Abstract
Recent evidences highlighted the presence of Lactococcus lactis during late cheese ripening. For this reason, the role of this microorganism, well known as dairy starter, should be reconsidered throughout cheese manufacturing and ripening. Thus, the main objective of this study was to develop a RT-qPCR protocol for the detection, quantification and determination of the viability of L. lactis in ripened cheese samples by direct analysis of microbial nucleic acids. Standard curves were constructed for the specific quantification of L. lactis in cheese matrices and good results in terms of selectivity, correlation coefficient and efficiency were obtained. Thirty-three ripened cheeses were analyzed and, on the basis of RNA analysis, twelve samples showed 106 to 108 CFU of L. lactis per gram of product, thirteen from 103 to 105 CFU/g, and in eight cheeses, L. lactis was not detected. Traditional plating on M17 medium led to loads ranging from 105 to 109 CFU/g, including the cheese samples where no L. lactis was found by RT-qPCR. From these cheeses, none of the colonies isolated on M17 medium was identified as L. lactis species. These data could be interpreted as a lack of selectivity of M17 medium where colony growth is not always related to lactococcal species. At the same time, the absence or low abundance of L. lactis isolates on M17 medium from cheese where L. lactis was detected by RT-qPCR support the hypothesis that L. lactis starter populations are mainly present in viable but not culturable state during ripening and, for this reason, culture-dependent methods have to be supplemented with direct analysis of cheese.
Introduction
The study of microbial ecology associated with dairy fermentations is fundamental to understand the bases of important traits of dairy products. Traditionally, microbial dynamics in dairy fermentations have been studied with methods based on cultivation on selective media followed by phenotypic and/or molecular characterization. These approaches highlighted the role and activity, in cheese manufacturing and ripening, of two microbial groups: starter lactic acid bacteria (LAB)(mainly Lactococcus lactis, Streptococcus thermophilus and Lactobacillus spp.), with primary function of producing sufficient lactic acid during cheese manufacturing to reduce the pH of the milk; and non-starter LAB (NSLAB) (mainly Lactobacillus spp., Pediococcus spp., Enterococcus spp. and Leuconostoc spp.), generally adventitious contaminants which grow later, during cheese ripening, with an impact on flavour development [1].
In the last years, approaches to study microorganisms in dairy products have undoubtedly changed. Culture-dependent approaches have shown limitations in terms of recovery rate, mainly related to the lack of knowledge of the real conditions under which most of bacteria are growing in their natural habitat, and the difficulty to develop media for cultivation accurately resembling these conditions [2], [3]. Thus, the cultivable populations may not totally represent the community, and the actual microbial diversity could be misinterpreted [4]. For these reasons, the trend is now towards the use of culture-independent methods because they are believed to overcome problems associated with selective cultivation and isolation of bacteria from dairy samples. The development of these techniques has revolutionized microbial ecology and their application in cheese microbiology is leading to new insights. In particular, in recent studies, some authors have hypothesized the presence of metabolically active starter populations throughout cheese ripening [5]–[8]. In particular, Rantsiou et al. [6] showed the presence of alive population of S. thermophilus and Lactococcus spp. in ripened Feta cheese by using the culture-independent technique FISH (Fluorescence in situ hybridization). Later, Dolci et al. [8] found metabolically active population of L. lactis in ripened Castelmagno PDO cheese by RT-PCR-DGGE (Denaturing Gradient Gel Electrophoresis). Thus, it should be considered the possibility that starter populations are in a viable but not culturable (VNC) state during cheese ripening and, for this reasons, culture-dependent methods are not able to highlight their presence. In support of this hypothesis, VNC state has been described for L. lactis as a physiological response to carbohydrate starvation [9], a situation that characterizes a cheese after fermentation [10]. The main objective of this study was to develop a RT-qPCR protocol for the detection, quantification and determination of the viability of L. lactis in ripened cheese samples by direct analysis of microbial nucleic acids. In parallel, a culture-dependent approach was carried out in order to investigate, in ripening conditions, the presence/absence of L. lactis cells able to grow on selective medium.
Materials and Methods
1 Optimization of quantitative PCR protocol for the detection of L. lactis
1.1 Primer design
The housekeeping gene tuf [7], encoding for the elongation factor tu [11], was chosen as target for the design of L. lactis specific primers. Tuf gene sequences of L. lactis subsp. lactis, subsp. cremoris and of other LAB species, commonly found in dairy products, were retrieved in GenBank (http://www.ncbi.nlm.nih.gov/genbank/)(Table 1) and aligned using the software Clustal W2, Multiple Sequence Alignment (http://www.ebi.ac.uk/Tools/msa/clustalw2/). For primer design, Primer3 software (http://primer3.ut.ee/) was employed. Finally, the BLAST search tool (Basic Local Alignment Search Tool, http://blast.ncbi.nlm.nih.gov/Blast.cgi) was used to check the in silico specificity of the selected primer pair Tuf2 forward (Tuf2f) (5′-TGA ACC ACA ATG GGT TGC TA-3′) and Tuf2 reverse (Tuf2r) (5′-TCG ACT GGA AGA AGG AGT GG -3′).
