Abstract
Alcohol-dependent individuals (ALC) have smaller hippocampi and poorer neurocognition than healthy controls. Results from studies on the association between alcohol consumption and hippocampal volume have been mixed, suggesting that comorbid or premorbid factors (i.e., those present prior to the initiation of alcohol dependence) determine hippocampal volume in ALC. We aimed to characterize the effects of select comorbid (i.e., cigarette smoking) and premorbid factors (brain-derived neurotrophic factor [BDNF] genotype [Val66Met rs6265]) on hippocampal volume in an ALC cohort followed longitudinally into extended abstinence. One hundred twenty-one adult ALC in treatment (76 smokers, 45 non-smokers) and 35 non-smoking light-drinking controls underwent quantitative magnetic resonance imaging, BDNF genotyping, and neurocognitive assessments. Representative subgroups were studied at 1 week, 1 month, and at an average of 7 months of abstinence. ALC had smaller hippocampi than healthy controls at all time points. Hippocampal volume at 1 month of abstinence correlated with lower visuospatial function. Smoking status did not influence hippocampal volume or hippocampal volume recovery during abstinence. However, only BDNF Val homozygotes tended to have hippocampal volume increases over 7 months of abstinence, and Val homozygotes had significantly larger hippocampi than Met carriers at 7 months of abstinence. These findings suggest that BDNF genotype, but not smoking status or measures of drinking severity, regulate functionally relevant hippocampal volume recovery in abstinent ALC. Future studies aimed at exploring genetic determinants of brain morphometry in ALC may need to evaluate individuals during extended abstinence after the acute environmental effects of chronic alcohol consumption have waned.
Keywords: abstinence, alcohol, BDNF, genetics, hippocampus, morphometry, MRI, neuroimaging, smoking, tobacco
Introduction
Recent brain imaging in alcohol-dependent individuals (ALC) has focused on the degree to which hippocampal morphometry relates to the direct neurotoxic effects of chronic alcohol consumption. Structural neuroimaging in adult treatment-seeking ALC demonstrated smaller hippocampal volumes within the first month of abstinence compared to healthy controls (Agartz, Momenan, Rawlings, Kerich, & Hommer, 1999; Jarrard, 1995; Pfefferbaum et al., 1995; Sullivan & Pfefferbaum, 2005; Wrase et al., 2008). Adolescents with a short history of alcohol abuse also have smaller hippocampi than age-matched healthy controls (De Bellis et al., 2000; Medina, Schweinsburg, Cohen-Zion, Nagel, & Tapert, 2007; Nagel, Schweinsburg, Phan, & Tapert, 2005). Importantly, the literature is mixed on the association of measurements of alcohol consumption and hippocampal volume. De Bellis et al. (2000) found that hippocampal size correlated positively with age of onset of alcohol dependence and correlated negatively with alcohol use duration, but other studies have found no such associations (Agartz et al., 1999; Gazdzinski et al., 2008; Nagel et al., 2005) or did not report on such a relationship (Pfefferbaum et al., 1995; Wrase et al., 2008). Together, these findings suggest that the observed hippocampal atrophy in adult ALC may be related to environmental factors other than alcohol consumption (such as chronic smoking, for example) or that hippocampal volume differences exist prior to the development of alcohol dependence (i.e., are premorbid).
Approximately 60–90% of ALC smoke cigarettes chronically with significant health risks (Giovino, 2002; Romberger & Grant, 2004). In our previous magnetic resonance studies of a small patient cohort (Gazdzinski et al., 2008), chronically smoking ALC (sALC) had smaller hippocampi during the first month of abstinence than non-smoking ALC (nsALC). Furthermore, both groups had hippocampal volume increases over this period, but only in nsALC did these increases correlate with improvements in visuospatial memory. Both preclinical and clinical studies suggest the hippocampus is involved in visuospatial memory (Devenport, Stidham, & Hale, 1989; Grant, 1987; Jarrard, 1995; Matthews, Simson, & Best, 1995; Munro, Saxton, & Butters, 2000; Vandergriff, Matthews, Best, & Simson, 1996).
Only 2 studies have explored the potential effects of premorbid factors on hippocampal volume by comparing alcohol-naïve adolescents with and without a family history of alcohol problems, and they found no significant effects of family history on hippocampal volume (Hanson et al., 2010; Hill et al., 2001). Although genetic factors account for > 50% of the variance in alcoholism liability (Goldman, Oroszi, & Ducci, 2005), and although the size of the hippocampus is hereditary (Sullivan, Pfefferbaum, Swan, & Carmelli, 2001), no study has explored specific functional genes that may affect hippocampal volume in ALC.
One candidate gene shown to affect brain morphology and cognition in other neurodegenerative diseases is brain-derived neurotrophic factor (BDNF). This neurotrophin is primarily active in the hippocampus and cerebral cortex (Hofer, Pagliusi, Hohn, Leibrock, & Barde, 1990); it supports survival of extant neurons and promotes neurogenesis (Ernfors, Kucera, Lee, Loring, & Jaenisch, 1995; Murer, Yan, & Raisman-Vozari, 2001). Carriers of the Val66Met (rs6265) single nucleotide polymorphism (SNP) (Met carriers) have impaired intracellular secretion and trafficking of BDNF relative to Val homozygotes (Chen et al., 2004; Egan et al., 2003). Healthy Met carriers have smaller hippocampi than Val homozygotes (Bueller et al., 2006; Molendijk et al., 2012; Ozsoy, Durak, & Esel, 2013). A recent quantitative neuroimaging study of adult recovering ALC from our laboratory (Mon et al., 2013) found no effect of BDNF genotype on neocortical gray matter cross-sectional volumes. However, cortical gray matter volume increased during the first month of abstinence in BDNF Val homozygotes only, not in Met carriers. The specific effects of BDNF genotype on hippocampal volume during abstinence from alcohol have not been investigated. The aims of this study were therefore to measure the effects of BDNF genotype and smoking on hippocampal structure and function in a large alcohol-dependent cohort followed further into abstinence than reported previously. We hypothesized that a) smoking ALC would demonstrate smaller hippocampi than non-smoking ALC up to 1 year into abstinence, b) BDNF Met carriers would exhibit less hippocampal volume recovery during abstinence than Val homozygotes, and c) hippocampal volume recovery would correlate with improvements in visuospatial memory.
Materials and Methods
Participants
One hundred and twenty-one alcohol-dependent individuals (ALC) were recruited from the substance abuse treatment programs at the VA Medical Center and Kaiser Permanente in San Francisco, and 35 healthy non-smoking light drinkers (nsLD) were recruited from the San Francisco Bay Area Community as controls. The ALC group consisted of current smokers (sALC, n = 76) and non-smokers (nsALC, n = 45). As the primary focus of the study was to identify determinants of hippocampal recovery during abstinence from alcohol, ALC participants were preferentially recruited for the study. All participants provided written informed consent and all study procedures were approved by The Institutional Review Boards of the University of California San Francisco and the San Francisco VA Medical Center.
