions of select anatomical components of the HD cell circuit cause deficits in spatial navigation tasks (Aggleton, Hunt, Nagle, and Neave, 1996; Clark, Rice, Akers, Candelaria-Cook, Taube, and Hamilton, 2013; Van Groen, Kadish, and Wyss, 2002; Wilton, Baird, Muir, Honey, and Aggleton, 2001) and on the directional signal carried by place cells (Calton, et al., 2003). These findings, along with the previously described evidence that spatial behavior is also disrupted by NMDA antagonists brings up the possibility that the effects of NMDA blockade on spatial navigation may be mediated at least partly by a disrupted HD system. We sought to test this possibility by examining the basic directional-specific activity of anterior thalamic HD cells following administration of low or high doses of the noncompetitive NMDA antagonist dizocilpine (MK-801).
Method
Subjects and Surgical Procedures
23 female Long-Evans rats 3-6 months of age served as subjects. The rats were singly housed in Plexiglas cages and maintained on a 16/8 hour light/dark cycle. Following recovery from surgery, water was available ad libitum but access to food was restricted as necessary to maintain body weights in the range of 85–90% of free feeding weights. All care and treatment of the animals was approved by the CSUS Institutional Animal Care and Use Committee and adhered to the APA ethical principles of animal use.
Standard surgical procedures were used for electrode implantation. Animals were anesthetized using a cocktail containing ketamine (30 mg/kg), xylazene (6mg/kg), and acepromazine (1mg/kg). An incision was made in the scalp to expose the skull and holes were drilled for the electrode and six anchor screws. The electrode was placed slightly dorsal to the right anterior dorsal nucleus (ADN) of the thalamus based on coordinates (1.5 mm posterior to bregma, 1.4 mm lateral to bregma, 3.6 mm dorsal to the dura) provided by Paxinos and Watson (1998). After placement of the electrode, the assembly was secured to the skull using orthopedic cement, the wound was packed with antibiotic ointment, and the incision was sutured closed. Animals were given a minimum of seven days of postoperative recovery before experimental sessions began.
Recording Apparatus
During recordings animals foraged for food pellets inside a wooden cylindrical enclosure (51 cm tall; 76 cm in diameter). The enclosure was painted gray, except for an approximate 100° arc of the inner wall that was painted white to provide a visible landmark. The enclosure was surrounded by a black curtain extending from the ceiling to the floor to hide visible landmarks outside of the enclosure. A white noise generator masked potential auditory cues and four lights arranged symmetrically on the ceiling provided illumination. A pellet dispenser mounted on the ceiling dropped food pellets (bio-serve; Frenchtown, NJ) at random intervals to encourage foraging behavior.
A data acquisition system (Neuralynx; Bozeman, MT) was used to monitor and record electrical signals from the brain and also to track the head position of the animal by monitoring the position of red and blue LEDs attached to the headstage. The electrodes consisted of a bundle of 16 25-μm diameter insulated nichrome wires wrapped around the pins of a custom connector that was potted in acrylic. The recording headstage (Neuralynx HS-18; Bozeman, MT) was connected by a recording cable to a motorized commutator which relayed the signal to the data acquisition system located in an adjoining room. Spikes on individual wires were amplified (20-50K), bandpass filtered (600-6000 Hz), and stored for offline sorting based on spike shape using custom software.
Daily screening for HD cells involved plugging the headstage onto the animal, placing the animal in the recording enclosure, and examining the electrical signal for direction-dependent cellular activity indicative of an isolated HD cell. If no HD cell was present, the electrode was lowered by 25-50 μm and the animal was returned to its homecage. If a HD cell was found the recording apparatus was prepared for data collection.
Drug Exposures
During experimental sessions, animals were administered the noncompetitive NMDA antagonist dizocilpine (MK-801) or isotonic saline. The drug was obtained from Tocris Bioscience (Ellisville, MO), and dissolved in isotonic saline. All injections were administered intraperitoneally at a final injection volume of 2 ml/kg.
Experimental Procedure
Figure 1 illustrates the basic design of the experiment. Each HD cell was recorded over four 8-minute recording sessions, during which the animal foraged for food pellets inside the recording enclosure. At the start of each session, the floor paper was changed to eliminate olfactory cues and the rat was given disorientation treatment by slowly it in a cardboard box for 30-60 s before placing the animal in the recording enclosure. All landmark manipulations occurred out of the view of the animal while the animal was within the cardboard box. In the first session (Pre-injection/Standard), the cell was recorded with the enclosure oriented so that the white landmark on the enclosure wall was centered in the north quadrant. Immediately following this was the Pre-injection/Rotated session, in which the cell was recorded following a 90 deg rotation of the enclosure so that the landmark was centered in the West quadrant. Following these baseline sessions, the animal was immediately given disorientation treatment, moved to another room, and injected with isotonic saline (Group Saline), MK-801 at a dose of 0.05 mg/kg (Group Low MK-801), or MK-801 at a dose of 0.1 mg/kg (Group High MK-801). Following the injection, the animal was returned to its homecage for 30 minutes to allow for drug absorption. When the experiment resumed the cell was recorded with the landmark oriented in the North position (the Post-injection/Standard session) and then again with the landmark oriented in the West position (the Post-injection/Rotated session).
Figure 1.
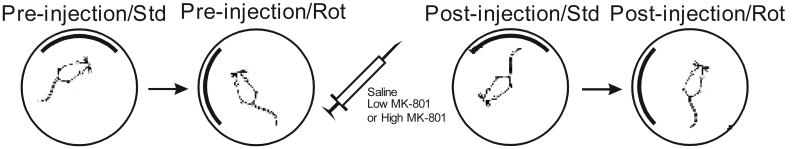
Overview of experimental procedure.
Analysis of Cellular Data
The directional specificity of recorded cells was qualitatively examined by constructing polar plots of firing rate (FR) by HD for each session. This was accomplished by dividing the 360° directional range into sixty 6° bins and then calculating the average firing rate of the recorded cell during the periods in which the head of the animal was oriented within each directional bin. In addition, cellular data from individual cells recorded in Pre-injection/Standard and Post-injection/Standard sessions were subjected to Rayleigh tests (Batschelet, 1981) to determine if spikes were significantly distributed in a non-random manner relative to HD, as would be expected if the cell was directionally tuned. The critical statistic of the Rayleigh test is the mean vector length, r, which varies between 0 and 1, with higher values indicating that the distribution of spike directions is clustered, i.e. distributed in a nonrandom fashion (Batschelet, 1981). In their raw form the spike/HD distributions are not suitable for this analysis as the test assumes that spikes have an equal chance of occurring in each directional bin and some directional bins will always be sampled more than others as the animal freely moves about during the recordings. To overcome this limitation, the number of spikes occurring in each directional bin was adjusted based on what would be expected if it had been sampled only as often as the fewest sampled bin (Calton and Taube, 2005). These adjusted spike/directional bin values were then subjected to Rayleigh tests using the CircStat MATLAB Toolbox for Circular Statistics (Berens, 2009).