Table 1. Accession number of the sequences used for the design of L. lactis Tuf2 specific primers and the species of LAB to which they belong.
| GenBank accession number | LAB species |
| CP000033.3 | Lactobacillus acidophilus NCFM |
| CP000416.1 | Lactobacillus brevis ATCC 367 |
| CP000423.1 | Lactobacillus casei ATCC 334 |
| FM177140.1 | Lactobacillus casei BL23 |
| CR954253.1 | Lactobacillus delbrueckii subsp. bulgaricus ATCC 11842 |
| CP000412.1 | Lactobacillus delbrueckii subsp. bulgaricus ATCC BAA-365 |
| AM295061.1 | Lactobacillus fermentum subsp. cellobiosus DSM 20055 |
| FJ825125.1 | Lactobacillus helveticus IMAU30062(C8402) |
| CP001617.1 | Lactobacillus plantarum JDM1 |
| AL935263.2 | Lactobacillus plantarum WCFS1 |
| AM406671.1 | Lactococcus lactis subsp. cremoris MG1363 |
| CP000425.1 | Lactococcus lactis subsp. cremoris SK11 |
| NC002662.1 | Lactococcus lactis subsp. lactis Il1403 |
| CP000422.1 | Pediococcus pentosaceus ATCC 25745 |
| CP000046.1 | Staphylococcus aureus subsp. aureus COL |
| CP000255.1 | Staphylococcus aureus subsp. aureus USA300_FPR3757 |
| CP000023.1 | Streptococcus thermophilus LMG 18311 |
| CP000024.1 | Streptococcus thermophilus CNRZ1066 |
| CP000419.1 | Streptococcus thermophilus LMD-9 |
| AE009948.1 | Streptococcus agalactiae 2603V/R |
| AL766847.1 | Streptococcus agalactiae NEM316 |
| CP000114.1 | Streptococcus agalactiae A909 |
| AE016830.1 | Enterococcus faecalis V583 |
| CP003351.1 | Enterococcus faecium Aus0004 |
1.2 Bacterial strains and DNA extraction
In order to optimize the amplification conditions and to assess the specificity of the qPCR protocol for L. lactis, the primers Tuf2f and Tuf2r, synthesized by Sigma-Aldrich (Milan, Italy), were used on target and non-target LAB commonly found in dairy products (Table 2). The bacterial strains were grown overnight at 37°C in M17 broth supplemented with lactose (5 g/L) (lactococci, enterococci and streptococci)(Oxoid, Milan, Italy) and in MRS broth (lactobacilli)(Oxoid). In addition, two strains of Staphylococcus aureus were cultivated in BHI broth (Oxoid) at 37°C. One millilitre of each culture was centrifuged at 20,844 g for 5 min, the supernatant discarded and the resulting pellet submitted to DNA extraction according to the protocol reported from Cocolin et al. [12]. DNA yield and quality were determined with the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
Table 2. Bacterial strains commonly found in dairy products and used in this study to optimize and assess the specificity of the qPCR protocol for L. lactis.
| Bacterial species | Strain codea |
| Enterococcus faecalis | BF 14D |
| Enterococcus faecalis | 551D |
| Enterococcus faecalis | 30FE3D |
| Enterococcus faecalis | 30FE4D |
| Enterococcus faecium | ATCC 19434TN |
| Enterococcus faecium | 37FE2D |
| Enterococcus faecium | 37FE3D |
| Enterococcus faecium | 571D |
| Enterococcus faecium | Ma F115D |
| Leuconostoc mesenteroides | Lm 24N |
| Lactobacillus brevis | CNRZ 734N |
| Lactobacillus casei | F30Bi4D |
| Lactobacillus casei | F150AeM5D |
| Lactobacillus casei shirota | F17MARD |
| Lactobacillus delbrueckii | c 91D |
| Lactobacillus delbrueckii | c 91D |
| Lactobacillus delbrueckii | c 92D |
| Lactobacillus delbrueckii | c 93D |
| Lactobacillus delbrueckii | c 94D |
| Lactobacillus helveticus | m 80N |
| Lactobacillus helveticus | m 81D |
| Lactobacillus paracasei | P 95D |
| Lactobacillus paracasei | P 96D |
| Lactobacillus paracasei | DSM 5622TN |
| Lactobacillus plantarum | 110 agD |
| Lactobacillus plantarum | a 810D |
| Lactobacillus plantarum | Lab7D |
| Lactobacillus plantarum | ATCC 4008TN |
| Lactococcus garvieae | DSM 20684N |
| Lactococcus lactis subsp. cremoris | Fc95D |
| Lactococcus lactis subsp. cremoris | Fc96D |
| Lactococcus lactis subsp. lactis | 39FL4D |
| Lactococcus lactis subsp. lactis | 39FL3D |
| Lactococcus lactis subsp. lactis | 39FL2D |
| Lactococcus lactis subsp. lactis | 39FL1D |
| Lactococcus lactis subsp. lactis | DSM 20481TN |
| Staphylococcus aureus | 661D |
| Staphylococcus aureus | 662D |
| Streptococcus thermophilus | LCM 3D |
| Streptococcus thermophilus | 6C6D |
| Streptococcus thermophilus | 7C6D |
| Streptococcus thermophilus | 8C6D |
| Streptococcus thermophilus | 4C10D |
| Streptococcus thermophilus | 4C16D |
| Streptococcus thermophilus | 6C16D |
| Streptococcus thermophilus | 10C7D |
| Streptococcus thermophilus | DSM 20617TN |
| Streptococcus thermophilus | DSM 20617TN |
D: strains from DISAFA Collection (University of Turin, Italy) isolated from dairy products; N: strains from International Collections.
