Abstract
Background
Systemic telomere length has been associated with measures of diastolic function, vascular stiffness and left ventricular mass mainly in smaller, patient-specific settings and not in a general population. In this study we describe the applicability of these findings in a large, representative population.
Methods and Results
Peripheral blood leukocyte telomere length (PBL TL) was measured using telomere restriction fragment analysis in the young to middle-aged (>2500 volunteers, ∼35 to 55 years old) Asklepios study population, free from overt cardiovascular disease. Subjects underwent extensive echocardiographic, hemodynamic and biochemical phenotyping. After adjusting for relevant confounders (age, sex, systolic blood pressure, heart rate, body mass index and use of antihypertensive drugs) we found no associations between PBL TL and left ventricular mass index (P = 0.943), ejection fraction (P = 0.933), peak systolic septal annular motion (P = 0.238), pulse wave velocity (P = 0.971) or pulse pressure (P = 0.999). In contrast, our data showed positive associations between PBL TL and parameters of LV filling: the transmitral flow early (E) to late (A) velocity ratio (E/A-ratio; P<0.001), the ratio of early (e′) to late (a′) mitral annular velocities (e′/a′-ratio; P = 0.012) and isovolumic relaxation time (P = 0.015). Interestingly, these associations were stronger in women than in men and were driven by associations between PBL TL and the late diastolic components (A and a′).
Conclusions
In a generally healthy, young to middle-aged population, PBL TL is not related to LV mass or systolic function, but might be associated with an altered LV filling pattern, especially in women.
Introduction
The progression of acquired cardiovascular diseases (CVD) throughout the human life can typically be tracked by a series of gradual changes in physical, chemical and biological parameters. Levels of systolic blood pressure (SBP), cholesterol, C-reactive protein (CRP), smoking status and sex have all been linked with an increased likelihood of adverse cardiovascular events [1]–[3]. Telomere length (TL), although not used in clinical practice, is one such parameter that has repeatedly been linked with cardiovascular health and disease development [4].
Telomeres are the nucleotide-protein complexes that shield the chromosomal ends from erosion caused by the end-replication problem during cell division and distinguishes them from double-stranded breaks to prevent chromosomal fusion [5]. Throughout the replicative lifespan of cells, their TL will decrease until a critical threshold is reached. Critically short telomeres will typically lead to a cell crisis resulting in senescence, apoptosis or immortalization [6]. TL is of particular interest because it potentially provides a cumulative measurement of stresses throughout life representing “biological age” [4].
Although there is still uncertainty about the mechanism(s) by which telomere biology and CVD pathogenesis affect each other, results from both molecular biology and epidemiology have repeatedly shown significant associations [7]–[11]. The same is true for cardiovascular risk factors such as insulin resistance, hypertension [12], smoking status [13], oxidative stress and inflammation [14].
Systemic TL has also been linked to LV structure and function but mostly in smaller, patient-specific settings and not in a general population [15]–[22]. Shorter TL can be found in heart failure (HF) patients [23], [24] and patients suffering from chronic HF have an increased morbidity if their telomeres are shorter [25]. However, reports on the association between TL and indicators of diastolic dysfunction show conflicting results [18], [19]. Similarly, a positive correlation has been described between LVM and PBL TL [20]–[22], but other studies did not detect a significant association between TL and LVM index or LV hypertrophy [18], [26], [27].
The population-based Asklepios Study offers the advantages of a large sample size and the measurement of numerous potential confounders of TL and CVD. We therefore investigated the relations between systemic TL and proven prognostic parameters [28]–[32] of vascular stiffness, cardiac stiffness, systolic function, diastolic function and ventricular mass, to shed light on the baseline state of these correlations.
Methods
Study Population
All data presented in this paper were collected during the first round of the Asklepios study on successful (cardiovascular) aging. The study comprises 2524 subjects approximately 35 to 55 years of age, free from overt cardiovascular disease or other significant pathologies at baseline. The full description of the study design, inclusion criteria, detailed methodology and population baseline characteristics have been published previously [33]. The study was conducted in concordance with the principles of the Declaration of Helsinki. All patients gave written informed consent and the study was approved by the Ghent University Ethical Committee. For the analyses reported here, we used the subset of 2509 patients for which reliable TL and all major TL confounder measurements (age, sex, paternal age at birth) were available (cf. De Meyer et al. [34]).
Biochemical analyses
All subjects were fasting, had refrained from smoking for at least 6 hours and were screened for active infection/inflammation before blood sampling. Conventional serum parameters were measured using commercial reagents according to the manufacturers' recommendations on a Modular P automated system (Roche Diagnostics, Mannheim, Germany), in an ISO 9002 certified reference laboratory [33]. Coefficient of variation of all tests was <3.0%. These parameters included Interleukin-6 (IL-6), C-reactive protein (CRP), oxidized low-density lipoprotein (ox-LDL), serum uric acid concentrations and brain natriuretic peptide precursor [33].
