Abstract
Dapper, Dishevelled-associated antagonist of β-catenin (DACT), is a key regulator of Wnt signaling pathway. The purpose of this study is to explore the epigenetic changes and the function ofDACT2 in human gastric cancer (GC). Eight human gastric cancer cell lines, 167 cases of primary gastric cancer and 8 cases of normal gastric mucosa were involved in this study. In addition, methylation Specific PCR (MSP), semi-quantitative RT-PCR, colony formation assay, flow cytometry assay, siRNA, immunofluorescence techniques and xenograft mice models were employed. The results indicate that DACT2 is frequently methylated in human primary gastric cancer (55.7%), and that methylation of DACT2 is associated with lost or reduction in its expression (X2 test, P<0.01). We found that DACT2 expression was regulated by promoter region hypermethylation. Methylation of DACT2 is associated with tumor differentiation, invasion and intravascular cancerous emboli (X2 test, P<0.05, P<0.05 and P<0.05). In gastric cancer patients treated with 5-FU and cisplatin, the five-year survival rates are higher in DACT2 methylated cases. DACT2 inhibits cell proliferation, migration and invasion in gastric cancer cells and suppresses gastric cancer xenografts in mice. Restoration of DACT2 expression inhibits both canonical and noncanonical WNT signaling in SGC7901 cells. Restoration of DACT2 expression sensitized gastric cancer cells to paclitaxel and 5-FU. In conclusion, DACT2 is frequently methylated in human gastric cancer and DACT2 expression is silenced by promoter region hypermethylation. DACT2 suppressed gastric cancer proliferation, invasion and metastasis by inhibiting Wnt signaling both in vitro and in vivo.
Keywords: Gastric cancer, DACT2, DNA methylation, Wnt signaling pathway, chemo-sensitivity
Introduction
Gastric cancer is one of the most common malignancies and the second leading cause of cancer-related death globally [1,2]. Both genetics and epigenetics seem to play important roles in gastric cancer [3-6]. Disruption of Wnt signaling pathway is most likely one of the important dysfunctional pathways responsible for gastric cancer pathogenesis [7]. Although accumulation of β-catenin is a hallmark of Wnt signaling activation, and occurs in more than 50% of these cancers, less than 30% of gastric cancers harbor β-catenin mutations [8-10]. APC is also rarely mutated in gastric cancer compared to colon cancer [10]. Thus, other components of Wnt signaling may play important roles in gastric cancer carcinogenesis.
Dapper was first isolated from Xenopus by Cheyettee et al. in a screen for proteins interacting with Dishevelled (Dvl), a key factor in the Wnt signaling pathway [11]. Human DACT1 and DACT2 were identified by bioinformatics in 2003 [12]. DACT1 is located on human chromosome 14q22.3 and has been reported frequently to be methylated in HCC. Human DACT3 is located on chromosome 19q13.32 and is regulated by histone modification in colorectal cancer [13,14]. Human DACT2 has been mapped to chromosome 6q27, and has been reported to have frequent loss of heterozygosity in human cancers [12,15-17]. DACT2 was also found to be frequently methylated in human lung cancer and hepatocellular cancer in our previous study [18,19]. In this study, we analyzed the epigenetic changes and the function of DACT2 in human gastric cancer.
Material and methods
Human tissue samples and cell lines
167 cases of human primary gastric cancer sample and 8 cases of normal gastric mucosa were collected from Chinese PLA General Hospital in Beijing or Ruijin Hospital in Shanghai. Snap-frozen fresh tissue samples were collected by surgery resection. All samples were collected under the guidelines approved by Chinese PLA General Hospital’s institutional review board and Ruijin Hospital’s institutional review board. The patients include 122 cases of male and 45 cases of female. The median age is 63.1 years old (range 28–89 years). All cancer samples were classified by TNM (UICC 2009) staging, including 64 cases of stage I and II, 103 cases of stage III and IV. Follow-up data of 72 patients treated with 5-flurouracil (5-FU) plus cisplatin after surgical resection is available, including 5-years survival data.
7 gastric cancer cell lines (NCI-N87, BGC823, AGS, MNK45, NUGC3, MGC803 and SGC7901) were previously established from primary gastric cancer, and maintained in 90% RPMI 1640 or DMEM (Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum. Cells were passaged 1:3 once total confluence (approximately 1 × 106 cells) was reached on a 75 cm2 culture flask (NEST Biotech., Jiangsu, China).
5-aza-2’-deoxycytidine treatment
Gastric cancer cell lines (NCI-N87, BGC823, AGS, MNK45, MGC803 and SGC7901) were split to low density (30% confluence) 12 hours before treatment. Cells were treated with 5-aza-2’-deoxycytidine (5-AZA) (Sigma, St. Louis, MO) at a concentration of 2 mM. Growth medium, conditioned with 5-AZA at 2 µM, was exchanged every 24 hours for total 96 hours treatment. At the end of treatment course, RNA was extracted from the cells as described below.
RNA isolation and semi-quantitative RT-PCR
Total RNA was isolated by Trizol reagent (Life Technologies, Gaithersburg, MD). Agarose gel electrophoresis and spectrophotometric analysis (A260: 280nm ratio) were used to evaluate RNA quality and quantity. RNA was stored at -80°C prior to use. 5 µg total RNA was used to syntheses first strand cDNA with random 6-mer primers and Superscript III-reverse transcriptase kit (Invitrogen, Carlsbad, CA). Following first strand synthesis, the reaction mixture was diluted to 100 μl by using water. Subsequently, 2.5 µl of diluted cDNA mixture was used for PCR amplification in a final 25 µl reaction volume. PCR amplification was carried out using primers: 5’-GGCTGAGACAACAGGACATCG-3’ (F) and 5’-GACCGTCGCTCATCTCGTAAAA-3’ (R). The primer set for DACT2 was designed to span intronic sequences between adjacent exons in order to control for genomic DNA contamination. A total of 33 cycles of amplification were performed for each of the RT-PCR experiments. As an internal control, GAPDH was amplified with 25 cycles to ensure cDNA quality and quantity for each RT-PCR. GAPDH primers were as follows: 5’-GACCACAGTCCATGCCATCAC-3’ (F) and 5’-GTCCACCACCCTGTTGCTGTA-3’ (R). Amplified products were analyzed on 1.5% agarose gels.
