Abstract
Background
Most prior studies evaluating subsequent malignant neoplasms (SMN) in patients with neuroblastoma are restricted to long-term survivors and/or their treatment exposures. This study investigates SMNs in patients diagnosed with neuroblastoma at our institution.
Methods
Records of 646 patients treated for neuroblastoma at St. Jude Children’s Research Hospital between 1961 and 2005 were reviewed. Data from patients with SMNs were analyzed and the 20-year and 30-year cumulative incidence of SMNs and standardized incidence ratio (SIR) were calculated.
Results
Twenty-one patients had a SMN. The 20- and 30-year cumulative incidences of a SMN were 2.6% ± 0.7% and 4.6% ± 1.1% respectively. The SIR was 8.3 (95% CI, 5.0–13.0). Five patients developed a SMN within 5 years from diagnosis. The median latency for the development of AML/MDS (n=4), sarcomas (n=7), and carcinomas (n=5) were 3.6 years, 9 years, and 24.2 years respectively. Nine patients died from their SMN, including all with AML/MDS.
Conclusions
Patients with neuroblastoma have an increased risk of secondary neoplasia. Modification of risk-adapted therapies will likely alter the affected patient population and the incidence of SMNs. Future studies are necessary to link SMNs to treatment exposures and to evaluate the risk of SMNs beyond 30 years from diagnosis.
Keywords: neuroblastoma, subsequent neoplasms, second malignancy
Introduction
Neuroblastoma is the most common pediatric extra-cranial solid malignancy,1 accounting for approximately 7.5% of all malignancies in children under 15 years of age.2 Some patients can be cured with surgery alone3 or with surgery and moderately intense chemotherapy.4 For patients with high risk disease, a multimodality treatment approach including intense chemotherapy, surgery, and radiation therapy have improved the 5-year survival rates from 5%5 to approximately 20–30%.6, 7 More recently, high-dose chemotherapy followed by hematopoietic cell rescue8 and immunotherapy 9 have further boosted survival rates to over 50%.
According to the US Surveillance Epidemiology and End Results (SEER) program,1 from 1975 until 1995 the incidence of childhood cancers increased while mortality decreased. Thus, there is an increasing number of childhood cancer survivors 10 at risk for long-term toxicities. In an analysis from the Childhood Cancer Survivor Study (CCSS) 11, the primary cause of death was recurrence of the original cancer. However, the second most common cause of death was a subsequent malignant neoplasm (SMN).
With an increasing number of children surviving the diagnosis of neuroblastoma, often due to very intense therapy, understanding the spectrum of late toxicities gains importance. The purpose of this study was to estimate the incidence of SMNs in consecutive patients treated over several decades for neuroblastoma at a pediatric cancer center and to describe the spectrum of subsequent malignancies identified.
Patients and Methods
Patients who were referred to St. Jude Children’s Research Hospital for treatment of newly diagnosed neuroblastoma from November 1961 to September 2005 were identified through the cancer registry. This retrospective study was approved by the institutional review board. For all patients in the cohort, age at diagnosis of neuroblastoma, location of the primary tumor, stage of disease, vital status and date of most recent follow-up were obtained.
The medical records of 21 patients with a SMN identified through the cancer registry were reviewed. Data extracted included patient history (family history, medical history prior to the diagnosis of neuroblastoma, and any known carcinogenic or radiation exposures of the patient or the patient’s parents), details of the clinical presentation [age at diagnosis, location of the primary tumor and metastases, stage, MYCN status (if known)] and delivered therapy (surgical procedures, chemotherapy exposures/doses, and location/dose of radiotherapy). Alkylator scores were determined as described.12 Pathology, imaging and urine catecholamines obtained at the time of diagnosis were reviewed to confirm the primary diagnosis of neuroblastoma for all patients who developed a subsequent malignancy. For each SMN, the date of diagnosis, histology, presenting symptoms, treatment, and outcome were obtained. Pathology reports of the subsequent malignancies were available for 18 patients (16 reviewed at our institution and 2 reports from outside institutions). Three patients (9, 14 and 16) did not have pathology reports available for review; however, all three had outside medical records describing their SMN. Radiation therapy records were reviewed to determine whether the SMN arose in a radiation field. For patients who elected to have genetic testing, data regarding TP53 mutations were obtained from the clinical record. No genetic testing was performed for this study.
Statistical Methods
The exact Wilcoxon rank sum test was used to compare age distributions among patients with and without second malignancies. Fisher’s exact test was used to compare gender and race distributions between patient groups.
Expected numbers of cancers were calculated using public use data from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute 13. The number of person-years of observation was compiled for age- and gender-defined subgroups and calculated as the time from diagnosis of neuroblastoma to diagnosis of SMN, death or the date of last contact, whichever was first. The standardized incidence ratio (SIR) was calculated as the ratio of the observed number of cases to the expected number of cases. SIRs are presented with 95% confidence intervals.14 The cumulative incidence of SMN was estimated. Death prior to development of a SMN was considered a competing event.