Telomere Length
For TL-analyses, whole blood was collected in EDTA tubes cooled to 4°C. DNA isolation was performed within 3 days of collection using the Puregene Genomic Purification Kit (Gentra Systems, Minnesota, USA). The DNA was long term stored at −80°C before TL measurement in duplicate. 5 µg was digested with 5U RsaI and 10U HinfI followed by gel electrophoresis, Southern blotting, radioactive hybridization of the telomeric fragments and weight markers, phospho-imaging and quantification (expressed as kbp: kilo base pairs) [14].
Echocardiographic and vascular examination
Blood pressure was recorded using bilateral triplicate measurements (1 min intervals) on a rested, sitting subject using a validated oscillometric Omron HEM device (Omron Healthcare Co. Ltd., Kyoto, Japan). Blood pressure values of these six readings were averaged and the mean value of systolic blood pressure (SBP) is used throughout this study. The subjects underwent a resting echocardiographic examination and a scan of the left and right carotid and femoral arteries (VIVID 7, GE Vingmed Ultrasound, Horten, Norway). Left ventricular (LV) internal dimensions were measured at end-diastole (LVEDD) with the area-length method. Sphericity was defined as LV width divided by LV length and is expressed as a percentage. Standard 2-D volumetric methods were used to calculate ejection fraction (EF) from end-diastolic and end-systolic LV volumes and to calculate LV mass (LVM). The LVM was scaled allometrically following the recommendations of Chirinos et al. [35] as LVM/(Height)1.7 to account for the effects of both obesity and blood pressure on LVM. We also scaled LVM to the body surface area (g/m2) [36].
Other cardiac and arterial measurements included the following: systolic (s′), and early (e′) and late (a′) diastolic septal mitral annulus pulsed wave tissue Doppler (TDI) velocities, pulsed wave Doppler early (E) and late (A) diastolic transmitral flow velocities, E-wave propagation velocity (Vpe) and carotid-femoral pulse wave velocity (PWV). PWV was calculated as follows:
| (1) |
In formula (1) ΔLS-F and ΔLS-C are the distances measured from sternal notch to femoral and carotid measuring sites respectively, ΔTQ-F and ΔTQ-C are the time delays between the start of the QRS complex and the onset of systolic flow in the femoral and carotid artery measured by pulse wave Doppler imaging (full methodology described in the online supplements of Rietzschel et al. [33]). CW Doppler recordings were used to measure isovolumic relaxation time (IVRT) as the interval from the closure spike of the aortic valve to onset of mitral flow.
Data analysis
Statistical analyses were performed in R 2.15.2. Continuous variables are reported as the mean value ± standard deviation. Means of groups were compared with Student (homoscedasticity) or Welch t-test (heteroscedasticity) as appropriate. To evaluate the contribution of the different confounders to the response variables under study we applied general linear models as implemented in the ‘glm’ function. We report both P-values and the estimated unstandardized effect sizes (b) for TL in these models.
Results
Baseline characteristics of the population are presented in Table 1.
Table 1. Baseline characteristics of the Asklepios study population.
| Variable | Women (n = 1291) | Men (n = 1218) | P-valuea | Population (n = 2509) |
| Age (years) | 45.9±6.0 | 46.1±5.9 | 0.316 | 46.0±6.0 |
