Abstract
Background
Obesity is associated with elevated coronary artery calcium (CAC), a marker of coronary atherosclerosis that is strongly predictive of cardiovascular events. we evaluated the effects of marked weight loss achieved through roux-en-Y gastric bypass surgery (GBS) on CAC scores.
Methods
We performed echocardiography and computed tomography of the heart in 149 subjects 6 years after enrollment in a prospective registry evaluating the cardiovascular effects of GBS. coronary calcium scores, left ventricular ejection fraction and left ventricular mass were measured.
Results
At baseline most coronary risk factors were similar between the GBS and nonsurgical groups including current smoking, systolic blood pressure, LDL-C, HDL-C, and TG. However, GBS patients were younger (4.7 years), less likely to be diabetic and less likely to be postmenopausal. At 6 years after enrollment, CAC score was significantly lower in patients who underwent GBS than those without surgery (p<0.01). GBS subjects had a lower likelihood of having measureable coronary calcium (odds ratio of CAC > zero = 0.39; 95% CI of (0.17, 0.90)). Significant predictors of zero CAC were GBS, female gender, younger age, baseline BMI, and baseline LDL-C. Substituting change in BMI for group status as a predictor variable showed that BMI change also predicted CAC (p=0.045). Changes in LDL-C did not predict the CAC differences between groups (p=0.67).
Conclusions
Sustained weight loss achieved through bariatric surgery is associated with less coronary calcification. This effect, which appears to be independent of changes in LDL-C, may contribute to lower cardiac mortality in patients with successful GBS.
Keywords: OBESITY, ATHEROSCLEROSIS, CORONARY CALCIUM, WEIGHT LOSS, BARIATRIC SURGERY
Introduction
The prevalence of obesity has doubled over the past 30 years with nearly 70% of adults in the United States now classified as overweight or obese.1 Severe obesity, defined as body mass index (BMI) > 35 kg/m2, is the fastest growing category of obesity.2 There is substantial evidence that obesity is a risk factor for the development of premature and accelerated atherosclerosis.3-7 A variety of mechanisms have been postulated to account for the pro-atherogenic effects of obesity, including the frequent coexistence of conventional coronary risk factors in obese individuals as well as putative atherogenic pathways that are independent of traditional risk factors.8-9 There is growing appreciation for the potential role of fat-secreted hormone signaling in obesity-related vascular disease.10-12
Atherosclerotic plaques may develop areas of calcification that are thought to represent healing of inflammatory foci or sites of intraplaque hemorrhage. The local inflammation may lead to transformation of smooth muscle cells into osteoblastic cells and/or activation of existing osteoblastic cells in the arterial wall.13 Calcified plaque can be readily detected and quantified using noncontrast computed tomography.14 Coronary artery calcium (CAC) scores are related to the total atherosclerotic burden in the coronary arterial tree.14 Coronary artery calcium scores of zero are associated with an extremely low 5 year cardiovascular event rate15 while higher CAC scores are strongly Associated with progressively higher event rates.16 Coronary calcium scores predict coronary events and long- term survival with much greater accuracy than framingham risk scores.17 Some clinicians have advocated the use of CAC scoring to decide on the use or intensity of lipid lowering therapies such as statins.18 Because of the noninvasive nature of the testing and the quantitative data that can be obtained, CAC scoring can be readily used to track progression or regression of calcified coronary atherosclerosis.5, 19, 20 Obesity appears to be a risk factor for more rapid progression of CAC scores over time3, 21, but it is unknown whether weight loss slows CAC development.
Bariatric surgery is the only effective method of producing rapid and 99 sustained weight loss in severe obesity.22 Hence, these procedures are being used with increasing frequency. Depending upon the type of procedure done, patients lose ~ 30-45% of their body weight by 2 years after surgery.22 This degree of weight loss has been associated with sustained improvements in systolic blood pressure, insulin resistance, hyperlipidemia and sleep disordered breathing.22-24 These beneficial changes in coronary risk factors should theoretically lead to a major reduction in cardiovascular event rates.25 Some published studies support this prediction.26
We measured CAC scores in 149 consecutive patients returning for 6-year follow up as part of a prospective registry examining the effects of gastric bypass surgery (GBS). We tested the hypothesis that GBS patients would have lower CAC scores than a matched group of nonsurgical patients with continued obesity.