Results
Patient Characteristics
From November 1, 1961 to September 30, 2005, 758 patients with neuroblastoma were identified of whom 646 met the inclusion criteria. Most of the ineligible patients were referred for treatment of recurrent neuroblastoma and lacked significant data points. Amongst these 646 patients, 21 (3.3%) developed a SMN.
Table 1 shows patient characteristics for the whole cohort (n=646) and for the subgroups of patients with and without a SMN after neuroblastoma. The median age at diagnosis of neuroblastoma for all patients was 25.5 months (range, birth to 19.4 years). There were no statistically significant differences in the distributions of age at diagnosis (p=0.19), gender (p=0.27), or race (black vs. white) (p=0.25) among the patients with and without a SMN. The distribution of stages by SMN was statistically significant (p=0.006). Two hundred ninety-one patients were alive at a median follow-up of 23.1 years from diagnosis (range, 2.1 – 47.9 years). Approximately 85% of survivors (246 of 291) have been seen or contacted within the last 2 years.
Table 1.
Patient Characteristics
| Population (n=646) | Patients without SMN (n=625) | Patients with SMN (n=21) | |
|---|---|---|---|
|
| |||
| Age at diagnosis | |||
| Median | 25.5 months | 25.9 months | 14.4 months |
| Range | birth – 19.4 years | birth – 19.4 years | 0.3 months – 8.7 |
|
| |||
| Gender | |||
| Male | 362 (56.0%) | 353 (56.5%) | 9 (42.9%) |
| Female | 284 (44.0%) | 272 (43.5%) | 12 (57.1%) |
|
| |||
| Race | |||
| White | 508 (78.6%) | 493 (78.9%) | 15 (71.4%) |
| Black | 116 (18.0%) | 110 (17.6%) | 6 (28.6%) |
| Hispanic | 8 (1.2%) | 8 (1.3%) | - |
| Unknown | 11 (1.7%) | 11 (1.8%) | - |
| Other | 3 (0.5%) | 3 (0.5%) | - |
|
| |||
| INSS Stage | |||
| INSS 1 | 86 (13.3%) | 83 (13.3%) | 3 (14.3%) |
| INSS 2A | 37 (5.7%) | 36 (5.7%) | 1 (4.8%) |
| INSS 2B | 55 (8.5%) | 48 (7.7%) | 7 (33.3%) |
| INSS 3 | 61 (9.4%) | 60 (9.6%) | 1 (4.8%) |
| INSS 4 | 372 (57.6%) | 363 (58.1%) | 9 (42.9%) |
| INSS 4S | 35 (5.4%) | 35 (5.6%) | 0 (0.0%) |
|
| |||
| Primary Site Group | |||
| Adrenal | 332 (51.4%) | 323 (51.7%) | 9 (42.9%) |
| Abdomen-Nonadrenal | 142 (22.0%) | 138 (22.1%) | 4 (19.0%) |
| Thoracic | 117 (18.1%) | 115 (18.4%) | 2 (9.5%) |
| Cervical | 22 (3.4%) | 19 (3.0%) | 3 (14.3%) |
| Pelvic | 19 (2.9%) | 17 (2.7%) | 2 (9.5%) |
| Nasal Cavity | 3 (0.5) | 2 (0.3%) | 1 (4.8%) |
| Unknown | 11 (1.7%) | 11 (1.8%) | 0 (0.0%) |
The medical histories of the 21 patients who developed second malignancies were reviewed. None of the patients who developed a SMN had an overgrowth syndrome. Although some patients had several family members with a diagnosis of cancer, none met the diagnostic criteria for Li-Fraumeni or Li-Fraumeni-like syndrome.15 None of the patients had neuroblastoma arising in both adrenal glands, a family history of neuroblastoma, congenital central hypoventilation syndrome, or Hirschsprung disease. The patient who developed a malignant peripheral nerve sheath tumor did not have evidence of neurofibromatosis, type 1. No TP53 mutations were identified in the peripheral blood of 2 patients (one who developed Ewing sarcoma and one who developed rhabdomyosarcoma), although several known polymorphic variants were detected.