| Weight (kg) | 66.7±12.7 | 82.0±12.4 | <2.2E-16 | 74.1±14.7 |
| Height (cm) | 163±6 | 176±7 | <2.2E-16b | 169±9 |
| Body Mass Index (kg/m2) | 25.1±4.6 | 26.5±3.7 | <2.2E-16b | 25.8±4.3 |
| Systolic Blood Pressure (mmHg) | 123±14 | 131±13 | <2.2E-16b | 127±14 |
| Pulse Pressure (mmHg) | 45.5±9.1 | 48.3±7.4 | <2.2E-16b | 46.9±8.4 |
| Pulse Wave Velocity (m/s) | 6.60±1.45 | 6.65±1.46 | 0.397 | 6.62±1.45 |
| Heart Rate (min−1) | 67.2±9.5 | 64.0±10.7 | 6.01E-15b | 65.6±10.2 |
| Used Antihypertensive Drugs | 145 (11.2%) | 118 (9.69%) | 0.284c | 263 (10.5%) |
| PBL TL (kbp) | 7.96±0.73 | 7.78±0.71 | 3.26E-09 | 7.87±0.73 |
| E (cm/s) | 78.9±14.2 | 70.6±13.0 | <2.2E-16b | 74.9±14.2 |
| A (cm/s) | 63.5±11.9 | 59.6±10.9 | <2.2E-16b | 61.6±11.6 |
| e′ (cm/s) | 9.41±2.13 | 8.63±1.83 | <2.2E-16b | 9.03±2.03 |
| a′ (cm/s) | 8.67±1.55 | 9.23±1.46 | <2.2E-16 | 8.94±1.53 |
| E/A | 1.29±0.32 | 1.22±0.29 | 2.65E-7b | 1.25±0.31 |
| e′/a′ | 1.13±0.36 | 0.970±0.29 | <2.2E-16b | 1.05±0.34 |
| E/e′ | 8.68±1.99 | 8.41±1.78 | 3.36E-4b | 8.55±1.90 |
| s′ (cm/s) | 7.91±1.13 | 7.93±1.20 | 0.756 | 7.92±1.16 |
| Vpe (m/s) | 79.4±22.1 | 71.9±19.5 | <2.2E-16d | 75.7±21.2 |
| DT (ms) | 167±29 | 170±30 | 9.29E-4 | 168±30 |
| Isovolumic Relaxation Time (ms) | 81.8±13.8 | 90.2±12.9 | <2.2E-16 | 85.9±14.0 |
| LV Ejection Fraction (%) | 64.8±6.7 | 62.5±6.4 | <2.2E-16 | 63.7±6.7 |
| LVEDD (mm) | 44.9±3.9 | 49.4±4.4 | <2.2E-16b | 47.1±4.7 |
| Sphericity (%) | 56.9±6.4 | 57.2±6.5 | 0.246 | 57.0±6.4 |
| Left Ventricular Mass (g) | 124±31 | 179±40 | <2.2E-16b | 151±45 |
| LVM index (g/m∧1.7) | 54.2±13 | 69.0±15.0 | <2.2E-16b | 61.3±15.9 |
| NTproBNP (pg/ml) | 81.3±64.1 | 36.4±39.2 | <2.2E-16b d | 59.5±58.0 |
: P-value for comparison between sexes using independent t-test (b:unequal variance) or chi-square test (c).
: Data was log transformed before statistical testing.
LVM: allometrically scaled Left Ventricular Mass index, PBL TL: Periferal Blood Leukocyte Telomere Length, E & A: peak transmitral flow velocities during early (E) and late (A) diastolic filling, e′ & a′: peak movement speed of mitral annulus during early (e′) and late (a′) diastolic filling, DT: transmitral Deceleration Time, NTproBNP: N-terminal prohormone of brain natriuretic peptide, LVEDD: Left Ventricular End-Diastolic Diameter, s′: peak systolic mitral annulus movement speed, Vpe: pulse propagation velocity in early diastole.
Diastolic function
Unadjusted models yielded positive linear associations between TL and E/A, e′/a′ (Fig. 1) and E/e′ (Table 2, Model 1). In successive (general linear) models, known major confounders of diastolic function were added, i.e. age and sex in Model 2, additionally heart rate (HR), systolic blood pressure (SBP) including use of antihypertensive drugs and body mass index (BMI) in Model 3. We did not remove non-significant independent variables from the proposed models in Table 2 for the individual response variables as removal of the non-significant terms did not alter the significance of the TL component. Addition of further potential confounders: LV sphericity, oxidative stress (oxidized-LDL cholesterol, serum uric acid) or inflammatory markers (high-sensitive CRP, IL-6), did not significantly alter the TL - diastolic dysfunction relationships when added to Model 3 as an independent variable (data not shown).
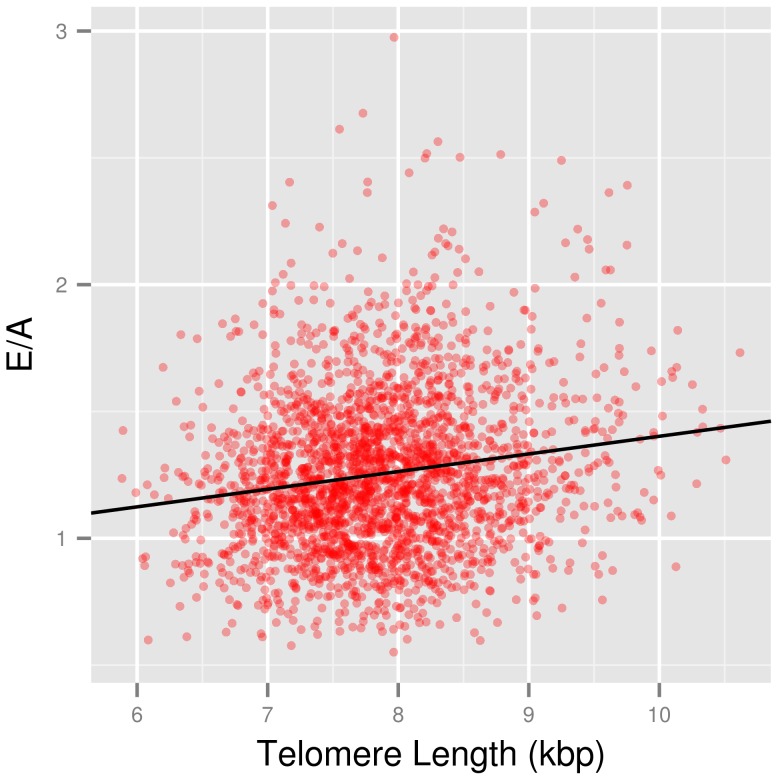
Figure 1. TL ∼ E/A.
