Abstract
Background
Contributors to fatal outcomes in TB/HIV co-infected patients actively undergoing TB treatment are poorly characterized. The aim was to assess factors associated with death in TB/HIV co-infected patients during the initial 6 months of TB treatment.
Methods
We conducted a hospital-based retrospective cohort study from January 2006 to December 2013 at the Yaoundé Central Hospital, Cameroon. We reviewed medical records to identify hospitalized co-infected TB/HIV patients aged 15 years and older. Death was defined as any death occurring during TB treatment, as per the World Health Organization's recommendations. We conducted logistic regression analysis to identify factors associated with a fatal outcome. Magnitudes of associations were expressed by adjusted odds ratio (aOR) with 95% confidence interval.
Results
The 337 patients enrolled had a mean age of 39.3 (standard deviation 10.3) years and 54.3% were female. TB treatment outcomes were distributed as follows: 205 (60.8%) treatment success, 99 (29.4%) deaths, 18 (5.3%) not evaluated, 14 (4.2%) lost to follow-up, and 1 (0.3%) failed. After exclusion of patients lost to follow-up and not evaluated, death in TB/HIV co-infected patients during TB treatment was associated with a TB diagnosis made before 2010 (aOR = 2.50 [1.31–4.78]; p = 0.006), the presence of other AIDS-defining diseases (aOR = 2.73 [1.27–5.86]; p = 0.010), non-AIDS comorbidities (aOR = 3.35 [1.37–8.21]; p = 0.008), not receiving cotrimoxazole prophylaxis (aOR = 3.61 [1.71–7.63]; p = 0.001), not receiving antiretroviral therapy (aOR = 2.45 [1.18–5.08]; p = 0.016), and CD4 cells count <50 cells/mm3 (aOR = 16.43 [1.05–258.04]; p = 0.047).
Conclusions
The TB treatment success rate among TB/HIV co-infected patients in our setting is low. Mortality was high among TB/HIV co-infected patients during TB treatment and is strongly associated with clinical and biological factors, highlighting the urgent need for specific interventions focused on enhancing patient outcomes.
Introduction
Tuberculosis (TB) due to Mycobacterium tuberculosis and human immunodeficiency virus (HIV) disease have been inextricably linked from the earliest years of the HIV/acquired immunodeficiency syndrome (AIDS) epidemic [1], [2]. Their dangerous synergy affects all aspects of both diseases, from pathogenesis and the epidemiologic profile, to clinical presentation, treatment, and prevention. This synergy also impacts larger societal issues with demographic, economic, and even political consequences [3].
The prevalence of HIV in Cameroon was estimated at 4.5% in 2012 [4]. In 2012, the number of HIV/AIDS related deaths reached 35,000 [4] with TB being the leading cause of death [5]–[7]. Based on an autopsy series in Botswana, 40 to 65% of HIV-infected patients with respiratory disease also harbored TB [8]. According to the World Health Organization (WHO), Cameroon which has a TB incidence rate of 124 cases per 100,000 inhabitants [9], is categorized as an intermediate TB burden country [10]. In 2013, 38% of TB patients in the country were infected with HIV [9].
In Cameroon, the treatment success rate in TB/HIV co-infected patients stands at 65% [9]. Despite improved treatment success rates, the death rate from TB in HIV co-infected persons remains very high. While some studies have found lower TB cure rates (58.8%) among TB/HIV patients compared to patients infected with TB alone (78.8%) [11], [12], other studies reported similar TB cure rates in those TB/HIV co-infected as compared to those infected with TB alone [13], [14]. Death rates in TB patients from sub-Saharan countries where HIV is highly prevalent have risen substantially over the last few decades [6]. A significant proportion of these deaths occur early in the course of treatment [7], [15]–[18] threatening the credibility of public health interventions to control TB in the eyes of the patients, health care providers, and the community. We therefore sought to evaluate the outcomes of TB treatment in TB/HIV co-infected patients with a focus on the factors associated with death during treatment.
Using a cohort design, we conducted an eight-year (2006–2013) hospital-based retrospective study at the Infectious Disease Unit (IDU) of the Yaoundé Central Hospital (YCH) to determine socio-demographical, clinical, and biological factors associated with death among TB/HIV co-infected patients undergoing TB treatment. We anticipate that data will contribute in improving the management of TB/HIV patients in Cameroon.
Materials and Methods
Design and setting
We conducted a hospital-based retrospective cohort study at the Infectious Disease Unit (IDU) of the Yaoundé Central Hospital (YCH) in Cameroon. The YCH is a tertiary-care hospital, which serves as a university teaching hospital for both undergraduate and postgraduate medical education. It offers specialized care for patients with infectious diseases along with various other pathologies, and has the greatest pool of HIV-infected patients in Yaoundé and its surrounding area. The IDU serves as the referral unit for the management of adults diagnosed with TB infection at the YCH. There are 238 Centers for Diagnosis and Treatment (CDT) of TB in Cameroon. The IDU houses one of the 22 TB CDTs in Yaoundé and thus treats and manages 10% of all TB cases diagnosed in the city, corresponding to 2% of all TB cases in Cameroon, per year [19]. All diagnosed TB cases are started on the standard TB treatment used in Cameroon [19]; outcomes are reported according to national TB control guidelines [19]. Patient medical information from initial consultation to discharge is recorded in a registry, the Tuberculosis Reporting Registry, which is the official system for mandatory reporting of all hospital TB cases.
Population
TB cases were identified via the Tuberculosis Reporting Register of the IDU of the YCH, the official system for mandatory reporting of all hospital TB cases. At diagnosis, TB reporting is mandatory nationwide in Cameroon for new cases or retreatment cases [19]. All patients reported from January 1, 2006 to June 30, 2013 were selected for inclusion in the study. This period was chosen in order to yield the maximum amount of data based on availability of the archives at the IDU of the YCH. Charts and data were located, retrieved, confirmed, and any identified duplicative files were consolidated. We included all TB/HIV co-infected patients who had undergone tuberculosis treatment with or without ART. The duration of follow-up for each patient was 6 months from initiation of the antituberculous therapeutic regimen, corresponding to the entire duration of TB treatment for new cases, and to the first 6 months of the 8-month regimen for retreated cases.
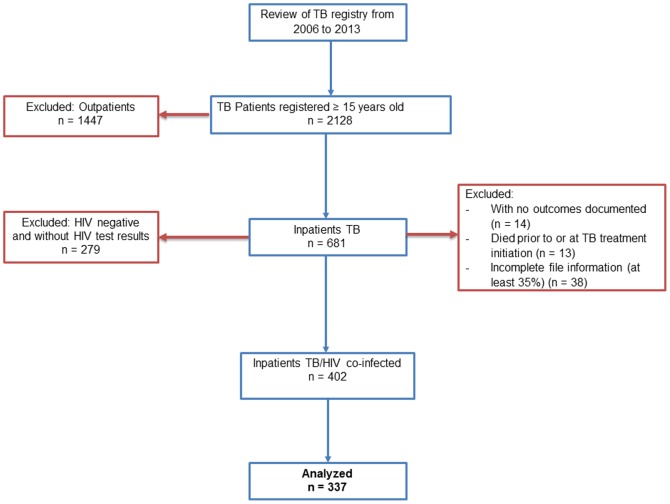
We screened 2,128 records from the TB Reporting Register, as schematized in Fig. 1, and included HIV-infected patients with TB, aged 15 years and older. We excluded outpatients, patients who died before initiation of TB treatment, or on the date TB treatment began, cases with missing information on follow-up and outcomes in the IDU/TB registers, and patients with incomplete file information (at least 35% of variables). Our study included only hospitalized patients because most of the outpatients fell under our exclusion criteria due to incomplete information in greater than 70%. After exclusions, 337 patients remained eligible for analysis and constituted our cohort of TB/HIV co-infected hospitalized subjects.
Figure 1. Enrollment of study participants.