The initial presentation of neuroblastoma and subsequent treatment of each patient who developed a SMN are provided in Table 2. All patients who developed a SMN had previously received chemotherapy, radiation therapy, or both. The 3 patients with an initial diagnosis of stage I disease received chemotherapy and/or radiation therapy for recurrent neuroblastoma prior to development of the SMN. All of the 19 patients who received chemotherapy were treated with cyclophosphamide (cumulative dosage range 384 – 28,510 mg/m2), 16 were exposed to doxorubicin (cumulative dosage range 71 – 463 mg/m2) and 10 to an epipodophyllotoxin [6 to etoposide (cumulative dosage range 1335 – 17484 mg/m2); 4 to teniposide (cumulative dosage range 494 – 1216 mg/m2)]. The 4 patients who developed treatment-related acute myeloid leukemia /myelodysplastic syndrome (AML/MDS) received cyclophosphamide (alkylator score ≥3 for 3 of the 4) as part of their initial therapy and 3 also received an epipodophyllotoxin. The patient with AML who was not exposed to an epipodophyllotoxin received 25 Gy of radiation to the abdomen and pelvis. Two of the four patients who developed AML/MDS had MLL gene rearrangement. The other two patients did not undergo this analysis. Of the 10 patients with a solid SMN who were exposed to radiation therapy, 6 developed a subsequent neoplasm within the radiation field with doses ranging from 15Gy to 39Gy.
Table 2.
Characteristics of 21 Patients with SMN after Neuroblastoma
| Pt # |
Sex | Race | Neuroblastoma | Second Malignancy | Outcome (yrs after SM diagnosis) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at Dx (yrs) |
Primary Site Group |
INSS Stage |
Year of Diagnosis |
Chemotherapy | Radiation Therapy |
Age at Diagnosis (years) |
Latency (years) |
Histologic diagnosis | Site | SMN within RT field? |
||||
| 1 | F | W | 0.1 | Adrenal | 4 | 1963 | Cy, V | None | 44.3 | 44.2 | Squamous cell carcinoma | Uterine cervix | No | Alive (3.7) |
| 2 | M | W | 0.2 | Abdomen-Nonadrenal | 4 | 1971 | V,Cy | Abdomen 33 Gy Pelvis 15.45 Gy |
27.1 | 26.9 | Hodgkin lymphoma | Inguinal Lymph Node | Yes | Alive (13.7) |
| 3 | M | AA | 1.7 | Adrenal | 4 | 1971 | V,Cy,D | Abdomen 29 Gy Mediastinum 28 Gy Skull 39 Gy |
10.8 | 9.0 | Malignant peripheral nerve sheath tumor | Frontal Lobe | Yes | Expired (0.8) |
| 4 | M | W | 0.02 | Thoracic | 4 | 1972 | V,Cy,D Imm | Abdomen 26.75 Gy Mediastinum 12.5 Gy |
29.7 | 29.7 | Adenocarcinoma | Colon | Yes | Expired (3.1) |
| 5 | F | AA | 1.2 | Cervical | 2B | 1973 | Cy | Lateral neck 35 Gy | 19.7 | 18.5 | Papillary carcinoma | Thyroid | Yes | Alive (19.6) |
| 6 | F | W | 4.0 | Pelvic | 3 | 1973 | V,Cy,D Imm | Abdomen/Pelvis 25 Gy | 9.5 | 5.4 | Acute myelomonocytic leukemia | N/A | Expired (0.9) | |
| 7 | M | W | 0.9 | Abdomen-Nonadrenal | 1 | 1976 | None | Paraspinal 23.25 Gy | 6.7 | 5.8 | Basal cell carcinoma | Back | Yes | Alive (29.4) |
| 8 | F | W | 1.1 | Adrenal | 1 | 1977 | None | Abdomen 24 Gy | 14.2 | 13.1 | Rhabdoid tumor | Posterior mediastinum | No | Expired (0.6) |
| 9 | M | W | 0.7 | Adrenal | 2B | 1977 | Cy,D Imm | None | 25.2 | 24.5 | Squamous cell carcinoma | Tongue | No | Alive (9.4) |
| 10 | F | W | 0.02 | Thoracic | 1 | 1980 | Cy,D | None | 10.7 | 10.7 | Mucoepidermoid carcinoma | Submandibular gland | No | Alive (21.4) |
| 11 | F | AA | 1.1 | Abdomen-Nonadrenal | 4 | 1981 | Cy,D,P,T | None | 26.2 | 25.0 | Clear cell carcinoma | Abdomen –unclear organ of origin | No | Expired (1.2) |
| 12 | M | W | 0.5 | Cervical | 2B | 1982 | Cy,D,P,T | None | 16.3 | 15.8 | Osteosarcoma | Maxilla | No | Alive (12.7) |