Scatterplot showing the unadjusted correlation between telomere length (TL) and the ratio of early (E) over late (A) mitral annulus movement speed (red) and the regression line (black).
Table 2. The association of TL (kbp) with different parameters of cardiovascular function using general linear models.
| Response variable (RV) | Model 1 | Model 2 | Model 3 | |
| E/A | b | 0.0696 | 0.0249 | 0.0264 |
| P | 2.83E-16 | 1.25E-03 | 8.09E-05 | |
| e′/a′ | b | 0.0764 | 0.0178 | 0.0182 |
| P | <2.2e-16 | 0.0281 | 0.0120 | |
| E (cm/s) | b | 1.91 | 0.195 | 0.226 |
| P | 1.09E-06 | 0.600 | 0.535 | |
| A (cm/s) | b | −1.60 | −0.860 | −0.905 |
| P | 5.37E-07 | 4.62E-03 | 4.51E-04 | |
| e′ (cm/s) | b | 0.397 | 0.050 | 0.043 |
| P | 8.93E-13 | 0.304 | 0.341 | |
| a′ (cm/s) | b | −0.211 | −0.068 | −0.079 |
| P | 5.77E-07 | 0.100 | 0.0403 | |
| E/e′ | b | −0.158 | −0.0214 | −0.0124 |
| P | 2.62E-3 | 0.677 | 0.794 | |
| DT (ms) | b | −1.86 | −0.123 | −0.302 |
| P | 2.24E-02 | 0.881 | 0.712 | |
| IVRT (s) | b | −2.70 | −0.941 | −0.900 |
| P | 2.32E-12 | 9.25E-03 | 0.0115 | |
| log(NTproBNP (pg/ml)) | b | 0.017 | −0.004 | −0.005 |
| P | 0.119 | 0.657 | 0.617 | |
| LVM index (g/m1.7) | b | −2.37 | −0.194 | 0.0228 |
| P | 5.41E-08 | 0.616 | 0.943 | |
| LVEDD (mm) | b | −0.497 | −0.164 | −0.124 |
| P | 1.11E-04 | 0.158 | 0.261 | |
| EF (%) | b | 0.0216 | −0,0183 | 0.0156 |
| P | 0.906 | 0.922 | 0.933 | |
| s′ (cm/s) | b | 0.018 | −0.033 | −0.038 |
| P | 0.579 | 0.315 | 0.238 |
Model 1: RV ∼ TL.
Model 2: RV ∼ TL+Age+Sex.
Model 3: Model 2+Systolic BP+Heart Rate+BMI+Used Antihypertensive Drugs.
E & A: peak transmitral flow velocities during early (E) and late (A) diastolic filling, e′ & a′: peak movement speed of mitral annulus during early (e′) and late (a′) diastolic filling, DT: transmitral Deceleration Time, IVRT: Isovolumic Relaxation Time, NTproBNP: N-terminal prohormone of brain natriuretic peptide, LVM: allometrically scaled Left Ventricular Mass index, LVEDD: Left Ventricular End-Diastolic Diameter, EF: Ejected Fraction of end-diastolic volume, s′: peak systolic mitral annulus movement speed, b: effect size (unstandardized), P: P-value of TL component.
In all models the positive association between E/A and TL remained significant (see Table 2, P≤0.002) with Model 3 accounting for approximately 43.2% of E/A variability (2.64% of the total variability could be attributed to TL). Examining the data separately by sex, we found that, upon adjustment for confounders (Model 3), the association between E/A and TL was significant in both women (b = 0.030, P = 0.001) and men (b = 0.023, P = 0.014). A similar approach was adopted for the e′/a′ ratio. Results of the linear models were included in Table 2 and demonstrated significance of the adjusted associations for e′/a′ (Model 3: b = 0.018, P = 0.012). Looking at both sexes separately, TL was a significant independent variable in women (b = 0.022, P = 0.032), but only borderline in men (b = 0.016, P = 0.098).
We further examined the components of these ratios (E/A and e′/a′) separately to determine whether the associations were attributable to one of both components or whether the ratios contained additional information beyond the terms they consist of. Surprisingly, the results indicated that neither E (b = 0.226, P = 0.535) nor e′ (b = 0.043, P = 0.341) were correlated with TL (Model 3). There was however a correlation of TL with A (b = −0.905, P<0.001) and a′ (b = −0.079, P = 0.040).