1.3 Amplification conditions
One-hundred nanograms of DNA extracted from each strain was used in qPCR in order to optimize the protocol. Different primer concentrations and annealing temperatures were tested to determine the most selective and efficient amplification conditions. The best results were obtained with Tuf2f and Tuf2r added at 400 nM and 50 nM, respectively, and using the SsoAdvancedTM SYBR@ Green Supermix (Bio-Rad, Hercules, CA, USA) in a final volume of 20 µL. Quantitative PCR assays were performed in Chromo4 Real Time PCR Detection System (Bio-Rad) with the software MJ OpticonMonitor version 3.1. The thermal cycle conditions were optimized as follows: an initial denaturation at 98°C for 2 min, 40 cycles at 95°C for 5 sec and 68.7°C for 30 sec, where the stringent annealing temperature value, and the cumulative annealing and extension steps were chosen to increase the selectivity of the protocol to the microorganism target L. lactis.
2 Construction of L. lactis standard curves
2.1 Standard curves from pure culture
Standard curves were constructed from ten-fold serial dilutions of an overnight pure culture of L. lactis subsp. lactis 39FL4 (DISAFA collection). One millilitre of each dilution was submitted to RNA extraction using the MasterPureTM Complete DNA and RNA Purification Kit (Epicentre, Madison, WI, USA) following the manufacturer's instructions, with the addition of lysozyme (50 mg/mL, Sigma, Milan, Italy) to improve cell wall lyses. Three microliters of TURBO - DNase (Ambion, Milan, Italy) were added to digest the DNA in the RNA samples, with an incubation of 3 h at 37°C. The absence of residual DNA, in the RNA samples, was checked by qPCR and, subsequently, reverse transcription (RT) was performed by using the M-MLV Reverse Transcriptase kit (Promega, Milan, Italy). RT was performed as follows: 5 µL of RNA were mixed with 1 µL of TUF2r primer (100 µM) in a reaction volume of 10 µl by addition of ultrapure water. The mix was treated at 75°C for 5 min for RNA denaturation and immediately placed on ice for 10 min. Five microliters of M-MLV RT Buffer (1×), 5 µl of dNTPs (10 µM each), 1 µl of M-MLV Reverse Transcriptase (8 U/µL) and 0.6 µL of RNasin ribonuclease inhibitor (20 U/µL) were added to the mix for a final volume of 25 µl by addition of ultrapure water. RT reaction was carried out at 42°C for 1 h in a Biorad DNA Engine thermal cycler (Bio-Rad) and the cDNA samples stored at −30°C. cDNA was subjected to qPCR, according to the amplification conditions described in paragraph 1.3. Standard curve was constructed by plotting the Ct values against the colony forming units (CFU)/mL of the overnight L. lactis subsp. lactis 39FL4 culture, evaluated by traditional plating on M17 agar supplemented with lactose (5 g/L)(Oxoid). The reactions were carried out in triplicate and mean values were considered for standard curve construction. The correlation coefficients (R2) and the efficiency (E) of the amplifications were calculated according to Rutledge and Cote [13].
2.2 Standard curves from inoculated cheese matrix and evaluation of L. lactis detection limit
Ten grams of grated cheese (Biraghi s.p.a., Cuneo, Italy) were contaminated with 10 mL of ten-fold serial dilutions of an overnight pure culture of L. lactis subsp. lactis 39FL4 and added of 30 mL of sterile Ringer solution (Oxoid). The samples were homogenized in a Stomacher (Interscience, Rockland, MA, USA) for 30 sec and 1 mL of each sample was subjected to RNA extraction by using the MasterPureTM Complete DNA and RNA Purification Kit (Epicentre). Ribonucleic acid samples were treated as reported above (paragraph 2.1) and each cDNA sample was submitted to qPCR. Standard curve was constructed by plotting the Ct values against CFU/g, evaluated by traditional plating on M17 agar supplemented with lactose (5 g/L), of the grated cheese inoculated with the overnight L. lactis subsp. lactis 39FL4 culture. The reactions were carried out in triplicate and mean values were considered for standard curve construction. The R2 and E of the amplifications were calculated according to Rutledge and Cote [13].