The effects of drug injection were determined on the following basic directional characteristics of recorded cells: peak firing rate, background firing rate, signal-to-noise, and directional information content. The peak firing rate (FRpeak) was the average firing rate corresponding to the preferred direction (i.e. the directional bin with the highest average firing rate across the session). The background firing rate (FRbgnd) was the average firing rate of the three directional bins centered at 180° opposite the preferred direction. Signal to noise was calculated using the ratio: (FRpeak- FRbgnd) / FRpeak, producing scores that ranged between 0 and 1. This formula was utilized instead of the more traditional FRpeak/FRbgnd, because several cells had very low background rates and others had background rates of zero making the statistic fluctuate widely and impossible to calculate for some cells using the latter formula. Directional Information Content is a measure of how many bits of HD information is conveyed by each spike (Skaggs, McNaughton, Gothard, & Markus, 1993) and was calculated by the following formula: Σ pi (λi/λ) log2 (λi/λ), where pi is the probability that the head pointed in the ith directional bin, λi is the mean firing rate for bin i, and λ is the mean firing rate across all directional bins.
The stability of the HD cell preferred directions between sessions provided an index of whether the landmark cues in the recording environment effectively controlled the directional specificity of the recorded cells. This analysis was performed using the cross-correlation method described by Taube & Burton (1995). In brief, the FR by HD function of one session was shifted in 6° increments while correlating this shifted function with the non-shifted function from the second session. The amount of shift required to produce the maximal Pearson r correlation between the two sessions is defined as the preferred direction shift between the sessions. These scores were then subjected to Rayleigh tests (Batschelet, 1981) to determine if the scores were distributed randomly (as would be the case if the preferred directions were not controlled by landmarks within the apparatus) or if the preferred direction shifts tended to cluster in a predictable direction (as would be the case if the preferred directions were controlled by the apparatus landmarks). Also calculated using these preferred direction shifts was the mean vector angle, m, which estimates the mean angle of the sample (Batschelet, 1981). To determine if the distribution of preferred direction shifts was different across drug conditions, we utilized an F-test of the concentration parameter of each distribution (Batschelet, 1981). This inferential test examines if two distributions of angular scores differ in their concentration around the mean vector angle (Batschelet, 1981), an expected finding if NMDA blockade reduces landmark control of the HD signal.
Analysis of Movement Behavior
Two measures were used to assess changes in animal movement following drug administrations. First, to provide an overall activity index of the animal, the recording enclosure was divided into eight pie-slice shaped regions and the number of times each minute the animal's head crossed between regions was computed. Second, the average movement speed of the animal for each session was calculated by assigning each 30 Hz video/spike record a movement speed score based on the distance the head of the animal moved between the start and end of the 1-second time period centered on that record, and the average of these movement speed scores was then calculated for the entire session.
Histology
At the completion of the experiments, animals were anesthetized deeply and a small anodal current (20 μA for 20 sec) was passed through one electrode wire to conduct a Prussian blue reaction. The animals were then perfused transcardially with saline followed by 10% formalin in saline and the brains were removed and placed in 10% formalin for at least 48 hr. The brains were then placed in a 10% formalin solution containing 2% potassium ferrocyanide for 24 hr and then reimmersed in 10% formalin (24 hr) before being sectioned (40 μm) in the coronal plane, stained with cresyl violet, and examined microscopically for localization of the recording sites. All recording electrodes were localized to the ADN or having passed through ADN.
Results
A total of 31 HD cells were recorded from the 23 rats, with 8 cells recorded in the Saline condition, 11 cells recorded in the Low MK-801 condition and 12 cells recorded in the High MK-801 condition. In the case when an animal had more than one cell recorded in the study (5 animals), each cell was recorded in separate sessions with at least 24 hours between cell recordings. Table 1 presents the recording history of those animals from which more than one cell was recorded.
Table 1. History of animals with multiple cell recordings.
| Rat | Fig. 4 Code | Condition |
|---|---|---|
| BL3 | A | High MK-801, High MK-801, High MK-801, Saline |
| BL6 | B | High MK-801, Saline |
| BL7 | C | High MK-801, High MK-801 |
| GR13 | D | Low MK-801, Low MK-801, Low MK-801 |
| KS24 | E | High MK-801, High MK-801 |
Note: The remaining unlisted animals (n = 18) had only a single cell recorded.
Effects of MK-801 on Behavior
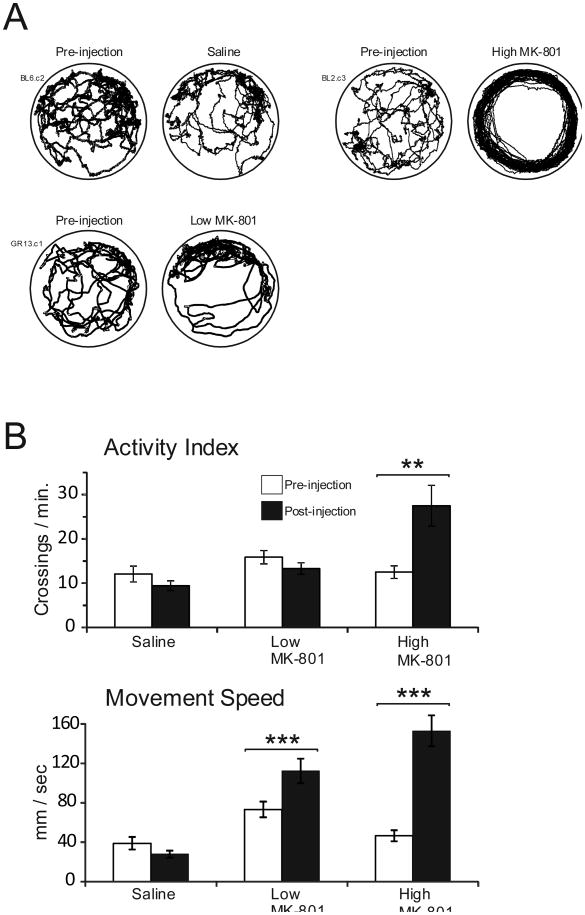
As other studies have commonly reported (e.g. Hill & Scorza, 2012; Koek, Woods, & Winger, 1988; Murata & Kawasaki, 1993) administration of MK-801 led to an alteration of the locomotion of the animals. In the case of the low dose, this effect appeared as a generalized increase in activity without obvious interruption of overt foraging behavior. Administration of the high dose of MK-801 produced a much more dramatic effect, with animals exhibiting hyperactive, stereotyped locomotion that typically involved continuous running along the inside perimeter of the recording cylinder. It is also notable that foraging behavior ceased following high dose MK-801 administration. Figure 2A presents example movement trajectories of the first two minutes of Pre-injection/Standard and Post-injection/Standard sessions in each of the three conditions, while Figure 2B displays the effect of injection on the overall activity index and on movement speed. Saline administration produced no significant effect on the activity index [t(7) = 0.501, p =.632] or on movement speed [t(7) = 1.10, p = .308]. Administration of the low dose of MK-801 marginally increased the activity index [t(10) = 2.21, p = .051] and significantly increased movement speed [t(10) = 5.25, p < .001]. Lastly, administration of the high dose of MK-801 significantly increased both the activity index [t(11) = 3.40, p =.006] as well as movement speed [t(11) = 7.72, p < .001].
Figure 2.
Drug effects on locomotor behavior. A. Movement trajectories of example animals for the first two minutes of sessions before and after injections. B. Mean boundary crossings and movement speed (+/- SEM) during the Pre-injection/Standard and Post-injection Standard sessions for each of the three conditions.