Of 121 ALC participants who received structural MRI, 117 also completed the neuropsychological assessment. The study design included 3 separate time points (TP): ALC participants were studied after 7 ± 3 days of abstinence (TP1), after 33 ± 9 days of abstinence (TP2), and after 213 ± 57 days of abstinence from alcohol (TP3). The sample was comprised of both “early starters” and “late starters”. “Early starters” entered the study at TP1 and were then assessed at TP2 and TP3, unless they were lost to follow-up or relapsed to drinking any amount of alcohol prior to their next assessment. Given the realities of clinical research and recruitment constraints, some individuals did not enter the study at TP1 (i.e., within 7 ± 3 days of stopping drinking) and instead entered the study at TP2 (i.e., within 33 ± 9 days of stopping drinking). These participants were classified as “late starters” and were then re-assessed at TP3, unless they were lost to follow-up or relapsed to drinking any amount of alcohol prior to TP3. Thus, individual participants could have data for any combination of TP1, TP2, and TP3, with the sample size at each TP determined by time of enrollment, ability to remain abstinent from alcohol, and attendance at follow-up assessments. The number of participants by group at each TP and the proportion of “early starters” and “late starters” can be found in Table 1. The sample did not differ on demographics or drinking severity measure at TP1, TP2, and TP3. The number of days abstinent at TP1, TP2, and TP3 were not different for nsALC and sALC (all p > 0.3). Of the 35 nsLD participants, 16 were re-studied at 290 ± 49 days after baseline assessments to confirm stability of imaging outcome measures over time.
Table 1.
Number of Participants Present at Each Time Point (TP).
| Time point |
nsALC | sALC | ALC (nsALC + sALC) |
nsLD | ||||
|---|---|---|---|---|---|---|---|---|
| Early starters |
Late starters |
Combined cohort |
Early starters |
Late starters |
Combined cohort |
|||
| TP1 | 35 | 0 | 35 | 49 | 0 | 49 | 84 | 35 |
| TP2 | 29 | 16 | 45 | 38 | 38 | 76 | 121 | 0 |
| TP3 | 19 | 7 | 16 | 11 | 10 | 21 | 37 | 16 |
Note: “Early Starters” entered the study at TP1 after 7 ± 3 days of abstinence from alcohol; “Late Starters” entered the study at TP2 after 33 ± 9 days of abstinence.
nsLD, non-smoking light drinking participant; nsALC, non-smoking alcohol-dependent participant; sALC, smoking alcohol-dependent participant.
All inclusion and exclusion criteria were reported previously (Durazzo, Gazdzinski, Banys, & Meyerhoff, 2004). Briefly, all ALC individuals met DSM-IV criteria for alcohol dependence, and had consumed > 150 standard alcohol-containing drinks (i.e., 13.6 g of pure ethanol) per month for > 8 years prior to enrollment into the study for males and > 80 drinks for > 6 years for females. All participants were free of general medical, neurologic, and psychiatric conditions known to influence hippocampal volume and neurocognition (e.g., schizophrenia, PTSD, dementia), except unipolar mood disorders, hypertension, and hepatitis C due to the high incidence of these conditions in alcohol- and tobacco-dependent populations (Fergusson, Goodwin, & Horwood, 2003; Gilman & Abraham, 2001; Paperwalla, Levin, Weiner, & Saravay, 2004). The proportion of ALC individuals with these conditions did not differ significantly by BDNF genotype or smoking status. Dependence on any illicit substance within 5 years of study was exclusionary.
Abstinence from alcohol and illicit substances was monitored during the study period. Between TP1 and TP2, all ALC participants were enrolled in either a residential or outpatient treatment program, where they were tested daily for substance use; they also received breathalyzer, urine toxicology, and a self-report questionnaire (timeline follow-back) on substance use at TP2. Abstinence between TP2 and TP3 was assessed by timeline follow-back and checking electronic medical records for positive breathalyzer or urine toxicology while in substance abuse treatment programs. Individuals who relapsed to any amount of alcohol or illicit substance use or participants who quit or initiated tobacco smoking during the study (n = 3) were excluded from analyses.
Psychiatric/Behavioral Assessment
At their first assessment, ALC participants completed the Structured Clinical Interview for DSM-IV Axis I disorders, Patient Edition, Version 2.0 (SCID-I/P; American Psychiatric Association, 1994) and standardized questionnaires assessing depressive (Beck Depression Inventory [BDI]; Beck, 1978) and anxiety symptomatologies (State-Trait Anxiety Inventory form Y-2 [STAI]; Spielberger et al., 1977), lifetime alcohol consumption (Lifetime Drinking History; Skinner & Sheu, 1982), lifetime substance use (in-house questionnaire assessing quantity and frequency of any substance use; Abé et al., 2013), and current level of nicotine dependence (Fagerstrom Tolerance Test for Nicotine Dependence [FTND]; Heatherton, Kozlowski, Frecker & Fagerstrom 1991). For smokers, the total number of cigarettes smoked per day and number of years of smoking at the current level were also recorded.
Neuroimaging Acquisition and Processing
Magnetic resonance imaging was performed on a 1.5T MR system (Siemens Vision, Iselin, NJ). Hippocampal volumes were obtained as described (Hsu et al., 2002), using a validated semi-automated high dimensional brain-warping algorithm (Medtronic Surgical Navigation Technologies, Louisville, CO). The algorithm utilized T1-weighted magnetization-prepared rapid gradient echo images acquired with TR/TE/TI = 10/7/300 ms, 15° flip angle, 1 mm × 1 mm in-plane resolution, and 1.5-mm thick coronal partitions oriented orthogonal to the long axes of the hippocampi as seen on scout images. Control points were placed at local landmarks of left and right hippocampi, and subsequent automated hippocampal morphometry used a fluid image-matching algorithm. Images acquired on the same participant at different TPs were co-registered to the participant’s initial acquisition to assure use of the same landmarks for hippocampal delineation across TPs (Hsu et al., 2002). Intracranial volume (ICV) was measured for each individual from T1-weighted images by summing the results of image segmentation into white matter, gray matter, and cerebrospinal fluid (Van Leemput, Maes, Vandermeulen, & Suetens, 1999). As ICV correlates with hippocampal volume (e.g., Whitwell, Crum, Watt, & Fox, 2001), ICV was used as covariate in cross-sectional analyses. Hippocampal volumes did not differ significantly between right and left hemispheres in any of the groups at either TP. Thus, bilateral hippocampal volumes averaged over both hemispheres are reported.
Genetic Analyses
Genomic DNA was isolated from blood samples of ALC at their first assessment. The BDNF SNP rs6265 was assayed using TaqMan genotyping assays from Applied Biosystems (Foster City, CA, USA). The SNP assays were performed using a reaction volume of 15 µL, which consisted of 7.5 µL of TaqMan 2 × universal master mix, 0.38 µL of 20 × TaqMan pre-designed SNP genotyping assay, 6.14 µL of nuclease-free water, and 1 µL genomic DNA. After PCR amplification as per manufacturer’s recommendations, SNP genotypes were determined by allelic discrimination using the ABI-7500 instrument (Applied Biosystems, Foster City, CA, USA). Genotype data were obtained from 67 unique ALC participants, 41 of whom participated at TP1, 63 at TP2, and 25 at TP3. Of this ALC sample, 67% were Val homozygotes, 32% were Val/Met heterozygotes, and 1% were Met homozygotes (within Hardy-Weinberg equilibrium, χ2 < 0.70, p > 0.40). Val/Met heterozygotes and Met homozygotes were combined into a Met carrier group for analysis.