The limit of detection of L. lactis was evaluated by RT-qPCR in grated cheese specifically inoculated with 5, 10, 50, 100, 1000 CFU/g of the microorganism. Commercial grated cheese was checked for the absence of lactococci before use.
3 Detection of L. lactis in commercial cheeses by culture-dependent and -independent methods
Thirty-three ripened cheeses (Table 3) were purchased from the Italian market. The cheeses were available for sale in a pre-packed format with an approximate weight of 100 g each.
Table 3. Quantification of L. lactis in the ripened cheeses analyzed in this study and comparison of the results obtained by using culture-independent and -dependent approaches.
| Culture-independent approach | Culture-dependent approach | |||
| Cheese sample and ripening time (day) | RT-qPCR | M17 agar plating | Molecular identification of the isolates | |
| LOG CFU/g cheese ± SD | LOG CFU/g ± SD | L. lactis RSA profile | Positive to HIS-PCR | |
| Asiago d'allevo PDO 120 d | * | 8.32±0.73 | 0/12 | 0/0 |
| Asiago PDO 90 d | 7.13±0.02 | 6.94±0.43 | 2/12 | 2/2 |
| Bra tenero PDO 45 d | * | 8.41±0.32 | 0/12 | 0/0 |
| Castelmagno 90 d | 5.34±0.03 | 6.58±0.24 | 4/12 | 0/4 |
| Castelmagno PDO 180 d | 6.17±0.05 | 5.40±0.43 | 2/12 | 1/2 |
| Fontal 60 d | 5.06±0.03 | 5.48±0.32 | 2/12 | 0/2 |
| Fontina PDO 120 d | 3.82±0.01 | 6.86±0.24 | 3/12 | 0/3 |
| Fontina PDO 120 d | 5.39±0.06 | 8.36±0.14 | 1/12 | 0/1 |
| Fontina PDO 120 d | 6.11±0.06 | 8.59±0.75 | 4/12 | 0/4 |
| Fontina PDO 120 d | 5.50±0.01 | 7.33±0.21 | 3/12 | 0/3 |
| Fontina PDO 120 d | 3.78±0.02 | 6.38±0.56 | 1/12 | 0/1 |
| Pecorino di Gallura | 6.97±0.04 | 9.48±0.42 | 4/12 | 0/4 |
| Fiore Sardo 120 d | 6.29±0.02 | 7.57±0.12 | 3/12 | 0/3 |
| Pecorino fioretto 120 d | 7.48±0.07 | 7.79±0.66 | 7/12 | 7/7 |
| Pecorino pastore 90 d | * | 5.70±0.55 | 1/12 | 0/1 |
| Pecorino romano 120 d | 5.56±0.04 | 6.88±0.46 | 5/12 | 0/5 |
| Pecorino sardo PDO 120 d | 4.96±0.07 | 7.76±0.78 | 2/12 | 0/2 |
| Pecorino toscano PDO 90 d | 7.58±0.04 | 6.51±0.54 | 0/12 | 0/0 |
| Raschera 60 d | * | 8.23±0.23 | 0/12 | 0/0 |
| Raschera PDO 90 d | 3.76±0.03 | 6.40±0.64 | 2/12 | 0/2 |
| Raschera PDO 90 d | 5.24±0.02 | 8.01±0.22 | 3/12 | 0/3 |
| Raschera PDO 60 d | * | 9.52±0.19 | 2/12 | 0/2 |
| Toma d'Oropa 45 d | * | 7.77±0.45 | 4/12 | 0/4 |
| Toma di Bra 45 d | * | 9.16±0.33 | 2/12 | 0/2 |
| Toma di capra 60 d | 8.47±0.01 | 8.59±0.56 | 12/12 | 6/12 |
| Toma di Lanzo 60 d | 7.06±0.01 | 8.15±0.16 | 6/12 | 1/6 |
| Toma di Lanzo 60 d | 4.55±0.02 | 8.88±0.24 | 3/12 | 0/3 |
| Toma di Lanzo 60 d | 4.82±0.02 | 8.92±0.21 | 4/12 | 3/4 |
| Toma di Lanzo 60 d | 6.90±0.02 | 8.60±0.31 | 5/12 | 1/5 |
| Toma piemontese PDO 60 d | 3.72±0.10 | 8.38±0.77 | 4/12 | 0/4 |
| Toma piemontese PDO 60 d | * | 9.19±0.13 | 3/12 | 0/3 |
| Toma piemontese PDO 60 d | 7.68±0.00 | 8.95±0.12 | 7/12 | 3/7 |
| Toma piemontese PDO 60 d | 7.40±0.02 | 7.45±0.48 | 4/12 | 0/4 |
*below quantification limit.
SD: standard deviation.
3.1 Detection of L. lactis in cheese samples by RT-qPCR assays
Ten grams of each cheese were homogenized in 40 mL of sterile Ringer solution (Oxoid) in a Stomacher (Interscience) for 1 min, and 1 mL of the homogenized suspension, in duplicate, was centrifuged at 20,844 g for 5 min to pellet the microbial cells for subsequent RNA extraction. The MasterPureTM Complete DNA and RNA Purification Kit (Epicentre) was used for nucleic acid extraction as reported above (2.1). Ribonucleic acid samples were treated with DNase and checked for the efficiency of the treatment, as reported in paragraph 2.1. RT was performed by using the M-MLV Reverse Transcriptase kit (Promega) (2.1).