DNA extraction and methylation specific PCR (MSP)
Genomic DNA from gastric cancer cell lines, 167 cases of gastric cancer, and 8 cases of normal gastric mucosa were isolated by proteinase K method. Then DNA was dissolved in low TE buffer and stored at -20°C. 2 μg genomic DNA was diluted in 50 μl of water. Then genomic DNA from primary gastric cancer and cell lines was bisulfite modified as previously described [20]. MSP primers were designed according to genomic sequences flanking presumed transcription start sites (TSS). Primers were synthesized (Invitrogen, Beijing) to detect bisulfite-induced changes affecting unmethylated (U) and methylated (M) alleles. MSP primer sequences are as follows: 5’-GCGCGTGTAGATTTCGTTTTTCGC-3’ (MF); 5’-AACCCCACGAACGACGCCG-3’ (MR); 5’-TTGGGGTGTGTGTAGATTTTGTTTTTTGT-3’ (UF) and 5’-CCCAAACCCCACAAACAACACCA-3’ (UR). The size of unmethylated PCR product is 161 bp and methylation PCR product is 152 bp. Each MSP reaction included approximately 100 ng of bisulfite-treated DNA, 25 pmoles of each primer, 100 pmoles dNTPs, 2.5 μl 10 × PCR buffer, and 1 unit of Taq Polymerase (Invitrogen, Carlsbad, CA) in a final reaction volume of 25 µl. Cycle conditions: 95°C × 5 min, 1 cycle; 35 cycles × (95°C × 30 sec, 62°C × 30 sec, 72°C × 40 sec); 72°C × 5 min, 1 cycle. Each PCR assay included a methylation control (in vitro methylated DNA, IVD), an unmethylated control (normal lymphocyte DNA, NL), and negative control (ddH2O). MSP products were analyzed using 2% agarose gel electrophoresis.
Immunohistochemistry (IHC)
Immunohistochemistry was performed according to previous report [18]. Rabbit anti-DACT2 antibody was diluted to 1/400 (OriGene Tech., MD, U.S.A.). The procedure was performed according to catalyzed signal amplification system instructions (ZSGB Biotech., Beijing, China).
The expression level of DACT2 was evaluated by both intensity and extent of the positive staining. The intensity of DACT2 expression was quantified using scores as follow: 0 = negative, 1 = weakly positive, 2 = moderately positive and 3 = strongly positive. The extent of DACT2 expression was quantified as percentage of positive staining areas in relation to whole tissue areas. The score standard is shown as follows: 0 = 0% reactivity, 1 = 1–10% reactivity, 2 = 11–50% reactivity, 3 = 51–80% reactivity and 4 points means samples is with >80% reactivity. The final IHC score was determined by multiplying intensity score by extent score, with the minimum score of 0 and maximum score of 12 points. So IHC scores are including 10–12: strong DACT2 expression (+++), 7–9: intermediate DACT2 expression (++), 3–6: weak DACT2 expression (+), and 0–2: negative DACT2 expression (-). Score of 3 points or greater was considered positive for DACT2 expression.
Construction of expression vectors
Full-length DACT2 cDNA (GenBank accession number NM_214462) was cloned by RT-PCR into pCMV6 vector (OriGene Tech., MD, USA).
RNA interference-mediated knockdown of DACT2
DACT2 siRNA were constructed by Gimma company in Shanghai, as follow: DACT2 8#( the complementary sequence with DACT2 mRNA ) 5’-GUCGGUUGAUGAGACUACUTT-3’ and 5’-AGUAGUCUCAUCAACCGACTT-3’; siRNA con vector (the random sequence as control that was not related to DACT2 mRNA) 5’-UUCUCCGAACGUGUCACGUTT-3’ and 5’-ACGUGACACGUUCGGAGAATT-3.
Transfection assay
Transient transfection was performed by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) or FuGENE 6 (Roche Applied Science, Indianapolis, IN) according to manufacturer’s instructions.
Colony formation assay
SGC7901 cells were seeded in 6-well culture plates 24 h before transfection. DACT2 expression or empty vector was transfected into SGC7901 cells. Cells were digested and reseeded at 1500 cells/ well in 6-well plates in triplicate after 36 h. Growth medium, conditioned with G418 (Invitrogen, Carlsbad, CA) at 400 µg/mL, was exchanged every 24 h. Clones were counted by 14 days after fixed with 75% ethanol for 30 min and stained with 0.2% crystal violet for visualization and counting.
Flow cytometry assay
The Cell phase was determined by quantitation of cellular DNA content with propidium iodide (PI) staining (KeyGen Biotech., Jiangsu, China). DACT2 expression or empty vectors were transfected into SGC7901 cells according to manufactory’s instruction (KeyGEN Biotech, Nanjing, China). After 48 h of transfection, cells were collected and then sorted by FACS Calibur (BD Biosciences, Franklin Lakes, NJ). Cell-cycle profiles were analyzed by WinMDI v. 2.9 software (Scripps Research Institute, La Jolla, CA).
Chemo-sensitivity assay
The cytotoxic effects of paclitaxel, cisplatin and 5-fluorouracil (5-FU) on SGC7901 cells were determined using Cell Counting Kit-8 (CCK-8). Briefly, cells were seeded at 4,000/well onto flat-bottomed 96-well culture plates and allowed to grow for 48 hours before the desired treatment. Cells were then detected with CCK-8 (Dojindo, Japan) according to the manufacturer’s instruction. Absorbance was read in a microplate reader at 450 nm. The half maximal inhibitory concentration (IC50) value was assessed by different concentrations of paclitaxel (0, 0.001, 0.01, 0.05, 0.1, 1 μM), cisplatin (0, 1, 2, 5, 10, 20 μM) and 5-FU (0, 1, 10, 100, 500, 1000 μM).
Cell invasion assay
The effect of DACT2 on cell invasion was detected by the Transwell assay (COSTAR transwell, Corning Incorporated, MA, USA). SGC7901 cells were transfected with empty vector or DACT2 vector. 3 × 105 cells were suspended in 500 µl of serum-free DMEM and loaded onto the upper compartment of an invasion chamber containing a polycarbonate membrane with an 8 µm pore size which was coated with a layer of extracellular matrix (ECM; Matrigel™, BD, NJ, USA). After 12 h of incubation, the invasive cells migrated through the ECM layer to the complete medium in the lower compartment. The invasive cells were stained with crystal violet and the number of invaded cells was counted in three independent high powered fields (× 100) readings with a light microscope. Statistical analysis was applied among the groups.
Transwell migration assay
The effect of DACT2 on cell migration was detected by using the Transwell assay in the absence of the ECM layer. SGC7901 cells were transfected with empty vector or DACT2 expression vector. After 48 h, DACT2 forced expression cells, empty vector transfected cells, or untransfected cells were harvested and suspended in the serum free DMEM. Cell suspensions were then placed into the upper well at a concentration of 1 × 104 cells/100 µl separately, while the complete medium with 10% fetal bovine serum was placed into the lower well (500 µl). The chamber was incubated for 6h. Non-migrated cells on the upper surface were scraped gently and washed out with PBS three times. DACT2 cells migrated to the lower surface of the membrane were stained with crystal violet and counted in three independent high powered fields (× 100) with light microscope. Statistical analysis was applied among groups.
DACT2 silenced and re-expressed SGC7901 cells xenograft mice
DACT2 re-expressed SGC7901 stable cell line (2 × 106 cells in 0.2 ml phosphate-buffered saline) was injected subcutaneously into the dorsal right flank of 4-week-old female Balb/c nude mice (n = 6), and DACT2 unexpressed SGC7901 cell line was injected subcutaneously into the dorsal left flank of the same nude mice (n = 6). 5 days after implantation, tumor volume was assessed every 2 days for 3 weeks. Tumor volume was calculated according to the following formula: V = L × W2/2 where V, volume (mm3); L, biggest diameter (mm); W, smallest diameter (mm). All procedures were approved by the Animal Ethics Committee of the Chinese PLA General Hospital.