Methods
Study population
The institutional review board of the University of Utah Health Sciences Center approved the protocol. All patients signed a written consent. The rationale and design of the utah obesity study have previously been reported. 27 This is a prospective, longitudinal study comparing a variety of metabolic, anthropometric, pulmonary, psychosocial and cardiovascular endpoints in severely obese subjects undergoing GBS vs. severely obese subjects treated without bariatric surgery. roux-en-Y GBS was done in all surgical patients. Patients in the nonsurgical reference group did not have any specific interventions mandated by the study protocol.
Experimental Protocol
Participants were examined at the Huntsman General Clinical Research Center. Data were obtained at the initial visit (Baseline), ~ 24 months later (2 years) and again ~ 6 years after initial enrollment. Clinical and echocardiographic findings from the 2-year and 6-year follow up visits have been reported previously.23, 28, 29 A group of consecutive subjects returning for 6-year follow up were invited to undergo computed tomography for CAC scoring. the only exclusion criterion was possible pregnancy.
Serum Chemistry
At each visit, following a 12-hour overnight fast, venous blood was drawn for measurement of 20 chemistry variables including glucose, insulin, glycosylated hemoglobin (HbA1c) and a lipid profile. Diabetes was defined as a fasting blood glucose level ≥126 mg/dl, an HbAIc level ≥6.5%, or taking antidiabetic medications. Hypertension was defined as a blood pressure ≥140/90 mmHg or taking antihypertensive medications.
Blood Pressure
Blood pressure was measured using automated equipment (Dinamap, Critikon, Tampa, FL). After 5 min of sitting in a quiet room, 3 consecutive bp readings were obtained from the right arm (2 min apart). The mean value was used for analysis.
Echocardiography
Standard 2-dimensional and Doppler echocardiograms were performed. Left ventricular dimensions were measured from 2-dimensional images according to american society for echocardiography recommendations.30 Left ventricular mass and ejection fraction were calculated from these measurements.
Computed tomography of the chest
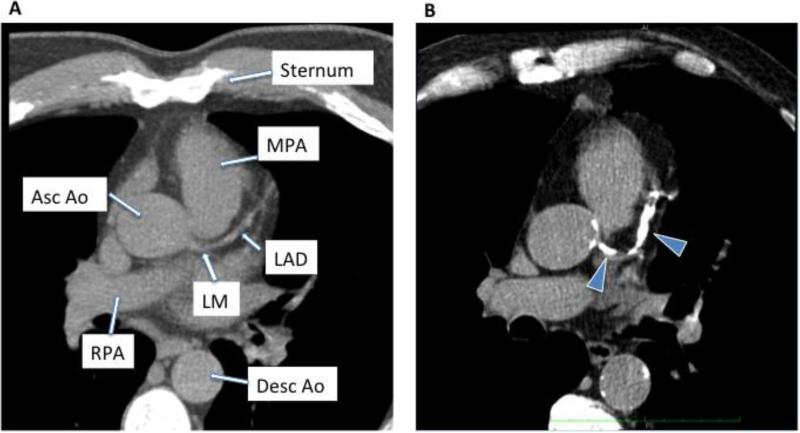
Electrocardiographic-gated, noncontrast, axial scanning of the chest was performed using prospective triggering with contiguous, nonoverlapping 3 mm slices (definition, siemens). The scan began at the bifurcation of the carina and continued to the bottom of the heart. The kVp was set at 120 mV. The scanner adjusted the tube current automatically. The CAC score was measured by 2 independent, blinded clinicians (TP and SEL) using siemens software. Both agatston and calcium volume scores were obtained (representative examples shown in figure 1).
Figure 1.
Examples of noncontrast computed tomography of the chest in a subject with no coronary calcium (A) and a subject with significant coronary calcification (arrowheads) seen in the left main (LM) coronary artery and left anterior descending (LAD) artery (B). Other structures shown include the main pulmonary artery (MPA), ascending aorta (asc ao), right pulmonary artery (RPA) and descending aorta (desc ao).
Statistical analysis
Continuous variables and changes in continuous variables were compared between groups using a student's t-test. Categorical variables were compared between groups by a chi-square test. Mean CAC score differences were compared using a nonparametric two-sample wllcoxon score test, median scores were compared by the median score test, and four coronary calcium categories were compared between groups by a mantel-haenszel chi-square test. Multiple logistic regression tested for the relationship of CAC>0 versus CAC=0 with surgery group after adjusting for baseline variables that might confound the relationship between CAC and GBS. Odds ratios for one standard deviation of each continuous covariate are presented. Clinically relevant variables that were significant in the univariable models were included in the final multivariable model.