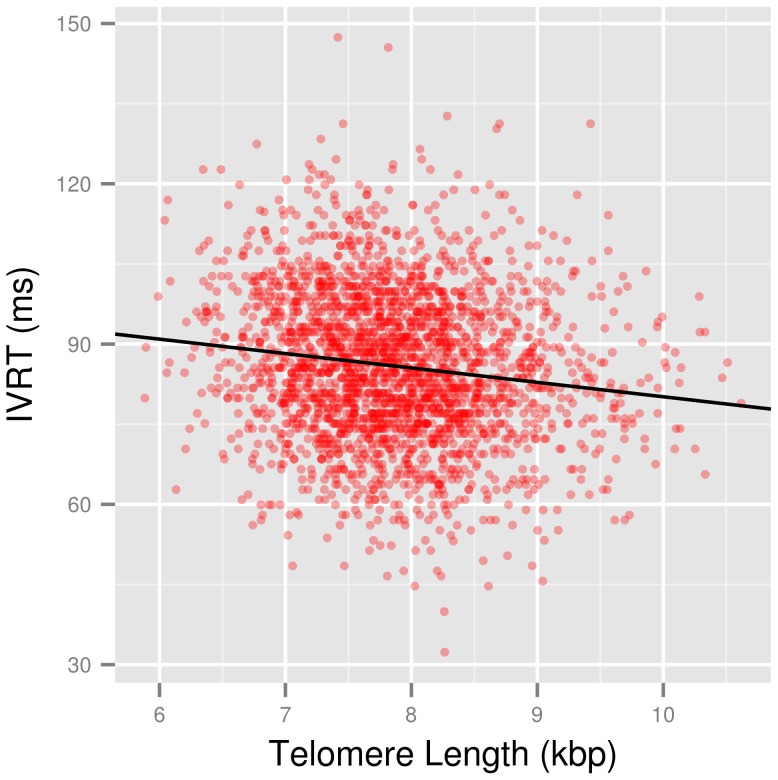
Accordingly, E/e′ was not correlated upon adequate adjustment (Model 3: b = 0.014, P = 0.775), neither were other indices of diastolic function: flow propagation velocity of the E-wave (Vpe; b = 0.212, P = 0.718), mitral inflow deceleration time (b = −0.302, P = 0.712) or duration of the atrial contraction (b = −0.028, P = 0.938). Only IVRT was inversely correlated with PBL TL (Fig. 2, Model 3: b = −0.900, P = 0.011). IVRT was also inversely correlated with PBL TL after age, sex and paternal-age adjustment (PBL TL as dependent variable; b = −2.92E-3, P = 0.010). Additionally, we found no significant association with brain natriuretic peptide (log(NT-proBNP): b = −0.005, P = 0.617) upon adjustment.
Figure 2. TL ∼ IVRT.
Scatterplot showing the unadjusted correlation between telomere length (TL) and isovolumic relaxation time (IVRT) (red) and the regression line (black).
Systolic function and LV structure
We could not document significant associations between TL and systolic function after adequate adjustment (Model 3, EF: b = 0.016, P = 0.933 and s′: b = −0.038, P = 0.238). Similarly no associations were found with LV structure assessed by LV end-diastolic diameter (LVEDD: b = −0.124, P = 0.261) and LV mass (b = −0.214, P = 0.801), body surface area adjusted LV mass (b = 0.010, P = 0.981) or allometrically height-adjusted LV mass (b = 0.023, P = 0.943).
Arterial stiffness
No significant partial correlation was found between PWV and age-adjusted TL (cf. supra), a result that remained unchanged after additional adjustments (Model 3, b = −0.001, P = 0.971). The same is true for pulse pressure (PP) in the Asklepios population (Model 3, b = 255E-6, P = 0.999).
Discussion
Our main finding is that we can extend a number of previously described associations between PBL TL and cardiovascular structure and function –usually detected in smaller, diseased cohorts - towards a middle-aged, apparently healthy population. After adjustment for confounders, we could not document an association with systolic function, cardiac structure or vascular stiffness. We do however document an intriguing association with certain parameters of LV filling.
LV filling and diastolic function
As mentioned in the introduction, previous reports on the association between TL and indicators of diastolic dysfunction have led to apparently conflicting results. Quartiles of telomere length were shown to correlate with diastolic dysfunction in CAD patients (evaluated by E/A and pulmonary vein flow) [18]. In contrast both E/A-ratio and diastolic dysfunction were not correlated with PBL TL in a study of elderly subjects (>85 years) [19]. This can potentially be explained by the very different nature of the populations under study. Our data showed a significant association between PBL TL and both the E/A-ratio and the e′/a′-ratio and with IVRT [37], but not with the E/e′-ratio (see Table 2 for details). E/e′ is mainly used as an indicator of elevated filling pressures, reflecting more advanced diastolic dysfunction not likely to be present in healthy subjects [38]. Concurrently, NT-proBNP, a biochemical marker reflecting elevated filling pressure and diastolic dysfunction [39], was not associated with PBL TL after correction for confounders (Model 3).
Analysis of the terms constituting the E/A and e′/a′ ratios in this population suggests that TL was associated with atrial contraction (A and a′), rather than with parameters that could reflect myocardial relaxation or filling pressure, such as E and e′, or reflect myocardial stiffness, such as shortened mitral deceleration time [40]. As previous publications only described correlations with E/A but not with the individual components, we cannot tell whether this was the case in other study populations.