Luciferase reporter assay
Cells were seeded at 5 × 104 cells/well in 24-well culture plates 24 h before transfection. pGL3-OT construct is a TCF/LEF–responsive reporter containing three consensus TCF binding sites. pRL-TK vector (Promega, Madison, WI) was used as a system control [18,19,21,22]. 100 ng/well pGL3-OT and 10 ng/well pRL-TK vector were transfected into SGC7901 cell line as basic luciferase reporter system [21]. 150 ng/well wild type or Ser45 mutation β-catenin vector was transfected into SGC7901 cell line as wild type or mutant β-catenin expression groups [23]. To explore the effect of DACT2 on β-catenin/TCF luciferase reporter activity, 100ng/well DACT2 vectors was transfected into wild type and mutant β-catenin expression SGC7901 cells.
Relative luciferase activity was measured by the Dual Luciferase Reporter Assay system (Promega) according to the manufacturer’s instruction 48 h after transfection. Each experiment was repeated for three times.
Western blot
To further validate the effect of DACT2 on Wnt signaling pathway, DACT2 expression construct or empty vector was transfected into SGC7901 cells. Cells were harvested and lysed in ice-cold Tris buffer (20 mmol/L Tris; pH 7.5) containing 137 mmol/L of NaCl, 2 mmol/L of EDTA, 1% Triton X, 10% glycerol, 50 mmol/L of NaF, 1 mmol/L of DTT, and a protease inhibitor cocktail (Roche Applied Science) after 48 hours of transfection. 45 µg of cell lysate was loaded into each lane. The protein lysates were then separated by SDS-PAGE and electroblotted onto PVDF membranes (Hybond-P, Amersham). After blocking with 5% nonfat milk and 0.1% Tween-20 in TBS, the membranes were incubated with rabbit anti-DACT2 (Abcam, MA, USA), rabbit anti-cyclinB1 (Bioworld Tech., MN, USA), rabbit anti-cdc2 (Bioworld Tech., MN, USA), rabbit anti-c-Myc (Bioworld Tech., MN, USA), rabbit anti-MMP-2 (Bioworld Tech., MN, USA), rabbit anti-MMP-9 (Bioworld Tech., MN, USA), rabbit anti-p-c-Jun (Epitomics, USA), rabbit anti-β-catenin (Epitomics, USA), p-β-catenin (CST, USA) or mouse anti-β-actin (Beyotime Biotech., China) antibodies. The blots were visualized using enhanced chemiluminescence (Pierce Bioscience, IL, USA).
Statistical analysis
Data were presented as mean ± SD. Statistical analysis was carried out by X2 test and t test. Kaplan-Meier was performed for survival curves. P<0.05 was considered significant difference.
Results
DACT2 expression was regulated by promoter region hypermethylation in gastric cancer
To explore the possibility of epigenetic regulation of DACT2 in human gastric cancer, 7 human gastric cancer cell lines were detected by semi-quantitative RT-PCR. DACT2 expression was discovered in NCI-N87, BGC823, AGS, MNK45, NUGC3 and MGC803 cell lines and no expression was found in SGC7901 cell line (Figure 1A). DACT2 was found completely methylated in SGC7901 cell line and partially methylated inBGC823 cell line, the other cells were unmethylated (Figure 1B). The results indicate that loss of DACT2 expression is correlated with promoter region hypermethylation in human gastric cancer cells. Induced expression of DACT2 by 5-AZA, a DNA methylation transferases (DNMTs) inhibitor, was revealed in SGC7901 cell line. But no expression change was found in the other cells before and after 5-AZA treatment. Above results indicate that DACT2 expression was regulated by promoter region methylation in human gastric cancer.
Figure 1.
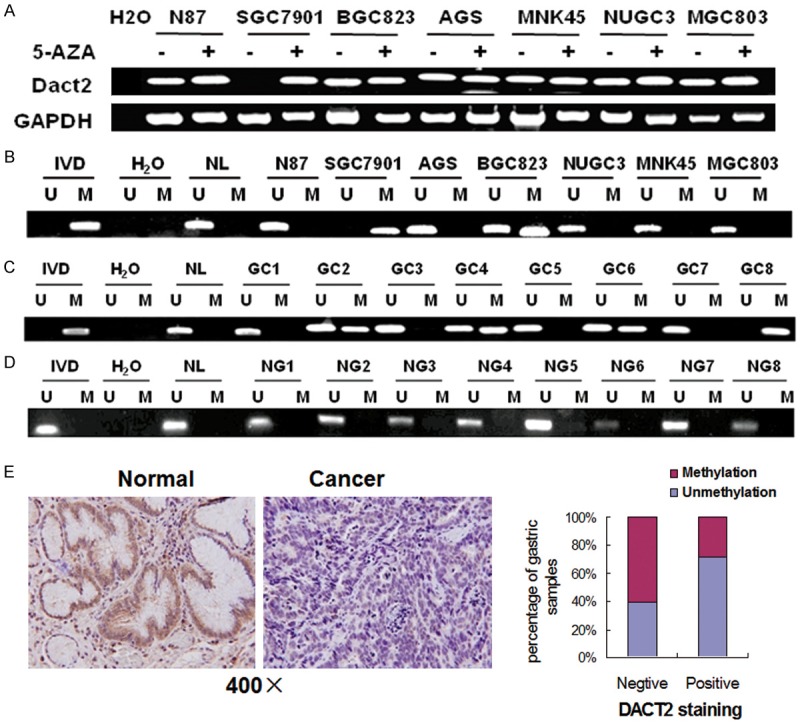
Dact2 is silenced by promoter region hypermethylation in gastric cancer. A. RT-PCR results of DACT2 expression: (-) untreated; (+) 5-AZA treated. H2O: double distilled water. GAPDH: internal control. B. Methylation status of DACT2 in gastric cancer cell lines. C. Representative MSP results: GC: gastric cancer; IVD: in vitro methylated DNA; NL: normal lymphocyte DNA; U: unmethylated alleles; M: methylated alleles. D. NG: normal gastric mucosa. E. IHC staining of DACT2 in gastric cancer and adjacent tissue (×400). DACT2 expression is associated with promoter region hypermethylation in reversely (X2 test, P = 0.049).
DACT2 is frequently methylated in human gastric cancer and methylation of Dact2 is associated with reduction of DACT2 expression in primary cancer samples
DACT2 was methylated in 55.7% (93/167) of primary gastric cancer (Figure 1C) and no methylation was found in 8 cases of normal human gastric mucosa (0/8) (Figure 1D). DACT2 methylation is associated with tumor differentiation (P<0.05), invasion (P<0.05), and intravascular cancerous emboli significantly (P<0.05, Table 1).
Table 1.