Neurocognitive Assessment
Neuropsychological testing (approximately 1.5 h) at each TP evaluated cognitive functions previously reported to be adversely affected by both alcohol-use disorders (Rourke & Grant, 2009) and chronic cigarette smoking (Durazzo & Meyerhoff, 2007; Durazzo, Meyerhoff, & Nixon, 2010). sALC were allowed to smoke ad libitum before and during neurocognitive testing to reduce potential confounds of nicotine withdrawal (for review, see Sacco, Bannon, & George, 2004). The following tests were administered: Wechsler Adult Intelligence Scale 3rd ed. (WAIS-III; Wechsler, 1997) - Digit Span (a measure of working memory), Symbol Search, and Digit Symbol (processing speed); and Brief Visuospatial Memory Test (BVMT) Revised (Benedict, 1997) - Total Recall (visuospatial learning) and Delayed Recall (visuospatial memory). Premorbid verbal intelligence was assessed using the American National Adult Reading Test (AMNART) (Grober & Sliwinski, 1991). Raw scores for all neurocognitive measures were converted to standardized scores via appropriate normative data adjusted for age.
Statistics
Multivariate analysis of covariance (MANCOVA) assessed differences between nsLD, nsALC, and sALC groups on age at enrollment, years of education, depression and anxiety symptomatologies, and ICV. A separate MANCOVA assessed differences between nsALC and sALC groups on drinking and smoking severity measures at baseline. Effect sizes (ES) were calculated using Cohen’s d (Cohen, 1992).
In cross-sectional analyses, univariate analysis of covariance (ANCOVA), followed by pairwise t tests, assessed differences in hippocampal volume by behavioral group (nsLD, nsALC, sALC), and within the combined alcohol-dependent group by BDNF genotype (Val homozygotes, Met carriers), separately at TP1, TP2, and TP3. Covariates (age, ICV, years of education, and average drinks per month over lifetime) were used where appropriate and only when accounting for significant variance.
Longitudinal analyses used linear mixed modeling (LMM) of hippocampal volumes from sALC and nsALC groups at all 3 TPs; covariate selection procedures identical to those in the cross-sectional analyses were utilized. Main effects for group, time, and the group-by-time interaction were tested. Group was defined by smoking status (nsALC vs. sALC) or BDNF genotype (Val homozygotes vs. Met carriers). If LMM demonstrated meaningful differences within the ALC groups by smoking status or genotype, an additional LMM analysis compared the implicated group to nsLD in order to assess the clinical significance of the findings. A simple effects model and percentage change analysis (i.e., [TPx hippocampal volume mean – TPy hippocampal volume mean]/TPx hippocampal volume mean) also assessed changes in the implicated group over the relevant time period. Hippocampal volumes of nsLD participants at baseline and follow-up were compared with paired t tests to test for relative stability of measurements over the mean 10-month test-retest interval. None of the controls changed their alcohol-use patterns or smoking behavior between TPs.
Associations between outcome measures were assessed by Spearman ranked correlations. At each TP, hippocampal volumes in the combined ALC group were correlated with 5 neurocognitive test measures, 5 drinking and 3 smoking severity measures, BDI, and STAI. Correlations between changes in hippocampal volume and changes in neurocognitive test performance between all TPs were also assessed. As groups did not differ by days abstinent at any TP, change scores were defined as: [e.g., for TP1-TP2 interval: (measure at TP2 - measure at TP1)/(measure at TP1)*100]. Significant correlations were then examined by smoking status (nsALC, sALC) and BDNF genotype (Val homozygote, Met carrier). We had an a priori hypothesis, based on a previous report that hippocampal volume correlates with visuospatial memory (Gazdzinksi et al., 2008). Therefore, alpha level was set at 0.05 for this comparison. Otherwise, strict Bonferroni correction was used to adjust alpha level for multiple comparisons for the remaining neurocognitive measures 0.05/5 = 0.01, drinking severity measures 0.05/5 = 0.01, and smoking measures 0.05/3 = 0.017. All statistical analyses were conducted with SPSS v21 (IBM Corporation, Armonk, NY) and R v3.0.1.
Results
Participant characterization
Detailed demographics and participant characteristics for all groups are given in Table 2. The ALC participants depicted are those studied at TP2 (n = 121); the corresponding characteristics of ALC “early starters” at TP1 (n = 84) and the remaining 37 ALC “late starters” who entered the study at TP2 did not differ from those of the entire sample. Groups differed on age [F(2,152) = 6.47, p = 0.002], years of education [F(2,152) = 26.5, p < 0.001], and AMNART intelligence scores [F(2,122) = 5.22, p = 0.007]. sALC had higher lifetime average drinks/month than nsALC (p < 0.001), earlier onset of heavy drinking (i.e., > 100 drinks per month in males and > 80 drinks per month in females; p = 0.002), and more regular drinking years (> 1 drink per month without meeting heavy drinking criteria; p = 0.035). sALC also tended to have more months of heavy drinking and higher average drinks/month in the year prior to enrollment (p < 0.10) than nsALC. The sALC group did not differ from the nsALC on BDI and STAI, nor on ICV. The sALC Fagerstrom score was 5.2 ± 1.9, indicating a moderate to high level of nicotine dependence; individuals smoked on average 19.1 ± 9.9 cigarettes per day.
Table 2.
Demographics, Alcohol, Cigarette, Psychiatric Histories in sample studied at TP2 (Mean ± SD)
| Measure | nsLD | nsALC | sALC |
p value (nsLD vs. nsALC) |
p value (nsLD vs. sALC) |
p value (nsALC vs. sALC) |
|---|---|---|---|---|---|---|
|
Total number of participants (female) |
35 (3) | 45 (7) | 76 (2) | – | – | – |
|
Age at enrollment (years) |
45.6 ± 9.9 | 53.6 ± 10.5 | 49.6 ± 9.0 | < 0.001 | 0.055 | 0.031 |
| Education (years) | 16.7 ± 2.4 | 14.6 ± 2.4 | 13.5 ± 1.8 | < 0.001 | < 0.001 | 0.007 |
| AMNART | 118.9 ± 6.6 | 114.5 ± 9.9 | 112.3 ± 8.6 | 0.058 | 0.002 | 0.245 |
|
Percent Caucasian/African American/Latino/other |
71/9/14/6 | 82/4/11/3 | 69/20/7/3 | – | – | – |
|
Number of days abstinent at TP1 |
– | 6.6 ± 3.8 | 6.5 ± 3.17 | – | – | 0.898 |
|
Number of days abstinent at TP2 |
– | 34.0 ± 9.5 | 32.4 ± 9.1 | – | – | 0.369 |
|
Number of days abstinent at TP3 |
– | 215.4 ± 39.5 | 209.5 ± 64.5 | – | – | 0.749 |
|
1-year average alcohol drinks/month |
15 ± 17 | 363 ± 195 | 434 ± 247 | < 0.001 | < 0.001 | 0.070 |
|
Life time average alcohol drinks/month |
16 ± 14 | 165 ± 101 | 259 ± 135 | < 0.001 | < 0.001 | < 0.001 |
|
Age-of-onset of heavy drinking (years) |
– | 27.3 ± 10 | 22.3 ± 6.5 | – | – | 0.002 |
|
Duration of heavy drinking (years) |
– | 21.7 ± 10.2 | 24.7 ± 8.3 | – | – | 0.099 |
|
Duration of regular drinking (years) |
26.9 ± 8.3 | 36.7 ± 11.1 | 32.8 ± 9.2 | < 0.0001 | 0.007 | 0.035 |
| FTND | - | - | 5.2 ± 1.9 | - | - | - |
| Cigarettes per day | - | - | 19.1 ± 9.9 | - | - | - |
|
Smoking duration (years) |
– | – | 27.2 ± 11.8 | |||
| BDI | 3.8 ± 3.9 | 8.8 ± 8.5 | 10.5 ± 7.2 | 0.004 | < 0.001 | 0.223 |
| STAI | 32.4 ± 7.8 | 44.8 ± 10.9 | 43.6 ± 11.2 | < 0.001 | < 0.001 | 0.581 |
| ICV | 1505 ± 144 | 1509 ± 6 | 1496 ± 8 | 0.914 | 0.728 | 0.615 |
FTND, Fagerstrom Tolerance Test for Nicotine Dependence; AMNART, American National Adult Reading Test; NA, not applicable; nsLD, non-smoking light drinking participant; nsALC, non-smoking alcohol-dependent participant; sALC, smoking alcohol-dependent participant; BDI, Beck Depression Inventory, STAI, State-Trait Anxiety Inventory. Heavy drinking is > 100 alcohol drinks per month in males and > 80 drinks per month in females. Regular drinking is > 1 drink per month without meeting heavy drinking criteria.