Data collection
Procedure
Data were extracted from: (i) the TB Reporting Register of the IDU-YCH, (ii) hospitalization registry of the IDU, (iii) medical records of hospitalized TB/HIV co-infected patients and (iv) TB yellow cards of TB/HIV co-infected patients. A TB yellow card is a physical record (on A4 format paper) used in Cameroon for documenting ongoing tuberculosis treatment and that is retained at health center and handled by caregivers [19]. It contains demographic and administrative information (name, sex, age, address, TB registry number) along with the type of TB (pulmonary versus extra-pulmonary) at the start of treatment, HIV status, and a calendar with check boxes for each day of taking anti-TB medication.
Socio-demographic data
We reviewed the patients' medical records and extracted socio-demographic data, including age, sex, marital status, highest level of education, and residence.
Clinical data
We collected data on the timing of TB and HIV diagnoses. The physician-in-charge of the CDT classified each patient's clinical TB presentation at the time of diagnosis according to national guidelines [19] adapted from the WHO Standard International Definitions [10]: smear-positive pulmonary TB (Acid-fast bacilli [AFB] originally found in at least two sputum specimens), smear-negative pulmonary TB (persistence of negative result of three sputum examinations after ten days of non-specific antibiotic treatment in a patient with clinical and radiographic signs suggesting pulmonary TB without other obvious cause), and extra-pulmonary TB (a patient in whom TB was found in organs other than the lungs [e.g. pleura, lymph nodes, abdomen, genitourinary tract, skin, joints and bones, meninges]). We considered as mixed form patients those who had extra-pulmonary disease associated with any form of pulmonary TB. At the IDU, most of the diagnoses of extra-pulmonary TB were based on clinical assessment (because of limited access to specific diagnostic tools) and on a favorable response to empirical anti-TB treatment.
National guidelines [19] adopted from the WHO Standard International Definitions [10] were used to classify TB status at the time of diagnosis. Each TB cases was classified as a new case or a retreatment case. Other clinical variables retrieved from patients' medical records included body weight, the occurrence of other AIDS-related opportunistic diseases, co-morbidities that were neither TB nor AIDS-related diseases, information on the different treatment outcomes, and whether or not the patient received Cotrimoxazole Prophylactic Therapy (CPT) and ART.
Biological data
We also reviewed patients' laboratory results and retrieved total white blood cell and CD4 cell counts, and hemoglobin level.
TB treatment outcomes
At the IDU of the YCH, all patients receiving TB treatment are reassessed at the end of months 2, 5 and 6 for new cases, and at the end of months 3, 5 and 8 in cases of retreatment. Thus, all information on treatment outcome was obtained from patients' medical records and their TB yellow cards. Outcomes were classified by the physician in-charge of the CDT using the definitions from the TB program in Cameroon [19] adapted from WHO international standard definitions [10] as follow: Cured (an initially sputum smear-positive patient whose sputum was smear-negative in the last month of treatment and on at least one previous occasion), Completed Treatment (a TB patient who completed treatment without evidence of failure but with no documentation that sputum smear or culture results in the last month of treatment and on at least one previous occasion were negative, either because tests were not done or because the results were unavailable), Died (from any cause during treatment), Failed (a patient whose sputum was initially smear-positive and remained sputum smear-positive at month 5 or later during TB treatment), Lost to follow-up (A patient who did not start treatment or whose treatment was interrupted for two consecutive months or more), and Not evaluated (a TB patient for whom no treatment outcome was assigned. This includes cases ‘transferred out’ to another CDT of TB as well as cases for whom the treatment outcome is unknown to the reporting unit). Successfully treated was a patient who was cured or who completed treatment.
Ethics statement
We obtained an ethical clearance from the Institutional Review Board of the Faculty of Medicine and Biomedical Sciences, University of Yaoundé 1, Cameroon. An authorization of the YCH was also obtained before data collection. The data were collected and analyzed anonymously.
Data analysis
Data was extracted, coded, entered, and analyzed using the Statistical Package for Social Science (SPSS) version 20.0 for Windows (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.). Continuous variables were expressed as the mean with standard deviation (SD) or median with interquartile range (IQR). Categorical variables were expressed as frequency with percentages (%). Frequency of TB treatment outcomes was analyzed using Windows Program for Epidemiologists version 11.25 and reported with Fisher's exact 95% confidence interval (95%CI).
To analyze predictors of death, we excluded in the first analysis, all patients who were lost to follow-up and not evaluated because we had no information on the outcome (death or survival). We also did a secondary analysis on attrition, excluding only not evaluated patients and considering lost to follow-up as death. In a third analysis, all continuous variables have not been categorized. Then, multiple imputation was used to handle missing data, creating a new data set which was the average of five data sets of imputed values [20], [21]. We included in the imputation model only variables assessed as risk factors of death. We therefore did not calculate imputed data for treatment outcome and we excluded those patients from all analysis as it was our primary outcome. We classified any death that occurred during TB treatment as a TB death, consistent with WHO guidelines [10]. We conducted univariate analysis comparing death and non-death patients concerning socio-demographic, clinical and biological characteristics. After this, we performed binary multivariate regression analyses to identify independent predictors of death. The initial multivariate logistic regression model included non colinear variables associated with death (as dependent variable) if the p-value was ≤0.20 in the univariate analysis. The final model was obtained by successively removing variables not associated at a p-value <0.05 only if the odds ratios for remaining variables were unchanged and taking interactions into account. Known risk factors such as weight, CD4 count, ART, or sampling variables such as year of inclusion were maintained systematically in the final model.
Results
Description of study population
Of the 681 TB inpatients, 402 (59.0%) were HIV co-infected and 279 (41.0%) were free of HIV infection or had negative HIV test results (Fig. 1). Of the 337 eligible patients, 180 (53.4%) were female. Mean age was 39.3 years (SD 10.3). Most of patients (n = 149, 44.2%) had a secondary education. There were mostly married patients (n = 155, 46.0%). Most patients resided in an urban area (n = 301, 89.3%). When considering the clinical TB presentation, most patients had extra-pulmonary TB alone (n = 125, 37.1%), followed closely by cases of smear-positive pulmonary TB alone (n = 119, 35.3%). The majority of our cases represented new diagnoses of tuberculosis (n = 297, 88.1%). Four fifths of patients received CPT and around two thirds received ART (Table 1).
Table 1. Socio-demographic, clinical, and biological characteristics of 337 patients co-infected with TB and HIV, Yaoundé Central Hospital, 2006–2013, Cameroon.
| Variables | |
| SOCIO-DEMOGRAPHIC | |
| Sex | |
| Male | 46.6% (n = 157) |
| Female | 53.4% (n = 180) |
| Age | |
| Range | 18–78 years |
| Mean | 39.3 years (10.3) |
| Median | 38 years (32–46) |
| Educational level | |
| No formal education | 14.2% (n = 48) |
| Primary | 14.2% (n = 48) |
| Secondary | 44.2% (n = 149) |
| University | 27.3% (n = 92) |
| Residence | |
| Rural | 10.7% (n = 36) |
| Urban | 89.3% (n = 301) |
| Marital status | |
| Married/Cohabitating | 46.0% (n = 155) |
| Single | 44.8% (n = 151) |
| Widowed | 6.8% (n = 23) |
| Divorced | 2.4% (n = 8) |
| CLINICAL | |
| TB presentation* | |
| SPP TB only | 35.3% (n = 119) |
| SNP TB only | 22.3% (n = 75) |
| EP TB only | 37.1% (n = 125) |
| Mixed (Pulmonary + EP TB) | 5.3% (n = 18) |
| Status at TB diagnosis | |
| New case | 88.1% (n = 297) |
| Relapse | 7.7% (n = 26) |
| Re-treatment after failure | 2.1% (n = 7) |
| Defaulter | 0.3% (n = 1) |
| Other | 1.8% (n = 6) |
| Weight1 | |
| Mean | 53.2 Kg (10.3) |
| Median | 53 Kg (45–60) |
| Known duration of HIV infection | |
| Mean | 8.4 Months (19.4) |
| Median | 0.95 Months (0.13–5.7) |
| Other AIDS-related non-TB disease | 15.7% (n = 50) |
| Other non-AIDS co-morbidity | 11.9% (n = 40) |
| Received prophylactic cotrimoxazole | 79.5% (n = 268) |
| Received antiretroviral therapy | 67.4% (n = 227) |
| LABORATORY VALUES | |
| White blood cell count2 | |
| Mean | 6471 cells/mm3 (5210) |
| Median | 5100 cells/mm3 (3300–7990) |
| Hemoglobin3 | |
| Mean | 8.3 g/dl (2.4) |
| Median | 8 g/dl (7–10) |
| CD4 cell count4 | |
| Mean | 121 cells/mm3 (109) |
| Median | 102 cells/mm3 (33–178) |
Data are % (n), mean (standard deviation) or median (interquartile range).