| 13 | F | W | 3.1 | Adrenal | 4 | 1983 | Cy,D,P,T | None | 27.3 | 24.2 | Renal cell carcinoma | Left kidney | No | Alive (3.8) |
| 14 | F | W | 8.7 | Adrenal | 4 | 1984 | Cy,D,P,T | None | 10.0 | 1.3 | Acute myelomonocytic leukemia | N/A | Expired (1.0) | |
| 15 | F | AA | 1.5 | Adrenal | 4 | 1989 | Cy,D,P,I,Car,E | None | 7.0 | 5.6 | Rhabdomyosarcoma (embryonal) | Thigh | No | Expired (0.4) |
| 16 | M | W | 0.9 | Pelvic | 2B | 1993 | Cy,D | None | 12.0 | 11.1 | Basal cell carcinoma | Chest | No | Alive (8.0) |
| 17 | F | W | 5.1 | Nasal Cavity | 2A | 1993 | V,Cy,D,I,E,Dac | Nasal cavity 30.5 Gy | 7.4 | 2.3 | Myelodysplastic syndrome [t(11;16)] | N/A | Expired (2.1) | |
| 18 | M | AA | 3.6 | Adrenal | 4 | 1997 | V,Cy,D,I,E,Top | None | 8.5 | 4.9 | Acute monocytic leukemia [t(9;11)(p22;q23)] | N/A | Expired (0.7) | |
| 19 | F | W | 3.3 | Adrenal | 2B | 2000 | Cy, D, E, Car | None | 12.3 | 9.0 | Gastrointestinal stromal tumor (GIST) | Stomach | No | Alive (0.9) |
| 20 | F | W | 6.1 | Cervical | 2B | 2003 | Cy,D,E,Car | Neck 30.6 Gy | 10.8 | 4.7 | Ewing sarcoma | Lumbar spine | No | Alive (4.0) |
| 21 | M | B | 2.2 | Abdomen-Nonadrenal | 2B - >4* | 2005 | Cy,D,P,E,Ir,Top,Car,M | Abdomen 23.4 Gy | 5.2 | 3.1 | Rhabdomyosarcoma (spindle cell) | Abdomen | Yes | Expired (3.5) |
Abbreviations: Dx, diagnosis; M, male; F, female; W, white; AA, African-American; V, vincristine; Cy, cyclophosphamide; D, doxorubicin; P, cisplatin; T, teniposide; I, ifosfamide; E, etoposide; Car, carboplatin; Dac, dactinomycin; Ir, irinotecan; M, melphalan; Imm, immunotherapy with BCG vaccine
developed widely metastatic disease after initial surgery for localized disease
Characteristics of Second Neoplasms
Of the 21 patients with SMNs (see Table 2), 11 were alive at the time of analysis. The median latency from diagnosis of neuroblastoma to diagnosis of a SMN was 10.7 years (range 1.3 to 44.2 years). The median latency from diagnosis of neuroblastoma to diagnosis of AML/MDS (n=4) was 3.6 years (range 1.3 to 5.4 years), of a sarcoma (n=7) was 9.0 years (range 3.1 to 15.8 years), and of a carcinoma (n=9) was 24.2 years (range 5.8 to 44.2 years). One additional patient developed Hodgkin lymphoma approximately 27 years after her neuroblastoma diagnosis.
Three patients developed a second small round blue cell tumor that was confirmed to be histologically different from the original tumor within 6 years of the initial diagnosis of neuroblastoma. One patient with high-risk neuroblastoma had a new abdominal mass noted at the completion of maintenance therapy. Although highly suspicious for recurrent neuroblastoma, biopsy of the mass revealed embryonal rhabdomyosarcoma (spindle cell variant) with expression of myogenin and muscle-specific actin. Two other patients presented with painful lesions that were thought to be recurrent neuroblastoma but on biopsy were found to be embryonal rhabdomyosarcoma (n=1) and Ewing sarcoma (n=1). The patient with Ewing sarcoma was previously treated for unresectable localized cervical neuroblastoma with chemotherapy and radiation therapy. Imaging evaluations for worsening back pain revealed a dumbbell-shaped mass at the L4 spinal. The biopsy demonstrated Ewing sarcoma and contained the EWS/FLI-1 fusion transcript.
Cumulative Incidence of Subsequent Neoplasms
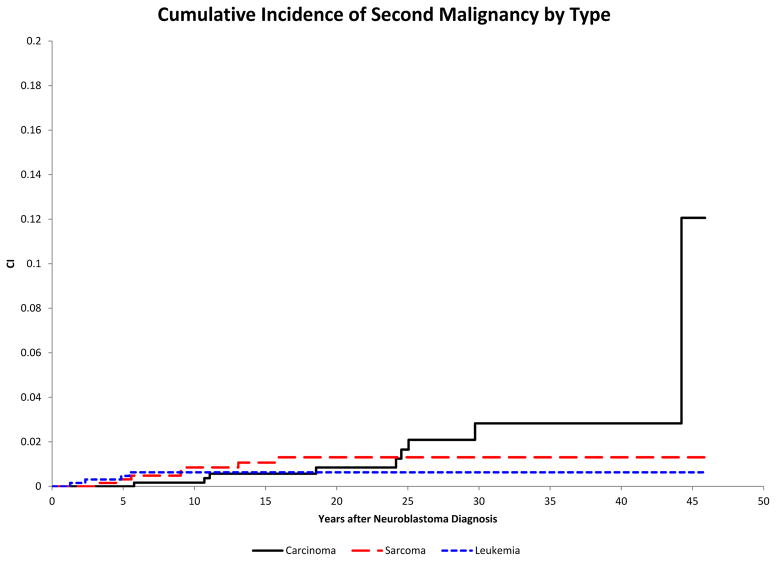
The 20-year and 30-year cumulative incidences of second malignancy were estimated to be 2.6% ± 0.7% and 4.6% ± 1.1%, respectively. The 20-year cumulative incidence of AML/MDS was 0.6%± 0.3%, of sarcomas was 1.3%± 0.5% and of carcinomas was 0.8%± 0.4% (Figure 1).