Drug Effects on Basic Directional Characteristics of HD Cells
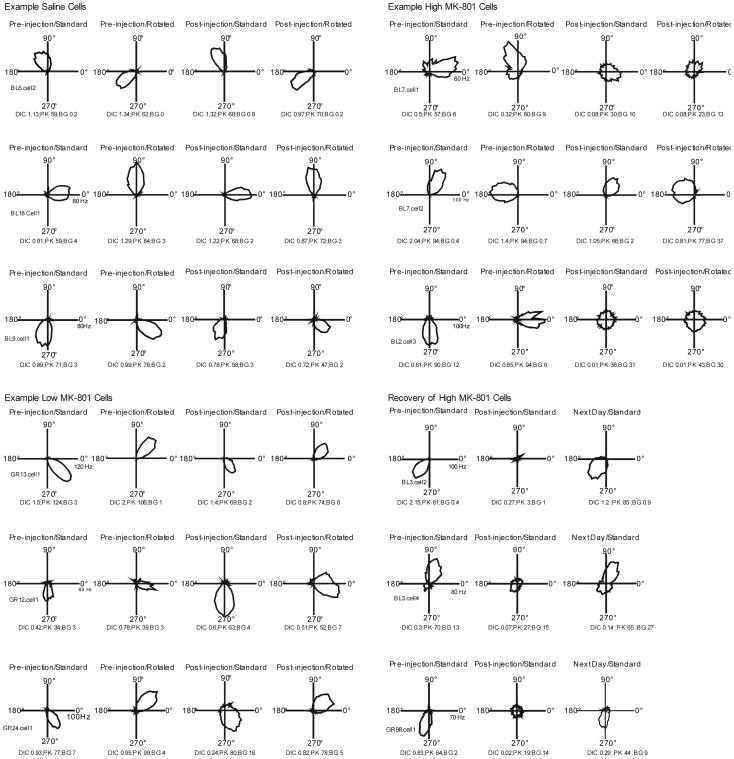
Figure 3 presents FR by HD tuning curves for three example cells in each condition. Prior to injection, all cells appeared to exhibit directional-specific activity characterized by an elevated firing rate over a narrow range of head directions, and this activity was controlled by the location of the landmarks in the recording enclosure as indicated by the corresponding change in preferred direction when the position of the landmark was shifted between Pre-injection/Standard and Pre-injection/Rotated conditions. Following injections, directional specificity was maintained for cells recorded in the Saline condition and was relatively unchanged for most cells following the low dose administration of the NMDA antagonist. In contrast, the directional-specific activity of cells was dramatically affected by administration of the high dose of MK-801. In these cells, drug administration led to dramatic decreases in peak FR accompanied by increases in background FR, to the extent that the FR by HD tuning curves exhibited little evidence of directional-specific activity. Surprisingly, despite this noticeable degradation of directional modulation after high dose drug administration, Rayleigh analyses indicated that all cells (including those recorded in high dose drug conditions) remained directionally tuned following drug administration, although the index of directionality, mean vector length, was significantly lower for high dose cells (described below).
Figure 3.
FR by HD tuning curves in the form of polar plots for three example cells from each condition. The bottom right panel shows the typical recovery of drug induced attenuation of directional specificity observed during recordings performed the next day. Cells were selected for display based on the amount of Post-Injection attenuation of the DIC measure shown in the session, with the first cell in each condition showing the typical DIC attenuation of that condition, the second cell showing the magnitude of attenuation typical of the lowest quartile of cells, and the third cell showing an effect typical of the largest quartile in the condition. The values shown at the bottom of each panel indicate DIC, Peak FR, and Background FR for that session.
Table 2 presents the means (± SEMs) on the basic measures of directional specificity calculated from the Pre-injection/Standard and Post-injection/Standard sessions of each condition. As expected, in the case of saline cells these measures did not change markedly following the injection, and while those in the Low MK-801 condition appeared to trend towards a weak attenuation of directional specificity, this effect was not significant on any of the measures. In contrast, cells recorded from animals in the High MK-801 condition showed significantly degraded responses in nearly all of the directional measures. Specifically, cells in the High MK-801 condition showed significant decreases in Peak FR, Signal-to-Noise, Directional Information Content, and Mean Vector Length. In five instances, High MK-801 cells were recorded the next day to determine if they had recovered from the attenuation of directional activity observed after drug administration (bottom right panel of Fig. 3). In each of these cases, the directional activity appeared to recover on the following day.
Table 2. Effect of Injection on Basic Directional Characteristics of HD Cells.
| Saline | Pre-injection | Post-injection | Pre vs. Post |
| Peak FR | 57.5 (± 4.4) | 54.3 (± 4.4) | t(7) = 0.93, p = .386 |
| Background FR | 3.7 (± .77) | 4.5 (± .93) | t(7) = 1.11, p = .302 |
| Signal-to-Noise | 0.927 (± .02) | 0.909 (±.023) | t(7) = 1.23, p = .259 |
| Directional IC | 0.675 (± .09) | 0.735 (± .42) | t(7) = 0.86, p = .417 |
| Mean Vector Length | 0.604 (± .05) | 0.594 (± .061) | t(7) = 0.29, p = .776 |
| Low MK-801 | Pre-injection | Post-injection | Pre vs. Post |
| Peak FR | 87.9 (± 16.0) | 81.1 (± 11.0) | t(10) = 0.84, p = .421 |
| Background FR | 4.7 (± 1.1) | 11.0 (± 3.6) | t(10) = 1.81, p = .100 |
| Signal-to-Noise | 0.921 (± .02) | 0.858 (± .04) | t(10) = 1.69, p = .122 |
| Directional IC | 0.935 (± .21) | 0.768 (± .22) | t(10) = 1.49, p = .167 |
| Mean Vector Length | 0.632 (± .06) | 0.557 (± .07) | t(10) = 1.34, p = .209 |
| High MK-801 | Pre-injection | Post-injection | Pre vs. Post |
| Peak FR | 77.9 (± 4.3) | 37.7 (± 9.4) | t(11) = 4.54, p < .001 |
| Background FR | 4.6 (± 1.3) | 19.7 (± 7.5) | t(11) = 2.01, p = .069 |
| Signal-to-Noise | 0.937 (± .02) | 0.501 (± .07) | t(11) = 6.50, p < .001 |
| Directional IC | 1.10 (± .18) | 0.147 (± .09) | t(11) = 6.45, p < .001 |
| Mean Vector Length | 0.664 (± .05) | 0.172 (± .05) | t(11) = 7.97, p < .001 |
Note: Values are means (± 1 SEM). Pre vs Post comparisons via a paired two-tailed t-test.