TB: tuberculosis.
*SPP: smear positive pulmonary, SNP: smear negative pulmonary, EP: extra pulmonary.
Data missing: there were 36 (10.7%) records without recorded weights.
Data missing: there were 18 (5.3%) records without recorded white blood cell counts.
Data missing: there were 18 (5.3%) records without recorded hemoglobin values.
Data missing: there were 28 (8.3%) records without recorded CD4 cell counts.
TB treatment outcomes
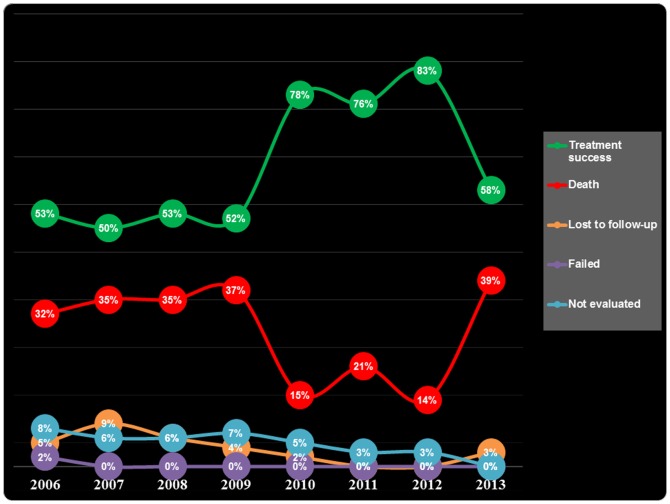
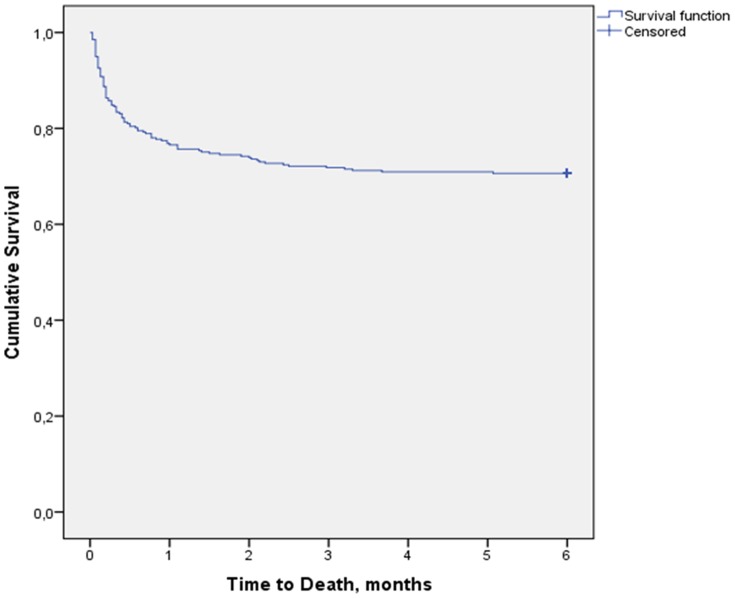
Among the 337 patients, TB treatment outcomes were distributed as follow: 134 (39.8%, 95%CI 34.5–45.2) completed treatment, 71 (21.1%, 95%CI 16.9–25.9) were cured, 99 (29.4%, 95%CI 24.6–34.6) died, 18 (5.3%, 95%CI 3.3–8.5) were not evaluated, 14 (4.2%, 95%CI 2.4–7.0) lost to follow-up and 1 (0.3%, 95%CI 0.02–1.9) failed. The overall treatment success rate (completed treatment or cured) was 60.8% (95% CI 55.4–66.0). Fig. 2 presents the trend of TB treatment outcomes in TB/HIV co-infected patients over the period from 2006 to 2013. In this figure, the trend of treatment success (green line) is symmetric to the trend of death (red line). After exclusion of not evaluated and lost to follow-up patients, among the remaining 305 patients who were followed for 6 months, the death rate was 32.5% (95%CI 27.2–38.0). Fig. 3 presents the Kaplan-Meier survival curve of the overall TB/HIV co-infected patients. We observed that after the 2nd month of TB treatment, the survival curve remains flat till the end of the 6th month. Death rates at one month, at two months, and at five months were 23.4%, 26.1% and 29.4% respectively.
Figure 2. Trend of TB treatment outcomes by year in TB/HIV co-infected patients from 2006 to 2013.
Figure 3. Kaplan-Meier survival curve of TB/HIV co-infected patients on TB treatment (N = 337).
Factors associated with death during TB treatment
In our first method of analysis (Table 2), in univariate analysis, factors associated with death during TB treatment were: TB diagnosis between 2006 and 2009, having body weight <50 Kgs, having a duration of known HIV infection ≥12 months, the presence of an AIDS-defining disease other than TB, the presence of an non-AIDS related comorbidity, not receiving CPT, not receiving ART, and having a CD4 cell count <50/mm3. In multivariate analysis, factors that remained associated with death during TB treatment were TB diagnosis between 2006 and 2009 (aOR: 2.50, 95%CI 1.31–4.78; p = 0.006), the presence of another AIDS-defining disease besides TB (aOR: 2.73, 95%CI 1.27–5.86; p = 0.010), the presence of another non-AIDS defining illness (aOR: 4.04, 95%CI 1.37–8.21; p = 0.008), not receiving CPT (aOR: 3.61, 95%CI 1.71–7.63; p = 0.001), not receiving ART (aOR: 2.45, 95%CI 1.18–5.08; p = 0.016), and having CD4 cell count <50/mm3 (aOR: 16.43, 95%CI 1.05–258.04; p = 0.047).
Table 2. Factors associated with death during TB treatment among TB/HIV co-infected patients, Yaoundé Central Hospital, 2006–2013, Cameroon.