The association of clinic-pathologic characteristics and methylation status of DACT2 in gastric cancer patients (n = 167)
| Clinical parameter | No. | Methylation status | P valuea | |
|---|---|---|---|---|
|
| ||||
| Methylated n = 93 (55.7%) | Unmethylated n = 74 (44.3%) | |||
| Age (year) | P = 0.4886 | |||
| <50 | 30 | 15 (50%) | 15 (50%) | |
| ≥50 | 137 | 78 (56.9%) | 59 (43.1%) | |
| Gender | P = 0.4958 | |||
| Male | 122 | 66 (54.1%) | 56 (45.9%) | |
| Female | 45 | 27 (60%) | 18 (40%) | |
| Differentiation | P = 0.0185* | |||
| Poorly | 113 | 70 (61.9%) | 43 (38.1%) | |
| Moderately/Well | 48 | 23 (47.9%) | 31 (52.1%) | |
| Tumor stage | P = 0.4497 | |||
| I/II | 64 | 38 (59.3%) | 26 (40.7%) | |
| III/IV | 103 | 55 (53.4%) | 48 (46.6%) | |
| T stage | P = 0.0461* | |||
| 1/2 | 29 | 21 (72.4%) | 8 (22.6%) | |
| 3/4 | 138 | 72 (52.2%) | 66 (47.8%) | |
| N stage | P = 0.8822 | |||
| Negative | 37 | 21 (56.8%) | 16 (43.2%) | |
| Positive | 130 | 72 (55.4%) | 58 (44.6%) | |
| Intravascular cancerous emboli | P = 0.0347* | |||
| Negative | 83 | 53 (63.9%) | 30 (36.1%) | |
| Positive | 84 | 40 (47.6%) | 44 (52.4%) | |
| Tumor size | P = 0.2187 | |||
| ≥5 cm | 110 | 65 (59.1%) | 45 (40.9 %) | |
| <5 cm | 57 | 28 (49.1%) | 29 (50.9%) | |
P values are obtained from X2 test;
Statistically significant, P < 0.05.
To evaluate the expression of DACT2 in primary gastric cancer, 42 cases of available paired gastric cancer and adjacent tissue sample were stained by IHC. DACT2 staining was mainly located in cytoplasm. Positive staining was found in 14 cases of gastric cancer and 32 cases of adjacent tissue (Figure 1E). The expression level is reduced significantly in cancer tissue (P<0.01). In 42 cases of cancer tissue samples, 21 cases were methylated (including 17 negative staining cases and 4 positive staining cases) and 21 cases (including 11 negative staining cases and 10 positive staining cases) were unmethylated. DACT2 expression is associated with promoter region hypermethylation in reversely (X2 test, P<0.05). Above results hint that DACT2 expression is regulated by promoter region hypermethylation in primary gastric cancer.
Restoration of DACT2 expression inhibits colony formation, migration and invasion of SGC7901 cells
To evaluate the effect of DACT2 on cell proliferation in gastric cancer, a colony formation assay was employed in SGC7901 cell line. As shown in Figure 2A, the clone number was reduced significantly in DACT2 re-expressed SGC7901 cells compared with control group (54.67±14.0 vs 113.67±22.5, P<0.05). The result suggests that DACT2 inhibits cell proliferation in gastric cancer.
Figure 2.
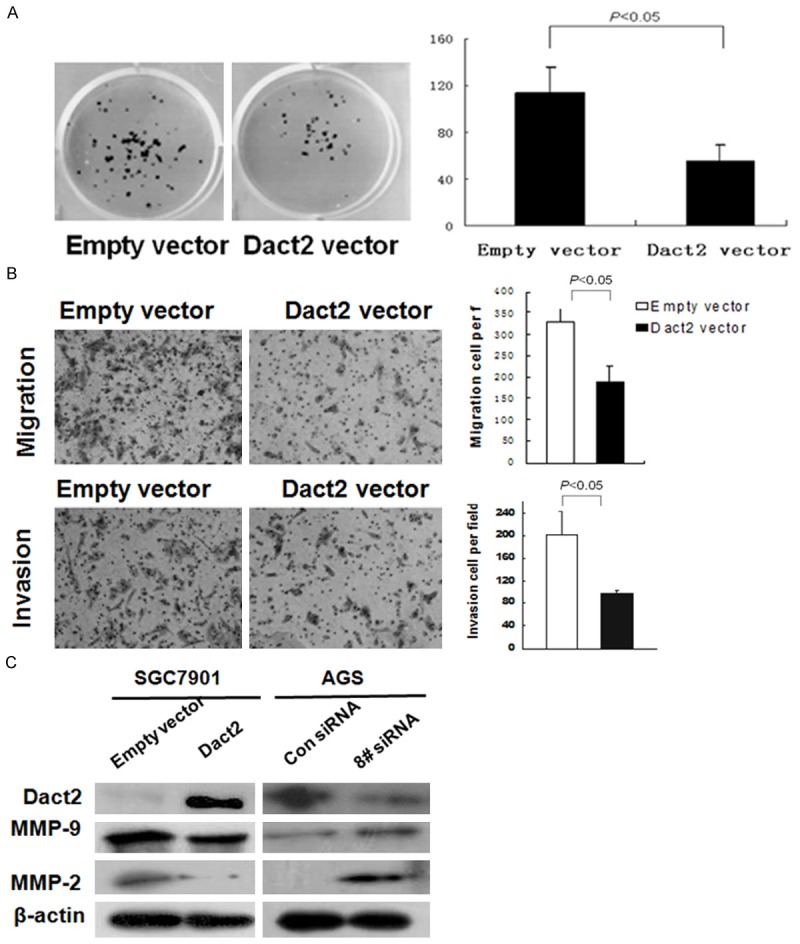
Colony formation, migration and invasion were inhibited by restoration of DACT2 expression in SGC7901 cells. And the migration and invasion were promoted by knock down of DACT2 in AGS cells. A. Colony formation results: the average number of colonies is presented in bar diagram. B. Restoration of DACT2 expression inhibits the migration and invasion in SGC7901 cells. C. Western Blot results: MMP-9, and MMP-2 expression were inhibited by restoration of DACT2 expression in SGC7901 cells and were increased by knock down of DACT2 expression in AGS cells.
To evaluate the effect of DACT2 on gastric cancer migration and invasion, the Transwell assay was employed. In the Transwell assay without ECM coating, the number of migrated cells in each high powered field was 189 ± 36 in the DACT2 re-expressed SGC7901 cells, and 327±33 in the empty vector group under the microscope (Figure 2B). The number of migrated cells is reduced significantly in DACT2 re-expressed SGC7901 cells compared to the empty vector group (P<0.05). The invasive cell number of each high powered field was 96±9 in the DACT2 expression group and 202±43 in the empty vector group (Figure 2B). The number of invasive cells was significantly reduced in the DACT2 re-expressed SGC7901 cells compared with the control group (P<0.05). These results indicate that DACT2 suppresses cellular invasion and migration in gastric cancer. The expression of MMP-2 and MMP-9, the major components related to cancer invasion and migration, was inhibited after re-expression of DACT2 in SGC7901 cells. This effect was further validated by knock down of DACT2 in DACT2 expressed AGS cells (Figure 2C). Above results suggest that DACT2 inhibits cell invasion and migration in gastric cancer.
