Abstract
Dysregulation of micro-RNAs has been shown to contribute to multiple tumorigenic processes, as well as to correlate with tumor progression and prognosis. miR-199a has been shown to be dysregulated in many different tumor types; however, the association between miR-199a and the clinicopathological features of osteosarcoma is unknown, and the target gene for miR-199a and the regulatory mechanism are also unknown. In this study, we demonstrated that miR-199a-3p is expressed at low levels in osteosarcoma cells, which may inhibit the migration and invasion of these tumor cells. The downregulation of miR-199a-3p expression is significantly correlated with the recurrence and lung metastasis of patients with osteosarcoma. Using multivariate Cox regression analysis, low level of expression of miR-199a-3p was shown to be an independent predictor for worse prognosis in osteosarcoma. Furthermore, we showed that miR-199a-3p mimics can decrease the expression of the mRNA and protein of the receptor tyrosine kinase AXL, and miR-199a-3p targets directly the 3’-UTR of AXL mRNA, suggesting that miR-199a-3p may downregulate the expression of the AXL gene to inhibit the progression of osteosarcoma. In patients’ osteosarcoma samples, we also showed a statistically significant inverse relation between the levels of miR-199a-3p and AXL, which is consistent with the results in osteosarcoma cell lines. Interestingly, miR-199a-3p mimics reduced the level of phosphorylation of AKT. Together with the previous data, we conclude that miR-199a-3p negatively contributes to the progression of osteosarcoma by downregulating the expression of AXL mRNA and protein. By this mechanism, a regulatory pathway comprised of miR-199a-3p and AXL may exist in osteosarcoma cells, which may as a result regulate the progression of osteosarcoma through the AKT pathway.
Keywords: Osteosarcoma, miR-199a-3p, AXL, migration, invasion, prognosis
Introduction
Osteosarcoma is the most common malignant bone tumor in children and adolescents, associated with both bone destruction and neoplastic bone formation. It has a high propensity for early metastasis; more than 30% patients with localized disease eventually develop distant metastasis after extensive chemotherapy and surgical treatment, and the 3-year survival after pulmonary metastasectomy is approximately 30% [1]. The molecular mechanism of the progression of osteosarcoma is still unknown; it is essential to understand the mechanism to provide the rationale for targeted therapies to be performed.
Micro-RNAs are ~20 nucleotides in length, endogenous, single-stranded, small non-coding RNAs that regulate gene expression by either partial or complete hybridization with the 3’-untranslated region (3’-UTR) of the target gene, resulting in either post-transcriptional gene silencing, mRNA degradation or translational repression of the target gene. Growing evidence suggests that aberrant expression of micro-RNAs play a pivotal role in tumor initiation and progression, acting as a promoter or suppressor depending on whether they target oncogenes or suppressor genes [2].
To date, many micro-RNAs have been shown to be associated with tumorigenesis and the development of osteosarcoma. Some are downregulated in osteosarcoma tissues and could inhibit cell development, proliferation, apoptosis, cell cycle, migration, invasion and metastasis by different target, such as miR-34b, miR-145, miR-335, and many others [3-7]. Some micro-RNAs produce the opposite effect by promoting the progression of osteosarcoma, for example, miR-128, miR-214, miR-181a, miR-199b-5p, etc [8-11]. In addition, the function of micro-RNAs varies in different tumors. miR-199a is one of the micro-RNAs participating in the regulation of different tumors. It was found to be downregulated in hepatocellular carcinoma [12], gastric cancer [13], bladder cancer [14], ovarian cancer [15], breast cancer, lung cancer and colorectal cancer [16], whereas it was up-regulated in acute myeloid leukemia [17], etc. Little is known about the expression and function of miR-199a in osteosarcoma. Thus far, only one report indicates that miR-199a-3p was down-regulated and could inhibit the proliferation and migration in osteosarcoma cells [18]. However, the precise role of miR-199a in osteosarcoma cells and the underlying mechanism by which miR-199a regulates the invasion and metastasis of osteosarcoma are still largely unknown.