p < 0.05,
p < 0.01
When the combined ALC group was stratified by BDNF genotype, an omnibus MANCOVA with Val homozygotes and Met carrier groups did not reveal differences in standard drinking measures (i.e., 1-year and lifetime average drinks/month, onset age of heavy drinking, or duration of drinking).
Hippocampal Volume Measures over Time in nsLD
Paired t test of change in hippocampal volume in 16 nsLD between baseline and follow-up (290 days) was not significant (p = 0.80), with an average difference in hippocampal volume of 0.5%. This demonstrates excellent test-retest reliability and/or little age-related hippocampal volume change over a period roughly equivalent to the TP1-TP3 interval in ALC (213 days).
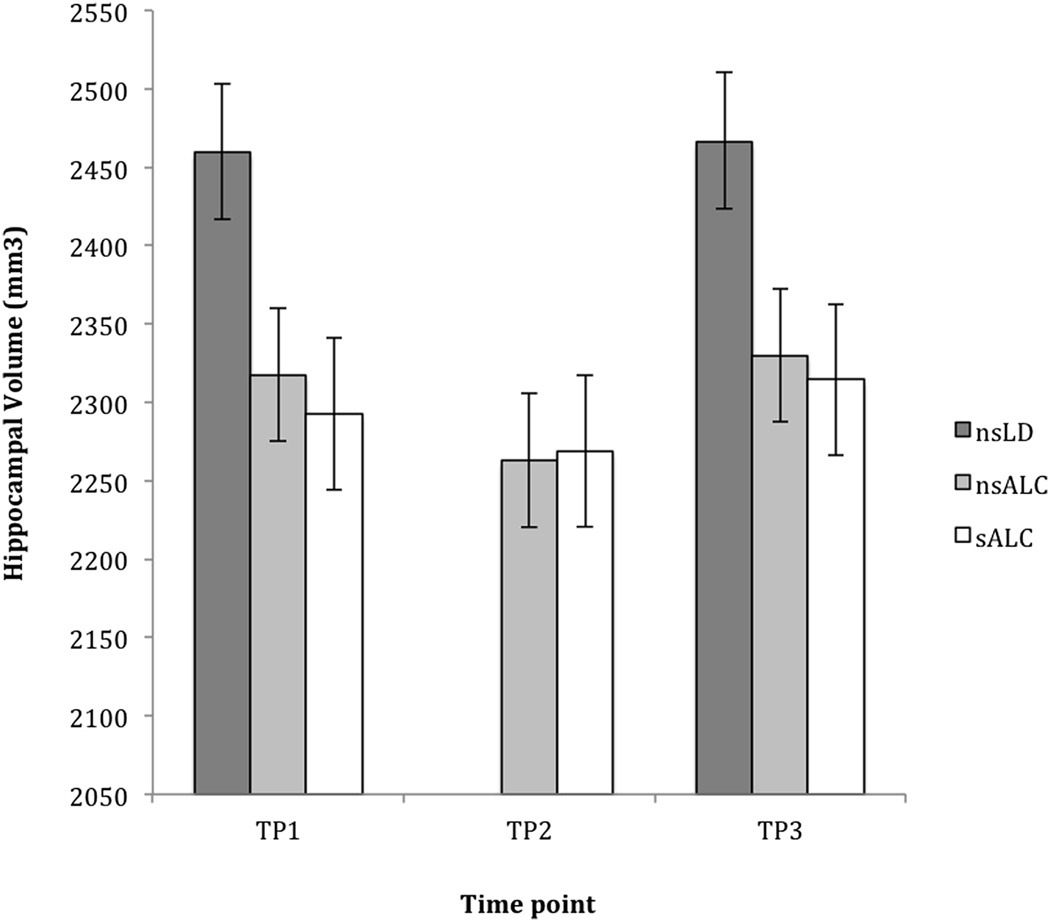
Hippocampal Volume Analyses by Smoking Status (see Figure 1)
Figure 1. Hippocampal Volume: Cross-Sectional Group Differences by Smoking Status at Three Time Points during Abstinence.
Cross-sectional differences in hippocampal volume (mean ± standard error) in non-smoking light-drinking controls (nsLD), non-smoking alcohol-dependent participants (nsALC), and smoking alcohol-dependent participants (sALC) during extended abstinence from alcohol. TP1 = 6.5 ± 3.4, TP2 = 33.2 ± 9.3, and TP3 = 212.5 ± 56.6 days abstinent from alcohol.
At TP1, ANCOVA comparing hippocampal volumes between the nsLD, nsALC, and sALC groups was significant [F(2,150) = 6.39, p = 0.002]. In planned pairwise comparisons, nsALC had 5.8% smaller hippocampi than nsLD (p = 0.033, ES = 0.52), while sALC had 6.8% smaller hippocampi than nsLD (p = 0.007, ES = 0.87). At TP2, ANCOVA was also significant [F(2,115) = 4.08, p = 0.019], with both nsALC and sALC having 8% smaller hippocampi than nsLD (both p < 0.002, ES > 0.68). An ANCOVA comparing hippocampal volume between nsALC and sALC groups at TP3 and nsLD at baseline showed a trend for significance [F(2,63) = 2.82, p = 0.067]. TP3 hippocampal volumes in both nsALC and sALC tended to be about 6% smaller than in nsLD (both p < 0.075, ES > 0.55). No significant hippocampal volume differences were observed between the nsALC and sALC groups at any TP.
Longitudinally, there were no significant main effects for smoking status or time and no statistically significant smoking status-by-time interaction (all p > 0.11) across all 3 TPs. Analyses conducted for TP1-TP2, TP2-TP3, and TP1-TP3 separately confirmed the findings for the analysis conducted over all TPs.
Hippocampal Volume Analysis by BDNF Genotype in Combined ALC Group
At TP1, ANCOVA comparing hippocampal volume between BDNF Val homozygotes and Met carriers showed a statistical trend with moderate effect size [F(1,39) = 2.44, p = 0.126; ES = 0.51], where Met carriers had 6.5% smaller hippocampi than Val homozygotes. Similarly at TP2, Met carriers had 4.2% smaller hippocampi than Val homozygotes (ES = 0.38), but this difference was not significant [F(1,60) = 1.69, p = 0.182]. At TP3, however, the group difference was significant [F(1,22) = 4.51, p = 0.016], with Met carriers having 9.9% smaller hippocampal volumes than Val homozygotes (ES = 1.11) (see Table 3).
