Abstract
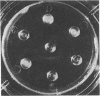
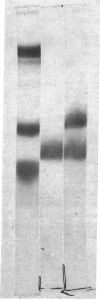
Soluble extracts from several regions of the mouse brain manifested regionally specific protein band patterns on polyacrylamide gel electrophoresis. In particular, one protein band appeared to occur uniquely in the olfactory bulb extracts where it was a quantitatively significant constituent of the soluble protein extract. This protein was purified to homogeneity by ammonium sulfate precipitation, DEAE-cellulose column chromatography, and polyacrylamide gel electrophoresis. The purified protein has a minimum molecular weight of about 20,000 by gel electrophoresis in the presence of sodium dodecyl sulfate. Precipitating antiserum prepared against this protein reacted only with the purified protein or with extracts of olfactory bulb, and not with extracts of other mouse tissues or brain regions. Quantitative immunoprecipitation assays indicate that this specific protein represents 1% of the total soluble protein in the mouse olfactory bulb. Soluble extracts of olfactory bulbs from several rodent species gave reactions of identity by agar gel diffusion with the antiserum to the mouse protein. It is suggested that this protein is an example of selective genetic expression within the central nervous system.
Keywords: mouse, immunoprecipitation, genetic expression, gel electrophoresis
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J., WEISSBACH H. Purification and properties of hydroxyindole-O-methyl transferase. J Biol Chem. 1961 Jan;236:211–213. [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969 Dec;137(4):433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Bennett G. S., Edelman G. M. Isolation of an acidic protein from rat brain. J Biol Chem. 1968 Dec 10;243(23):6234–6241. [PubMed] [Google Scholar]
- Cheal M. L., Sprott R. L. Social olfaction: a review of the role of olfaction in a variety of animal behaviors. Psychol Rep. 1971 Aug;29(1):195–243. doi: 10.2466/pr0.1971.29.1.195. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Davies W. E. The disc electrophoretic separation of proteins from various parts of the guinea pig brain. J Neurochem. 1970 Mar;17(3):297–303. doi: 10.1111/j.1471-4159.1970.tb02216.x. [DOI] [PubMed] [Google Scholar]
- Hahn W. E., Laird C. D. Transcription of nonrepeated DNA in mouse brain. Science. 1971 Jul 9;173(3992):158–161. doi: 10.1126/science.173.3992.158. [DOI] [PubMed] [Google Scholar]
- Hartman B. K., Udenfriend S. A method for immediate visualization of proteins in acrylamide gels and its use for preparation of antibodies to enzymes. Anal Biochem. 1969 Sep;30(3):391–394. doi: 10.1016/0003-2697(69)90132-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moore B. W., Cicero T. J., Perez V. J., Cowan W. M. Levels of two brain-specific proteins during degeneration and development. UCLA Forum Med Sci. 1971;14:481–489. [PubMed] [Google Scholar]
- Moulton D. G., Beidler L. M. Structure and function in the peripheral olfactory system. Physiol Rev. 1967 Jan;47(1):1–52. doi: 10.1152/physrev.1967.47.1.1. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]