Using the online miRBase target database research tool (http://www.mirbase.org) [19,20], we have identified that the receptor tyrosine kinase (RTK) AXL may be one of the putative target genes of miR-199a. As a member of the TAM family of RTKs, AXL is a 140-kDa protein that is activated either by the growth-arrest-specific gene 6 (Gas6) or by homophilic interactions, activating different signaling molecules. Overexpression of the AXL receptor has been identified in multiple malignancies and is observed in both primary tumors and metastatic lesions [21]. In our previous work, we have already shown that AXL is highly expressed in osteosarcoma cells, and significantly correlated with the recurrence and lung metastasis of patients with osteosarcoma. Furthermore, high expression of activated AXL was an independent predictor for worse prognosis in osteosarcoma [22]. Hence, it was very interesting and important to know the exact expression and clinicopathological significance of miR-199a in osteosarcoma, as well as the molecular interactions between miR-199a and AXL. In this study, we evaluated the role of miR-199a-3p in osteosarcoma, and attempted to identify the regulatory mechanism of miR-199a-3p on the potential target gene AXL.
Materials and methods
Human osteosarcoma cell lines in culture
The normal fibroblast line NIH3T3 was purchased from the laboratory animal center of Sun Yat-Sen University. The osteosarcoma cell lines MG63, U2OS, SaOS2, HOS and 143B were previously maintained in our laboratory. Most cell lines were grown in RPMI 1640 with 10% fetal bovine serum (HyClone, Logan, UT) and 100 U/ml penicillin/streptomycin solution (Gibco, Grand Island, NY, USA), except for the U2OS cells that were maintained in Dulbecco’s modified Eagle’s minimal essential medium (DMEM) with 12% fetal bovine serum and 100 U/ml penicillin/streptomycin solution (Gibco, USA). All the cell lines were incubated at 37°C in a humidified 5% CO2 atmosphere.
Human osteosarcoma samples and paired normal tissues
Paired osteosarcoma tissues and adjacent normal muscle tissues were collected from the archives of the 1st Affiliated Hospital of Sun Yat-Sen University. A total of 33 cases of for malin-fixed, paraffin-embedded (FFPE) biopsy specimens of primary non-metastatic osteosarcoma obtained between 1999 and 2008 were analyzed. A total of 30 cases of fresh samples of primary osteosarcoma and paired non-tumor tissues from surgical resection were frozen in liquid nitrogen and stored at -80°C for RNA extraction. This study was approved by the Institutional Research Ethics Committee and informed consent of the patients who had been diagnosed at our hospital was obtained
Transfection of miR-199a-3p mimics or AXL siRNA
When cells were harvested at log phrase, 3×103 or 2×105 cells were seeded into wells of a 96-well plate or 6-well plate, and incubated overnight. After 4 hours in medium without serum, cells were transfected using X-treme reagent (Roche, Indianapolis, IN, USA) with miR-199a-3p mimics, or small interference RNA targeting AXL (si-AXL), or negative control (RIBIO, Guangzhou, CHINA) at a final concentration as 50 nM. Transfection complexes were prepared according to the manufacturer’s instructions. After 6 hours, cells were maintained in medium with serum. Transfection efficiency was evaluated by qRT-PCR.
Total RNA extraction
For fresh tissues and cell lines, total RNA was isolated by using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Briefly, all samples were treated with Trizol followed by chloroform. The mixture was centrifuged at 14000× g for 10 min at 4°C and 700 µl 75% ethanol was added to the aqueous layer. Finally, the purified RNA was diluted with 30 µl of RNase-free water. With regard to FFPE specimens, before TRIzol reagent, 20 sections of 5 microns from each tissue were deparaffinized with xylene, washed with ethanol, and dried. The lysis buffer and proteinase K were then added to the dried sections for 5 hours or overnight.
qRT-PCR for quantification of miR-199a-3p and AXL
The relative levels of miR-199a-3p and AXL mRNAs were detected by qRT-PCR using the Step One Real-time PCR System (Applied Biosystems). For the detection of AXL and GAPDH mRNAs, reverse transcription was performed using PrimeScript RT Master Mix (TaKaRa, RR036A, JAPAN), and qRT-PCR was carried out using SYBR Premix Ex Taq II (TaKaRa, RR820A). For the detection of miR-199a-3p and the universal small nuclear RNA U6 (RNU6B), reverse transcription was performed using One Step PrimeScript miRNA cDNA Synthesis Kit (TaKaRa, RR716), and qRT-PCR was performed using SYBR Premix Ex TaqII (TaKaRa, RR716). qRT-PCR primer sequences were as follows: the specific forward primers of miR-199a-3p and RNU6B were 5’-ACAGUAGUC UGCACAUUGGUUA-3’ and 5’-ACGCAAATTCGTGAAGCGTT-3’. The specific reverse primers of miR-199a-3p and RNU6B were universal. The AXL sense primer was 5’-TCAAGGTGGCTGTGAAGACGA-3’, and antisense 5’- CGTTCAGAACCCTGGAAACA GAC-3’, the GAPDH sense primer 5’-GCACCGTCAAGGCTGAGAA C-3’, and antisense 5’-TGGTGAAGACGCCAGTGGA-3’. All primer sequences were purchased from TaKaRa Inc. The relative expression levels of miR-199a -3p and AXL mRNAs were normalized to that of the internal control RNU6B and GAPDH mRNAs using the 2-ΔΔCt method. Each sample was assayed in triplicate.