Drug Effects on Landmark Control and Stability of the HD Signal
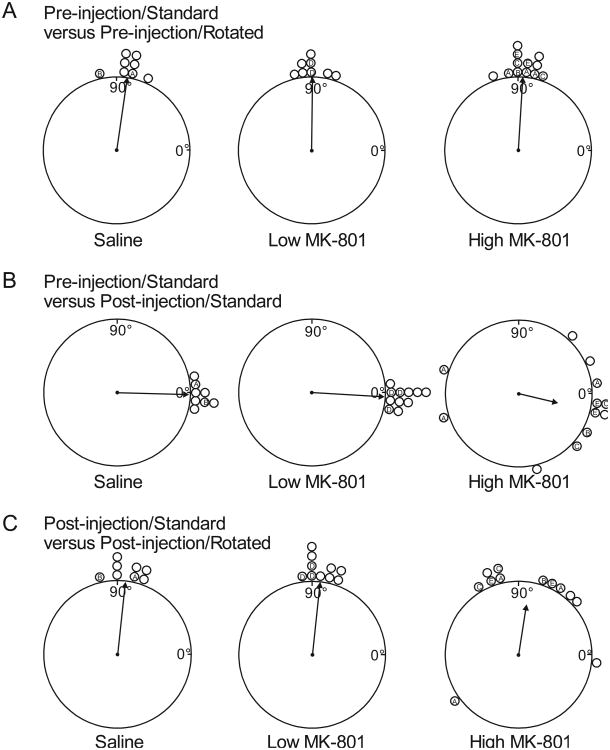
The ability of landmark cues to control the preferred direction of HD cells following NMDA blockade was examined by calculating the directional shift in the FR by HD tuning curves between sessions. Figure 4 presents polar plots of the directional shifts between sessions. As is typical of HD cells, prior to drug administration, cells in all conditions were controlled by the visual landmarks in the recording enclosure. This is indicated by the finding that shifts between Pre-injection/Standard and Pre-injection/Rotated sessions were centered on 90° for all three conditions, the same direction and distance in which the landmarks were rotated between those sessions.
Figure 4.
Scatter diagrams showing the amount of angular shift of FR by HD functions between sessions for each recorded cell. A. Amount of angular shift observed in Pre-injection/Rotated sessions relative to Pre-injection/Standard sessions. B. Amount of angular shift observed in Post-injection/Standard sessions relative to Pre-injection/Standard sessions. C. Amount of angular shift observed in Post-injection/Rotated sessions relative to Post-injection/Standard sessions. The arrow on each plot denotes the observed mean vector angle (m) of the shifts of each condition. The length of the arrow denotes the mean vector length (r), with a length of 1.0 (no variability in shift scores) represented by a vector spanning the radius of the plot. The letters inside shift indicators denote cells recorded from the same animals. Angular shift data from three Pre-injection/Rotated Low MK-801 cells and one Post-injection/Rotated Low MK-801 cell was not available due to equipment problems during data collection.
The effect of drug administration on landmark control can be assessed by examining the directional shifts between Pre-injection/Standard and Post-injection/Standard sessions (Figure 4B). Because in these session comparisons the apparatus landmarks were at the same positions between the two sessions, the directional shifts between the sessions would be clustered around the zero position if the landmarks in the apparatus continued to effectively control directional-specific activity following injections. As would be expected, shifts were clustered around zero in the case of the Saline condition, and this tendency continued for the Low MK-801 condition. In contrast, while the shifts for High MK-801 condition still seemed clustered around zero, the distribution of shifts appeared more variable. A Rayleigh analysis of the directional shifts within each condition showed that for all three conditions the distribution of shifts were nonrandom [mean vector lengths (r) = 0.99, 1.0, and 0.55; mean vector angles (m) = -1.5°,-3.3°, and -13.3° for Saline, LowMK-801, and High MK-801, respectively; ps < .05]. Concentration tests performed between Saline and each drug condition to determine if drug administration led to a change in the amount of clustering around the central angle showed no differences between Saline and LowMK-801 [F(7,10) = 1.87, p = .33] but significant decreases in concentration relative to the Saline condition for High MK-801 [F(11,7) = 54.5, p < .001] condition.
Figure 4C presents polar plots of directional shifts between the Post-injection/Standard and Post-injection/Rotated sessions for all three conditions. In this comparison, because the landmarks in the apparatus were rotated by 90° between the two sessions we would expect cells to show directional shifts of 90° if the HD cell remained tuned to the landmarks following landmark rotation. As in the previous comparison, directional control by the landmarks in the apparatus appeared normal in saline and Low MK-801 conditions and moderately diminished in the High MK-801 condition. While Rayleigh tests indicated that the distribution of directional shifts was nonrandom within each of the three conditions (rs = .99, .99, and .69; ms = 84.0°, 84.0°, and 81.1° for Saline, Low MK-801, and High MK-801, respectively), significant concentration decreases were found for High MK-801 [F(11,7) = 19.8, p < .001] relative to saline. Altogether, these measures support the conclusion that despite the large degradation of basic directional characteristics following high dose NMDA blockade, what remained of the directional specificity of these cells typically continued to be controlled by the landmarks in the apparatus.
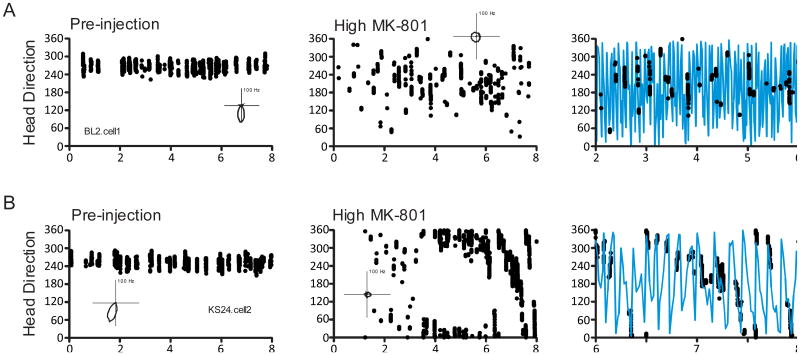
Observed Instances of Directional Instability
As described above, while in many cases the higher dose of MK-801 produced a large degradation of basic directional characteristics (i.e. decreases in peak firing rate accompanied by increases in background rate) leading to the overall effect of the directional signal being “noisier”, this attenuated directional specificity often appeared somewhat stable and controlled by the landmarks in the recording enclosure (e.g. the predictable shifts between sessions illustrated in Figure 4). In a few instances, however, cells from the High MK-801 condition appeared to remain directionally sensitive but exhibited unstable preferred directions, i.e. preferred directions that shifted from one moment to the next, within the recording session. This effect can be illustrated by calculating the “instantaneous preferred direction” of the cell across the duration of the recording session (see the Figure 5 caption). Using this procedure, the majority of cells showed degraded but stable directional tuning following administration of high dose NMDA antagonist. An example of this pattern is shown by the plots of Figure 5A, where in the Pre-injection session the instantaneous preferred directions were localized to a narrow range of head directions, and following drug administration these periods of high activity were concentrated in a wider directional range with an increase in extraneous spikes, but the overall directional range was still relatively consistent over the course of the recorded session. In contrast, the panels of Figure 5B were produced from a cell that seemed to show directional dependency but an unstable preferred direction following administration of the high dose of MK-801. The activity of this cell following drug administration seemed constrained to isolated bursts that show a gradual shift in head direction within the session. When the time scale is expanded to better reveal the burst activity and head direction traces are added (third panel of Figure 5B) it becomes apparent that the burst activity is gradually shifting in a counterclockwise direction as the animal moved about the enclosure. This evidence of unstable preferred directions following drug administration was found in three High MK-801 cells (27%). None of the saline or Low MK-801 cells exhibited this pattern of activity. This effect was somewhat but not entirely animal specific, as one rat (KS24) showed the effect in both of its two recorded cells, but the other cell was recorded from a rat (BL3) that also had several cells that did not show the effect. It is also notable that this latter cell (BL3.cell2) is responsible for producing each of the largest outliers in the preferred direction shifts shown in Figures 4B and 4C. To assess whether these unstable cells were largely responsible for the observed degraded basic directional characteristics of the High MK-801 condition, we reanalyzed the basic tuning characteristics after omitting these three cells, and the tuning characteristics remained significantly degraded (all ps < .05), supporting the notion that this is an additional effect beyond that observed in the majority of cells and is not statistically responsible for the overall impairment of the HD system in the high dose condition.