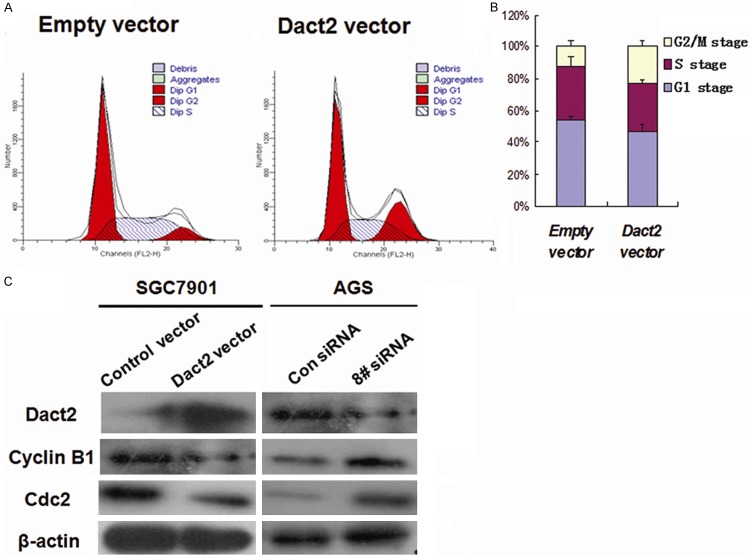
Re-expression of DACT2 induced G2/M phase arrest in gastric cancer cells
The effect of DACT2 on the cell cycle was evaluated by flow cytometry. In SGC7901 cells transfected with an empty vector, the percentage of G0/1 phase was 44.1±5.95%, S phase was 44.1±5.95 and G2/M phase was 6.63±3.37%. In contrast, in DACT2 re-expressed SGC7901 cells, the ratio of G0/1 phase was 43.92±3.01%, S phase was 34.13±2.21% and G2/M phase was 21.96±5.21% (Figure 3A). The ratio of S phase was reduced significantly (34.13±2.21% vs. 44.1±5.95%, P<0.01), and the ratio of G2/M phase was increased significantly after restoration of DACT2 expression (21.96±5.21% vs 6.63±3.37%, P<0.01, Figure 3B). These results indicate that DACT2 reduced S phase and induced G2/M phase arrest in SGC7901 cells.
Figure 3.
Cell phase distribution and G2/M phase related protein expression before and after re-expression DACT2 (A) Restoration of DACT2 expression reduced S phase and increased G2/M phase cells. (B) The ratio of each phase is presented by bar diagram. (C) The expression of cdc2 and cyclinB1 in DACT2 unexpressed and expressed SGC7901 cells or before and after siRNA knock down DACT2 in AGS cells.
To further validate the effect of DACT2 on G2/M phase, cdc2 and cyclinB1 (G2/M check point proteins) were analyzed in DACT2 unexpressed SGC7901 cells and DACT2 expressed AGS cells. As shown in Figure 3C, the expression of cdc2 and cyclinB1 was suppressed after restoration of DACT2 expression in SGC7901 cells and their expression was enhanced when a knock down of DACT2 in AGS cells occurred. These results further validate that DACT2 may induce G2/M arrest in gastric cancer.
DACT2 inhibits both canonical Wnt/β-catenin signaling and noncanonical Wnt/c-Jun signaling in gastric cancer
As shown in Figure 4A, the activity of TCF/LEF luciferase reporter was inhibited by co-transfecting DACT2 with wild type or mutant type of β-catenin in SGC7901 cells. The expression ofβ-catenin and c-myc, the Wnt signaling downstream genes, were reduced and the level of p-β-catenin (phosphorylated β-catenin) was increased in DACT2 re-expressed SGC7901 cells. The effect of DACT2 on Wnt/β-catenin signaling was further validated by knocking down DACT2 in DACT2 constant expressed AGS cells. The level of p-β-catenin was reduced and the expression ofβ-catenin and c-myc were increased by knocking down DACT2 in AGS cells (Figure 4B). These results suggest that DACT2 inhibits Wnt/β-catenin signaling in gastric cancer.
Figure 4.
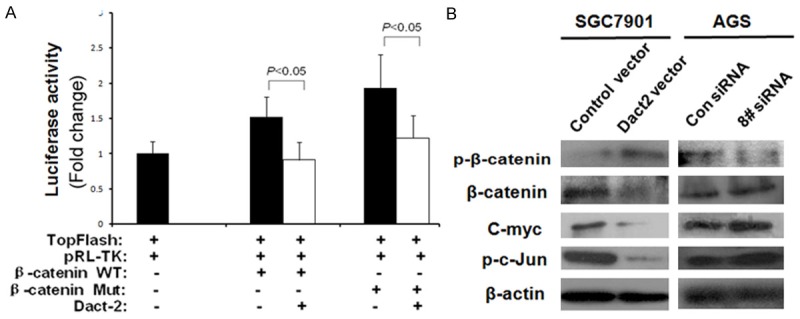
DACT2 inhibits both Wnt/β-catenin and Wnt/c-Jun signaling pathway. A. Results of TCF/LEF luciferase reporter assay. TopFlash: TCF/LEF-responsive reporter; pRL-TK: internal control; WT: wild-type; Mut: mutant. B. p-β-catenin, β-catenin, C-myc and p-c-Jun expression. p-β-catenin: phosphorylated β-catenin; p-c-Jun: phosphorylated c-Jun. Con siRNA: siRNA control; 8# siRNA: DACT2 siRNA #8.
Since DACT2 inhibits TCF/LEF activity under overexpression of both wild type and mutant type β-catenin in SGC7901 cells, it is possible that DACT2 may be involved in both the canonical Wnt/β-catenin and the noncanonical Wnt/c-Jun pathway [11]. To validate if DACT2 involved the noncanonical Wnt/c-Jun signaling, c-Jun was detected by western blot in DACT2 unexpressed and re-expressed SGC7901 cells as well as in AGS cells before and after knock down of DACT2. As shown in Figure 4B, the expression of c-Jun was decreased after restoration of DACT2 expression in SGC7901 cells and increased by knocking down DACT2 in AGS cells. These results suggest that DACT2 is involved in the noncanonical Wnt/c-Jun signaling.
DACT2 suppressed gastric cancer growth in xenograft mice
To further understand the effect of DACT2 on gastric cancer, DACT2 re-expressed and unexpressed SGC7901 xenograft mice were employed in this study (Figure 5A). As shown in Figure 5B, 5C, the volume of gastric cancer is significantly different in DACT2 re-expressed and unexpressed SGC7901 xenograft mice (14.33±7.05 mm3 vs. 307.46±140.42 mm3, t test, P<0.05). The weight of the tumor is also significantly different in DACT2 re-expressed and unexpressed SGC7901 xenograft mice (25.05±2.0 mg vs 365.67±20.02 mg, t test, P<0.01) (Figure 5C). DACT2 expression was validated by IHC in DACT2 expressed SGC7901 xenografts (Figure 5D). These results suggest that DACT2 suppresses SGC7901 xenograft growth in mice.