Flow cytometry for detection of AXL protein
At 48 hours post-transfection, the osteosarcoma cells (MG63, U2OS, HOS and 143B) were harvested, washed three times with cold PBS. Then, the IC fixation buffer (eBioscience, San Diego, CA, USA) was added for 15 mins. After centrifugation, the cells were permeabilized using the permeabilization buffer (PB) (eBioscience, USA) for 15 mins. The cells in suspension were then incubated with PB containing rabbit monoclonal antibodies against AXL (1:100 dilution) (Cell Signaling Technology, Beverly, MA, USA) or IgG (negative control) at room temperature. After 30 mins, the cells were washed twice with PB and incubated in sheep anti-rabbit antibodies IgG-FITC (BD Pharmingen, San Diego, CA, USA) for 30 mins at room temperature. After washing twice with PB, the labeled cells were analyzed by flow cytometry at an excitation wavelength of 488 nm (Beckman Coulter, Fullerton, CA, USA). All experiments were carried out independently at least three times.
MTT assay for the viability of osteosarcoma cell lines
The four osteosarcoma cells MG63, U2OS, HOS and 143B were transfected with miR-199a-3p mimics or AXL siRNA, for 24 h, 48 h and 72 h. They were incubated in 200 µl of 5 mg/ml solution of MTT (Sigma-Aldrich, St. Louis, Missouri, USA) at 37°C for 4 h, and then lysed in 200 µl of dimethylsulfoxide at room temperature for 15 min. The absorbance of each wells was read at 490 nm using ELISA microplate reader (TECAN, Salzburg, Austria). Each experiment was performed in triplicate.
Wound healing assays in vitro
The cell cultures (MG63 and U2OS) in 6-well plate at 90% confluence were scratched to create two parallel wounds by using a 10 µl pipette tip when the cells treated. Then, the cells were washed with PBS and incubated in serum-free medium at 37°C for 24 h and 48 h. Pictures were taken at 0, 8, 12, 24 h and 0, 12, 24, 48 h, respectively, using a Nikon Eclipse TE2000-5 microscope. Three separate experiments were performed.
Migration and invasion assays in vitro
Cell migration and invasion assays were carried out in Transwell chambers with 8 μm pore size (Corning Costar, Cambridge, MA, USA) as previously described [22]. Matrigel™ Matrix (BD Biosciences, Palo Alto, CA, USA) was diluted 1:7 using serum-free basal medium and 50 µl Matrigel Matrix dilution was added to the upper chamber of Transwell inserts. Moreover, 100 µl MG63 (2×105/ml), U2OS (4×105/ml), HOS (2×105/ml), and 143B (1×105/ml) cell suspensions from different groups were seeded in the upper chambers pre-coated and pre-uncoated with Matrigel Matrix in 24-well plates and cultured in serum-free basal medium. Medium without FBS was also added to the lower chambers. After 24 h, cells were removed using cotton swabs in the upper chambers. The inserts was washed three times with PBS, and cells that migrated to and invaded the bottom surface of the insert were fixed with 4% paraformaldehyde and stained using 1% crystal violet. The migrating and invading cells were counted under the microscope from randomly selected five fields and photomicrographs were taken (magnification ×200).
Western blot
MG63 and U2OS cells were transfected with miR-199a-3p mimics for 24 h. Total proteins was isolated, and the protein concentration was measured. Western Blotting was performed as described previously [22]. Primary antibodies against P-AKT (Ser 473) (Cell Signaling Technology, USA) were used, and antibodies against GAPDH were used as a loading control. The hybridization signals were detected using Femto Maximum Sensitivity chemiluminescent substrate (Thermo Scientific, Hudson, NH, USA).