Figure 1.
Cumulative incidence of second malignant neoplasms (SMNs) in survivors of neuroblastoma (y-axis) by histologic class of SMN (acute myeloid leukemia/myelodysplastic syndrome (AML/MDS), sarcoma, and carcinoma) relative to time since diagnosis of neuroblastoma in years (x-axis).
Risk of SMN Compared to the General Population
Based on the total of 21 SMNs in our neuroblastoma cohort and the expected number of cases of 2.27, according to age- and gender-specific SEER rates the SIR was 9.3 (95% CI, 5.7 – 14.2). Excluding the 2 cases with basal cell carcinoma, the SIR was 8.3 (95% CI, 5.0 – 13.0).
Discussion
This study describes the incidence, clinical features, and outcome of subsequent malignant neoplasms in a large cohort of patients initially treated for neuroblastoma. This study is not limited to patients who developed subsequent malignancies 5 years from their neuroblastoma diagnosis,16, 17 who were treated for high-risk disease,18–20 or who had a specific SMN histology21, 22 but includes the entire spectrum of patients with neuroblastoma treated at a single institution. Notable findings include the identification of 5 SMNs within 5 years of diagnosis (3 of which were treatment-related MDS/AML), the second report of Ewing sarcoma as a SMN following neuroblastoma,23 further confirmation of Hodgkin lymphoma,16, 24 renal cell carcinoma,16, 25 soft tissue sarcoma,16, 17, 19, 22, 26, 27 and osteosarcoma17, 18, 21 as SMNs after neuroblastoma treatment, and demonstration of an increased incidence of SMNs versus that expected in an age-matched population.
One important clinical point identified in this cohort is the concurrent presentation of recurrent neuroblastoma and a SMN. Our study includes 2 patients (one with acute monocytic leukemia and one with rhabdomyosarcoma) who developed a SMN within 15 months of completing treatment for their neuroblastoma. Additionally, 2 other patients had symptoms and imaging that were concerning for recurrent neuroblastoma but were proven by biopsy to be Ewing sarcoma and rhabdomyosarcoma. These four cases highlight the importance of an increased suspicion of SMN in this patient population also at risk for recurrent neuroblastoma.
In our cohort of patients, one of the SMNs appeared to arise within the residual mass, in agreement with other reports of rhabdomyosarcomas19, 22 and a malignant peripheral nerve sheath tumor19 arising within a residual mass after treatment for neuroblastoma. Additionally, one patient with a large residual mass 25 years after the diagnosis of neuroblastoma had a significant delay in diagnosis of clear cell carcinoma because many of her symptoms were attributed to the residual mass. The management of residual masses in neuroblastoma is complex, since it has been well-described that some neuroblastic tumors resolve spontaneously or remain stable for years without progression, thus requiring no intervention.28 Furthermore, patients with low and intermediate risk neuroblastoma have excellent outcomes, even if residual tumor remains evident at the end of therapy.29 These findings, along with the report of excellent outcome in intermediate-risk patients with reduced chemotherapy,4 may result in more patients having a residual mass at the end of treatment. In caring for these survivors, physicians should be aware of the potential risk for development of a SMN within the residual mass.
Bone sarcomas are one of the most frequent SMNs in childhood cancer survivors, the vast majority of which are osteosarcoma.30–34 This is most likely related to the known elevated risk in patients with hereditary retinoblastoma,30, 34 Li Fraumeni syndrome,15 and in patients treated with radiation therapy.27, 35, 36 Interestingly, the single patient in our cohort who developed a subsequent osteosarcoma had not received radiation. However, this patient was treated with alkylating agents, which have been implicated in the development of osteosarcoma.33 While osteosarcoma arising as a SMN after treatment of childhood cancer has been frequently described, Ewing sarcoma as a SMN is less common.37, 38 In an extensive review of the literature, there is one report of a case of Ewing sarcoma in a patient previously treated for neuroblastoma.23 Our patient is the second patient ever described. Although none of our patients met the criteria for Li-Fraumeni or Li-Fraumeni-like syndrome, it is possible that there are unrecognized genetic factors that predispose these patients to malignancy.