3.2 Detection of L. lactis in cheese samples by traditional plating and species-specific PCR
The cheese samples, homogenized as described above, were serially diluted and plated on M17 agar media (Oxoid) supplemented with lactose (5 g/L), supposed to be selective for lactic acid cocci. The plates were incubated, aerobically, at 37°C for 48 h and, after counting, 12 randomly selected colonies were isolated for each sample. A total of 396 isolates were grown overnight at 37°C in M17 broth (Oxoid) supplemented with lactose (5 g/L). One millilitre of each culture was transferred to a 1.5 mL screw cap tubes containing 0.3 g of glass beads with a diameter of 0.5 mm and centrifuged at 20,844 g for 5 min. The supernatant was discarded and the resulting pellet was stored at −20°C until DNA extraction as previously reported [12].
The identification of L. lactis isolates was carried out by combining PCR 16S–23S rRNA gene spacer analysis (RSA) [13] and L. lactis specific PCR, based on the amplification of a portion of the histidine biosynthesis operon (His-PCR) [14]. On the basis of RSA electrophoretic patterns, the isolates showing a single band were checked by His-PCR to confirm their belonging to L. lactis species.
Results
4 Specificity of the qPCR protocol to L. lactis strains
The specificity of the primer couple Tuf2f and Tuf2r was assessed by qPCR using the genomic DNA of the bacterial strains reported in Table 2. The fluorescence signal was detected for the subspecies L. lactis subsp. lactis and subsp. cremoris at Ct values of, on average, 14.37 (±0.12) and 15.69 (±0.31), respectively; strains belonging to species phylogenetically related to L. lactis and/or commonly found in dairy products were not amplified within the 40 cycles set in the amplification protocol. The melting curve of the amplicons obtained from qPCR for L. lactis strains was studied and a single peak was observed for each reaction; the absence of secondary amplification products confirmed the high specificity of the protocol optimized.
5 Standard curves and L. lactis detection limit
In order to quantify L. lactis, standard curves were constructed both from pure cultures and using grated cheese, simulating a long ripened cheese matrix, inoculated with pure cultures of L. lactis. Lactococci were not found in the grated matrices before inoculation by both cultivation on M17 medium (<5 cfu/g) and direct RT-qPCR (<10 cfu/g).
A quantification limit of 102 CFU/mL and CFU/g was obtained for L. lactis RNA extracted from pure culture and grated cheese, respectively, and the linearity range was spanning from 102 to 108 CFU/mL or g, covering 6 orders of magnitude. A good linear correlation between the Ct values and L. lactis cell loads was obtained for the standard curves constructed, showing R2 values of 0.997 and 0.996 for inoculated cheese and pure cultures, respectively; and E values of 99,3% and 93,1% for inoculated cheese and pure cultures, respectively. The standard curves with the relative equation, R2 and E are reported in Fig. 1. The results obtained in terms of quantification limit, efficiency of amplification and coefficient of correlation derived by three independent experiments. Standard deviations have not been reported in Fig. 1 because of their low values (data not shown).
Figure 1. Standard curves obtained by RT-qPCR for L. lactis inoculated in cheese matrix (A) and L. lactis pure culture (B).
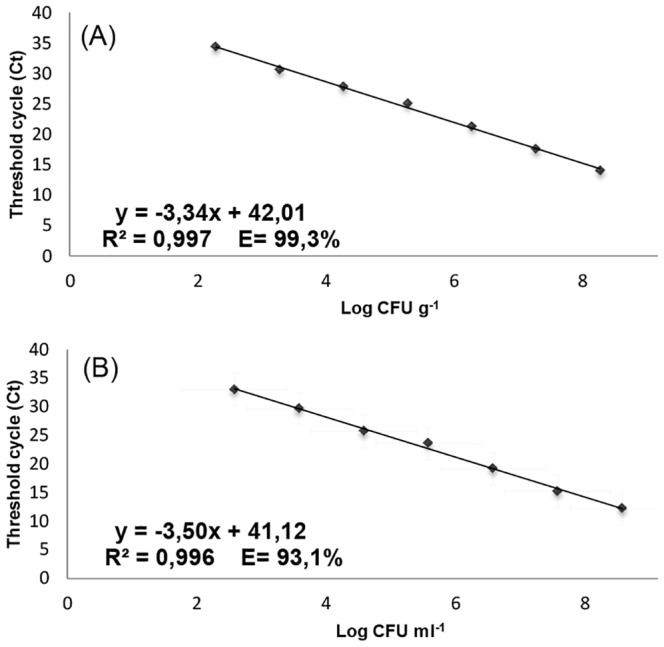
The limit of detection of L. lactis in grated cheese, evaluated by RT-qPCR, corresponded to 10 CFU/g.