| Variables, n (%) | Dead n = 99 | Alive n = 206 | Total N = 305§ | Univariate analysis | Multivariate analysis | ||
| Crude OR (CI 95%) | p-value | Adjusted OR (CI 95%) | p-value | ||||
| SOCIO-DEMOGRAPHIC | |||||||
| Sex | |||||||
| Male | 52 (52.5) | 96 (46.6) | 148 (48.5) | 1.27 (0.78–2.05) | .333 | ||
| Female | 47 (47.5) | 110 (53.4) | 157 (51.5) | Ref | |||
| Age (years) | |||||||
| ≥47 | 26 (26.3) | 47 (22.8) | 73 (23.9) | 1.22 (0.62–2.39) | .567 | ||
| 39–46 | 20 (20.2) | 58 (28.2) | 78 (25.6) | 0.76 (0.38–1.52) | .435 | ||
| 33–38 | 28 (28.3) | 46 (22.3) | 74 (23.3) | 1.34 (0.69–2.61) | .390 | ||
| 18–32 | 25 (25.3) | 55 (26.7) | 80 (26.2) | Ref | |||
| Level of education | |||||||
| Primary/No formal | 30 (30.3) | 56 (27.2) | 86 (28.2) | 1.16 (0.69–1.97) | .571 | ||
| Secondary/University | 69 (69.7) | 150 (72.8) | 219 (71.8) | Ref | |||
| Marital status | |||||||
| Alone (single/widowed/divorced) | 60 (60.6) | 103 (50.0) | 163 (53.4) | 1.54 (0.95–2.50) | .083 | 1.44 (0.78–2.65) | .242 |
| Married/Cohabiting | 39 (39.4) | 103 (50.0) | 142 (46.6) | Ref | |||
| Residence | |||||||
| Rural | 13 (13.1) | 21 (10.2) | 34 (11.1) | 0.75 (0.40–1.57) | .447 | ||
| Urban | 86 (86.9) | 185 (89.8) | 271 (88.9) | Ref | |||
| CLINICAL | |||||||
| Year of TB diagnosis | |||||||
| 2006–2009 | 70 (70.7) | 106 (51.5) | 176 (57.7) | 2.28 (1.37–3.80) | .002 | 2.50 (1.31–4.78) | .006 |
| 2010–2013 | 29 (29.3) | 100 (48.5) | 129 (42.3) | Ref | |||
| TB clinical presentation* | |||||||
| Mixed (Pulmonary + EP TB) | 8 (8.1) | 7 (3.4) | 15 (4.9) | 2.91 (1.86–4.56) | .973 | ||
| EP TB only | 41 (41.4) | 69 (33.5) | 110 (36.1) | 1.51 (1.20–1.91) | .859 | ||
| SNP TB only | 19 (19.2) | 51 (24.8) | 70 (23.0) | 0.95 (0.72–1.25) | .485 | ||
| SPP TB only | 31 (31.3) | 79 (38.3) | 110 (36.1) | Ref | |||
| Status at TB diagnosis | |||||||
| Retreatment case | 11 (11.1) | 27 (13.1) | 38 (12.5) | 0.83 (0.61–1.12) | .227 | ||
| New case | 88 (88.9) | 179 (86.9) | 267 (87.5) | Ref | |||
| Body weight (Kg) | |||||||
| ≤50 | 51 (51.5) | 63 (30.6) | 114 (37.4) | 2.38 (1.44–3.93) | .001 | 1.75 (0.94–3.29) | .079 |
| >50 | 39 (39.4) | 117 (56.8) | 156 (51.1) | Ref | |||
| Missing | 9 (9.1) | 26 (12.6) | 35 (11.5) | - | |||
| Duration of known HIV infection | |||||||
| ≥12 months | 13 (13.1) | 39 (18.9) | 52 (17.0) | 0.65 (0.49–0.85) | .002 | 0.73 (0.32–1.68) | .460 |
| <12 months | 86 (86.9) | 167 (81.1) | 253 (83.0) | Ref | |||
| Presence of another AIDS-related non-TB disease | |||||||
| Yes | 27 (27.3) | 23 (11.2) | 50 (16.4) | 2.98 (2.32–3.84) | <.0001 | 2.73 (1.27–5.86) | .010 |
| No | 72 (72.7) | 183 (88.8) | 255 (83.6) | Ref | |||
| Presence of another non-AIDS comorbidity | |||||||
| Yes | 20 (20.2) | 17 (8.3) | 37 (12.1) | 2.81 (2.12–3.74) | <.0001 | 3.35 (1.37–8.21) | .008 |
| No | 79 (79.8) | 189 (91.7) | 268 (87.9) | Ref | |||
| Cotrimoxazole prophylactic therapy | |||||||
| No | 32 (32.3) | 31 (15.0) | 63 (20.7) | 2.70 (2.14–3.40) | <.0001 | 3.61 (1.71–7.63) | .001 |
| Yes | 67 (67.7) | 175 (85.0) | 242 (79.0) | Ref | |||
| Antiretroviral therapy | |||||||
| No | 37 (37.4) | 60 (29.1) | 97 (31.8) | 1.45 (1.18–1.79) | .0004 | 2.45 (1.18–5.08) | .016 |
| Yes | 62 (62.6) | 146 (70.9) | 208 (68.2) | Ref | |||
| LABORATORY VALUES | |||||||
| White blood cell level (cell/mm3) | |||||||
| <4,000 | 38 (38.4) | 61 (29.6) | 99 (32.5) | 1.67 (0.98–2.84) | .061 | 1.32 (0.68–2.58) | .417 |
| >10,000 | 14 (14.1) | 26 (12.6) | 40 (13.1) | 1.39 (0.67–2.87) | .375 | 0.54 (0.20–1.46) | .220 |
| 4,000–10,000 | 40 (40.4) | 110 (53.4) | 150 (49.2) | Ref | |||
| Missing | 7 (7.1) | 9 (4.4) | 16 (5.2) | - | |||
| Hemoglobin level (g/dl) | |||||||
| <8 | 39 (39.4) | 61 (29.6) | 100 (32.8) | 1.55 (0.91–2.64) | .107 | 1.33 (0.70–2.54) | .380 |
| ≥8 | 53 (53.5) | 135 (65.5) | 188 (61.6) | Ref | |||
| Missing | 7 (7.1) | 10 (4.9) | 17 (5.6) | - | |||
| CD4 cell count (cell/mm3) | |||||||
| <50 | 56 (56.6) | 44 (21.4) | 100 (32.8) | 8.14 (0.75–88.89) | .082 | 16.43 (1.05–258.04) | .047 |
| 50–199 | 31 (31.3) | 100 (48.5) | 131 (43.0) | 2.09 (0.20–21.71) | .519 | 4.17 (0.29–59.8) | .275 |
| 200–350 | 4 (4.0) | 33 (16.0) | 37 (12.1) | 1.09 (0.06–19.28) | .950 | 1.40 (0.06–31.52) | .821 |
| >350 | 1 (1.0) | 10 (4.9) | 11 (3.6) | Ref | |||
| Missing | 7 (7.1) | 19 (9.2) | 26 (8.5) | - | |||
From the 337 patients, we have excluded all patients who were lost to follow-up (n = 14) and not evaluated (n = 18).
*SPP: smear positive pulmonary, SNP: smear negative pulmonary, EP: extra pulmonary.
TB: tuberculosis.
All missing data were imputed.
In the analysis on attrition (Table 3), in univariate analysis, factors associated with death/lost to follow-up (LTFU) during TB treatment were: being single, a TB diagnosis between 2006 and 2009, having mixed form of TB at clinical presentation, having body weight <50 Kgs, the presence of an AIDS-defining disease other than TB, the presence of an non-AIDS related comorbidity, and not receiving CPT. In multivariate analysis, factors associated with death/LTFU during TB treatment were TB diagnosis between 2006 and 2009 (aOR: 3.0, 95%CI 1.57–5.70; p = 0.001), the presence of another AIDS-defining disease besides TB (aOR: 2.61, 95%CI 1.20–5.70; p = 0.016), the presence of another non-AIDS defining illness (aOR: 4.04, 95%CI 1.37–8.21; p = 0.008), not receiving CPT (aOR: 3.91, 95%CI 1.86–8.21; p<0.001), not receiving ART (aOR: 2.64, 95%CI 1.29–5.38; p = 0.008), and having CD4 cell count <50/mm3 (aOR: 11.14, 95%CI 1.82–68.21; p = 0.011).