Relaxation and stiffness induce opposite effects on mitral E and A and hence on E/A [40]. The present data do not provide sufficient evidence for an independent association between PBL TL and LV relaxation or LV stiffness in the general middle-aged population. Although there is an association with longer IVRT after correction for heart rate and blood pressure, it is difficult to attribute this to myocardial relaxation without a persisting association with the best validated determinants e′ and Vpe [41]. The persisting associations with atrial contraction flow velocity (A) and with the simultaneously occurring annular velocity (a′) then most likely affects compound measures such as E/A and e′/a′. One could speculate that increased IVRT and enhanced atrial contraction represent an early and subtle delay of myocardial relaxation, which is not yet apparent in other measurements. We therefore would describe the findings as correlation between PBL TL and altered filling pattern without sufficient evidence for diastolic dysfunction. It is noteworthy though that the associations with PBL TL were stronger in women than in men, an interesting finding in light of the increased occurrence of diastolic HF in women [42].
LV systolic function, structure and vascular stiffness
In this population without overt cardiac disease, we report that PBL TL was not associated with minor changes in systolic parameters such as EF and tissue Doppler movement speed of the septal mitral annulus (s′). Increased LV mass correlates with increased all-cause and cardiovascular mortality [43] and a positive correlation has been described between LVM and PBL TL [20]–[22]. However, our findings do not support an association between PBL TL and LVM, body surface area scaled LVM or height-scaled LVM in the context of a relatively young population. Two other studies also failed to detect an association between TL and LVM index or LV hypertrophy in two older populations (∼65 years) [18], [26]. These findings and the fact that the former studies do not agree on whether normotensive or hypertensive patients show a TL – LVM correlation, lead us to the conclusion that there is likely an as of yet unidentified confounder at work. A third study found no cross-sectional or longitudinal associations between PBL LTL and cardiac measurements including LVM (adjusted for body surface area) [27].
With respect to vascular stiffness, no significant associations between PWV (or PP) and TL were found in either sex. Previous studies have reported this association to be significant in men [15], [44]. The discordance may be attributable to age and health characteristics of the respective populations. Indeed these populations were featured by a higher mean age (∼10 years) and a higher mean PWV (60% higher) compared to the Asklepios study albeit with a different measurement protocol for PWV.
Limitations
The Asklepios data set does not yet include any longitudinal information thus limiting it to all the drawbacks associated with cross-sectional study designs. Particularly claims of causality can not be made with cross-sectional data alone. Despite the extensive characterization of the Asklepios study, there are some descriptors of CV function which were not measured (e.g. pulmonary venous flow). We cannot exclude the involvement of these factors.
Mechanistic insights
There are two general models in which TL is tied to cardiovascular health. The first states that telomere shortening is a primary driver of (cardiovascular) ageing. In support of this model our findings indicate that the associations between some parameters of LV filling and telomere length, are not limited to (chronic) HF patients, but may already be present in a young to middle-aged, apparently healthy population and are more pronounced in women. It is tempting to speculate that telomere biology could be mechanistically involved in the early pathogenesis of diastolic dysfunction and possibly HF by extension. As a matter of fact, in mice, knock-out of the telomere elongating enzyme telomerase, resulted in shortened telomere length over several generations which was associated with the development of overt chronic HF [45]. However, telomere biology in mice cannot be easily transposed to humans and additional experiments would be absolutely necessary to pinpoint the exact mechanisms.
In our data, TL was clearly correlated with E/A, e′/a′ and IVRT but not with other indices of diastolic function. We further explored the relationship between PBL TL and other cardiac and hemodynamic parameters such as sphericity of the ventricle, Vpe, duration of the A-wave and deceleration time (data not shown). No significant associations were found that could help provide clues as to the mechanism by which PBL TL and diastolic function might be linked.
The second model states that TL is merely an epiphenomenon, an indicator influenced by conditions in the ageing body. Accelerated telomere attrition in subjects with mildly impaired diastolic function could also be caused by oxidative stress and inflammation, two factors that are known to affect diastolic function as well as telomere length [14], [23], [46]–[48]. In this case it might be expected that the addition of oxidative stress and inflammation would cause reduced significance for TL. However, additional markers (CRP, oxidized LDL, IL-6 and serum uric acid) did not substantially alter the significance of the PBL TL component relative to Model 3 in Table 2 (data not shown). It should be noted though that these markers only reflect point measurements of oxidative stress and inflammation, which are variable by nature, whereas telomere length has been hypothesized to reflect their cumulated effects (reviewed in De Meyer et al. [4]).
Our data, at present, is insufficient to determine the more likely model. Some of the non-replicated associations might still become apparent with ageing, assuming that a certain threshold of telomere attrition needs to be reached before it has a measurable effect on cardiovascular stiffness or visa versa.
Conclusions
Our results show that several parameters of cardiovascular structure and function which have been associated with TL, fail to replicate in a well-phenotyped middle-aged population sample. However, PBL TL is associated with subtle changes in certain parameters of LV filling in this population and these associations are more apparent in women. Further investigation of the underlying biological mechanisms is warranted to provide insights into the relationship between PBL TL, diastolic function and cardiovascular health.