6 Viability of L. lactis in ripened cheeses
6.1 Culture-independent approach
Cheese samples on the market (Table 3) were analyzed to assess the applicability of RT-qPCR protocols for the direct quantification of L. lactis in dairy matrices, and to investigate the viability of this microorganism in ripened products.
Twenty-five samples, on a total of thirty-three, resulted positive for the presence of viable cells of L. lactis, as detected by RT-qPCR. The interpolation of the Ct values obtained, in the standard curve equation from inoculated cheese (Fig. 1A), resulted in L. lactis loads ranging from 103 to 108 CFU/g for RNA analysis (Table 3). The highest values were obtained for some of the Toma, Pecorino and Asiago cheese type samples, with loads higher than 107 CFU/g. In Fontina cheese, L. lactis counts ranged from 103 to 106 CFU/g, while in Castelmagno, L. lactis was detected with values of 105 and 106 CFU/g. In Raschera, L. lactis was found with loads of 103 and 105 CFU/g. On the contrary, eight samples belonging to Asiago, Bra, Pecorino, Raschera and Toma cheese type, were negative for L. lactis viability.
6.2 Culture-dependent approach
The cheese samples purchased from the market were subjected to traditional plating on M17 medium and the values ranged from 105 to 109 CFU/g (Table 3). A total of 396 colonies were randomly selected, purified and subjected to molecular identification; 78 isolates showed a RSA electrophoretic profile characterized by an unique band migrating approximately at 390 bp, characteristic of L. lactis species [15]. Species-specific His-PCR was necessary to finally establish the taxonomic position of the isolates of which only 24, spread among eight cheese samples (Table 3), resulted to belong to L. lactis species.
Discussion
The importance to monitor cheese microbial populations has been considered by different authors and, now, the literature is rich in papers focused on this topic [16]. In particular, it has been investigated the role of LAB, during the most effective technological phases, when it is important to have certain microbial activities to achieve the expected quality of the final product.
The primary role of starter LAB in cheese is considered a dogma in dairy microbiology. They produce high amount of lactic acid, causing milk acidification, and represent a bio-catalytic potential for cheese-ripening reactions, through the liberation of hydrolytic intracellular enzymes following autolysis [1]. Feirtag and McKay [17] first reported this phenomenon for lactococci and associated their autolytic activity to enhanced flavour development in cheese.
As reported in the introduction, recent studies have highlighted the presence, throughout cheese ripening, of alive cells of L. lactis by culture-independent techniques based on FISH [6], RT-PCR-DGGE [8] and RT-qPCR [18]. These evidences impose a more careful understanding of the role of L. lactis, in the ripening process, not only in terms of autolytic activity, but also as metabolically active cells.
In the present study, we investigated the presence of L. lactis populations in different ripened cheeses available on the market. In accordance with Desfossés-Foucault et al. [18] and supporting the first evidences cited above [6], [8], the results confirmed the presence of viable cells of L. lactis in cheeses, at the end of the ripening time. Thirty-three cheeses were analyzed. On the basis of RT-qPCR results, twelve samples showed 106 to 108 CFU of L. lactis per gram of product, and thirteen from 103 to 105 CFU/g. In eight cheeses, L. lactis was not found (L. lactis detection limit in grated cheese: 10 CFU/g), thus, the microorganism was considered not involved in the ripening of these products. Traditional plating on M17 medium led to loads ranging from 105 to 109 CFU/g, including cheese samples were no L. lactis was found by RT-qPCR. In these cheeses, none of the colonies isolated on M17 medium was identified as L. lactis. These data could be interpreted as a lack of selectivity of M17 medium where colony growth is not always related to lactococcal species. Probably, lactococci are able to grow on M17 medium when they are abundant and not stressed, as for example during milk and curd fermentation. Differently, during the ripening process, it is known that NSLAB increase in number and prevail on lactococcal populations, which are often out-competed by the numerically more abundant lactobacilli. Nevertheless, in this work, a few isolates were identified as L. lactis by His-PCR. They were obtained from eight cheese samples with loads higher than 107 CFU/g, detected by RT-qPCR, except for two samples characterized by values of 104 and 106 CFU/g. These data could be explained with the relative high abundance of L. lactis in these cheeses and, thus, its capability to compete with the rest of microbiota and multiply on synthetic media. On the basis of the results obtained, and as reported by other authors [19], alternative cultural approaches should be better considered. Currently, M17 is the medium mainly used for lactococci cultivation, but new formulations for the isolation of LAB from cheese have been recently studied as, for example, cheese agar [19], which was used to recover minority populations from milk, whey starter and fresh curd of Parmigiano Reggiano, hardly estimable on traditional media. Thus, as future prospective, for a more reliable and effective recovery of lactococci, in particular L. lactis, during cheese ripening, the optimization and formulation of specific nutritional conditions should be better investigated.