Results
We enrolled 65 GBS patients and 84 nonsurgical patients. Baseline clinical characteristics of the cohort for this study are shown in table 1. At enrollment, the nonsurgical subjects were slightly older than subjects who had gastric bypass surgery (47 vs. 43 years, p=0.007), weighed less (125 vs. 134 kg, p=0.04) and had lower glucose levels and less diabetes. Insulin levels, however, did not significantly differ between groups. Blood pressures, lipids, LV mass and ejection fraction, and smoking status also did not significantly differ between groups at baseline.
Table 1.
Baseline characteristics of the study cohort.
| Nonsurgical (n=84) | Gastric Bypass Surgery (n=65) | p value | |
|---|---|---|---|
| Age (years) | 47.5±11.2 | 42.8±9.1 | 0.007 |
| Gender (%male) | 25 | 18 | 0.34 |
| Body mass index (kg/m2) | 44.0±6.6 | 47.1±7.6 | 0.010 |
| Weight (kg) | 125.4±25.5 | 134.0±25.2 | 0.04 |
| Current smoker (%) | 4.8 | 12.5 | 0.09 |
| Menopause (% of females) | 54.0 | 32.1 | 0.02 |
| Diabetes (%) | 35.7 | 16.9 | 0.011 |
| Hypertension (%) | 47.6 | 35.4 | 0.13 |
| Systolic blood pressure (mmHg) | 133.7+18.8 | 128.8±19.7 | 0.12 |
| Diastolic blood pressure (mmHg) | 78.9±14.2 | 76.1±14.2 | 0.23 |
| LDL cholesterol (mg/dl) | 120.4±33.5 | 119.7±34.2 | 0.90 |
| HDL cholesterol (mg/dl) | 45.1±9.7 | 45.1±12.4 | 0.99 |
| Triglycerides | 182.4±90.8 | 204.8±132.5 | 0.22 |
| (mg/dl) | |||
| Glucose (mg/dl) | 113.9±28.7 | 99.4±25.7 | 0.002 |
| HbA1c (%) | 6.1±1.03 | 5.6±0.60 | 0.0002 |
| Insulin (μU/ml) | 15.5+8.3 | 15.9±9.9 | 0.77 |
| Left ventricular mass (g)* | 188.6+59.1 | 190.5±68.7 | 0.87 |
| Left ventricular ejection fraction (%)* | 65.2±8.1 | 66.8±9.8 | 0.35 |
| Years Education | 13.8±2.3 | 14.2±2.2 | 0.29 |
| Income category† | 3.3+1.3 | 3.8±1.2 | 0.014 |
For left ventricular mass and ejection fraction at baseline there were only 57 and 51 measurements available.
Six categories of income: 1=<10K; 2=10-29K; 3=30-49K; 4=50-69K; 5=70-99K;6=≥100K
At the time of the 6-year visit, patients in the GBS group had lost a mean of 40.5 kg body weight vs. a gain of 1.3 kg (p < 0.001) in the control group. Metabolic risk factors for coronary artery disease significantly improved between baseline and year 6 in gbs compared with control subjects: LDL cholesterol (−1.3 vs. +21.9 mg/dl), HDL cholesterol (+9.4 vs. −2.8 mg/dl), serum triglycerides (−69.5 vs. +9.6 mg/dl), fasting glucose (− 18.9 vs. + 1.5 mg/dl) and systolic blood pressure (−8.4 vs. +1.8 mmhg) (all p<0.01). Left ventricular mass declined from baseline to 6 years in the GBS (p=0.02), but not the control subjects (p=0.82). However, the difference in the change between groups did not reach statistical significance. Left ventricular ejection fraction did not significantly change in either group between baseline and year 6.
Table 2 shows the mean, median, and CAC distribution in the two study groups. Seventy-two percent of the GBS group had no measurable CAC compared with 49% in the control group, with the overall distributions of CAC across four categories significantly different between groups (p=0.003). Because the greatest difference in CAC distribution percentages between the two groups occurred in the 0 CAC category, further logistic regressions were run using presence or absence of CAC as the dependent variable to assess the influence of additional covariates on CAC scores.