Table 3.
Hippocampal Volume [mm3] (Mean ± SD) at Each Time Point (TP) during Abstinence by BDNF Genotype
| Val/Val | Met carriers | % Difference | p value | ES (Cohen’s d) | |
|---|---|---|---|---|---|
| TP1a | 2354 ± 302 | 2201 ± 301 | 6.5% | 0.126 | 0.51 |
| TP2b | 2318 ± 278 | 2221 ± 250 | 4.2% | 0.182 | 0.38 |
| TP3c | 2393 ± 211 | 2156 ± 218 | 9.9% | 0.016* | 1.11 |
p < .05
Subset of 26 Val, 15 Met Carriers
Subset of 42 Val, 21 Met Carriers
Subset of 16 Val, 9 Met Carriers
An ANCOVA revealed no smoking × genotype interaction at TP1, TP2, or TP3 (all F < 0.24, p > 0.629), demonstrating that within Val homozygotes or Met carriers at each TP, hippocampal volumes did not differ significantly as a function of smoking status.
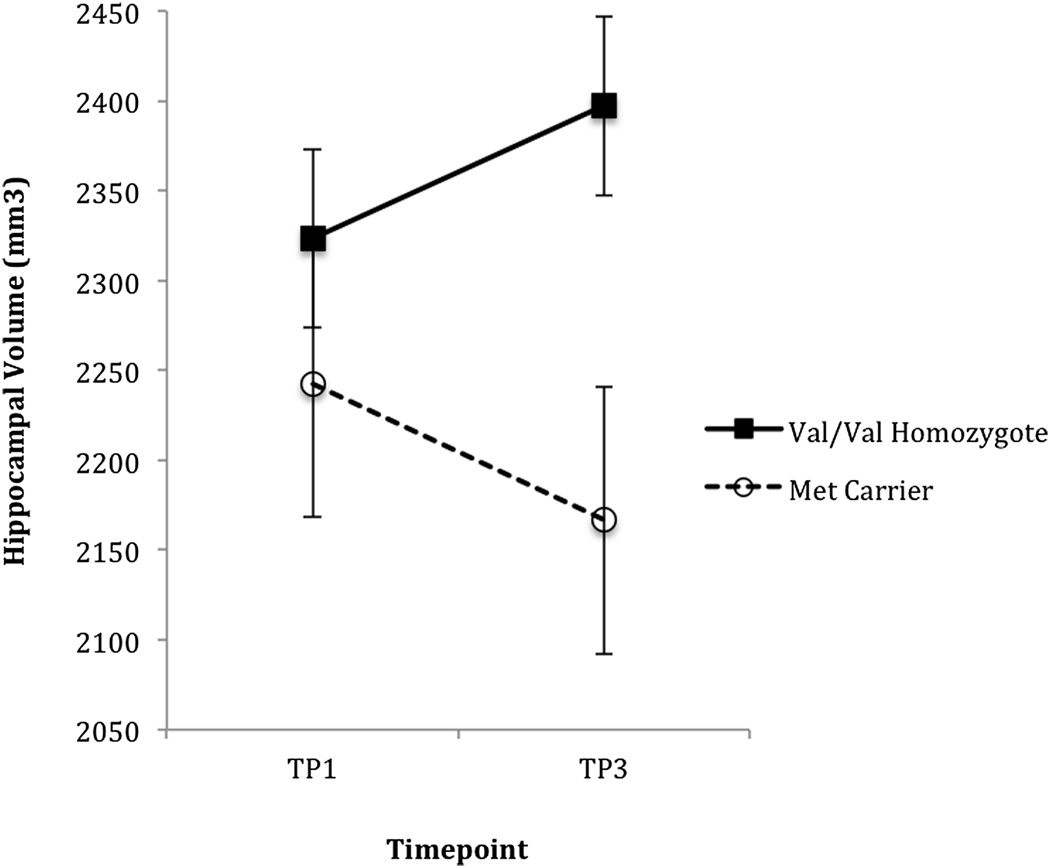
Longitudinally within ALC, LMM revealed no statistically significant main effects of genotype or time and no significant genotype-by-time interaction for hippocampal volume recovery between TP1-TP2 or between TP2-TP3 (all p > 0.15). However, over the longer interval between TP1-TP3, LMM revealed a trend for a genotype-by-time interaction [F(1,19) = 4.04, p = 0.086], with Val homozygotes demonstrating greater hippocampal volume recovery than Met carriers (see Fig. 2). A separate LMM involving TP1 and TP3 data from ALC Val homozygotes and test-retest data from nsLD revealed a main effect of group [F(1,58) = 4.53, p = 0.036], demonstrating that ALC Val homozygotes had smaller hippocampi than controls averaged over time. There was also a weak trend for a group-by-time interaction [F(1,58) = 4.54, p = 0.130], demonstrating that ALC Val homozygotes tended to have greater hippocampal volume changes than nsLD over time. There was no main effect for time (p = 0.218). In addition, a simple effects model showed a nonsignificant increase [F(1,19) = 2.603, p = 0.135] of hippocampal volume in Val homozygotes between TP1 and TP3. Furthermore, in ALC Val homozygotes, hippocampal volume increased 54.26 mm3 (2.5%) between TP1 and TP3, whereas hippocampal volume in controls increased by only 8.68 mm3 (0.4%), corresponding to a 6.25-fold greater hippocampal volume increase in ALC Val homozygotes between TP1 and TP3 than in controls over a comparable time interval.
Figure 2. Longitudinal Hippocampal Volume Recovery by BDNF Genotype.
Longitudinal differences in hippocampal volume recovery (mean ± standard error) in BDNF Val66Met (rs6265) polymorphism carriers (Met Carrier) and non-carriers (Val Homozygotes) during extended abstinence from alcohol. TP1 = 6.2 ± 3.6 and TP3 = 213.9 ± 51.0 days abstinent from alcohol. Closed symbols: Val homozygotes; open symbols: Met Carriers. The figure depicts a genotype × time interaction (p = 0.086).
Associations among Outcome Measures
In those 117 ALC participants at TP2 who had both structural and cognitive measures (sALC and nsALC combined), hippocampal volume correlated with visuospatial memory (rho = 0.234, p = 0.01) and visuospatial processing (rho = 0.202, p = 0.03). In 79 ALC participants at TP1, hippocampal volume tended to correlate with visuospatial memory (rho = 0.210, p = 0.063). Hippocampal volume did not correlate with any of the neurocognitive measures in the much smaller ALC samples at TP3. Partial correlations including AMNART or age did not change the strengths of these correlations appreciably. Drinking measures did not correlate with hippocampal volumes at any TP.
