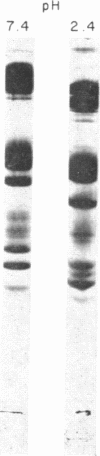
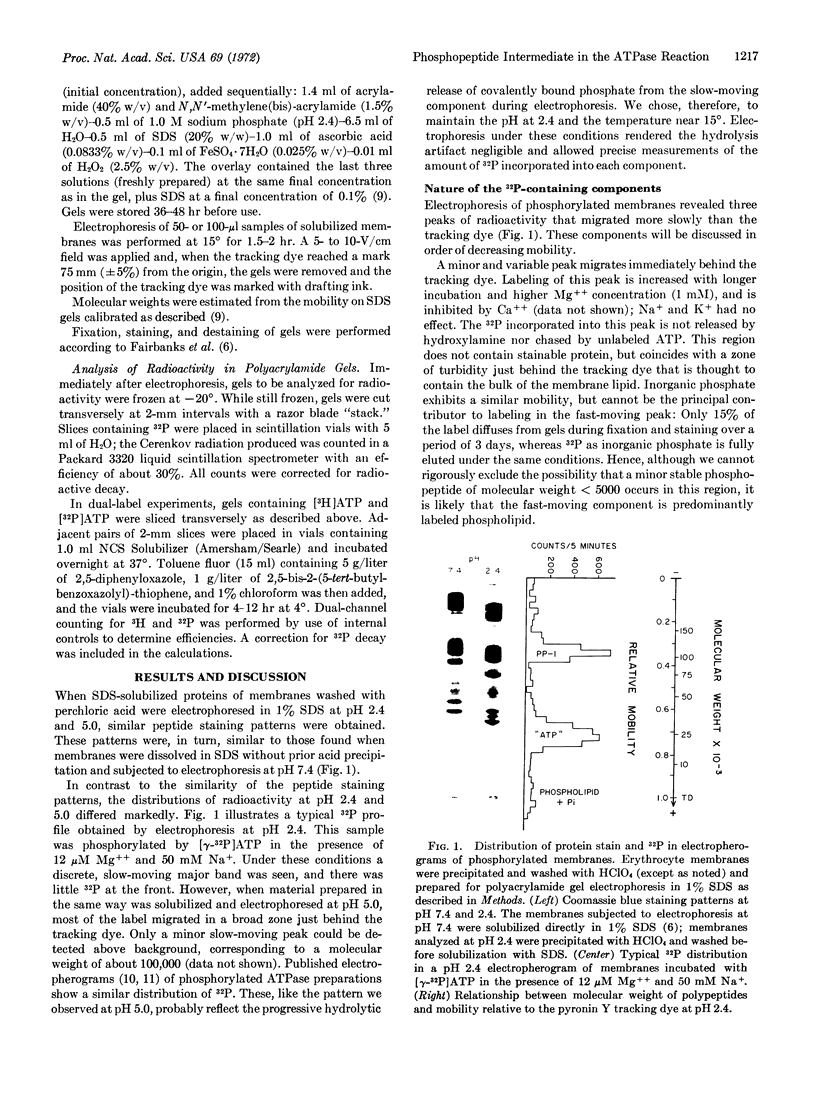
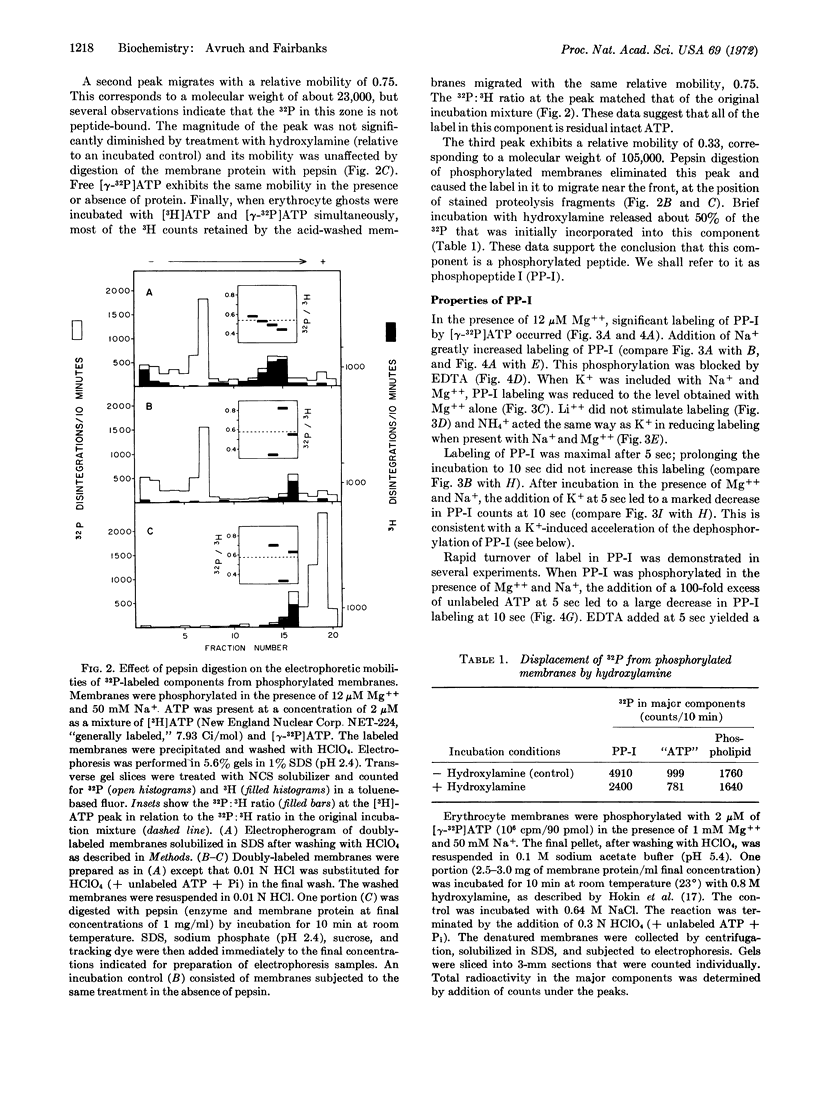
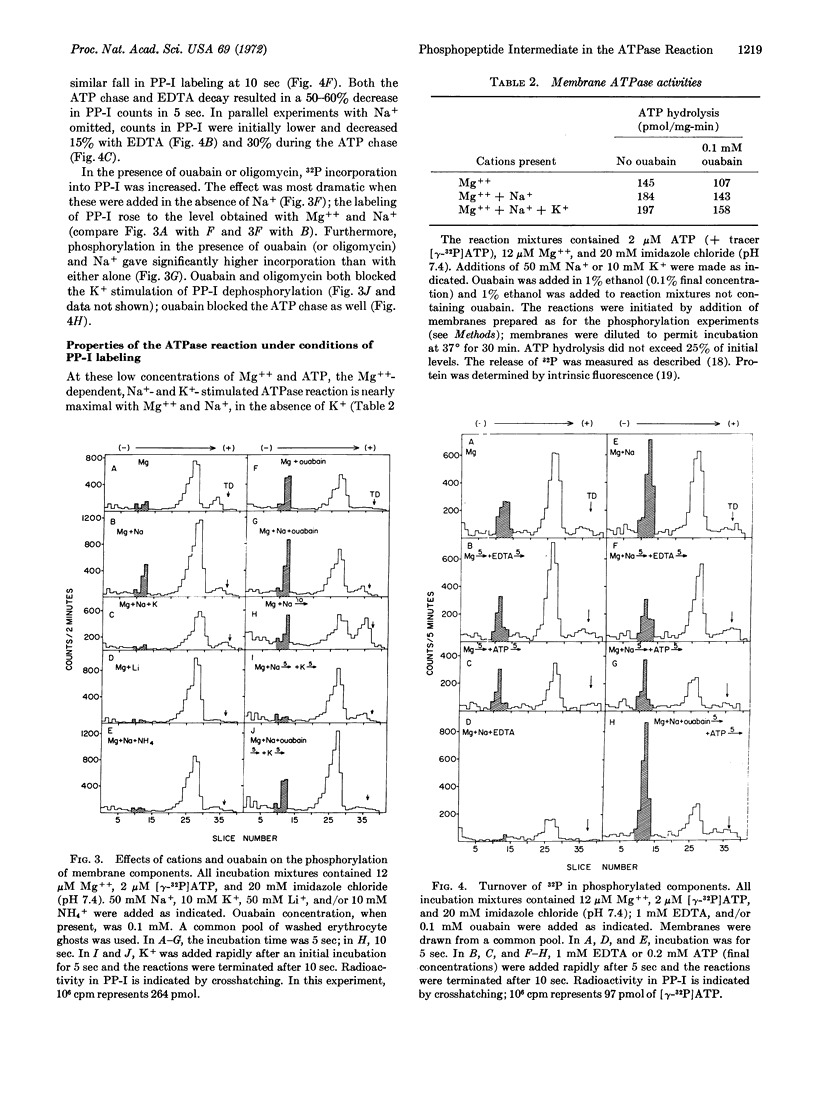
Abstract
Human erythrocyte membranes are phosphorylated by [γ-32P]ATP in association with the Mg++-dependent, Na+ and K+-stimulated ATPase (EC 3.1.6.3) reaction. To delineate the membrane species involved, phosphorylated membranes were analyzed by polyacrylamide gel electrophoresis in sodium dodecyl sulfate, under conditions that minimize hydrolysis of acyl phosphate linkages. Three radioactive components were detected, of which only one was a phosphopeptide, of apparent molecular weight 105,000. The phosphate bound to this peptide undergoes rapid turnover and is discharged by hydroxylamine. In the presence of Mg++, the phosphorylation of this peptide is specifically stimulated by Na+ and blocked by ethylene diamine tetraacetate; its dephosphorylation is stimulated by K+ and blocked by ouabain. We conclude that this phosphopeptide is an intermediate in the Mg++-dependent, Na+- and K+-stimulated ATPase reaction of the erythrocyte membrane.