Table 2.
Computed tomographic results at 6 year follow up.
| Coronary Artery Calcium Score | No Gastric Bypass Surgery (n=84) | Gastric Bypass Surgery (n=65) | P value |
|---|---|---|---|
| Mean±SD (range) | 103±325 (0-2516) | 30±109 (0-645) | 0.002* |
| Median (IQR) | 1 (0-32) | 0(0-1) | 0.004† |
| Calcium score category | 0.003‡ | ||
| 0 | 49% (n=41) | 72% (n=47) | |
| 1-10 | 18% (n=15) | 14% (n=9) | |
| 11-100 | 17% (n=14) | 8% (n=5) | |
| >100 | 17% (n=14) | 6% (n=4) | |
Data shown in table are Agatston scores.
Wilcoxon two-sample score test
Median score test
Mantel-Haenszel chi-square test
Table 3 shows the significant covariates that predict the presence or absence of CAC (>0 vs. 0). Only gender, age at CAC measurement, baseline BMI, and baseline LDL-C significantly predicted the presence of CAC. after adjustment for these covariates, the odds ratio (95% ci) of having > 0 CAC for the GBS versus the control group was 0.39 (0.17, 0.90). Baseline systolic blood pressure, insulin, HDL-C, smoking status, income level, marital status, or education level were not significant in the logistic model. Despite diabetes prevalence being significantly different at baseline between groups, it did not significantly predict the presence of CAC and the group difference remained significant (p=0.034) with diabetes in the model. Replacing diabetes with either glucose or HbA1c in the logistic model showed that neither of these variables significantly predicted CAC. Neither baseline nor change in lv mass or ejection fraction predicted CAC. A stepwise logistic model was used forcing into the model gender, age, baseline BMI and baseline LDL-C and allowing variables representing changes from baseline to the 6-year exam to enter, including changes in LDL-C, triglycerides, HDL-C, systolic and diastolic blood pressure, glucose, insulin and HbAic. None of these change variables entered the logistic model and did not further explain CAC. When group status was replaced by BMI change and LDL-C change in the logistic model, greater BMI loss was associated with an odds ratio for elevated CAC of 0.61 for a 1 sd decrease in BMI of 8.7 kg/m2 (95% ci, 0.38-0.99; p=0.045). The odds ratio for LDL-C change was not significant (or 1.11; ci 0.68-1.82 for a 1 sd decrease in LDL-C of 36.7 mg/dl; p=0.67).
Table 3.
Logistic Regression Coefficients for the Presence or Absence of cac
| Variable | Odds Ratios (95% Confidence Intervals | p-value |
|---|---|---|
| Gender (F vs. M) | 0.22 (0.08, 0.57) | 0.002 |
| age at cac measurement (for 1 SD of 10.4 y) | 2.8 (1.8,4.4) | <0.001 |
| Baseline BMI (for 1 SD of 7.2 kg/m2) | 1.6(1.1,2.5) | 0.020 |
| Baseline LDL-C (for 1 SD of 33.7 mg/dl) | 1.7(1.1,2.6) | 0.011 |
| Group (GBS vs. Controls) | 0.39 (0.17, 0.90) | 0.027 |
Discussion
Severe obesity is increasingly being treated with bariatric surgery. The cost effectiveness of this approach will be largely determined by the ability of the surgical procedure to prevent adverse, long-term sequelae of obesity. In addition to other known benefits of bariatric surgery, surgical weight loss is predicted to reduce cardiovascular events as there are dramatic and sustained improvements in nearly all of the known nc no coronary risk factors.23, 25, 28 The results of the current study support the hypothesis that the marked weight loss and improvement in risk factors after bariatric surgery may slow the progression of coronary atherosclerosis, thus resulting in lower CAC scores in GBS compared to nonsurgical obese patients after 6 years.