Figure 5.
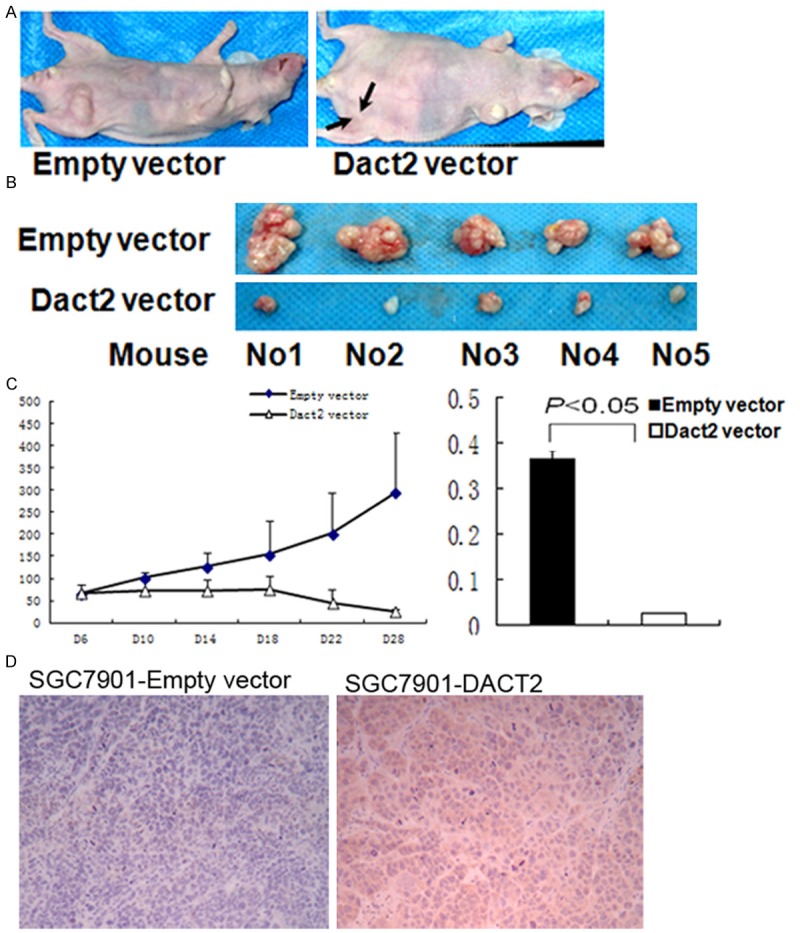
DACT2 suppressed SGC7901 cell xenograft growth. A and B. DACT2 unexpressed and expressed SGC7901 cell xenograft. C. Tumor growth curve in DACT2 vector and Empty vector groups. D and E. Diagrams show the volume and weight in DACT2 expressed and unexpressed SGC7901 cell xenografts.
DACT2 methylation is related to 5-FU and cisplatin sensitivity in human primary gastric cancer, and DACT2 sensitizes SGC7901 cells to paclitaxel
In 72 cases of gastric cancer patients with available follow-up data, the five-year survival rates were 53% in DACT2 methylated and 33% in unmethylated patients, which was treated by 5-FU plus cisplatin after surgical resection (Figure 6A). It suggests that DACT2 methylation may be a sensitive marker for 5-FU and/or cisplatin in gastric cancer. Further analysis was performed to investigate the sensitivity of 5-FU or cisplatin in gastric cancer cells. As shown in Figure 6B, the IC50 of 5-FU are 62.75±4.47 μM and 48.18±3.23 μM in DACT2 unexpressed and re-expressed SGC7901 cells, resectively (P<0.05). The IC50 of cisplatin are 12.75 ± 1.01 μM and 16.78 ± 0.65 μM in DACT2 unexpressed and re-expressed SGC7901 cells. DACT2 unexpressed SGC7901 cell is more sensitive than DACT2 expressed SGC7901cell to cisplatin (P<0.01).
Figure 6.
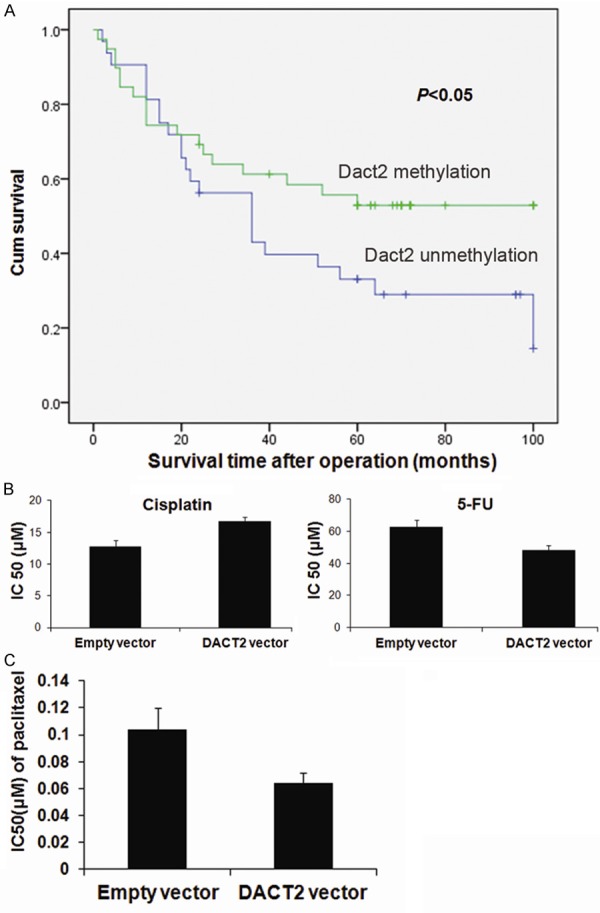
The predictive value of DACT2 methylation in chemo-sensitivity. A. The five-year survival rates in Dact2 methylated and unmethylated patients. B. The sensitivity of SGC7901 cells to 5-FU and cisplatin before and after re-expression of DACT2. The cell viability was measured by CCK-8 after treatment with different concentration of cisplatin for 48h. C. The sensitivity of DACT2 unexpression and expressed SGC7901 cell to paclitaxel.
Paclitaxel exerts its cytotoxic activity through enhancement and stabilization of the microtubule assembly reducing their dynamicity leading to an arrest of the cell cycle at the G2/M phase. As G2/M arrest was induced by DACT2 in SGC7901 cells, the sensitivity of paclitaxel in SGC7901 cells was detected before and after restoration of DACT2 expression (Figure 6C). The IC50 of paclitaxel is lower in DACT2 re-expressed SGC7901 cells than in control group after 48 hours treatment (0.064±0.0076 μM vs 0.10±0.016 μM, P< 0.01). Above results indicate that DACT2 sensitized SGC7901 cells to paclitaxel. Paclitaxel and 5-FU are more sensitive to DACT2 expressed SGC7901 cells than DACT2 unexpressed SGC7901 cells, but cisplatin is more sensitive to DACT2 unexpressed SGC7901 cells than expressed DACT2 SGC7901 cells.