Not surprisingly, all patients in our cohort who developed a SMN had previously received chemotherapy and/or radiation therapy for the treatment of neuroblastoma. All of the patients with a subsequent AML/MDS had been treated with an epipodophyllin, alkylating agent, or both. Epipodophyllin exposure is a well-known risk factor for the development of treatment-related leukemias,39 although debate surrounds the importance of cumulative dose33 versus schedule of administration.40 Likewise, radiation is well known to play a role in the development of secondary sarcomas26, 32, 36 and carcinomas.33 We observed carcinomas and sarcomas both within previously irradiated tissue as well as in patients who had never received radiation therapy. Due to the small number of patients in our cohort with SMNs and the wide range of SMN histologies and exposures, this study lacks the power to clearly define the relationship between exposures and development of specific SMNs neuroblastoma. However, it is noteworthy that no patient treated with surgery alone developed a second malignancy.
The incidence of SMNs in our neuroblastoma population was 9.3 times higher than the expected incidence in an age and gender-matched population. This increased incidence is within the range of reported median SIRs for SMNs in neuroblastoma survivors of 8 – 10.4.16, 17, 24 When cases of non-melanoma skin cancers are excluded, the SIR was 8.3. Since ours is not a population-based study, there may be a bias towards more intensive therapy at our large referral center. The range of latency periods noted in our study ranging from the lowest for AML/MDS and the highest for carcinomas is similar to that reported by the CCSS33 in which AML has the shortest latency period at 6.1 years and breast cancer the longest at 15.7 years. Bone and soft tissue sarcomas are intermediate at 9.6 and 10.6 years, respectively. 33 In our study, all of the SMNs that occurred 20 or more years after diagnosis were carcinomas. As survivors of neuroblastoma continue to age, further studies are needed to better understand the risk for the development of late SMNs in this population.
Over the past two decades, significant changes have been made to the treatment approach for patients with low-, intermediate- and high-risk neuroblastoma. Patients with low- and intermediate-risk disease have experienced a significant reduction in therapy. In our study, 10 patients with stage 1, 2A or 2B (low-risk or intermediate-risk) disease developed a SMN. These patients were all treated decades ago and if treated in the modern era using the current risk-based stratification system would have received significantly less therapy, possibly diminishing the risk of a SMN. Additionally, two of the stage 4 patients in the study were infants and by current risk-adapted treatment guidelines would have received less therapy, including reduction in chemotherapy for both patients and elimination of radiation therapy for one. Thus our estimates of the risk of SMN for low-risk patients may exceed the actual risk for patients treated with contemporary therapy. Although therapy has been reduced for low- and intermediate-risk groups, significant intensification of therapy has occurred in the high- risk group with consequent improvement in long-term survival. The effects of increased chemotherapy dose intensity, tandem bone marrow transplants, altered radiotherapy regimens, immunologic therapy and MIBG therapy on the risk of SMN are unknown. It is therefore possible that our study underestimates the risk of SMNs for high-risk patients with neuroblastoma treated in the most recent era.
In summary, our study demonstrates that the risk of developing a SMN after the diagnosis of neuroblastoma is increased compared to the general population. The latency periods differ depending on the type of SMN with most AML/MDS developing in the first 5 years after diagnosis, sarcomas 5–15 years following diagnosis and carcinomas more than 15 years after diagnosis. The incidence of carcinomas continues to increase with age. As highlighted in this study, we must continue to evaluate the risk of SMN as treatment strategies change. As treatment intensity is reduced in the low- and intermediate-risk groups moving forward we may see a reduction in SMNs in this population. However, equally important is the fact that intensification of treatment and addition of new therapeutic agents for high-risk patients may be accompanied by higher SMN risks. Given the young age of patients diagnosed with neuroblastoma and the improving survival rates, it will be important investigate whether the incidence of carcinomas continues to increase as this population ages. Additionally, we must closely monitor future survivors, especially those of high-risk disease, who may be at increased risk for developing SMNs.
Acknowledgments
This study was supported in part by Cancer Center Support Grant CA21765 from the US National Institutes of Health (NIH), and by the American Lebanese Syrian Associated Charities (ALSAC). These supporting entities were not involved in the design, conduct or reporting of this study. The authors appreciate the help of Joanna Hwang, Valerie McPherson, and Tad McKeon for data management
Footnotes
Conflicts of Interest
The authors have no financial or personal relationships that would potentially influence the design, conduct or reporting of this study.