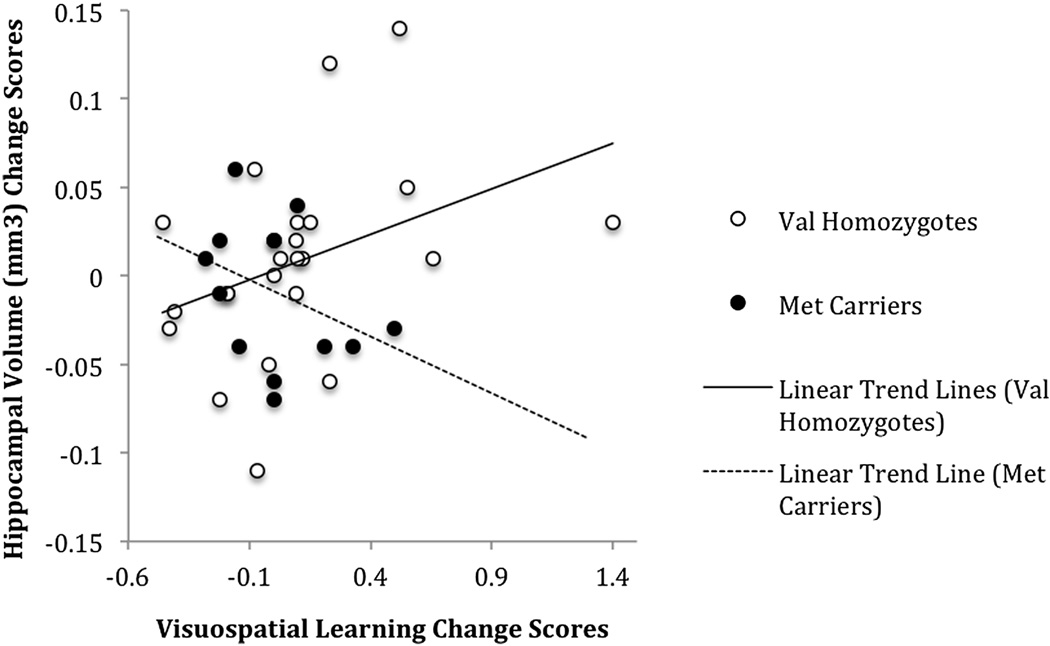
Hippocampal volume changes did not correlate significantly with change in neurocognition between TP1 and TP3 or between TP2 and TP3. However, between TP1 and TP2, hippocampal volume change correlated with changes in visuospatial memory (rho = 0.286, p = 0.024) and visuospatial learning (rho = 0.259, p = 0.042), which is a replication of a previous finding in a similar, but smaller non-independent cohort (sample including approximately 20% of the present cohort) (Gazdzinksi et al., 2008). The correlation between changes in hippocampal volume and visuospatial memory scores was significant and positive in BDNF Val homozygotes (rho = .512, p = .012), but it was not significant in Met carriers (rho = −.352, p = 0.238) (see Fig. 3).
Figure 3. Correlations Between Hippocampal Volume Change and Change in Visuospatial Memory between TP1 and TP2 as a Function of BDNF Genotype.
Change measures for Val homozygotes and Met carriers, respectively, between TP1 = 6.4 ± 3.4 and 6.5 ± 3.1 days and TP2 = 33.8 ± 9.9 and 32.8 ± 9.1 days abstinent from alcohol. Open circles: Val homozygotes; solid circles: Met carriers. The correlation of the change measures was significant in BDNF Val homozygotes (rho = .512, p = .012), but not in Met carriers (rho = −.352, p = 0.238). Linear regression fits are depicted.
Discussion
This study in treatment-seeking abstinent alcohol-dependent individuals reports on the effects of cigarette smoking and BDNF genotype on the structural and functional recovery of the hippocampus. Both nsALC and sALC participants exhibited persistent decrements in hippocampal volume for an average of 213 days of abstinence from alcohol when compared to nsLD controls. The volume loss was independent of lifetime alcohol consumption history, and hippocampal volume in the combined ALC group did not recover significantly over the abstinence period evaluated. Contrary to our a priori hypothesis and a preliminary report (Gazdzinski et al., 2008), smoking status did not significantly affect hippocampal volumes or their recoveries with abstinence in ALC. However, when the ALC participants were stratified based on presence or absence of the BDNF Val66Met polymorphism (rs6265), longitudinal analysis revealed that long-term abstinent Val homozygotes had smaller hippocampi than controls across time. Val homozygotes also tended to have larger hippocampal volume increases over time compared to both Met carriers and controls. Consequently, these differing recovery rates appeared to lead to significantly larger hippocampi in Val homozygotes compared to Met carriers at about 7 months of abstinence. Furthermore and as hypothesized, hippocampal volume cross-sectionally and its recovery correlated with improvements in visuospatial functions in the combined ALC group. When stratified on BDNF genotype, a moderate-to-strong correlation was found between hippocampal volume recovery and improvements in visuospatial memory only in Val homozygotes. The genotype findings and the functionally relevant long-term hippocampal volume increases complement our previous report (Mon et al., 2013) that similarly describes increases in lobar cortical and subcortical gray matter nuclei in Val homozygotes, but not in Met carriers, during the first month of abstinence from alcohol.
The hippocampal volume loss observed in recently abstinent ALC is consistent with previous cross-sectional reports (Agartz et al., 1999; Pfefferbaum et al., 1995; Wrase et al., 2008). A new finding is our demonstration of the persistence of this volume loss up to an average of 7 months of abstinence. A previous study in our lab (which used the same hippocampal volume determination method, albeit with a sample including approximately 20% of the present cohort) demonstrated reduced hippocampal volume in sALC compared to nsALC, and hippocampal volume recovery was present in both groups during the first month of abstinence from alcohol (Gazdzinski et al., 2008). With the much larger sample of this analysis, we were unable to replicate these earlier effects of smoking status on hippocampal volume and its recovery. However, smoking status has consistently been associated with morphometric differences in other brain regions (Durazzo et al., 2010).
Explanations for the lack of hippocampal volume recovery observed in both sALC and nsALC in the present study could include the presence of irreversible damage secondary to cumulative neurotoxic effects from alcohol (Harper & Kril, 1990; Harper, Kril, & Daly, 1987) and cigarette smoking, the presence of premorbid or comorbid factors that regulate hippocampal volume (Nagel et al., 2005), or a combination of these. The fact that several measures of drinking severity did not correlate with hippocampal volume is consistent with previous reports (Agartz et al., 1999; Gazdzinski et al., 2008; Nagel et al., 2005; Sullivan & Pfefferbaum, 2005) and suggests that premorbid factors account for some, if not all of the differences in hippocampal volume observed. One candidate premorbid factor associated with neuroplasticity and investigated in this study, the BDNF Val66Met (rs6265) SNP, was recently shown to regulate tissue-type dependent brain volume recovery in multiple brain regions (Mon et al., 2013): Val homozygotes exhibited increases of frontal, parietal, temporal, caudate, and thalamic gray matter, whereas Met carriers exhibited increases of frontal, temporal, and parietal white matter during the 1st month of abstinence from alcohol. In that study, differential rates of recovery by genotype during the first month of abstinence did not lead to significant cross-sectional differences in brain volumes, and we asserted that a longer period of abstinence might be required to observe the cross-sectional effects of BDNF on regional brain volumes. Our current hippocampal volume findings are consistent with this assertion, with higher rates of hippocampal volume recovery in Val homozygotes compared to Met carriers leading to cross-sectional differences in hippocampal volume at a mean of 7 months of abstinence. This suggests that extended abstinence may be required to detect BDNF effects on brain tissue volumes, as BDNF effects may be masked earlier in abstinence by acute alcohol exposure-related neuroplasticity.