Discussion
In this study, we found DACT2 expression was absent or reduced in human gastric cancer. This result is similar to our previous reports regarding DACT2 expression in human lung cancer and hepatic cancer [18,19]. DACT2 is located on human chromosome 6q27 [12]. This region is frequently lost in various human cancers, including gastric cancer [15-17]. Chen et al reported that LOH on chromosome 6q exists in 80% of Chinese gastric cancer, suggesting the presence of tumor suppressor genes in 6q [24]. As reported previously, DACT2 expression is mainly regulated by promoter region hypermethylation and not by mutation in human lung cancer [18,19]. In this study, we also found that DACT2 was frequently methylated in human gastric cancer and its expression was silenced by promoter region hypermethylation. It suggests that methylation of DACT2 is a potential gastric cancer detection biomarker. DACT2 methylation was also significantly associated with tumor differentiation (P<0.05), invasion (P<0.05) and intravascular cancerous emboli (P<0.05). These findings indicate that methylation of DACT2 promotes gastric cancer development and invasion. To analyze the effect of DACT2 on gastric carcinogenesis further, colony formation assay, flow cytometry and a Transwell assay were all employed. Restoration of DACT2 expression inhibited colony formation, induced G2/M arrest, and inhibited cell migration and invasion in a SGC7901 cell line. Tumor growth was also inhibited by DACT2 expression in mice xenografted with SGC7901 cells. All of the above results suggest that DACT2 is a tumor suppressor in human gastric cancer.
In the canonical Wnt/β-catenin pathway, reduced degradation of β-catenin will increase translocation of β-catenin into the nucleus, and activate TCF/LEF and downstream targets [25-28]. C-myc and MMPs are downstream targets of Wnt/β-catenin signaling. In our study, re-expression of DACT2 inhibited TCF/LEF activity, and the effect was tempered by co-transfection of β-catenin with DACT2 in SGC7901 cells. The downstream targets, c-myc and MMPs, were reduced. Phosphorylated β-catenin was, however, increased. These results suggest that DACT2 is involved in the canonical Wnt/β-catenin pathway in gastric cancer. Dpr2 proteins are conserved from zebrafish to human in four conserved domains: an NH2-terminal leucine-zipper domain, two serine-rich domains, and a binding motif for PDZ domain at the C-terminus [11,12,27,29-31]. Only the PDZ-binding motif was identified to bind Dishevelled whereas the function of the other domains remains unclear [11]. Cheyette et al. reported that Dpr inhibits noncanonical Wnt/c-Jun signaling in Xenopus [11]. Su et al. found that mouse DACT2 antagonize TGF-βsignaling [32].Our previous study demonstrated that DACT2 inhibits Wnt signaling in human lung and hepatic cancer [27,33]. This study indicates that DACT2 inhibits noncanonical Wnt/c-Jun signaling in human gastric cancer. These results suggest that the function of DACT2 may be partially different in different species or different tissues. The ratio of G2/M phase is increased after restoration of DACT2 expression in SGC7901 cells. As reported before, G2/M phase cells is sensitive to paclitaxel [34]. Our results indicate that DACT2 re-expressed SGC7901 cells are more sensitive to paclitaxel, but DACT2 unexpressed SGC7901 cells are more sensitive to cisplatin. While, in primary gastric cancer patients treated by 5-FU plus cisplatin, the five-year survival rates were higher in Dact2 methylated than in unmethylated patients. The IC50 of 5-FU is very high in both DACT2 unexpressed and re-expressed SGC7901 cells, The IC50 of cisplatin and paclitaxel is very low in DACT2 unexpressed and re-expressed SGC7901 cells. Cisplatin may play a more important role than 5-FU in combined 5-FU and cisplatin therapy in gastric cancer. Our results suggest that DACT2 methylation is a sensitive marker of cisplatin and a marker of insensitivity for paclitaxel. DACT2 methylation is associated with tumor differentiation (P<0.05), invasion (P<0.05) and intravascular cancerous emboli (P<0.05). These results suggest that DACT2 may involve gastric cancer development and progression. Functional studies further validated that DACT2 inhibited colony formation, cell migration and invasion in gastric cancer cells. In vivo, DACT2 seemed to suppress gastric cancer xenograft. All of these above results suggest that DACT2 is a tumor suppressor in human gastric cancer. Our results further reveal that DACT2 suppresses gastric cancer growth through both the canonical Wnt/β-catenin pathway and the noncanonical Wnt/c-Jun Signaling.
In conclusion, DACT2 is frequently methylated in human gastric cancer and DACT2 expression was silenced by promoter region hypermethylation. Methylation of DACT2 promotes gastric cancer growth, invasion and metastasis both in vitro and in vivo. Methylation of DACT2 activated both the canonical Wnt/β-catenin signaling and noncanonical Wnt/c-Jun signaling in gastric cancer. In 5-FU plus cisplatin treated gastric cancer patients, the five-year survival rates was higher in DACT2 methylated patients. Restoration of DACT2 expression sensitized paclitaxel and 5-FU to gastric cancer cells.
Acknowledgements
This work was supported by grants from the National Basic Research Program (973 Program No. 2012CB934002, 2010CB912802), National High-tech R&D Program (863 Program No. SS2012AA020314, SS2012AA020821, SS2012AA020303), National Key Scientific instrument Special Programme of China (Grant No. 2011YQ03013405) and National Science Foundation of China (Grant No. 81121004, 81071953, 81161120432).
Disclosure of conflict of interest
JGH is a consultant to MDxHealth. The other authors declare no conflict of interest.