Reference List
- 1.Ries LAG, Percy CL, Bunin GR. Introduction. In: Ries LAG, Smith MA, Gurney JG, et al., editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. Bethesda, MD: National Cancer Institute, SEER Program; 1999. pp. 1–16. [Google Scholar]
- 2.Goodman MT, Gurney JG, Smith MA, et al. Sympathetic Nervous System Tumors. In: Ries LAG, Smith MA, Gurney JG, et al., editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. Bethesda, MD: National Cancer Institute, SEER Program; 1999. pp. 65–72. [Google Scholar]
- 3.Perez CA, Matthay KK, Atkinson JB, et al. Biologic variables in the outcome of stages I and II neuroblastoma treated with surgery as primary therapy: a children's cancer group study. J Clin Oncol. 2000;18:18–26. doi: 10.1200/JCO.2000.18.1.18. [DOI] [PubMed] [Google Scholar]
- 4.Baker DL, Schmidt ML, Cohn SL, et al. Outcome after reduced chemotherapy for intermediate- risk neuroblastoma. N Engl J Med. 2010;363:1313–1323. doi: 10.1056/NEJMoa1001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowman LC, Hancock ML, Santana VM, et al. Impact of intensified therapy on clinical outcome in infants and children with neuroblastoma: the St Jude Children's Research Hospital experience, 1962 to 1988. J Clin Oncol. 1991;9:1599–1608. doi: 10.1200/JCO.1991.9.9.1599. [DOI] [PubMed] [Google Scholar]
- 6.Matthay KK, Reynolds CP, Seeger RC, et al. Long-Term Results for Children With High-Risk Neuroblastoma Treated on a Randomized Trial of Myeloablative Therapy Followed by 13-cis-Retinoic Acid: A Children's Oncology Group Study. J Clin Oncol. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson AD, Pinkerton CR, Lewis IJ, et al. High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: a randomised trial. Lancet Oncol. 2008;9:247–256. doi: 10.1016/S1470-2045(08)70069-X. [DOI] [PubMed] [Google Scholar]
- 8.George RE, Li S, Medeiros-Nancarrow C, et al. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. J Clin Oncol. 2006;24:2891–2896. doi: 10.1200/JCO.2006.05.6986. [DOI] [PubMed] [Google Scholar]
- 9.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 11.Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2001;19:3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 12.Tucker MA, D'Angio GJ, Boice JD, Jr, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317:588–593. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 13.Horner MJ, Ries LAG, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2006. National Cancer Institute; 2009. [Google Scholar]
- 14.Breslow NE, Day NE. Statistical Methods in Cancer Research:Volume II - The Design and Analysis of Cohort Studies. Lyon, France: International Agency for Research on Cancer; 1987. p. 69. [PubMed] [Google Scholar]
- 15.Birch JM, Alston RD, McNally RJ, et al. Relative frequency and morphology of cancers in carriers of germline TP53 mutations. Oncogene. 2001;20:4621–4628. doi: 10.1038/sj.onc.1204621. [DOI] [PubMed] [Google Scholar]
- 16.Laverdiere C, Liu Q, Yasui Y, et al. Long-term outcomes in survivors of neuroblastoma: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101:1131–1140. doi: 10.1093/jnci/djp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubino C, Adjadj E, Guerin S, et al. Long-term risk of second malignant neoplasms after neuroblastoma in childhood: role of treatment. Int J Cancer. 2003;107:791–796. doi: 10.1002/ijc.11455. [DOI] [PubMed] [Google Scholar]
- 18.Flandin I, Hartmann O, Michon J, et al. Impact of TBI on late effects in children treated by megatherapy for Stage IV neuroblastoma. A study of the French Society of Pediatric oncology. Int J Radiat Oncol Biol Phys. 2006;64:1424–1431. doi: 10.1016/j.ijrobp.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Garaventa A, Gambini C, Villavecchia G, et al. Second malignancies in children with neuroblastoma after combined treatment with 131I-metaiodobenzylguanidine. Cancer. 2003;97:1332–1338. doi: 10.1002/cncr.11167. [DOI] [PubMed] [Google Scholar]
- 20.Laverdiere C, Cheung NK, Kushner BH, et al. Long-term complications in survivors of advanced stage neuroblastoma. Pediatr Blood Cancer. 2005 doi: 10.1002/pbc.20331. [DOI] [PubMed] [Google Scholar]
- 21.Iwata A, Hirota T, Konno K, et al. Osteosarcoma as a second malignancy after treatment for neuroblastoma. Pediatr Hematol Oncol. 2001;18:465–469. doi: 10.1080/088800101750476050. [DOI] [PubMed] [Google Scholar]