The absence or low abundance of L. lactis isolates on M17 medium, support the thesis hypothesized by other authors [9] that L. lactis starter populations are mainly present in VNC state during cheese ripening and, for this reason, culture-dependent methods are not able to detect their presence and have to be complemented with direct analysis in cheese. These considerations can be especially corroborated from the results obtained in eight cheese samples where the difference, in terms of microbial load, between RT-qPCR and plating data, was lower than 102 CFU/g and, thus, the absence of L. lactis growth, on M17, could not be justifiable with the prevalence of NSLAB.
For some of the cheeses analysed, experiments were performed in order to “resuscitate” L. lactis VNC cells and preliminary results highlighted that different carbon sources, in cultural media, affect differently their growing ability (data not shown); in particular, enrichment in medium with high percentage of glucose (2.5%) seemed to stimulate the attitude of the cells to become culturable again (data not shown). Recent researches were focused on these aspects and highlighted the presence of VNC L. lactis cells in ripened cheese products [18], [20]. Differently, Flórez and colleagues [5] found abundance of L. lactis isolates on M17 from the analysis of Spanish cheese, but they did not specify the distribution of the isolates among milk, curd and cheese samples. In addition, Desfossés-Foucault et al. [18] recently described that some species may be unable to grow even if they are in a viable and metabolically active state in the food matrix. Thus, culture-independent methods allow to overcome biases associated to the culturing step. The detection of microbial populations from total DNA or RNA extracted directly from food matrices can give a more realistic and reliable “picture” of cheese microbiota.
In order to monitor the presence and viability of L. lactis throughout cheese manufacturing and ripening, a highly selective qPCR protocol was optimized. The detection of L. lactis with respect to other LAB species, which normally colonize ripened cheeses, was reached by selective primer design on tuf gene codyfing a GTP binding protein and widespread in eubacteria genomes [21]. Tuf gene has been generally recognized as a housekeeping gene [21]; moreover, its stability was confirmed by studying its expression throughout L. lactis growth curve (data not shown).
SYBR green fluorescent chemistry was chosen and good results were obtained, in terms of specificity, correlation coefficient and efficiency, by increasing the stringency of the thermal cycle and using primers in unbalanced concentration. In particular, the high (compared to primer melting temperature) annealing temperature, used in qPCR and RT-qPCR protocols, allowed the specific detection of L. lactis and no fluorescent signal was detected when the protocol was applied to the other LAB species. Thus, tuf gene represented a suitable target for the specific detection and quantification of L. lactis as also highlighted by other authors [20]. Moreover, the efficiency of the protocols was improved by the choice of nucleic acid extraction protocols specifically designed for the treatment of fatty matrices [22], highlighting, once again, how this step heavily influence the performance of the subsequent amplification. The high quality of the extracted RNA and the set amplification conditions allowed to obtain standard curves with a good linearity range covering 6 orders of magnitude, from 102 to 108 CFU/g.
Other authors optimized qPCR protocols to detect L. lactis in milk [3], under simulated conditions of cheese manufacture [23], in ultrafiltered milk cheese models [7], [24] and in the manufacturing of raw milk soft cheeses [20]. The only study dealing with the monitoring of active population of L. lactis in cheese ripening by RT-qPCR has been focused on Cheddar cheese [18], for which undefined starter cultures containing L. lactis subsp. lactis and L. lactis subsp. cremoris are commonly used. In particular, the authors evaluated the impact of milk heat treatments and ripening temperatures on lactococcal starter and NSLAB throughout maturing of Cheddar cheese and the results showed that lactococci remained dominant throughout the ripening process.
The results presented in this study, however, do not shed light into the possible contribution of L. lactis in terms of organoleptic characteristics of the final cheese product. Thus, as future prospective, it will be important to investigate the role, in terms of metabolic activities, of this microorganism during cheese ripening. In particular, it will be interesting to understand which L. lactis functions are being carried out in each specific phase of the production, with the final aim of improving technological processes and cheese quality. A major improvement in our knowledge about the activity of this microorganism could be harnessed to control cheese ripening and the transfer of this knowledge to dairy industry could lead to the selection of new L. lactis strains with specific metabolic traits.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Beresford TP, Fitzsimons NA, Brennan NL, Cogan TM (2001) Recent advances in cheese microbiology. Int Dairy J 11:259–274. [Google Scholar]