Although our previous morphometric findings were not fully replicated, this larger cohort study is in agreement with our previously reported correlations between hippocampal volume recovery and improvements in visuospatial memory in a non-independent cohort that included approximately 20% of the present cohort (Gazdzinski et al., 2008). Although potentially clinically meaningful, this finding should be interpreted with caution until replicated in a fully independent sample. In addition, hippocampal volume correlated significantly with visuospatial memory at TP2 and at trend level at TP1. Lack of significant correlations at TP3 may be due to a significantly smaller sample size corresponding to lack of power, while the enduring acute effects of alcohol at TP1 may obscure the association between hippocampal volume and visuospatial memory in early abstinence. The neurocognitive findings are consistent with the existing literature on neurocognitive effects of chronic alcohol exposure with selective deficits observed in spatially mediated memory tasks (Devenport et al., 1989; Grant, 1987; Jarrard, 1995; Matthews et al., 1995; Munro et al., 2000; Vandergriff et al., 1996). Stratification by BDNF genotype revealed that the correlation between hippocampal volume recovery and visuospatial memory was driven by the Val homozygous group. This finding is consistent with healthy Met carriers showing impaired function on a number of neurocognitive tests (Egan et al., 2003; Hariri et al., 2003; Miyajima et al., 2008; Raz et al., 2009; Tsai, Hong, Yu, & Chen, 2004).
Limitations for the generalizability of our findings include a predominantly male and Caucasian study cohort; as such, gender and race effects could not be assessed. Another limitation is small subgroup sample sizes, particularly at TP3. The subset of our cohort with genetic data (n = 67) is relatively small for genetic analyses, but is quite large for neuroimaging datasets and, by extension, is a rather large sample for exploring neuroimaging/genotype associations. However, assembling large longitudinal cohorts with complete behavioral, neurocognitive, neuroimaging, and genotype data remains a challenge, especially when considering relatively low long-term abstinence rates and attrition in studies of abstinent substance users (Sullivan & Pfefferbaum, 2013; Thygesen, Johansen, Keiding, Giovanucci, & Grønbaek, 2008; Torvik, Rognmo, & Tambs, 2012). An additional potential limitation is the effects the quite different sample sizes of the ALC group (n = 121) and nsLD control group (n = 35) may have had on limiting power to detect group differences. However, the magnitude of effect sizes between ALC and controls is not strongly affected by sample size. In addition, in all our group comparisons, all critical model assumptions were met, i.e., homogeneity of variances of predictors across groups, normally distributed residual errors, and no outliers in either group. A deliberate decision to focus on recruiting more ALC than control participants, because of logistical and financial limitations, also allowed us to assemble a large cohort with reasonable statistical power for analysis of our primary hypotheses on determinants of hippocampal recovery during abstinence from alcohol. Other limitations included not having measures of nutrition and exercise and including only a single genetic polymorphism out of many that can potentially influence this complex phenotype. For example, the effects of BDNF on hippocampal volume, its functional activity, and memory performance in healthy controls have been shown to be affected by other genes (Kauppi, Nilsson, Persson, & Nyberg, 2014; Richter-Schmidinger et al., 2011). On the other hand, we made sincere efforts to exclude threats to validity of our analyses by excluding participants with medical, neurologic, and psychiatric disorders previously shown to affect hippocampal volume, and by including pertinent covariates in our analyses.
In conclusion, alcohol dependence is associated with decrements in hippocampal volume that persist at a mean of 213 days of abstinence, and smaller volumes relate to poorer visuospatial functioning in short-term abstinence. Smoking status does not appear to affect significantly hippocampal volume or hippocampal volume recovery as assessed with the morphometrics employed. Collectively, our analyses demonstrate that BDNF Val homozygosity appears to facilitate recovery of hippocampal volume and associated visuospatial function during long-term abstinence from alcohol, with BDNF genotypic differences in hippocampal volume observed in protracted abstinence only. BDNF genotype was not associated with neurocognitive function or substance use variables per se, underscoring the importance of studying relevant intermediate phenotypes as demonstrated here. Our findings also suggest that BDNF genotype effects on hippocampal volume and function in early abstinence, as well as their short-term improvements, are overshadowed/masked by environmental factors (such as the acute neurotoxic consequences of long-term chronic alcohol consumption). Therefore, future studies aimed at exploring genetic determinants of brain morphometry/function and its changes in alcohol dependence cannot employ actively drinking individuals, but they rather need to evaluate long-term abstinent individuals in whom environmental influences on brain structure/function have waned.
Acknowledgments
The authors thank all participants who volunteered for this study. The work was supported by grants from the National Institutes of Health (AA10788 to DJM; DA025202 to DJM; DA24136 to TCD) and by the use of resources and facilities at the San Francisco Veterans Administration Medical Center, and administered by the Northern California Institute for Research and Education. MEH was supported by a training grant (R25 MH060482 to Carol A. Mathews) that allowed for protected research time during psychiatric residency. We thank Dr. Stefan Gazdzinski for his contribution to MR data acquisition, as well as Mary Rebecca Young, Kathleen Altieri, Ricky Chen, and Drs. Peter Banys and Ellen Herbst of the VA Substance Abuse Day Hospital, and Dr. David Pating, Karen Moise and their colleagues at the Kaiser Permanente Chemical Dependency Recovery Program in San Francisco for their valuable assistance with recruiting participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No author reports any associated financial interests in the research or potential conflicts of interest.