Table 3. Factors associated with death/lost to follow-up during TB treatment among TB/HIV co-infected patients, Yaoundé Central Hospital, 2006–2013, Cameroon.
| Variables, n (%) | Dead+LTFUn = 113 | Alive n = 206 | Total N = 319§ | Univariate analysis | Multivariate analysis | ||
| Crude OR (CI 95%) | p-value | Adjusted OR (CI 95%) | p-value | ||||
| SOCIO-DEMOGRAPHIC | |||||||
| Sex | |||||||
| Male | 54 (47.8) | 96 (46.6) | 150 (53.0) | 0.95 (0.60–1.51) | .839 | ||
| Female | 59 (52.2) | 110 (53.4) | 169 (47.0) | Ref | |||
| Age (years) | |||||||
| ≥47 | 26 (23.0) | 47 (22.8) | 73 (22.9) | 1.05 (0.54–2.02) | .886 | ||
| 39–46 | 25 (22.1) | 58 (28.2) | 83 (26.0) | 0.82 (0.43–1.57) | .543 | ||
| 33–38 | 33 (29.2) | 46 (22.3) | 79 (24.8) | 1.36 (0.72–2.57) | .341 | ||
| 18–32 | 29 (25.7) | 55 (26.7) | 84 (26.3) | Ref | |||
| Level of education | |||||||
| Primary/No formal | 35 (31.0) | 56 (27.2) | 91 (28.5) | 1.20 (0.72–1.99) | .474 | ||
| Secondary/University | 78 (69.0) | 150 (72.8) | 228 (71.5) | Ref | |||
| Marital status | |||||||
| Alone (single/widowed/divorced) | 70 (69.1) | 103 (50.0) | 173 (54.2) | 1.63 (1.02–2.60) | .041 | 1.67 (0.91–3.04) | .097 |
| Married/Cohabiting | 43 (38.1) | 103 (50.0) | 146 (45.8) | Ref | |||
| Residence | |||||||
| Rural | 13 (11.5) | 21 (89.8) | 34 (10.7) | 1.15 (0.55–2.38) | .717 | ||
| Urban | 100 (88.5) | 185 (10.2) | 285 (89.3) | Ref | |||
| CLINICAL | |||||||
| Year of TB diagnosis | |||||||
| 2006–2009 | 82 (72.6) | 106 (51.5) | 188 (58.9) | 2.50 (1.52–4.10) | <.001 | 3.0 (1.57–5.70) | .001 |
| 2010–2013 | 31 (27.4) | 100 (48.5) | 131 (41.1) | Ref | |||
| TB clinical presentation* | |||||||
| Mixed (Pulmonary + EP TB) | 10 (8.8) | 7 (3.4) | 17 (5.3) | 2.89 (1.02–8.18) | .045 | 1.74 (0.47–6.48) | .407 |
| EP TB only | 43 (38.1) | 69 (33.5) | 112 (35.1) | 1.26 (0.74–2.17) | .398 | 1.49 (0.76–2.91) | .241 |
| SNP TB only | 21 (18.6) | 51 (24.8) | 72 (22.6) | 0.83 (0.44–1.58) | .577 | 0.48 (0.21–1.06) | .069 |
| SPP TB only | 39 (34.5) | 79 (38.3) | 118 (37.0) | Ref | |||
| Status at TB diagnosis | |||||||
| Retreatment case | 12 (10.6) | 27 (13.1) | 39 (12.2) | 0.79 (0.38–1.62) | .517 | ||
| New case | 101 (89.4) | 179 (86.9) | 280 (87.8) | Ref | |||
| Body weight (Kg) | |||||||
| ≤50 | 56 (49.6) | 63 (30.6) | 119 (37.3) | 2.11 (1.27–3.50) | .004 | 1.46 (0.79–2.69) | .226 |
| >50 | 47 (41.6) | 117 (56.8) | 164 (51.4) | Ref | |||
| Missing | 10 (8.8) | 26 (12.6) | 36 (11.3) | - | |||
| Duration of known HIV infection | |||||||
| ≥12 months | 14 (12.4) | 39 (18.9) | 53 (16.6) | 1.65 (0.85–3.19) | .136 | 1.31 (0.59–2.90) | .513 |
| <12 months | 99 (87.6) | 167 (81.1) | 266 (83.4) | Ref | |||
| Presence of another AIDS-related non-TB disease | |||||||
| Yes | 29 (25.7) | 23 (11.2) | 52 (16.3) | 2.74 (1.50–5.03) | .001 | 2.61 (1.20–5.70) | .016 |
| No | 84 (74.3) | 183 (88.8) | 267 (83.7) | Ref | |||
| Presence of another non-AIDS comorbidity | |||||||
| Yes | 21 (18.6) | 17 (8.3) | 38 (11.9) | 2.54 (1.28–5.04) | .008 | 3.34 (1.40–7.99) | .007 |
| No | 92 (81.4) | 189 (91.7) | 281 (88.1) | Ref | |||
| Cotrimoxazole prophylactic therapy | |||||||
| No | 36 (31.9) | 31 (15.0) | 67 (21.0) | 2.64 (1.52–4.57) | .001 | 3.91 (1.86–8.21) | <.001 |
| Yes | 77 (68.1) | 175 (85.0) | 252 (79.0) | Ref | |||
| Antiretroviral therapy | |||||||
| No | 43 (38.1) | 60 (29.1) | 103 (32.3) | 1.50 (0.92–2.43) | .104 | 2.64 (1.29–5.38) | .008 |
| Yes | 70 (61.9) | 146 (70.9) | 216 (67.7) | Ref | |||
| LABORATORY VALUES | |||||||
| White blood cell level (cell/mm3) | |||||||
| <4,000 | 41 (36.3) | 61 (29.6) | 102 (32.0) | 1.49 (0.88–2.51) | .135 | 1.26 (0.65–2.43) | .499 |
| >10,000 | 17 (15.0) | 26 (12.6) | 43 (13.5) | 1.57 (0.78–3.17) | .206 | 0.71 (0.29–1.76) | .453 |
| 4,000–10,000 | 47 (41.6) | 110 (53.4) | 157 (49.2) | Ref | |||
| Missing | 8 (7.1) | 9 (4.4) | 17 (5.3) | - | |||
| Hemoglobin level (g/dl) | |||||||
| <8 | 42 (37.2) | 61 (29.6) | 103 (32.3) | 1.45 (0.90–2.35) | .129 | 1.28 (0.70–2.34) | .426 |
| ≥8 | 63 (55.8) | 136 (66.0) | 199 (62.4) | Ref | |||
| Missing | 8 (7.1) | 9 (4.4) | 17 (5.3) | - | |||
| CD4 cell count (cell/mm3) | |||||||
| <50 | 62 (54.9) | 44 (21.4) | 106 (33.2) | 3.81 (0.89–16.24) | .069 | 11.14 (1.82–68.21) | .011 |
| 50–199 | 36 (31.9) | 100 (48.5) | 136 (42.6) | 1.03 (0.22–4.74) | .967 | 2.59 (0.44–15.44) | .283 |
| 200–350 | 4 (3.5) | 33 (16.0) | 37 (11.6) | 0.44 (0.07–2.90) | .379 | 0.61 (0.09–4.13) | .604 |
| >350 | 3 (2.7) | 10 (4.9) | 13 (4.1) | Ref | |||
| Missing | 8 (7.1) | 19 (9.2) | 27 (8.5) | - | |||
From the 337 patients, we have excluded all patients who were not evaluated (n = 18).
*SPP: smear positive pulmonary, SNP: smear negative pulmonary, EP: extra pulmonary.
LTFU: lost to follow-up, TB: tuberculosis.
All missing data were imputed.
In the third analysis (S1 Data), integrating age, body weight, duration of known HIV infection, white blood cell level, hemoglobin level, and CD4 cell count without categorization, factors associated with death were TB diagnosis between 2006 and 2009 (aOR: 2.55, 95%CI 0.81–2.64; p = 0.003), the presence of another AIDS-defining disease besides TB (aOR: 2.39, 95%CI 1.14–5.02; p = 0.022), the presence of another non-AIDS defining illness (aOR: 2.83, 95%CI 1.22–.60; p = 0.016), not receiving CPT (aOR: 3.31, 95%CI 1.61–6.80; p = 0.001), not receiving ART (aOR: 2.41, 95%CI 1.20–4.84; p = 0.014), and CD4 cell count (aOR [1 cell decrease]: 1.01, 95%CI 1.006–1.013; p<0.0001).