Abbreviations
- DACT2
Dapper Dishevelled-associated antagonist of β-catenin
- GC
Gastric Cancer
- MSP
methylation specific PCR
- RT-PCR
reverse transcription PCR
- 5-AZA
5-aza-2’-deoxycytidine
References
- 1.Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477–490. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Gigek CO, Chen ES, Calcagno DQ, Wisnieski F, Burbano RR, Smith MA. Epigenetic mechanisms in gastric cancer. Epigenomics. 2012;4:279–294. doi: 10.2217/epi.12.22. [DOI] [PubMed] [Google Scholar]
- 4.Panani AD. Cytogenetic and molecular aspects of gastric cancer: clinical implications. Cancer Lett. 2008;266:99–115. doi: 10.1016/j.canlet.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 5.Milne AN, Carneiro F, O’Morain C, Offerhaus GJ. Nature meets nurture: molecular genetics of gastric cancer. Hum Genet. 2009;126:615–628. doi: 10.1007/s00439-009-0722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudler P. Genetic aspects of gastric cancer instability. ScientificWorldJournal. 2012;2012:761909. doi: 10.1100/2012/761909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theodosiou NA, Tabin CJ. Wnt signaling during development of the gastrointestinal tract. Developmental Biology. 2003;259:258–271. doi: 10.1016/s0012-1606(03)00185-4. [DOI] [PubMed] [Google Scholar]
- 8.Cheng XX, Wang ZC, Chen XY, Sun Y, Kong QY, Liu J, Li H. Correlation of Wnt-2 expression and beta-catenin intracellular accumulation in Chinese gastric cancers: relevance with tumour dissemination. Cancer Lett. 2005;223:339–347. doi: 10.1016/j.canlet.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Oshima H, Matsunaga A, Fujimura T, Tsukamoto T, Taketo MM, Oshima M. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology. 2006;131:1086–1095. doi: 10.1053/j.gastro.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Woo DK, Kim HS, Lee HS, Kang YH, Yang HK, Kim WH. Altered expression and mutation of beta-catenin gene in gastric carcinomas and cell lines. Int J Cancer. 2001;95:108–113. doi: 10.1002/1097-0215(20010320)95:2<108::aid-ijc1019>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Cheyette BN, Waxman JS, Miller JR, Takemaru K, Sheldahl LC, Khlebtsova N, Fox EP, Earnest T, Moon RT. Dapper, a Dishevelled-associated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Dev Cell. 2002;2:449–461. doi: 10.1016/s1534-5807(02)00140-5. [DOI] [PubMed] [Google Scholar]
- 12.Katoh M. Identification and characterization of human DAPPER1 and DAPPER2 genes in silico. Int J Oncol. 2003;22:907–913. [PubMed] [Google Scholar]
- 13.Yau TO, Chan CY, Chan KL, Lee MF, Wong CM, Fan ST, Ng IO. HDPR1, a novel inhibitor of the WNT/beta-catenin signaling, is frequently downregulated in hepatocellular carcinoma: involvement of methylation-mediated gene silencing. Oncogene. 2005;24:1607–1614. doi: 10.1038/sj.onc.1208340. [DOI] [PubMed] [Google Scholar]
- 14.Jiang X, Tan J, Li J, Kivimae S, Yang X, Zhuang L, Lee PL, Chan MT, Stanton LW, Liu ET, Cheyette BN, Yu Q. DACT3 is an epigenetic regulator of Wnt/beta-catenin signaling in colorectal cancer and is a therapeutic target of histone modifications. Cancer Cell. 2008;13:529–541. doi: 10.1016/j.ccr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li BC, Chan WY, Li CY, Chow C, Ng EK, Chung SC. Allelic loss of chromosome 6q in gastric carcinoma. Diagn Mol Pathol. 2003;12:193–200. doi: 10.1097/00019606-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Bignone PA, Lee KY, Liu Y, Emilion G, Finch J, Soosay AE, Charnock FM, Beck S, Dunham I, Mungall AJ, Ganesan TS. RPS6KA2, a putative tumour suppressor gene at 6q27 in sporadic epithelial ovarian cancer. Oncogene. 2007;26:683–700. doi: 10.1038/sj.onc.1209827. [DOI] [PubMed] [Google Scholar]
- 17.Amiel A, Mulchanov I, Elis A, Gaber E, Manor Y, Fejgin M, Lishner M. Deletion of 6q27 in chronic lymphocytic leukemia and multiple myeloma detected by fluorescence in situ hybridization. Cancer Genet Cytogenet. 1999;112:53–56. doi: 10.1016/s0165-4608(98)00255-6. [DOI] [PubMed] [Google Scholar]
- 18.Jia Y, Yang Y, Brock MV, Zhan Q, Herman JG, Guo M. Epigenetic regulation of DACT2, a key component of the Wnt signalling pathway in human lung cancer. J Pathol. 2013;230:194–204. doi: 10.1002/path.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Yang Y, Liu X, Herman JG, Brock MV, Licchesi JD, Yue W, Pei X, Guo M. Epigenetic regulation of the Wnt signaling inhibitor DACT2 in human hepatocellular carcinoma. Epigenetics. 2013;8:373–382. doi: 10.4161/epi.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shih IM, Yu J, He TC, Vogelstein B, Kinzler KW. The beta-catenin binding domain of adenomatous polyposis coli is sufficient for tumor suppression. Cancer Res. 2000;60:1671–1676. [PubMed] [Google Scholar]
- 22.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 23.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 24.Chen P, Chu YL, Song TS, Lei L, Wang JS, Wang K, Ni L, Niu YY. [Inheritance instability of chromosome 6q in patients with gastric cancer] . Sichuan Da Xue Xue Bao Yi Xue Ban. 2006;37:852–855. [PubMed] [Google Scholar]
- 25.Jia Y, Yang Y, Zhan Q, Brock MV, Zheng X, Yu Y, Herman JG, Guo M. Inhibition of SOX17 by microRNA 141 and methylation activates the WNT signaling pathway in esophageal cancer. J Mol Diagn. 2012;14:577–585. doi: 10.1016/j.jmoldx.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Jia Y, Yang Y, Brock MV, Cao B, Zhan Q, Li Y, Yu Y, Herman JG, Guo M. Methylation of TFPI-2 is an early event of esophageal carcinogenesis. Epigenomics. 2012;4:135–146. doi: 10.2217/epi.12.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolligs FT, Bommer G, ouml , ke B. Wnt/Beta-Catenin/Tcf Signaling: A Critical Pathway in Gastrointestinal Tumorigenesis. Digestion. 2002;66:131–144. doi: 10.1159/000066755. [DOI] [PubMed] [Google Scholar]
- 28.Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24:2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher DA, Kivimae S, Hoshino J, Suriben R, Martin PM, Baxter N, Cheyette BN. Three Dact gene family members are expressed during embryonic development and in the adult brains of mice. Dev Dyn. 2006;235:2620–2630. doi: 10.1002/dvdy.20917. [DOI] [PubMed] [Google Scholar]
- 30.Waxman JS. Zebrafish Dapper1 and Dapper2 play distinct roles in Wnt-mediated developmental processes. Development. 2004;131:5909–5921. doi: 10.1242/dev.01520. [DOI] [PubMed] [Google Scholar]
- 31.Gillhouse M, Wagner Nyholm M, Hikasa H, Sokol SY, Grinblat Y. Two Frodo/Dapper homologs are expressed in the developing brain and mesoderm of zebrafish. Dev Dyn. 2004;230:403–409. doi: 10.1002/dvdy.20060. [DOI] [PubMed] [Google Scholar]
- 32.Su Y, Zhang L, Gao X, Meng F, Wen J, Zhou H, Meng A, Chen YG. The evolutionally conserved activity of Dapper2 in antagonizing TGF-beta signaling. FASEB J. 2007;21:682–690. doi: 10.1096/fj.06-6246com. [DOI] [PubMed] [Google Scholar]
- 33.Kopp CW, Siegel JB, Hancock WW, Anrather J, Winkler H, Geczy CL, Kaczmarek E, Bach FH, Robson SC. Effect of porcine endothelial tissue factor pathway inhibitor on human coagulation factors. Transplantation. 1997;63:749–758. doi: 10.1097/00007890-199703150-00023. [DOI] [PubMed] [Google Scholar]
- 34.Lenhard RE, Osteen RT, Gansler TS American Cancer Society. Clinical oncology. Atlanta, Ga.: American Cancer Society; 2001. [Google Scholar]