- 22.Mann GS, Byrne AT, Nadel HR, et al. Embryonal rhabdomyosarcoma as a second malignancy following multimodal therapy for advanced-stage neuroblastoma. Pediatr Radiol. 2008;38:1017–1020. doi: 10.1007/s00247-008-0905-y. [DOI] [PubMed] [Google Scholar]
- 23.Jimenez M, Leon P, Castro L, et al. Second tumors in pediatric oncologic patients. Report of 5 cases. Rev Med Univ Navarra. 1995;40:72–77. [PubMed] [Google Scholar]
- 24.Haupt R, Garaventa A, Gambini C, et al. Improved survival of children with neuroblastoma between 1979 and 2005: a report of the Italian Neuroblastoma Registry. J Clin Oncol. 2010;28:2331–2338. doi: 10.1200/JCO.2009.24.8351. [DOI] [PubMed] [Google Scholar]
- 25.Medeiros LJ, Palmedo G, Krigman HR, et al. Oncocytoid renal cell carcinoma after neuroblastoma: a report of four cases of a distinct clinicopathologic entity. Am J Surg Pathol. 1999;23:772–780. doi: 10.1097/00000478-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Bisogno G, Sotti G, Nowicki Y, et al. Soft tissue sarcoma as a second malignant neoplasm in the pediatric age group. Cancer. 2004;100:1758–1765. doi: 10.1002/cncr.20159. [DOI] [PubMed] [Google Scholar]
- 27.Paulino AC, Fowler BZ. Secondary neoplasms after radiotherapy for a childhood solid tumor. Pediatr Hematol Oncol. 2005;22:89–101. doi: 10.1080/08880010590896459. [DOI] [PubMed] [Google Scholar]
- 28.Kushner BH, LaQuaglia MP, Cheung NK. Rethinking management of localized neuroblastoma. J Clin Oncol. 1993;11:1832–1834. [PubMed] [Google Scholar]
- 29.Matthay KK, Sather HN, Seeger RC, et al. Excellent outcome of stage II neuroblastoma is independent of residual disease and radiation therapy. J Clin Oncol. 1989;7:236–244. doi: 10.1200/JCO.1989.7.2.236. [DOI] [PubMed] [Google Scholar]
- 30.Garwicz S, Anderson H, Olsen JH, et al. Second malignant neoplasms after cancer in childhood and adolescence: a population-based case-control study in the 5 Nordic countries. The Nordic Society for Pediatric Hematology and Oncology. The Association of the Nordic Cancer Registries. Int J Cancer. 2000;88:672–678. doi: 10.1002/1097-0215(20001115)88:4<672::aid-ijc24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 31.Inskip PD, Ries LAG, Cohen RJ, et al. New Malignancies Following Childhood Cancer. In: Curtis RE, Freedman DM, Ron E, et al., editors. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973–2000. Bethesda, MD: National Cancer Institute; 2006. pp. 465–481. NIH Publ. No. 05–5302. [Google Scholar]
- 32.Meadows AT, Friedman DL, Neglia JP, et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2356–2362. doi: 10.1200/JCO.2008.21.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 34.Smith MB, Xue H, Strong L, et al. Forty-year experience with second malignancies after treatment of childhood cancer: analysis of outcome following the development of the second malignancy. J Pediatr Surg. 1993;28:1342–1348. doi: 10.1016/s0022-3468(05)80325-2. [DOI] [PubMed] [Google Scholar]
- 35.Tucker MA, D'Angio GJ, Boice JD, Jr, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317:588–593. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 36.Newton WA, Jr, Meadows AT, Shimada H, et al. Bone sarcomas as second malignant neoplasms following childhood cancer. Cancer. 1991;67:193–201. doi: 10.1002/1097-0142(19910101)67:1<193::aid-cncr2820670132>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 37.Spunt SL, Rodriguez-Galindo C, Fuller CE, et al. Ewing sarcoma-family tumors that arise after treatment of primary childhood cancer. Cancer. 2006;107:201–206. doi: 10.1002/cncr.21962. [DOI] [PubMed] [Google Scholar]
- 38.Cope JU, Tsokos M, Miller RW. Ewing sarcoma and sinonasal neuroectodermal tumors as second malignant tumors after retinoblastoma and other neoplasms. Med Pediatr Oncol. 2001;36:290–294. doi: 10.1002/1096-911X(20010201)36:2<290::AID-MPO1067>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 39.Smith MA, Rubinstein L, Anderson JR, et al. Secondary leukemia or myelodysplastic syndrome after treatment with epipodophyllotoxins. J Clin Oncol. 1999;17:569–577. doi: 10.1200/JCO.1999.17.2.569. [DOI] [PubMed] [Google Scholar]
- 40.Pui CH, Ribeiro RC, Hancock ML, et al. Acute myeloid leukemia in children treated with epipodophyllotoxins for acute lymphoblastic leukemia. N Engl J Med. 1991;325:1682–1687. doi: 10.1056/NEJM199112123252402. [DOI] [PubMed] [Google Scholar]