Discussion
In this retrospective tertiary-care hospital-based study in Cameroon, the TB treatment success rate among TB/HIV co-infected patients is low (60.8%), possibly reflecting a high death rate (29.4%). However, we noted an improvement in the treatment success rates over time, from 2006 to 2013. Moreover, co-infected patients in our setting demonstrated lower rates of LTFU and failure as compared to that reported in other studies [7], [9], [17], [22]–[24]. The present study reveals several factors associated with death in TB/HIV co-infected patients ongoing TB treatment at the IDU of the YCH. They include four clinical factors: the presence of another opportunistic disease, the presence of another non-AIDS related comorbidity, not receiving CPT, and not receiving ART; and one biological factor: having a CD4 cell count <50/mm3. The study did not identify an association with any socio-demographic factors. A TB diagnosis between 2006 and 2009 was also a factor associated with death within 6 months of TB treatment initiation. The same factors were found when we added LTFU to death.
Death rate
The overall death rate in our cohort of patients was 29.4% (95%CI 24.6–34.6), which is notably higher than that reported in previous studies including inpatients and outpatients in Cameroon (9.9% [95%CI 7.4–12.8] and 7.4% [95%CI 3.6–13.1]) [11], [23], in Nigeria (11.1% [95%CI 7.0–16.5]) [25], and in India (15.0%, 95%CI 14.4–15.9) [26]. Most patients requiring hospitalization for TB therapy (as is the case in our cohort) are generally sicker. They present with advanced infection and severe immune deficiency, such that in the absence of rapid intervention, death becomes imminent. Most of the deaths in our cohort occurred within the first 4 weeks after TB treatment initiation. This is congruent with findings from several studies where death in TB/HIV co-infected patients tended to occur early in the course of TB treatment [15], [16], [18], [27]. The fact that patients, especially those suspected of having extra-pulmonary TB were often unable to pay for and obtain needed diagnostic testing contributed to delays in TB diagnosis and treatment. A number of possible explanations may underlie the observed increased mortality among our co-infected patients. The location and extent of TB is influenced by the degree of host immunosuppression, often increasing the difficulty of diagnosis and hence delaying treatment initiation, resulting in higher mortality [28]. Immunological studies have also shown that host responses to Mycobacterium tuberculosis enhance HIV replication [29], [30], thus accelerating the natural progression of HIV and further depressing cellular immunity. From the year 2006 to 2009, the death rate in our study population was stable, remaining between 32% and 35%. It dropped to about 15% in 2010 and later rose to about 39% in 2013. Again, a great proportion of patients in the 2013 cohort were still on TB treatment and their outcomes had not yet being assigned to them at the time of data collection data for the present study. Another contributing factor was several occurrences of inadequate supplies and stock-outs of anti-tuberculosis drugs at the IDU in the year 2013.
Death rates in TB patients particularly in the high HIV prevalence populations of sub-Saharan African countries including Cameroon, have risen substantially over the last decades [6]. It is worth noting that HIV infection has been shown to be an independent predictor of death in TB patients during TB treatment [7], [31], [32] and may represent the major reason why the 29.4% overall mortality rate in our cohort of patients was as high as it was. Sileshi and colleagues [16] in Northwest Ethiopia similarly found that the risk of death during TB treatment is higher in patients treated as inpatients compared to those treated at an outpatient health center. As mentioned, a likely reason is that those who are cared for in the inpatient setting have more extensive disease. As a result, the more severely ill hospitalized patients have a greater mortality, as compared to the less ill health center outpatients. This observation also likely explains the reason for the high incidence of death in our study population treated at the IDU of the YCH. The symmetric trend of success rate and death suggest that the frequency of death rate was possibly related to success rate but not to other outcomes of TB treatment. The overall death rate in our cohort of patients is also notably higher than that reported in another major center of tuberculosis treatment in Yaoundé (Jamot hospital) among inpatients (10–12%) [7], [24]. This is possibly due to fact that Jamot hospital is a specialized center focusing on management of TB compared to IDU-YCH. Also, among patients followed for six months, death rate was higher as compared to the death rate reported with inclusion of LTFU and not evaluated patients.
Factors associated with death
The presence of other AIDS-related opportunistic diseases, co-morbidities, and other non-AIDS related conditions was associated with mortality. The finding that opportunistic infections and other co-morbid conditions are associated with a lethal outcome during treatment is consistent with a study carried out in Malaysia [27] and in Northern India [33]. TB is known to be the most common opportunistic infection and the leading cause of death in HIV patients worldwide, especially in resource-limited settings [5], [6]. Thus, when other AIDS-related opportunistic diseases and other co-morbid conditions are added to the burden of TB and when TB complicates the course of AIDS, the immune system weakens and death becomes inevitable in spite of anti-tuberculosis treatments. We cannot rule out the ineffectiveness of CPT and patients not receiving CPT as potential underlying contributors to the risk of death among those with other AIDS-related opportunistic diseases.
Patients not receiving CPT were more likely to die than those taking CPT. In line with this finding, studies from Sub-Saharan Africa have shown that not taking CPT was significantly associated with mortality [16], [34]–[36]. In Cameroon, CPT is indicated in all cases of TB/HIV co-infection in both adults and children, irrespective of the CD4 cell count level and WHO clinical stage [19]. All the patients in our cohort were thus eligible to CPT. Mwaungulu and colleagues in Malawi [36] found that overall TB mortality rate was reduced from 37% to 29% when patients received CPT. In their study, although TB mortality rate was unchanged over 2 years in HIV-negative patients, it decreased from 43% to 24% in HIV-infected patients. The study concluded that TB/HIV patients should systematically receive CPT. In our study, however, patients who died shortly after being diagnosed with TB and HIV may not have had the chance to initiate CPT. This may have led us to overestimate the benefit of CPT.
Not receiving ART has been well documented as a risk factor for death among TB/HIV co-infected patients in several studies [16], [26], [27], [37]. Our results are in agreement with the observation, as not receiving ART was an independent predictor of death in our cohort of TB/HIV co-infected patients undergoing TB treatment. The widespread use of antiretroviral therapy beginning in 1996 has markedly improved the survival of HIV-infected patients in both developing and developed countries by reducing the number of deaths from opportunistic infections [4]. In TB/HIV co-infected patients, introduction of ART is not an easy decision because of issues related to immune reconstitution inflammatory syndrome. However, Sileshi and colleagues in Ethiopia demonstrated that HIV-associated TB patients will have better survival if highly active ART and TB treatment are started concurrently [16], a finding consistent with the Camelia study in which the death rate was lower among patients receiving ART 2 weeks after TB treatment initiation compared to those receiving ART 8 weeks later [38]. This is also in line with recommendations from the Ministry of Public Health in Cameroon [19], [39].
The present study further revealed that TB/HIV co-infected patients with low CD4 counts <50 cells/mm3 were more likely to die than those with CD4 cell counts >350 cells/mm3. This striking observation is consistent with a study in Northwest Ethiopia, which showed that TB/HIV co-infected patients with a CD4 count of <50 cells/mm3 had a 13% increased risk of death compared to patients with CD4 counts greater than or equal to 200 cells/mm3. Other studies have reported that the highest death rates occurred in TB/HIV co-infected patients with the lowest CD4 counts [7], [27], [37], [40]. HIV infection impairs cell-mediated immunity, largely through depletion of CD4 lymphocytes. The impaired immunity leads to increased number of cases of primary TB and reactivation of TB in HIV-infected people [41]. In addition, the depletion of CD4 cells can, because of the emergence of associated illness, result in interruption of TB treatment, a risk factor for the development of drug resistant TB, the treatment of which is costly and complex, especially in the setting of HIV-infection further contributing to an increasing risk of death [17].