Keywords: sodium transport, acyl phosphate, polyacrylamide gel electrophoresis
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Bader H., Post R. L., Bond G. H. Comparison of sources of a phosphorylated intermediate in transport ATPase. Biochim Biophys Acta. 1968 Jan 3;150(1):41–46. doi: 10.1016/0005-2736(68)90006-0. [DOI] [PubMed] [Google Scholar]
- Blostein R. Relationships between erythrocyte membrane phosphorylation and adenosine triphosphate hydrolysis. J Biol Chem. 1968 Apr 25;243(8):1957–1965. [PubMed] [Google Scholar]
- Blostein R. Sodium-activated adenosine triphosphatase activity of the erythrocyte membrane. J Biol Chem. 1970 Jan 25;245(2):270–275. [PubMed] [Google Scholar]
- Fahn S., Koval G. J., Albers R. W. Sodium-potassium-activated adenosine triphosphatase of Electrophorus electric organ. I. An associated sodium-activated transphosphorylation. J Biol Chem. 1966 Apr 25;241(8):1882–1889. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hokin L. E., Sastry P. S., Galsworthy P. R., Yoda A. Evidence that a phosphorylated intermediate in a brain transport adenosine triphosphatase is an acyl phosphate. Proc Natl Acad Sci U S A. 1965 Jul;54(1):177–184. doi: 10.1073/pnas.54.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan E. M., Raymond S. Gel electrophoresis: a new catalyst for acid systems. Anal Biochem. 1969 Feb;27(2):205–211. doi: 10.1016/0003-2697(69)90024-4. [DOI] [PubMed] [Google Scholar]
- Kyte J. Phosphorylation of a purified (Na + +K + ) adenosine triphosphatase. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1259–1265. doi: 10.1016/s0006-291x(71)80008-6. [DOI] [PubMed] [Google Scholar]
- POST R. L., SEN A. K., ROSENTHAL A. S. A PHOSPHORYLATED INTERMEDIATE IN ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT ACROSS KIDNEY MEMBRANES. J Biol Chem. 1965 Mar;240:1437–1445. [PubMed] [Google Scholar]
- Resch K., Imm W., Ferber E., Wallach D. F., Fischer H. Quantitative determination of soluble and membrane proteins through their native fluorescence. Naturwissenschaften. 1971 Apr;58(4):220–220. doi: 10.1007/BF00591853. [DOI] [PubMed] [Google Scholar]
- SAMAHA F. J., GERGELY J. NA - AND K-STIMULATED ATPASE IN HUMAN STRIATED MUSCLE. Arch Biochem Biophys. 1965 Jan;109:76–79. doi: 10.1016/0003-9861(65)90289-4. [DOI] [PubMed] [Google Scholar]
- SEN A. K., POST R. L. STOICHIOMETRY AND LOCALIZATION OF ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT IN THE ERYTHROCYTE. J Biol Chem. 1964 Jan;239:345–352. [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Uesugi S., Dulak N. C., Dixon J. F., Hexum T. D., Dahl J. L., Perdue J. F., Hokin L. E. Studies on the characterization of the sodium-potassium transport adenosine triphosphatase. VI. Large scale partial purification and properties of a lubrol-solubilized bovine brain enzyme. J Biol Chem. 1971 Jan 25;246(2):531–543. [PubMed] [Google Scholar]