Figure 5.
HD by Time scatterplots of instantaneous preferred directions. Instantaneous preferred directions (scatter points) were defined as the HDs during which the smoothed firing rate of the cell reached at least three standard deviations above the mean firing rate of the session. A. Scatterplots from a cell that showed attenuated directional specificity but did not show unstable preferred directions within the High MK-801 drug session. B. Scatterplots from a cell that showed a slow drift in preferred direction during the High MK-801 session. The third panel in each row shows data on an expanded time scale with HD (blue traces) added. Panel insets show polar plots of FR by HD functions of each cell for the session.
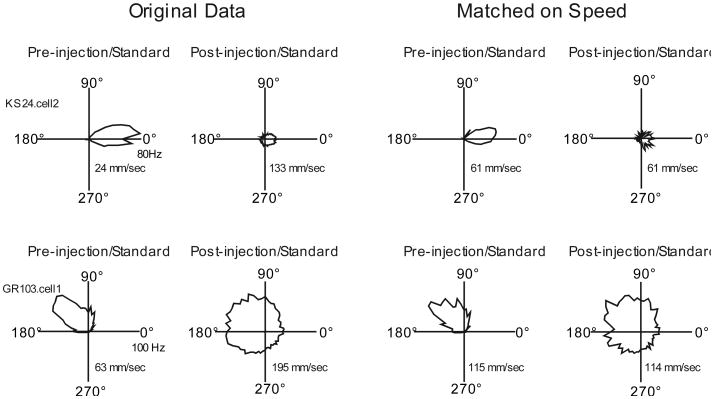
Relationship of movement speed with impaired directional signal
As mentioned previously, the animals in the High MK-801 condition became quite hyperactive following drug administration, exhibiting dramatic increases in the overall activity index as well as average running speed. This behavioral change may be important because the instantaneous movement characteristics of the animal, including angular head velocity, and movement speed have been shown to affect the activity of HD cells (Blair & Sharp, 1995; Taube, 1995). To assess whether this movement effect might account for the impaired directional signal following high dose drug administration, the previously described analysis of HD cell basic directional characteristics was repeated in High MK-801 cells after matching pre- and post-injection cellular data on movement speed. To accomplish this, the video/spike records of each session was rank ordered on movement speed from slowest to fastest and then the slowest pre-injection samples and fastest post-injection samples were eliminated in equal numbers until the average movement speed was equivalent between the two sessions. Figure 6 shows tuning curves from several example cells before and after this data transformation, illustrating that there appeared to be only modest effects of this transformation on the overall directional specific activity of recorded cells. Following this matching on movement speed, High MK-801 cells continued to show drug-induced degradation of most directional measures including DIC [Pre-injection mean = 1.15 ± .18 bits, Post-injection mean = 0.27 ± .13; t(10) = 7.76 bits, p < .001] Peak FR [Pre-injection mean = 88.5 ± 6.4 Hz, Post-injection mean = 45.4 ± 10.5 Hz; t(10) = 4.09, p = .002], Signal to Noise [Pre-injection mean = 0.94 ±.02, Post-injection mean = 0.60 ± .06; t(10) = 5.65, p < .001], and mean vector length [Pre-injection mean = 0.71 ± .06, Post-injection mean = 0.20 ± .05; t(10) = 10.1, p < .001], despite the fact that the procedure eliminated the differences in running speed [Pre-injection mean = 92.5 ± 11.9 mm/sec, Post-injection mean = 92.5 ± 11.8 mm/sec; t(11) = 0.2, p > .05], as well as crossings [Pre-injection mean = 16.1 ± 4.2 crossings/min, Post-injection mean = 11.3 ± 2.1 crossings/min; t(10) = 1.20, p > .05]. In summary, this additional analysis provides evidence that our observed impairment of the HD system following high dose drug administration was not simply an artifact of the drug-induced movement alteration.
Figure 6.
Example HD by FR tuning curves from High MK-801 sessions before and after matching Pre- and Post- injection sessions on average movement speed. Inset shows average movement speed for that session.
Discussion
The present study is the first published finding of the effects of NMDA blockade on the HD system. We found that systemic application of the noncompetitive NMDA antagonist MK-801 produced dose-dependent effects on most directional characteristics of anterior thalamic HD cells. The lower dose of MK-801 produced no significant effects on the basic directional-specific activity of these cells and also did not affect the stability of the directional signal relative to the landmarks in the recording enclosure. One potential limitation of this lack of drug effect observed in the low dose condition is the relatively small sample size. It is possible that with a larger sample the relative modest effects observed in the low dose condition would have been significant.
In contrast, the higher dose affected nearly all aspects of the directional signal, including peak firing rates, signal to noise ratio, directional information content, and mean vector length. Despite the dramatic weakening of the directional signal following high dose NMDA blockade, the attenuated directional signal of HD cells was still somewhat controlled by the landmarks in the apparatus in most cells. In a minority of cells, however, high dose NMDA blockade seemed to lead to unstable directional specific activity with preferred directions gradually drifting from one preferred direction to another within recording sessions. The qualitative difference between this latter effect and the others is considerable, as the other effects of NMDA antagonism could be interpreted as the directional signal simply becoming “noisier” thereby affecting the overall strength of the signal but not necessarily the stability of the spatial representation imbedded within the signal. The finding that NMDA blockade leads to unstable directional signals in the HD system would likely be a much more disruptive effect on any behavioral processes supported by this system.
Motor Disruption as a Potential Mechanism for the Observed Effects
It is a common observation that animals receiving systemic administration of MK-801 exhibit abnormal locomotor behavior characterized by hyperactivity, ataxia, and behavioral disorganization (e.g., Hill & Scorza, 2012; Koek, et al., 1988; Murata & Kawasaki, 1993). In fact, the motor effects of MK-801 are so disruptive at the higher ends of the dosing spectrum that some researchers have concluded that it is not possible to dissociate the potential cognitive effects of this drug from the motor disturbances in the case of certain tasks such as the Morris Water Maze (e.g., Ahlander, Misane, Schott, & Ogren, 1999). While it is clear that gross motor impairment and behavioral disorganization can interfere with the physical performance of a spatial task such as a water maze, it is less obvious that this behavioral disruption in-of-itself would necessarily impact the directional signal carried by the HD system, especially considering the relatively simple foraging task utilized in the present study. On the other hand, some studies have found evidence that the movement state of the animal can affect the HD system. In their efforts to assess the relative contributions between vestibular, motor, and proprioceptive cues in the generation and maintenance of the HD signal, a number of researchers have performed recordings during restraint and/or passive rotation of the animal through different head directions. The commonly reported effects of these treatments are decreases in the preferred firing rate by 20-50%, without changes in the preferred directions of recorded cells (Bassett, Zugaro, Muir, Golob, Muller, & Taube, 2005; Taube, 1995; Zugaro, Tabuchi, Fouquier, Berthoz, & Wiener, 2001).