Interestingly, we found that a diagnosis of TB in the period from 2006 to 2009 (before 2010) was significantly associated with death as compared to a diagnosis made during other periods of our study. In 2010, Cameroon introduced updated national guidelines on ART based on the WHO recommendations and guidance [39], designed to improve the quality and enhance the delivery of care. From 2006 to 2009, ART was prescribed in the presence of active TB and CD4 count ≤350 cell/mm3 and can be deferred once CD4 count ≥200 cell/mm3. Since 2010, ART has been prescribed for persons with HIV and active TB regardless of the level of CD4 cell count. It is therefore important to prescribe ART to all TB/HIV co-infected patients as soon as possible after the TB diagnosis is established.
Certain risk factors for death we identified in our cohort are identical with those in a general HIV-infected population without TB [42]–[44]. This is explained by the fact that the emergence of TB in the setting of HIV reflects HIV-related immunosuppression and TB infection can therefore be considered a stage of HIV infection.
Limitations
Our study has some limitations. Data about other potentially confounding biomedical predictors for death in our population, such as drug resistance, serum albumin level, time from HIV diagnosis to initiation of ART, and adherence to medication were not available and were thus excluded from the analysis [38], [45]–[47]. Data related to these potential predictors were incomplete or absent in many patient records, underscoring the significant challenges of performing ‘real life’ retrospective clinical research in Sub-Saharan Africa. For example, due to patients' lack of money, some diagnostic and laboratory testing were not performed. Furthermore, our study was limited to patients hospitalized during the study period, representing about one third of all patients with tuberculosis followed at the center during that time and two thirds of all TB/HIV co-infected patients aged 15 years and above. Our results may, therefore, not be generalizable to all patients with TB/HIV, particularly those receiving their treatment on an ambulatory basis. Exclusion of patients who were transferred out may have also confounded our results slightly since patients lost to follow-up probably included individuals dying at home without their death being reported. Duration of known HIV infection is distinct from the duration of HIV infection and therefore underestimates the true duration of HIV infection. We also acknowledge that body mass index would have provided more information that weight alone, unfortunately, we did could not get the height of patients in medical records.
Conclusions
The death rate was high in our cohort. TB diagnosis before 2010, the presence of other AIDS-related diseases and non AIDS-related co-morbid conditions, not receiving CPT, not receiving ART, and having CD4 cell counts <50 cells/mm3 were identified as potential factors of death during TB treatment among TB/HIV co-infected patients.
We recommend that clinicians initiate TB treatment well before CD4 cells fall below 50 cells/mm3; and that they prescribe CPT for all TB/HIV co-infected patients. We also call for more clinical and scientific research in the area of TB/HIV co-infection on a larger scale, including both inpatients and outpatients in resource limited settings to assess the generalizability of our findings. For health policy makers, we advocate reinforcing training of health personnel on the diagnosis and management of TB/HIV co-infection, making TB and HIV drugs and cotrimoxazole consistently available to all those requiring them, in order to avoid stock-outs, and strengthening TB and HIV collaborative treatment activities in order to generate the knowledge that is needed for better care of TB/HIV co-infected patients.
Supporting Information
Factors associated with death during TB treatment among TB/HIV co-infected patients, Yaoundé Central Hospital, 2006–2013, Cameroon (sensitivity analysis integrating continuous data).
(DOCX)
Data of the 337 patients included in the study.
(PDF)
Acknowledgments
This study was conducted as part of AAA's MD degree requirement. We acknowledge and thank the personnel of the Infectious Disease Unit of Yaoundé Central Hospital for their support during data collection.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper as Supplementary file S2.
Funding Statement
The authors have no support or funding to report.
References
- 1. Sunderam G, McDonald RJ, Maniatis T, Oleske J, Kapila R, et al. (1986) Tuberculosis as a manifestation of the acquired immunodeficiency syndrome (AIDS). JAMA 256:362–366. [PubMed] [Google Scholar]
- 2. Quinn TC, Mann JM, Curran JW, Piot P (2001) AIDS in Africa: an epidemiologic paradigm. 1986. Bull World Health Organ 79:1159–1167. [PMC free article] [PubMed] [Google Scholar]
- 3. Friedland G, Churchyard GJ, Nardell E (2007) Tuberculosis and HIV coinfection: current state of knowledge and research priorities. J Infect Dis 196 Suppl 1: S1–3. [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS (2013) Global Report: UNAIDS report on the global AIDS epidemic 2013. UNAIDS. Available: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. Accessed 2014 Nov 24.
- 5.World Health Organization (2011) Global tuberculosis control: WHO report 2011. Geneva, Switzerland: World Health Organization. Available: http://apps.who.int/iris/bitstream/10665/44728/1/9789241564380_eng.pdf?ua=1. Accessed 2014 Nov 24.
- 6. Harries AD (2000) Issues facing TB control (6). Tuberculosis control in sub-Saharan Africa in the face of HIV and AIDS. Scott Med J 45:47–50 discussion 51. [DOI] [PubMed] [Google Scholar]
- 7. Kuaban C, Pefura E, Bava D, Onana I (2011) Early mortality in new patients on treatment for smear positive pulmonary tuberculosis in Yaounde-Cameroon. Health Sciences and Diseases 12. [Google Scholar]
- 8. Ansari NA, Kombe AH, Kenyon TA (2002) Pathology and causes of death in a group of 128 predominantly HIV-positive patients in Botswana, 1997–1998. Int J Tuberc Lung Dis 6:55–63. [PubMed] [Google Scholar]
- 9.Programme National de lutte contre la Tuberculose (2014) Plan stratégique de lutte contre la tuberculose au Cameroun 2015–2019. Yaoundé, Cameroon: Programme National de lutte contre la Tuberculose. Available: http://www.pnlt.cm/index.php/documentation/plan-strategique-national/doc_download/18-plan-strategique-natinal-tuberculose-cameroun. Accessed 2014 Nov 24.
- 10.World Health Organization (2013) Global tuberculosis report 2013. Geneva, Switzerland: World Health Organization. Available: http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf. Accessed 2014 Nov 24.
- 11. Sume GE, Hoshen M, Bita G, Kabore S, Nzima VN (2009) Treatment outcome of TB/HIV positive and negative smear positive pulmonary tuberculosis patients treated using daily self-administered therapy in a Cameroonian district hospital. East Afr Med J 86:469–475. [DOI] [PubMed] [Google Scholar]
- 12. Banerjee A, Moyo S, Salaniponi F, Harries A (1997) HIV testing and tuberculosis treatment outcome in a rural district in Malawi. Trans R Soc Trop Med Hyg 91:707–708. [DOI] [PubMed] [Google Scholar]
- 13. van den Broek J, Mfinanga S, Moshiro C, O'Brien R, Mugomela A, et al. (1998) Impact of human immunodeficiency virus infection on the outcome of treatment and survival of tuberculosis patients in Mwanza, Tanzania. Int J Tuberc Lung Dis 2:547–552. [PubMed] [Google Scholar]
- 14. El-Sony AI, Khamis AH, Enarson DA, Baraka O, Mustafa SA, et al. (2002) Treatment results of DOTS in 1797 Sudanese tuberculosis patients with or without HIV co-infection. Int J Tuberc Lung Dis 6:1058–1066. [PubMed] [Google Scholar]
- 15. Zachariah R, Spielmann MP, Harries AD, Salaniponi FML (2002) Moderate to severe malnutrition in patients with tuberculosis is a risk factor associated with early death. Trans Roy Soc Trop Med Hyg 96:291–294. [DOI] [PubMed] [Google Scholar]
- 16. Sileshi B, Deyessa N, Girma B, Melese M, Suarez P (2013) Predictors of mortality among TB-HIV Co-infected patients being treated for tuberculosis in Northwest Ethiopia: a retrospective cohort study. BMC Infect Dis 13:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Connolly C, Reid A, Davies G, Sturm W, McAdam KP, et al. (1999) Relapse and mortality among HIV-infected and uninfected patients with tuberculosis successfully treated with twice weekly directly observed therapy in rural South Africa. AIDS 13:1543–1547. [DOI] [PubMed] [Google Scholar]
- 18. Shaweno D, Worku A (2012) Tuberculosis treatment survival of HIV positive TB patients on directly observed treatment short-course in Southern Ethiopia: a retrospective cohort study. BMC Res Notes 5:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Programme National de lutte contre la Tuberculose (2012) Guide pour les personnels de santé. Yaoundé, Cameroon: Ministry of Public Health of Cameroon. Available: http://www.pnlt.cm/index.php/documentation/rapports-d-activites/cat_view/4-guidetechnique?orderby=dmdatecounter&ascdesc=DESC. Accessed 2014 Nov 24.