In the 1980's, medical treatments for hypercholesterolemia were extremely limited. The program on surgical control of Hypercholesterolemia (posch) used ileal diversion to lower serum cholesterol levels.31 This procedure is related to current gastric bypass surgeries, although ileal diversion produces more pronounced malabsorption. The patients studied in POSCH were not necessarily overweight and the surgery was not intended to be a means of weight loss. This relatively extreme form of lipid lowering treatment, when applied to a group of patients with known coronary artery disease, was found to be associated with slower progression of coronary arterial luminal narrowing as assessed by serial invasive angiography.31
Invasive angiography is not an acceptable method for longitudinal study of atherosclerosis in asymptomatic patients. Fortunately, newer, noninvasive methods of tracking atherosclerosis are now available. Coronary calcium scoring gives reproducible, quantitative measures of calcified plaque that are highly predictive of future coronary events. Coronary calcium scoring is currently considered to be appropriate in asymptomatic patients with intermediate risk factors for coronary events, or selected lower risk patients such as those with a family history of premature coronary artery disease.32 Calcification of atherosclerotic plaques is thought to be, at least in part, a response to injury, inflammation or hemorrhage.13 As such, the presence of coronary calcium suggests prior “active” plaques. In the short term, increased calcification of plaques may actually represent a healing process. perhaps, this explains the surprising lack of effect of statin therapy on progression of 90 CAC in most short to intermediate term studies (< 1 year) of this therapy.20, 33 However, if progression of atherosclerosis is truly slowed, then over a longer period of time, effective therapy should reduce the appearance of new coronary calcification, a process that tends to continue inexorably in most patients with CAC present at the time of a baseline examination. Only 1 randomized, long-term study has suggested that CAC progression may be slowed by a pharmacological intervention. The women's health Initiative randomized women with prior hysterectomy, age 50-59, to estrogen therapy or placebo.34 At seven years after enrollment, the women randomized to estrogen had significantly lower CAC scores than those randomized to placebo.34 In that study, like ours, baseline CAC scores were not available. The findings from the women's health study, in conjunction with the present data, support the concept that it will typically require studies with long time frames (likely > 5 years) in order to demonstrate whether an intervention alters CAC progression. The mechanistic pathways by which GBS or weight loss are independently associated with zero CAC, will require additional investigation. It is tempting to speculate that improvements in traditional coronary risk factors and more novel risk markers such as adipocytokines or other inflammatory markers could be involved.
Despite the fact that long studies will be necessary, using CAC as a marker of atherosclerosis progression has many compelling advantages. The test is quick, painless, low cost and increasingly available. The results of CAC scoring are quantitative and have good reproducibility. A high degree of reader training and skill is not required for accurate interpretation. The rate of progression of CAC is relatively high in the general population, estimated at 25 agatston units/year in participants of the mesa study.19 Thus, it is feasible to detect changes in the rate of progression studying smaller study populations. Calcium scores have very high predictive value for cardiac events and cardiovascular mortality35, and hence, have been proposed as a useful surrogate for hard clinical endpoints. It will almost certainly take more patients and longer studies to prove whether weight loss interventions definitively change both CAC scores and clinical outcomes.
Limitations
There are 2 major limitations of this study: 1) this is a cross sectional sample that includes only a subset (n=149) of the subjects enrolled in the study, and 2) we do not have baseline CAC scores so we cannot assess changes in CAC over time. The reasons for this are that CAC scoring was not part of the original protocol. It was not widely available at the inception of this longitudinal study, which began in 2001 and was conceived prior to that. Unlike many studies of bariatric urgery, we did include a reference group of subjects with continued obesity that were enrolled and followed concurrently. This allows us to draw inferences about differences in CAC scores at follow up, even though this parameter was not evaluated initially. Conclusions based on differences between groups at follow up are predicated on the fact that the groups were relatively well matched at baseline in terms of coronary risk factors. Fortunately this is the case. We cannot exclude the possibility that the lower CAC scores in the patients who underwent GBS are the result of a sampling error rather than a result of weight loss. However, it seems plausible that GBS with subsequent weight loss does alter the course of coronary atherosclerosis given the profound and sustained improvements in coronary risk factors that persist for years after GBS.25, 28 No other single treatment has produced this breadth and magnitude of coronary risk factor attenuation. Nonetheless, prospective data will be needed before it can be concluded that GBS and its related beneficial effects fundamentally alters atherosclerosis.
Implications
Bariatric surgery continues to be the most effective means of producing sustained weight loss and reduction in coronary risk factors in severely obese patients. The use of bariatric procedures is increasing rapidly and new procedures such as the gastric sleeve operation are being introduced. The cost of bariatric surgery occurs at the time of the procedure while many of the cost benefits, such as potential reductions in cardiovascular morbidity, are delayed. Our study supports the hypothesis that there is substantial long-term cardiovascular benefit from surgical weight loss procedures independent of traditional risk factors such as lowering cholesterol.