While we do not completely discount the effects of locomotor impairment on our findings, we think it unlikely that this effect can account for a majority of our effects for several reasons. First, the studies described above relating the HD signal to the movement state of the animal found these attenuating effects using restraint and/or slow passive rotation of the animal during recordings. In addition to the potential inhibition of proprioceptive, motor, and efferent copy signals produced by restraint, slow passive movements of the animal will reduce the overall sampling of head directions, likely leading to a distorted tuning function given that the function is calculated from the average firing rate at each different head direction. In contrast, our high dose drug effects were accompanied by behavioral hyperactivity and, if anything, our animals overly sampled the full directional range compared to controls. In addition, our observed attenuation of the HD signal (a reduction in peak FR of greater than 50% and a reduction in DIC of greater than 80%) appeared larger than those found in restraint/passive movement studies, and our occasional finding of preferred directions shifting within sessions was never reported by these other studies. Also, the disruptive effects of restraint/passive rotation on the HD system are not a universal finding, as a more recent study by Shinder and Taube (2011) utilizing a surgically implanted device for securing the animal's head to a turntable and additional restraints to produce more complete immobilization than any previous study found very little effects of this treatment on the directional signal of HD cells. Finally, even after matching pre-injection and high dose behavioral epochs on running speed we continued to find a dramatic attenuation of the directional signal following administration of the high dose, providing evidence that the attenuation was not simply an artifact of the enhanced movement speed during drugged sessions. In sum, while it is true that our effects of NMDA blockade on the HD system cannot be completely dissociated from the locomotor effects (a problem shared by many other published studies of MK-801 on spatial cognition) several reasons suggest that our effects cannot be largely explained by movement disruption.
Present Findings Relative to Reports of MK-801 Induced Spatial Impairment
Over the past several decades a number of studies have found spatial deficits in rats following administration of MK-801. Table 3 provides an overview of many of these studies. Given these deficits, and the common view that the HD cell network contributes to the neural circuitry used for spatial navigation, the next logical question is whether our findings have identified a potential mechanism by which MK-801 causes spatial deficits. In support of this possibility, as presented in Table 3 many behavioral studies report spatial deficits following administration of MK801 at doses equal or greater than the dose at which our effects on the HD system were apparent (e.g., Butelman, 1989; Ward et al., 1990; Uekita & Okaichi, 2005). Even more convincing, some studies testing multiple doses of MK-801 have found spatial deficits at our high dose 0.1 mg/kg but no or reduced effects at our ineffective dose of .05 mg/kg (Filliat & Blanchet, 1995; Van der Staay et al., 2011;Whishaw & Auer, 1989). On the other hand, a few studies do report spatial impairment at our ineffective dose of .05 mg/kg (Robinson et al., 1989; Whishaw and Auer, 1989), suggesting that an attenuated HD system was not necessary to produce spatial impairment in these studies.
Table 3. Summary of Pharmacological effects of MK-801 on Reference Memory.
| Reference | Task | Strain | Sex and Weight | Route and Dose (mg/kg) | Significant Effects |
|---|---|---|---|---|---|
| Ahlander et al (1999) | MWM | Sprague Dawley | M (300-350 g) | s.c. 0.01, 0.03, 0.05, 0.1 | ↑Escape latency and ↓ number of crossings over platform (.05 and .1) |
| Filliat & Blanchet (1995) | MWM | Wistar | M (200-220 g) | i.p. 0.05, 0.1, 0.2 | ↑Escape latency (0.2); No quadrant bias during probe trials (0.1 and 0.2) |
| Ramirez-Amaya et al. (2001) | MWM | Wistar | M (250-300 g) | i.p. 0.05 | ↑ Escape latency and ↓ crossings |
| Robinson, Crooks, Shinkman, & Gallagher (1989) | MWM | Long-Evans | M (300-350 g) | s.c. 0.01, 0.05, 0.08 | ↑ Escape latency (0.05 and 0.08) |
| Stuchlik, Rezacova, Vales, Bubenikova, & Kubik (2004) | MWM | Long-Evans | M (250-300 g) | i.p. 0.1, 0.2 | ↑Escape latency (low and high); low dose showed improvement over time |
| Uekita & Okaichi (2005) | MWM | Albino Wistar | M (2 months old) | i.p. 0.1 | ↑ Escape latency |
| Van der Staay, Rutten, Erb, & Blokland (2011) | MWM | Wistar | M (200-250 g) | s.c. .05, .07, .10 | Escape latency and swim distance ↑linearly with dose. All conditions showed learning on probe trial except highest dose. |
| Whishaw & Auer (1989) | MWM | Long-Evans | F (200-250 g) | i.v., 0.05, 0.1 | ↑Errors and escape latency, larger effect at larger dose. |
| Pitkanen, Sirvio, MacDonald, Niemi, Ekonsalo, & Riekkinen (1995) | MWM & RAM | Wistar | M (250-300 g) | i.p. 0.1 | ↑ Escape latency (water maze); ↑ task completion time and number of errors in week three test sessions (radial arm maze) |
| Butelman (1989) | RAM | Lister Hooded | M (250-350 g) | i.p. 0.1, 0.2 | ↓Efficiency values |
| Caramanos & Shapiro (1994) | RAM | Sprague-Dawley | F (250-500 g) | i.p. 0.0625 | ↑Reference memory errors and did not show improvement with training |
| Manahan-Vaughan, vonHaebler, Winter, Juckel, & Heinemann (2008) | RAM | Wistar | M (260-280 g) | i.p. 0.5 | ↑Reference memory errors at day 8-10 through study duration |
| Shapiro & Caramanos (1990) | RAM | Sprague-Dawley | M (230 – 270 g) | i.p. 0.0625 | ↑ Reference memory errors |
| Shapiro & O'Connor (1992) | RAM | Sprague-Dawley | F (230-270 g) | i.p. 0.0625 | ↑ Reference memory errors |
| Ward, Mason & Abraham (1990) | RAM | Sprague-Dawley | M (220-300 g) | i.p. 0.1, 0.5 | ↑ Reference memory errors; larger effect at higher dose |
Notes. MWM = Morris Water Maze; RAM = Radial Arm Maze
One possible explanation for these occasional findings of impaired spatial behavior in the seemingly absence of HD system impairment is that there is a qualitative difference in the nature of the HD system impairment by low and high doses of NMDA antagonists. Whereas higher doses of the drug produce overt degradation of the basic directional signal carried by the HD system as shown in our study, lower doses may leave the directional signal intact but instead impair the consolidation of directional information necessary to learn a spatial relationship in a navigational task. This possibility is supported by findings that while higher doses of NMDA antagonists may impair both acquisition and performance of spatial tasks, lower doses may impair acquisition but spare previously acquired performance (Robinson et al., 1989; Shapiro & Caramanos, 1990; Whishaw & Auer, 1989). In addition, this pattern of disruption is consistent with the findings of Kentros, Hargreaves, Hawkins, Kandel, Shapiro, & Muller (1998) that NMDA blockade led to place cells showing impairment in the experience-dependent consolidation of a new environment without greatly altering the basic spatial characteristics of the place cells in a familiar environment. As in the case of place cells, the directional signal carried by the HD system also shows consolidation in new environments (Dudchenko and Zinyuk, 2005) and so it would be interesting to determine if this plasticity can be altered by NMDA antagonists. The finding that spatial learning is most impaired by NMDA blockade when the training occurs in an unfamiliar environment (Caramanos & Shapiro, 1994; Uekita & Okaichi, 2005) is consistent with the possibility that the acquisition of a spatial task is dependent on the consolidation of the environment into the neural circuitry supporting spatial behavior.