Acknowledgments
We thank F. Van Hoeke, B. Leydens, F. Brusselmans, F. De Block, D. Broucke and the residents and general practitioners of Erpe-Mere and Nieuwerkerken for their invaluable efforts. Memberships of the Asklepios Investigators may be consulted in the Acknowledgements of Rietzschel et al. 2007 [33].
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data from the Asklepios study are not publicly available due to patient privacy concerns. Individuals seeking access may contact Ernst.Rietzschel@UGent.be.
Funding Statement
The Asklepios Study is supported by Research Foundation Flanders (FWO, http://www.fwo.be/en/) research grants G.0427.03 and G.0838.10N. SD is supported by the agency for Innovation by Science and Technology (IWT, http://www.iwt.be/english/welcome) grant SB101371. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, et al. (2008) General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 117:743–753. [DOI] [PubMed] [Google Scholar]
- 2. Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR (2008) C-Reactive Protein and Parental History Improve Global Cardiovascular Risk Prediction The Reynolds Risk Score for Men. Circulation 118:2243–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, et al. (2003) Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 24:987–1003. [DOI] [PubMed] [Google Scholar]
- 4. De Meyer T, Rietzschel ER, De Buyzere ML, Van Criekinge W, Bekaert S (2011) Telomere length and cardiovascular aging: The means to the ends? Ageing Research Reviews 10:297–303. [DOI] [PubMed] [Google Scholar]
- 5. de Lange T (2005) Shelterin: the protein complex that shapes and safeguards human telomeres. Genes & Development 19:2100–2110. [DOI] [PubMed] [Google Scholar]
- 6. Qian YJ, Chen XB (2010) Tumor suppression by p53: making cells senescent. Histology and Histopathology 25:515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang E, Harley CB (1995) Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci U S A 92:11190–11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, et al. (2002) Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105:1541–1544. [DOI] [PubMed] [Google Scholar]
- 9. Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, et al. (2007) Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet 369:107–114. [DOI] [PubMed] [Google Scholar]
- 10. Willeit P, Willeit J, Brandstatter A, Ehrlenbach S, Mayr A, et al. (2010) Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arterioscler Thromb Vasc Biol 30:1649–1656. [DOI] [PubMed] [Google Scholar]
- 11. De Meyer T, Rietzschel ER, De Buyzere ML, Langlois MR, De Bacquer D, et al. (2009) Systemic telomere length and preclinical atherosclerosis: the Asklepios Study. Eur Heart J 30:3074–3081. [DOI] [PubMed] [Google Scholar]
- 12. Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, et al. (2006) Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell 5:325–330. [DOI] [PubMed] [Google Scholar]
- 13. O'Donnell CJ, Demissie S, Kimura M, Levy D, Gardner JP, et al. (2008) Leukocyte telomere length and carotid artery intimal medial thickness: the Framingham Heart Study. Arterioscler Thromb Vasc Biol 28:1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bekaert S, De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, et al. (2007) Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell 6:639–647. [DOI] [PubMed] [Google Scholar]
- 15. Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, et al. (2001) Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension 37:381–385. [DOI] [PubMed] [Google Scholar]
- 16. Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, et al. (2000) Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension 36:195–200. [DOI] [PubMed] [Google Scholar]
- 17. Nawrot TS, Staessen JA, Holvoet P, Struijker-Boudier HA, Schiffers P, et al. (2010) Telomere length and its associations with oxidized-LDL, carotid artery distensibility and smoking. Frontiers in bioscience 2:1164–1168. [DOI] [PubMed] [Google Scholar]
- 18. Farzaneh-Far R, Cawthon RM, Na B, Browner WS, Schiller NB, et al. (2008) Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arterioscler Thromb Vasc Biol 28:1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Collerton J, Martin-Ruiz C, Kenny A, Barrass K, von Zglinicki T, et al. (2007) Telomere length is associated with left ventricular function in the oldest old: the Newcastle 85+ Study. Eur Heart J 28:172–176. [DOI] [PubMed] [Google Scholar]
- 20. Vasan RS, Demissie S, Kimura M, Cupples LA, White C, et al. (2009) Association of leukocyte telomere length with echocardiographic left ventricular mass: the Framingham Heart Study. Circulation 120:1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuznetsova T, Codd V, Brouilette S, Thijs L, Gonzalez A, et al. (2010) Association between left ventricular mass and telomere length in a population study. Am J Epidemiol 172:440–450. [DOI] [PubMed] [Google Scholar]
- 22. Huber M, Treszl A, Wehland M, Winther I, Zergibel I, et al. (2012) Genetic variants implicated in telomere length associated with left ventricular function in patients with hypertension and cardiac organ damage. J Mol Med (Berl) 90:1059–1067. [DOI] [PubMed] [Google Scholar]
- 23. van der Harst P, van der Steege G, de Boer RA, Voors AA, Hall AS, et al. (2007) Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol 49:1459–1464. [DOI] [PubMed] [Google Scholar]