Acknowledgments
Funding: The study was supported by the National Institute of Health (R01 DK055006 and M01 RR000064) and the Amundsen Endowed Professorship, University of Utah.
Footnotes
Disclosures: None of the authors have any conflicts of interest to disclose.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. Jama. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Sturm R. Increases in morbid obesity in the USA: 2000-2005. Public Health. 2007;121:492–6. doi: 10.1016/j.puhe.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassidy AE, Bielak LF, Zhou Y, Sheedy PF, 2nd, Turner ST, Breen JF, Araoz PA, Kullo IJ, Lin X, Peyser PA. Progression of subclinical coronary atherosclerosis: does obesity make a difference? Circulation. 2005;111:1877–82. doi: 10.1161/01.CIR.0000161820.40494.5D. [DOI] [PubMed] [Google Scholar]
- 4.Folsom AR, Stevens J, Schreiner PJ, McGovern PG. Body mass index, waist/hip ratio, and coronary heart disease incidence in African Americans and whites. Atherosclerosis Risk in Communities Study Investigators. Am J Epidemiol. 1998;148:1187–94. doi: 10.1093/oxfordjournals.aje.a009608. [DOI] [PubMed] [Google Scholar]
- 5.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2007;115:2722–30. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 6.Lee CD, Jacobs DR, Jr., Schreiner PJ, Iribarren C, Hankinson A. Abdominal obesity and coronary artery calcification in young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2007;86:48–54. doi: 10.1093/ajcn/86.1.48. [DOI] [PubMed] [Google Scholar]
- 7.Kramer CK, von Muhlen D, Gross JL, Barrett-Connor E. A prospective study of abdominal obesity and coronary artery calcium progression in older adults. J Clin Endocrinol Metab. 2009;94:5039–44. doi: 10.1210/jc.2009-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ROCHA VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 9.Meyers MR, Gokce N. Endothelial dysfunction in obesity: etiological role in atherosclerosis. Curr Opin Endocrinol Diabetes Obes. 2007;14:365–9. doi: 10.1097/MED.0b013e3282be90a8. [DOI] [PubMed] [Google Scholar]
- 10.Maahs DM, Ogden LG, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, Hokanson JE, Ehrlich J, Eckel RH, Rewers M. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111:747–53. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- 11.Martin SS, Qasim AN, Rader DJ, Reilly MP. C-reactive protein modifies the association of plasma leptin with coronary calcium in asymptomatic overweight individuals. Obesity. 2012;20:856–61. doi: 10.1038/oby.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunita E, Yamamoto H, Kitagawa T, Ohashi N, Utsunomiya H, Oka T, Horiguchi J, Awai K, Kihara Y. Association between plasma high-molecular-weight adiponectin and coronary plaque characteristics assessed by computed tomography angiography in conditions of visceral adipose accumulation. Circ J. 2012;76:1687–96. doi: 10.1253/circj.cj-11-1442. [DOI] [PubMed] [Google Scholar]
- 13.Serrano CV, Jr., Oranges M, Brunaldi V, de MSA, Torres TA, Nicolau JC, Ramires JA. Skeletonized coronary arteries: pathophysiological and clinical aspects of vascular calcification. Vasc Health Risk Manag. 2011;7:143–51. doi: 10.2147/VHRM.S16328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Shareghi S, Ahmadi N, Young E, Gopal A, Liu ST, Budoff MJ. Prognostic significance of zero coronary calcium scores on cardiac computed tomography. J Cardiovasc Comput Tomogr. 2007;1:155–9. doi: 10.1016/j.jcct.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Hou ZH, Lu B, Gao Y, Jiang SL, Wang Y, Li W, Budoff MJ. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5:990–9. doi: 10.1016/j.jcmg.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O'Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46:807–14. doi: 10.1016/j.jacc.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 18.Martin SS, Blaha MJ, Blankstein R, Agatston A, Rivera JJ, Virani SS, Ouyang P, Jones SR, Blumenthal RS, Budoff MJ, Nasir K. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation. 2014;129:77–86. doi: 10.1161/CIRCULATIONAHA.113.003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, Blumenthal RS, Detrano RC, Bild DE, Guerci AD, Liu K, Shea S, Szklo M, Post W, Lima J, Bertoni A, Wong ND. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2013;61:1231–9. doi: 10.1016/j.jacc.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Priester TC, Litwin SE. Measuring progression of coronary atherosclerosis with computed tomography: searching for clarity among shades of gray. J Cardiovasc Comput Tomogr. 2009;3(Suppl 2):S81–90. doi: 10.1016/j.jcct.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Wee CC, Girotra S, Weinstein AR, Mittleman MA, Mukamal KJ. The relationship between obesity and atherosclerotic progression and prognosis among patients with coronary artery bypass grafts the effect of aggressive statin therapy. J Am Coll Cardiol. 2008;52:620–5. doi: 10.1016/j.jacc.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 23.Adams TD, Pendleton RC, Strong MB, Kolotkin RL, Walker JM, Litwin SE, Berjaoui WK, LaMonte MJ, Cloward TV, Avelar E, Owan TE, Nuttall RT, Gress RE, Crosby RD, Hopkins PN, Brinton EA, Rosamond WD, Wiebke GA, Yanowitz FG, Farney RJ, Halverson RC, Simper SC, Smith SC, Hunt SC. Health outcomes of gastric bypass patients compared to nonsurgical, nonintervened severely obese. Obesity. 2010;18:121–30. doi: 10.1038/oby.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219–34. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 25.Benraouane F, Litwin SE. Reductions in cardiovascular risk after bariatric surgery. Curr Opin Cardiol. 2011;26:555–61. doi: 10.1097/HCO.0b013e32834b7fc4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, Ahlin S, Anveden A, Bengtsson C, Bergmark G, Bouchard C, Carlsson B, Dahlgren S, Karlsson J, Lindroos AK, Lonroth H, Narbro K, Naslund I, Olbers T, Svensson PA, Carlsson LM. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 27.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF. Overweight, Obesity, and Mortality in a Large Prospective Cohort of Persons 50 to 71 Years Old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 28.Adams TD, Davidson LE, Litwin SE, Kolotkin RL, LaMonte MJ, Pendleton RC, Strong MB, Vinik R, Wanner NA, Hopkins PN, Gress RE, Walker JM, Cloward TV, Nuttall RT, Hammoud A, Greenwood JL, Crosby RD, McKinlay R, Simper SC, Smith SC, Hunt SC. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308:1122–31. doi: 10.1001/2012.jama.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owan T, Avelar E, Morley K, Jiji R, Hall N, Krezowski J, Gallagher J, Williams Z, Preece K, Gundersen N, Strong MB, Pendleton RC, Segerson N, Cloward TV, Walker JM, Farney RJ, Gress RE, Adams TD, Hunt SC, Litwin SE. Favorable changes in cardiac geometry and function following gastric bypass surgery: 2-year follow-up in the Utah obesity study. J Am Coll Cardiol. 2011;57:732–9. doi: 10.1016/j.jacc.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka pa, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Buchwald H, Matts JP, Fitch LL, Campos CT, Sanmarco ME, Amplatz K, Castaneda-Zuniga WR, Hunter DW, Pearce MB, Bissett JK, et al. Changes in sequential coronary arteriograms and subsequent coronary events. Surgical Control of the Hyperlipidemias (POSCH) Group. JAMA. 1992;268:1429–33. [PubMed] [Google Scholar]
- 32.Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P, Rubin GD. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Journal of cardiovascular computed tomography. 2010;4:407, E1–33. doi: 10.1016/j.jcct.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 33.McEvoy JW, Blaha MJ, Defilippis AP, Budoff MJ, Nasir K, Blumenthal RS, Jones SR. Coronary artery calcium progression: an important clinical measurement? A review of published reports. J Am Coll Cardiol. 2010;56:1613–22. doi: 10.1016/j.jacc.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 34.Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, Kuller LH, Cochrane BB, Hunt JR, Ludlam SE, Pettinger MB, Gass M, Margolis KL, Nathan L, Ockene JK, Prentice RL, Robbins J, Stefanick ML. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 35.Budoff MJ, Nasir K, McClelland RL, Detrano R, Wong N, Blumenthal RS, Kondos G, Kronmal RA. Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2009;53:345–52. doi: 10.1016/j.jacc.2008.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]