Highlights.
Systemic NMDA blockade disrupted the HD signal in a dose dependent manner.
MK-801 at 0.1 mg/kg greatly weakened HD cell directional-specific activity.
This may provide a mechanism by which MK-801 interferes with spatial learning.
Acknowledgments
This research was supported by NIH grant 1R15NS071470-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Hunt PR, Nagle S, Neave N. The effects of selective lesions within the anterior thalamic nuclei on spatial memory in the rat. Behavioral Brain Research. 1996;81:189–198. doi: 10.1016/s0166-4328(96)89080-2. [DOI] [PubMed] [Google Scholar]
- Ahlander M, Misane I, Schott PA, Ogren SO. A behavioral analysis of the spatial learning deficit induced by the NMDA receptor antagonist MK-801 (Dizocilpine) in the rat. Neuropsychopharmacology. 1999;21:414–426. doi: 10.1016/S0893-133X(98)00116-X. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Niewoehner B, Lyon L, Romberg C, Schmitt WB, Taylor A, Sanderson DJ, Cottam J, Sprengel R, Seeburg PH, Kohr G, Rawlins JNP. NMDA receptor subunit NR2A is required for rapidly acquired spatial working memory but not incremental spatial reference memory. Journal of Neuroscience. 2008;28:3623–3630. doi: 10.1523/JNEUROSCI.3639-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett JP, Zugaro MB, Muir GM, Golob EJ, Muller RU, Taube JS. Passive movements of the head do not abolish anticipatory firing properties of head direction cells. Journal of Neurophysiology. 2005;93:1304–1316. doi: 10.1152/jn.00490.2004. [DOI] [PubMed] [Google Scholar]
- Batschelet E. Circular Statistics in Biology. London: Academic; 1981. [Google Scholar]
- Berens P. CircStat: A MATLAB toolbox for circular statistics. Journal of Statistical Software. 2009;31 Retrieved from: http://www.jstatsoft.org/v31/i10. [Google Scholar]
- Blair HT, Sharp PE. Anticipatory head direction signals in anterior thalamus: Evidence for a thalamocortical circuit that integrates angular head motion to compute head direction. Journal of Neuroscience. 1995;15:6260–6270. doi: 10.1523/JNEUROSCI.15-09-06260.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Butelman ER. A novel NMDA antagonist, MK-801, impairs performance in a hippocampal-dependent spatial learning task. Pharmacology, Biochemistry, and Behavior. 1989;34:13–16. doi: 10.1016/0091-3057(89)90345-6. [DOI] [PubMed] [Google Scholar]
- Cain DP, Saucier D, Hall J, Hargreaves EL, Boon F. Detailed behavioral analysis of water maze acquisition under APV or CNQX: Contribution of sensorimotor disturbances to drug induced acquisition deficits. Behavioral Neuroscience. 1996;110:86–102. doi: 10.1037//0735-7044.110.1.86. [DOI] [PubMed] [Google Scholar]
- Calton JL, Stackman RW, Goodridge JP, Archey WB, Dudchenko PA, Taube JS. Hippocampal place cell instability after lesions of the head direction cell network. Journal of Neuroscience. 2003;23:9719–9731. doi: 10.1523/JNEUROSCI.23-30-09719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calton JL, Taube JS. Degradation of head direction cell activity during inverted locomotion. Journal of Neuroscience. 2005;25:2420–2428. doi: 10.1523/JNEUROSCI.3511-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramanos Z, Shapiro ML. Spatial memory and N-Methyl-D-Aspartate receptor antagonists APV and MK-801: Memory impairments depend on familiarity with the environment, drug dose, and training duration. Behavioral Neuroscience. 1994;108:30–43. doi: 10.1037//0735-7044.108.1.30. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Rice JP, Akers KG, Candelaria-Cook FT, Taube JS, Hamilton DA. Lesions of the dorsal tegmental nuclei disrupt control of navigation by distal landmarks in cued, directional, and place variants of the Morris water task. Behavioral Neuroscience. 2013;127:566–581. doi: 10.1037/a0033087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Butcher SP, Morris RGM. The NMDA receptor antagonist D-2-amino-5-phosphonopentanoate (D-AP5) impairs spatial learning and LTP in vivo at intracerebral concentrations comparable to those that block LTP in vitro. Journal of Neuroscience. 1992;12:21–34. doi: 10.1523/JNEUROSCI.12-01-00021.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdikman D, Moser EI. A manifold of spatial maps in the brain. Trends in Cognitive Sciences. 2010;14:561–569. doi: 10.1016/j.tics.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Zinyuk LE. The formation of cognitive maps of adjacent environments: Evidence from the head direction system. Behavioral Neuroscience. 2005;119:1511–1523. doi: 10.1037/0735-7044.119.6.1511. [DOI] [PubMed] [Google Scholar]
- Filliat P, Blanchet G. Effects of TCP on spatial memory: Comparison with MK-801. Pharmacology, Biochemistry and Behavior. 1995;51:429–432. doi: 10.1016/0091-3057(95)00002-e. [DOI] [PubMed] [Google Scholar]
- Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. Spatial representation in the entorhinal cortex. Science. 2004;305:1258–1264. doi: 10.1126/science.1099901. [DOI] [PubMed] [Google Scholar]
- Hill XL, Scorza MC. Role of the anterior thalamic nucleus in the motor hyperactivity induced by systemic MK-801 administration in rats. Neuropharmacology. 2012;62:2440–2446. doi: 10.1016/j.neuropharm.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behavioral and Neural Biology. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- Keith JR, Rudy JW. Why NMDA receptor-dependent long-term potentiation may not be a mechanism of learning and memory: reappraisal of the NMDA receptor blockade strategy. Psychobiology. 1990;18:251–257. [Google Scholar]
- Kentros C, Hargreaves E, Hawkins RD, Kandel ER, Shapiro M, Muller RV. Abolition of long-term stability of new hippocampal place cell maps by NMDA receptor blockade. Science. 1998;280:2121–2126. doi: 10.1126/science.280.5372.2121. [DOI] [PubMed] [Google Scholar]
- Koek W, Woods JH, Winger GD. MK-801, a proposed noncompetitive antagonist of excitatory amino acid neurotransmission, produces phencyclidine-like behavioral effects in pigeons, rats, and rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 1988;245:969–974. [PubMed] [Google Scholar]
- Manahan-Vaughan D, von Haebler D, Winter C, Juckel G, Heinemann U. A single application of MK801 causes symptoms of acute psychosis, deficits in spatial memory, and impairment of synaptic plasticity in rats. Hippocampus. 2008;18:125–134. doi: 10.1002/hipo.20367. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Gerrard JL, Gothard K, Jung MW, Knierim JJ, Kudrimoti H, Qin Y, Skaggs WE, Suster M, Weaver KL. Deciphering the hippocampal polyglot: The hippocampus as a path integration system. Journal of Experimental Biology. 1996;199:173–185. doi: 10.1242/jeb.199.1.173. [DOI] [PubMed] [Google Scholar]