- 24. Wong LS, Huzen J, de Boer RA, van Gilst WH, van Veldhuisen DJ, et al. (2011) Telomere length of circulating leukocyte subpopulations and buccal cells in patients with ischemic heart failure and their offspring. PLoS ONE 6:e23118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van der Harst P, de Boer RA, Samani NJ, Wong LSM, Huzen J, et al. (2010) Telomere length and outcome in heart failure. Annals of Medicine 42:36–44. [DOI] [PubMed] [Google Scholar]
- 26. Fyhrquist F, Silventoinen K, Saijonmaa O, Kontula K, Devereux RB, et al. (2011) Telomere length and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE Study. J Hum Hypertens 25:711–718. [DOI] [PubMed] [Google Scholar]
- 27. Masi S, D'Aiuto F, Martin-Ruiz C, Kahn T, Wong A, et al. (2014) Rate of telomere shortening and cardiovascular damage: a longitudinal study in the 1946 British Birth Cohort. Eur Heart J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oh JK, Ha JW, Redfield MM, Ujino K, Seward JB, et al. (2004) Triphasic mitral inflow velocity with middiastolic filling: Clinical implications and associated echocardiographic findings. Journal of the American Society of Echocardiography 17:428–431. [DOI] [PubMed] [Google Scholar]
- 29. Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA (1997) Doppler tissue imaging: A noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. Journal of the American College of Cardiology 30:1527–1533. [DOI] [PubMed] [Google Scholar]
- 30. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, et al. (2006) Expert consensus document on arterial stiffness: methodological issues and clinical applications. European Heart Journal 27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 31. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, et al. (2010) Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vlachopoulos C, Aznaouridis K, Stefanadis C (2010) Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 33. Rietzschel ER, De Buyzere ML, Bekaert S, Segers P, De Bacquer D, et al. (2007) Rationale, design, methods and baseline characteristics of the Asklepios Study. Eur J Cardiovasc Prev Rehabil 14:179–191. [DOI] [PubMed] [Google Scholar]
- 34. De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Van Criekinge W, et al. (2007) Paternal age at birth is an important determinant of offspring telomere length. Hum Mol Genet 16:3097–3102. [DOI] [PubMed] [Google Scholar]
- 35. Chirinos JA, Rietzschel ER, De Buyzere ML, De Bacquer D, Gillebert TC, et al. (2009) Arterial load and ventricular-arterial coupling: physiologic relations with body size and effect of obesity. Hypertension 54:558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, et al. (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 37. Brecker SJ, Lee CH, Gibson DG (1992) Relation of left ventricular isovolumic relaxation time and incoordination to transmitral Doppler filling patterns. Br Heart J 68:567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bhella PS, Pacini EL, Prasad A, Hastings JL, Adams-Huet B, et al. (2011) Echocardiographic indices do not reliably track changes in left-sided filling pressure in healthy subjects or patients with heart failure with preserved ejection fraction. Circulation Cardiovascular imaging 4:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lubien E, DeMaria A, Krishnaswamy P, Clopton P, Koon J, et al. (2002) Utility of B-natriuretic peptide in detecting diastolic dysfunction: comparison with Doppler velocity recordings. Circulation 105:595–601. [DOI] [PubMed] [Google Scholar]
- 40. Stoddard MF, Pearson AC, Kern MJ, Ratcliff J, Mrosek DG, et al. (1989) Left ventricular diastolic function: comparison of pulsed Doppler echocardiographic and hemodynamic indexes in subjects with and without coronary artery disease. J Am Coll Cardiol 13:327–336. [DOI] [PubMed] [Google Scholar]
- 41. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, et al. (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22:107–133. [DOI] [PubMed] [Google Scholar]
- 42. Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA (2005) Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation 112:2254–2262. [DOI] [PubMed] [Google Scholar]
- 43. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP (1990) Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322:1561–1566. [DOI] [PubMed] [Google Scholar]
- 44. Wang YY, Chen AF, Wang HZ, Xie LY, Sui KX, et al. (2011) Association of shorter mean telomere length with large artery stiffness in patients with coronary heart disease. Aging Male 14:27–32. [DOI] [PubMed] [Google Scholar]
- 45. Leri A, Franco S, Zacheo A, Barlucchi L, Chimenti S, et al. (2003) Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. Embo Journal 22:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends in Biochemical Sciences 27:339–344. [DOI] [PubMed] [Google Scholar]
- 47. Giordano FJ (2005) Oxygen, oxidative stress, hypoxia, and heart failure. Journal of Clinical Investigation 115:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Anand IS, Latini R, Florea VG, Kuskowski MA, Rector T, et al. (2005) C-reactive protein in heart failure - Prognostic value and the effect of valsartan. Circulation 112:1428–1434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data from the Asklepios study are not publicly available due to patient privacy concerns. Individuals seeking access may contact Ernst.Rietzschel@UGent.be.