References
- Abé C, Mon A, Hoefer ME, Durazzo TC, Pennington DL, Schmidt TP, et al. Metabolic abnormalities in lobar and subcortical brain regions of abstinent polysubstance users: magnetic resonance spectroscopic imaging. Alcohol and Alcoholism. 2013;48:543–551. doi: 10.1093/alcalc/agt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Archives of General Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression Inventory. Philadelphia, PA: Center for Cognitive Therapy; 1978. [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta JK. BDNF VAL66Met allele is associated with reduced hippocampal volume in healthy subjects. Biological Psychiatry. 2006;59:812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, et al. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. The Journal of Neuroscience. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, et al. Hippocampal volume in adolescent-onset alcohol use disorders. The American Journal of Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- Devenport L, Stidham J, Hale R. Ethanol and spatial localization. Behavioral Neuroscience. 1989;103:1259–1266. doi: 10.1037//0735-7044.103.6.1259. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcoholism: Clinical and Experimental Research. 2004;28:1849–1860. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ. Neurobiological and neurocognitive effects of chronic cigarette smoking and alcoholism. Frontiers in Bioscience. 2007;12:4079–4100. doi: 10.2741/2373. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ. Chronic cigarette smoking: implications for neurocognition and brain neurobiology. International Journal of Environmental Research and Public Health. 2010;7:3760–3791. doi: 10.3390/ijerph7103760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Kucera J, Lee KF, Loring J, Jaenisch R. Studies on the physiological role of brain-derived neurotrophic factor and neurotrophin-3 in knockout mice. The International Journal of Developmental Biology. 1995;39:799–807. [PubMed] [Google Scholar]
- Fergusson DM, Goodwin RD, Horwood LJ. Major depression and cigarette smoking: results of a 21-year longitudinal study. Psychological Medicine. 2003;33:1357–1367. doi: 10.1017/s0033291703008596. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Yeh PH, Hardin D, Banys P, Meyerhoff DJ. Chronic cigarette smoking modulates injury and short-term recovery of the medial temporal lobe in alcoholics. Psychiatry Research. 2008;162:133–145. doi: 10.1016/j.pscychresns.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Abraham HD. A longitudinal study of the order of onset of alcohol dependence and major depression. Drug and Alcohol Dependence. 2001;63:277–286. doi: 10.1016/s0376-8716(00)00216-7. [DOI] [PubMed] [Google Scholar]
- Giovino GA. Epidemiology of tobacco use in the United States. Oncogene. 2002;21:7326–7340. doi: 10.1038/sj.onc.1205808. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nature Reviews Genetics. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Grant I. Alcohol and the brain: neuropsychological correlates. Journal of Consulting and Clinical Psychology. 1987;55:310–324. doi: 10.1037//0022-006x.55.3.310. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Journal of Clinical and Experimental Neuropsychology. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Nagel BJ, Spadoni AD, Gorlick A, Tapert SF. Hippocampal volumes in adolescents with and without a family history of alcoholism. The American Journal of Drug and Alcohol Abuse. 2010;36:161–167. doi: 10.3109/00952991003736397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. The Journal of Neuroscience. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Kril J, Daly J. Are we drinking our neurones away? British Medical Journal (Clinical Research and Education) 1987;294:534–536. doi: 10.1136/bmj.294.6571.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Kril JJ. Neuropathology of alcoholism. Alcohol and Alcoholism. 1990;25:207–216. doi: 10.1093/oxfordjournals.alcalc.a044994. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, et al. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biological Psychiatry. 2001;49:894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. The EMBO Journal. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YY, Schuff N, Du AT, Mark K, Zhu X, Hardin D, et al. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. Journal of Magnetic Resonance Imaging. 2002;16:305–310. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE. What does the hippocampus really do? Behavioural Brain Research. 1995;71:1–10. doi: 10.1016/0166-4328(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Kauppi K, Nilsson LG, Persson J, Nyberg L. Additive genetic effect of APOE and BDNF on hippocampus activity. Neuroimage. 2014;89:306–313. doi: 10.1016/j.neuroimage.2013.11.049. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Simson PE, Best PJ. Acute ethanol impairs spatial memory but not stimulus/response memory in the rat. Alcoholism: Clinical and Experimental Research. 1995;19:902–909. doi: 10.1111/j.1530-0277.1995.tb00965.x. [DOI] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology and Teratology. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima F, Ollier W, Mayes A, Jackson A, Thacker N, Rabbitt P, et al. Brain-derived neurotrophic factor polymorphism Val66Met influences cognitive abilities in the elderly. Genes, Brain, and Behavior. 2008;7:411–417. doi: 10.1111/j.1601-183X.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- Molendijk ML, van Tol MJ, Penninx BW, van der Wee NJ, Aleman A, Veltman DJ, et al. BDNF val66met affects hippocampal volume and emotion-related hippocampal memory activity. Translational Psychiatry. 2012;2:e74. doi: 10.1038/tp.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Gazdzinski S, Hutchison KE, Pennington D, Meyerhoff DJ. Brain-derived neurotrophic factor genotype is associated with brain gray and white matter tissue volumes recovery in abstinent alcohol-dependent individuals. Genes, Brain, and Behavior. 2013;12:98–107. doi: 10.1111/j.1601-183X.2012.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro CA, Saxton J, Butters MA. The neuropsychological consequences of abstinence among older alcoholics: a cross-sectional study. Alcoholism: Clinical and Experimental Research. 2000;24:1510–1516. [PubMed] [Google Scholar]
- Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Progress in Neurobiology. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsoy S, Durak AC, Esel E. Hippocampal volumes and cognitive functions in adult alcoholic patients with adolescent-onset. Alcohol. 2013;47:9–14. doi: 10.1016/j.alcohol.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Paperwalla KN, Levin TT, Weiner J, Saravay SM. Smoking and depression. The Medical Clinics of North America. 2004;88:1483–1494. doi: 10.1016/j.mcna.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Raz N, Dahle CL, Rodrigue KM, Kennedy KM, Land SJ, Jacobs BS. Brain-derived neurotrophic factor Val66Met and blood glucose: a synergistic effect on memory. Frontiers in Human Neuroscience. 2008;2:12. doi: 10.3389/neuro.09.012.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Schmidinger T, Alexopoulos P, Horn M, Maus S, Reichel M, Rhein C, et al. Influence of brain-derived neurotrophic-factor and apolipoprotein E genetic variants on hippocampal volume and memory performance in healthy young adults. Journal of Neural Transmission. 2011;118:249–257. doi: 10.1007/s00702-010-0539-8. [DOI] [PubMed] [Google Scholar]
- Romberger DJ, Grant K. Alcohol consumption and smoking status: the role of smoking cessation. Biomedicine & Pharmacotherapy. 2004;58:77–83. doi: 10.1016/j.biopha.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Rourke SB, Grant I. The neurobehavioral correlates of alcoholism. In: Grant I, Adams K, editors. Neuropsychological Assessment of Neuropsychiatric and Neuromedical Disorders. 3rd edn. New York: Oxford University Press; 2009. pp. 398–454. [Google Scholar]
- Sacco KA, Bannon KL, George TP. Nicotinic receptor mechanisms and cognition in normal states and neuropsychiatric disorders. Journal of Psychopharmacology. 2004;18:457–474. doi: 10.1177/0269881104047273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. Journal of Studies on Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Self-Evaluation Questionnaire. Redwood City, CA: Mind Garden; 1977. [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology. 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neuropsychology and neuroimaging studies in alcohol-dependence. Revue de Neuropsychologie. 2013;5:187–199. [Google Scholar]
- Sullivan EV, Pfefferbaum A, Swan GE, Carmelli D. Heritability of hippocampal size in elderly twin men: equivalent influence from genes and environment. Hippocampus. 2001;11:754–762. doi: 10.1002/hipo.1091. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Hong CJ, Yu YW, Chen TJ. Association study of a brain-derived neurotrophic factor (BDNF) Val66Met polymorphism and personality trait and intelligence in healthy young females. Neuropsychobiology. 2004;49:13–16. doi: 10.1159/000075333. [DOI] [PubMed] [Google Scholar]
- Thygesen LC, Johansen C, Keiding N, Giovanucci E, Grønbaek M. Effects of sample attrition in a longitudinal study of the association between alcohol intake and all-cause mortality. Addiction. 2008;103:1149–1159. doi: 10.1111/j.1360-0443.2008.02241.x. [DOI] [PubMed] [Google Scholar]
- Torvik FA, Rognmo K, Tambs K. Alcohol use and mental distress as predictors of non-response in a general population health survey: the HUNT study. Social Psychiatry and Psychiatric Epidemiology. 2012;47:805–816. doi: 10.1007/s00127-011-0387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leemput K, Maes F, Vandermeulen D, Suetens P. Automated model-based tissue classification of MR images of the brain. IEEE Transactions on Medical Imaging. 1999;18:897–908. doi: 10.1109/42.811270. [DOI] [PubMed] [Google Scholar]
- Vandergriff JL, Matthews DB, Best PJ, Simson PE. Effect of ethanol and diazepam on spatial and nonspatial tasks in rats on an 8-arm radial arm maze. Alcoholism: Clinical and Experimental Research. 1996;19(Suppl.):64A. [Google Scholar]
- Whitwell JL, Crum WR, Watt HC, Fox NC. Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. American Journal of Neuroradiology. 2001;22:1483–1489. [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, et al. Amygdala volume associated with alcohol abuse relapse and craving. The American Journal of Psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]