- Moser EI, Moser MB. A metric for space. Hippocampus. 2008;18:1142–1156. doi: 10.1002/hipo.20483. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Steele RJ, Bell JE, Martin SJ. N-methyl-D-aspartate receptors, learning and memory: chronic intraventricular infusion of the NMDA receptor antagonist D-AP5 interacts directly with the neural mechanisms of spatial learning. European Journal of Neuroscience. 2013;37:700–717. doi: 10.1111/ejn.12086. [DOI] [PubMed] [Google Scholar]
- Murata S, Kawasaki K. Common and uncommon behavioural effects of antagonists for different modulatory sites in the NMDA receptor/channel complex. European Journal of Pharmacology. 1993;239:9–15. doi: 10.1016/0014-2999(93)90969-o. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map: preliminary evidence from unit activity in the freely-moving rat. Brain Research. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford, UK: Clarendon; 1978. [Google Scholar]
- Olton DS, Walker JA, Gage FH. Hippocampal connections and spatial discrimination. Brain Research. 1978;139:295–308. doi: 10.1016/0006-8993(78)90930-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Pitkänen M, Sirviö J, MacDonald E, Niemi S, Ekonsalo T, Riekkinen P., Sr The effects of D-cycloserine and MK-801 on the performance of rats in two spatial learning and memory tasks. European Neuropsychopharmacology. 1995;5:457–463. [PubMed] [Google Scholar]
- Place R, Lykken C, Beer Z, Suh J, McHugh TJ, Tonegawa S, Eichenbaum H, Sauvage MM. NMDA signaling in CA1 mediates selectively the spatial component of episodic memory. Learning and Memory. 2012;19:164–169. doi: 10.1101/lm.025254.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Amaya V, Balderas I, Sandoval J, Escobar ML, Bermúdez-Rattoni F. Spatial long-term memory is related to mossy fiber synaptogenesis. The Journal of Neuroscience. 2001;21(18):7340–7348. doi: 10.1523/JNEUROSCI.21-18-07340.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GS, Crooks GB, Shinkman PG, Gallagher M. Behavioral effects of MK-801 mimic deficits associated with hippocampal damage. Psychobiology. 1989;17:156–164. [Google Scholar]
- Shapiro ML, Caramanos Z. NMDA antagonist MK-801 impairs acquisition but not performance of spatial working and reference memory. Psychobiology. 1990;18:231–243. [Google Scholar]
- Shapiro ML, O'Connor C. N-Methyl-D-Aspartate receptor antagonist MK-801 and spatial memory representation: Working memory is impaired in an unfamiliar environment but not in a familiar environment. Behavioral Neuroscience. 1992;106:604–612. doi: 10.1037//0735-7044.106.4.604. [DOI] [PubMed] [Google Scholar]
- Shapiro ML, Caramanos Z. NMDA antagonist MK-801 impairs acquisition but not performance of spatial working and reference memory. Psychobiology. 1990;18:231–243. [Google Scholar]
- Sharp PE, Blair HT, Cho J. The anatomical and computational basis of the rat head-direction cell signal. Trends in Neurosciences. 2001;24:289–294. doi: 10.1016/s0166-2236(00)01797-5. [DOI] [PubMed] [Google Scholar]
- Shinder ME, Taube JS. Active and passive movement are encoded equally by head direction cells in the anterodorsal thalamus. Journal of Neurophysiology. 2011;106:788–800. doi: 10.1152/jn.01098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solstad T, Boccara CN, Kropff E, Moser MB, Moser EI. Representation of geometric borders in the entorhinal cortex. Science. 2008;322:1865–1868. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- Steele RJ, Morris RGM. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion f the NMDA-Antagonist D-AP5. Hippocampus. 1999;9:118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Stuchlik A, Vales K. Systemic administration of MK-801, a non-competitive NMDA-receptor antagonist, elicits a behavioral deficit of rats in the Active Allothetic Place Avoidance (AAPA) task irrespectively of their intact spatial pretraining. Behavioral Brain Research. 2005;159:163–171. doi: 10.1016/j.bbr.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Stuchlik A, Rezacova L, Vales K, Bubenikova V, Kubik S. Application of a novel Active Allothetic Place Avoidance task (AAPA) in testing a pharmacological model of psychosis in rats: comparison with the Morris Water Maze. Neuroscience letters. 2004;366:162–166. doi: 10.1016/j.neulet.2004.05.037. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Rodriguez AJ. The role of the fornix/fimbria and some related subcortical structures in place learning and memory. Behavioral Brain Research. 1989;32:265–277. doi: 10.1016/s0166-4328(89)80059-2. [DOI] [PubMed] [Google Scholar]
- Taube JS. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. Journal of Neuroscience. 1995;15:70–86. doi: 10.1523/JNEUROSCI.15-01-00070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Burton HL. Head direction cell activity monitored in a novel environment and during a cue conflict situation. Journal of Neurophysiology. 1995;74:1953–1971. doi: 10.1152/jn.1995.74.5.1953. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Uekita T, Okaichi H. NMDA antagonist MK-801 does not interfere with the use of spatial representation in a familiar environment. Behavioral Neuroscience. 2005;119:548–556. doi: 10.1037/0735-7044.119.2.548. [DOI] [PubMed] [Google Scholar]
- Van der Staay FJ, Rutten K, Erb C, Blokland A. Effects of the cognition impairer MK-801 on learning and memory in mice and rats. Behavioural Brain Research. 2011;220:215–229. doi: 10.1016/j.bbr.2011.01.052. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Kadish I, Wyss JM. Role of the anterodorsal and anteroventral nuclei of the thalamus in spatial memory in the rat. Behavioural Brain Research. 2002;132:19–28. doi: 10.1016/s0166-4328(01)00390-4. [DOI] [PubMed] [Google Scholar]
- Ward L, Mason SE, Abraham WC. Effects of the NMDA antagonists CPP and MK-801 on radial arm maze performance in rats. Pharmacology, Biochemistry, and Behavior. 1990;35:785–790. doi: 10.1016/0091-3057(90)90359-P. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Auer RN. Immediate and long-lasting effects of MK-801 on motor activity, spatial navigation in a swimming pool and EEG in the rat. Psychopharmacology. 1989;98:500–507. doi: 10.1007/BF00441949. [DOI] [PubMed] [Google Scholar]
- Wilton LAK, Baird AL, Muir JL, Honey RC, Aggleton JP. Loss of the thalamic nuclei for “head direction” impairs performance on spatial memory tasks in rats. Behavioral Neuroscience. 2001;115:861–869. doi: 10.1037//0735-7044.115.4.861. [DOI] [PubMed] [Google Scholar]
- Zugaro MB, Tabuchi E, Fouquier C, Berthoz A, Wiener SI. Active locomotion increases peak firing rates of anterodorsal thalamic head direction cells. Journal of Neurophysiology. 2001;86:692–702. doi: 10.1152/jn.2001.86.2.692. [DOI] [PubMed] [Google Scholar]