- 20. Rubin D, Schenker N (1991;Apr Multiple imputation in health-care databases: an overview and some applications. Statistics in medicine 10:585–598. [DOI] [PubMed] [Google Scholar]
- 21.Little RJ, Rubin DB (1987) Statistical Analysis With Missing Data: Wiley.
- 22. Kuaban C, Bercion R, Koulla-Shiro S (1997) HIV seroprevalence rate and incidence of adverse skin reactions in adults with pulmonary tuberculosis receiving thiacetazone free anti-tuberculosis treatment in Yaounde, Cameroon. East Afr Med J 74:474–477. [PubMed] [Google Scholar]
- 23. Pefura Yone EW, Kuaban C, Kengne AP (2012) HIV testing, HIV status and outcomes of treatment for tuberculosis in a major diagnosis and treatment centre in Yaounde, Cameroon: a retrospective cohort study. BMC Infect Dis 12:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yone EW, Kuaban C, Kengne AP (2012) [Impact of HIV infection on the evolution of tuberculosis among adult patients in Yaounde, Cameroon]. Rev Pneumol Clin 68:338–344. [DOI] [PubMed] [Google Scholar]
- 25. Ifebunandu NA, Ukwaja KN, Obi SN (2012) Treatment outcome of HIV-associatedtuberculosis in a resource-poor setting. Trop Doct 42:74–76. [DOI] [PubMed] [Google Scholar]
- 26. Shastri S, Naik B, Shet A, Rewari B, De Costa A (2013) TB treatment outcomes among TB-HIV co-infections in Karnataka, India: how do these compare with non-HIV tuberculosis outcomes in the province? BMC Public Health 13:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ismail I, Bulgiba A (2013) Predictors of death during tuberculosis treatment in TB/HIV co-infected patients in Malaysia. PLoS One 8:e73250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizzardi GP, Pantaleo G (1999) The immunopathogenesis of HIV-sa1 infection. In: Armstrong D, Cohen J, editors. Infectious diseases. London: Harcourt. pp.1–12.
- 29. Nakata K, Rom WN, Honda Y, Condos R, Kanegasaki S (1997) Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication in the lung. Am J Respir Crit Care Med 155:996–1003. [DOI] [PubMed] [Google Scholar]
- 30. Zhang Y, Nakata K, Weiden M, Rom WN (1995) Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication by transcriptionalactivation at the long terminal repeat. J Clin Invest 95:2324–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Connolly C, Davies GR, Wilkinson D (1998) Impact of human immunodeficiency virus epidemic on mortality among adults with tuberculosis in rural South Africa. Int J Tuberc Lung Dis 2:919–925. [PubMed] [Google Scholar]
- 32. Harries AD, Nyangulu DS, Kang'Ombe C, Ndalama D, Glynn JR, et al. (1998) Treatment outcome of an unselected cohort of tuberculosis patients in relation to human immunodeficiency virus serostatus in Zomba hospital, Malawi. Trans Roy Soc Trop Med Hyg 92:343–347. [DOI] [PubMed] [Google Scholar]
- 33. Kantipong P, Murakami K, Moolphate S, Aung MN, Yamada N (2012) Causes of mortality among tuberculosis and HIV co-infected patients in Chiang Rai, Northern Thailand. HIV/AIDS – Research and Palliative Care 4:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harries AD, Zachariah R, Lawn SD (2009) Providing HIV care for coinfected tuberculosis patients: a perspective from sub-Saharan Africa. Int J Tuberc Lung Dis 13. [PubMed]
- 35. Witor SZ (1999) Efficacy of trimethroprim-sulphamethoxazole (co-trimoxazole) prophylaxis to decrease morbidity and mortality in HIV-infected patients with tuberculosis in Abidjan, Cote d'Ivoire: a randomized controlled trial. The Lancet 353:1469–1475. [DOI] [PubMed] [Google Scholar]
- 36. Mwaungulu FB, Floyd S (2004;May Cotrimoxazole prophylaxis reduces mortality in human immunodeficiency virus-positive tuberculosis patients in Kagora District, Malawi Bull WorldHealth Organ. 82:354–363. [PMC free article] [PubMed] [Google Scholar]
- 37. Kirenga BJ, Levin J, Ayakaka I, Worodria W, Reilly N, et al. (2014) Treatment outcomes of new tuberculosis patients hospitalized in Kampala, Uganda: a prospective cohort study. PLoS One 9:e90614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blanc FX, Sok T, Laureillard D, Borand L, Rekacewicz C, et al. (2011) Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 365:1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National AIDS Control Committtee (2010) National Guidelines for the Management of HIV Infection in Adults and Adolescents. Yaoundé, Cameroon: Ministry of Public of Health.
- 40. Mungrue K, Beharry A, Kalloo J, Mahabir S, Maraj T, et al. (2009) Trends in HIV/TB coinfection in Trinidad and Tobago for the period 1998–2007. J Int Assoc Physicians AIDS Care (Chic) 8:170–175. [DOI] [PubMed] [Google Scholar]
- 41.Hoffmann C (2007) Opportunistic Infections: In Bernd Sebastian Kamps, ed. HIV Medicine. Paris: Flying Publisher. pp.389–477.
- 42. Kohli R, Lo Y, Howard AA, Buono D, Floris-Moore M, et al. (2005) Mortality in an urban cohort of HIV-infected and at-risk drug users in the era of highly active antiretroviral therapy. Clin Infect Dis 41:864–872. [DOI] [PubMed] [Google Scholar]
- 43. Dou ZH, Zhao Y, He Y, He WS, Ji GP, et al. (2009) [A retrospective cohort study on reduction of AIDS mortality among patients enrolled in national-free antiretroviral treatment programme in two cities in China]. Zhonghua Yu Fang Yi Xue Za Zhi 43:1091–1095. [PubMed] [Google Scholar]
- 44. Sun DY, Wang Q, Yang WJ, Zhu Q, Wang Z (2012) [Survival analysis on AIDS antiretroviral therapy in Henan province during 2003–2009]. Zhonghua Liu Xing Bing Xue Za Zhi 33:181–184. [PubMed] [Google Scholar]
- 45. Marcy O, Laureillard D, Madec Y, Chan S, Mayaud C, et al. (2014) Causes and determinants of mortality in HIV-infected adults with tuberculosis: an analysis from the CAMELIA ANRS 1295-CIPRA KH001 randomized trial. Clin Infect Dis 59:435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cox H, Hughes J, Daniels J, Azevedo V, McDermid C, et al. (2014) Community-based treatment of drug-resistant tuberculosis in Khayelitsha, South Africa. Int J Tuberc Lung Dis 18:441–448. [DOI] [PubMed] [Google Scholar]
- 47. Alvarez-Uria G, Midde M, Pakam R, Naik PK (2013) Diagnostic and Prognostic Value of Serum Albumin for Tuberculosis in HIV Infected Patients Eligible for Antiretroviral Therapy: Datafrom an HIV Cohort Study in India. Bioimpacts 3:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Factors associated with death during TB treatment among TB/HIV co-infected patients, Yaoundé Central Hospital, 2006–2013, Cameroon (sensitivity analysis integrating continuous data).
(DOCX)
Data of the 337 patients included in the study.
(PDF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper as Supplementary file S2.