- 2. Ercolini D (2004) PCR-DGGE fingerprinting: novel strategies for detection of microbes in food. J Microbiol Methods 56:297–314. [DOI] [PubMed] [Google Scholar]
- 3. Ndoye B, Rasolofo EA, LaPointe G, Roy D (2011) A review of the molecular approaches to investigate the diversity and activity of cheese microbiota. Dairy Sci Technol 91:495–524. [Google Scholar]
- 4. Temmerman R, Huys G, Swings J (2004) Identification of lactic acid bacteria: culture-dependent and culture-independent methods. Trends Food Sci Technol 15:348–359. [Google Scholar]
- 5. Flórez AB, Mayo B (2006) Microbial diversity and succession during the manufacture and ripening of traditional, Spanish, blue-veined Cabrales cheese, as determined by PCR-DGGE. Int J Food Microbiol 110:165–171. [DOI] [PubMed] [Google Scholar]
- 6. Rantsiou K, Urso R, Dolci P, Comi G, Cocolin L (2008) Microflora of Feta cheese from four Greek manufacturers. Int J Food Microbiol 126:36–42. [DOI] [PubMed] [Google Scholar]
- 7. Ulve VM, Monnet C, Valence F, Fauquant J, Falentin H, et al. (2008) RNA extraction from cheese for analysis of in situ gene expression of Lactococcus lactis . J Appl Microbiol 105:1327–1333. [DOI] [PubMed] [Google Scholar]
- 8. Dolci P, Alessandria V, Rantsiou K, Bertolino M, Cocolin L (2010) Microbial diversity, dynamics and activity throughout manufacturing and ripening of Castelmagno PDO cheese. Int J Food Microbiol 143:71–75. [DOI] [PubMed] [Google Scholar]
- 9. Ganesan B, Stuart MR, Weimer BC (2007) Carbohydrate starvation causes a metabolically active but nonculturable state in Lactococcus lactis . Appl Environ Microbiol 73:2498–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stuart MR, Chou LS, Weimer BC (1999) Influence of Carbohydrate Starvation and Arginine on Culturability and Amino Acid Utilization of Lactococcus lactis subsp. lactis . Appl Envir Microbiol 65:665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ke D, Boissinot M, Huletsky A, Picard FJ, Frenette J, et al. (2000) Evidence for horizontal gene transfer in evolution of elongation factor Tu in enterococci. J Bacteriol 182:6913–6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cocolin L, Manzano M, Cantoni C, Comi G (2001) Denaturing gradient gel electrophoresis analysis of the 16S rRNA gene V1 region to monitor dynamic changes in the bacterial population during fermentation of Italian sausages. Appl Environ Microbiol 67:5113–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jensen Ma, Webster Ja, Straus N (1993) Rapid identification of bacteria on the basis of polymerase chain reaction-amplified ribosomal DNA spacer polymorphisms. Appl Environ Microbiol 59:945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Corroler D, Mangin I, Desmasures N, Gueguen M (1998) An ecological study of lactococci isolated from raw milk in the camembert cheese registered designation of origin area. Appl Environ Microbiol 64:4729–4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fortina MG, Ricci G, Acquati A, Zeppa G, Gandini A, et al. (2003) Genetic characterization of some lactic acid bacteria occurring in an artisanal protected denomination origin (PDO) Italian cheese, the Toma piemontese. Food Microbiol 20:397–404. [Google Scholar]
- 16. Jany J-L, Barbier G (2008) Culture-independent methods for identifying microbial communities in cheese. Food Microbiol 25:839–848. [DOI] [PubMed] [Google Scholar]
- 17. Feirtag JM, McKay LL (1987) Thermoinducible Lysis of Temperature-Sensitive Streptococcus cremoris Strains. J Dairy Sci 70:1779–1784. [Google Scholar]
- 18. Desfossés-foucault É, Lapointe G, Roy D (2013) International Journal of Food Microbiology Dynamics and rRNA transcriptional activity of lactococci and lactobacilli during Cheddar cheese ripening. Int J Food Microbiol 166:117–124. [DOI] [PubMed] [Google Scholar]
- 19. Neviani E, De Dea Lindner J, Bernini V, Gatti M (2009) Recovery and differentiation of long ripened cheese microflora through a new cheese-based cultural medium. Food Microbiol 26:240–245. [DOI] [PubMed] [Google Scholar]
- 20. Achilleos C, Berthier F (2013) Quantitative PCR for the specific quantification of Lactococcus lactis and Lactobacillus paracasei and its interest for Lactococcus lactis in cheese samples. Food Microbiol 36:286–295. [DOI] [PubMed] [Google Scholar]
- 21. Ventura M, Canchaya C, Meylan V, Klaenhammer TR, Zink R (2003) Analysis, characterization, and loci of the tuf genes in Lactobacillus and Bifidobacterium species and their direct application for species identification. Appl Environ Microbiol 69:6908–6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mertens K, Freund L, Schmoock G, Hänsel C, Melzer F, et al. (2014) Comparative evaluation of eleven commercial DNA extraction kits for real-time PCR detection of Bacillus anthracis spores in spiked dairy samples. Int J Food Microbiol 170:29–37. [DOI] [PubMed] [Google Scholar]
- 23. Taïbi A, Dabour N, Lamoureux M, Roy D, LaPointe G (2011) Comparative transcriptome analysis of Lactococcus lactis subsp. cremoris strains under conditions simulating Cheddar cheese manufacture. Int J Food Microbiol 146:263–275. [DOI] [PubMed] [Google Scholar]
- 24. Cretenet M, Laroute V, Ulvé V, Jeanson S, Nouaille S, et al. (2011) Dynamic analysis of the Lactococcus lactis transcriptome in cheeses made from milk concentrated by ultrafiltration reveals multiple strategies of adaptation to stresses. Appl Environ Microbiol 77:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.