Construction of 3’-UTR-Dual-Luciferase plasmid and reporter assays
The miR-199a-3p sequence was obtained from miR-Base (http://www.microrna.sanger.ac.uk) and the target gene was predicted using bioinformatics software including Targetscan (www.targetsan.org). A fragment of 3’-UTR of AXL (1863 bp) containing the putative miR-199a-3p binding site was amplified by polymerase chain reaction (PCR) using the primers shown in Figure 4A. The PCR product was subcloned into a pmiR-RB-REPORT™ vector immediately downstream to the luciferase gene sequence. A pmiR-RB-REPORT™ construct containing 3’-UTR of AXL with a mutant seed sequence of miR-199a-3p was also synthesized. All constructs were verified by DNA sequencing. 293T cells were plated in 96-well clusters, and cotransfected with 100 ng constructs with or without miR-199a-3p mimics (50 nM/well). The osteosarcoma cell lines 143B and MG63 were also co-transfected with miR-199a-3p mimics and AXL wt-3’-UTR (100 ng/well) by using X-treme Gene reagent (50 nM/well) (Roche, USA). Mimic control and AXL mut-3’-UTR were used as positive and negative controls, respectively. After 24 hours, Firefly and Renilla luciferase activities were measured sequentially by using the dual luciferase assay kit (Promega, Madison, Wisc, USA) following the specification on Tecan Infinite F500 (Tecan Systems, San Jose, CA, USA). The Firefly luciferase activities were normalized with those of Renilla luciferase and expressed as relative Rluc/Luc Ratio. Every experiment was performed in triplicate.
Figure 4.
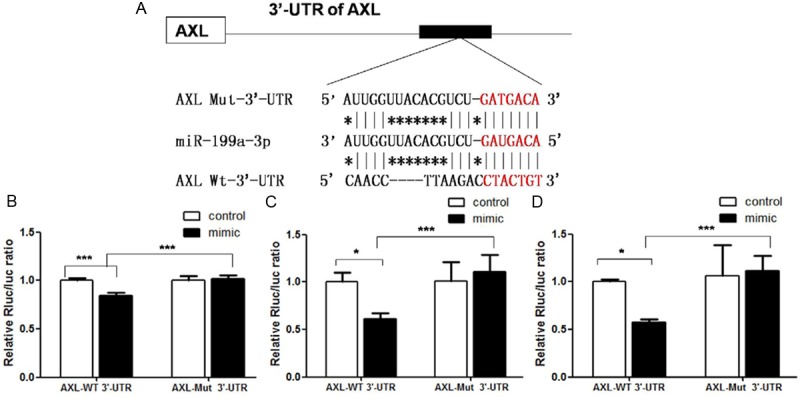
A. Sequence of the binding site of miR-199a-3p to the 3’-UTR of AXL in a luciferase reporter gene (wt-AXL) assay and its mutant variant (mut-AXL); B. The wt-AXL vector (or mut-AXL) and miR-199a-3p mimics were co-transfected into 293T cells, and the luciferase activity of miR-199a-3p mimic-transfected cells were significantly reduced compared with control cells; C. The wt-AXL vector and miR-199a-3p mimics or control osteosarcoma cell line 143B. miR-199a-3p-mediated suppression of luciferase activity was abolished by the mutant putative binding site; D. Luciferase activity in the osteosarcoma cell line MG63, the result in MG63 was as the same as in 143B (*P < 0.05, ***P < 0.001). Taken together, these data suggest that the 3’-UTR of AXL is a functional target for miR-199a-3p.
Statistical analysis
For statistical analysis of microarray data, the control and test sample groups were compared using t-tests. T-values were calculated for each miRNA, and P-values were computed from the theoretical t-distribution. miRNAs with P-values below a critical P-value (typically 0.05) were selected for cluster analysis. Disease-free survival (DFS) and overall survival (OS) was calculated and univariate and multivariate survival analyses were performed as previously described [22]. Correlations between miR-199a-3p and AXL and the clinicopathological features were evaluated by chi-square or ANOVA testing. Pearson analyses were used to test correlations between miR-199a-3p and AXL expression in osteosarcoma tissues. Statistical analyses were conducted using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA) with a 2-sided significance level of P < 0.05.
Results
miR-199a-3p is downregulated in osteosarcoma cell lines and has no effect on cells’ viability
We detected the levels of miR-199a-3p expression in osteosarcoma tissues and the cell lines MG63, U2OS, 143B, HOS, and SaOS-2. The no-rmal mesenchymal cell line NIH3T3 and paired normal muscle tissue from osteosarcoma patients were used as normal controls. The expression of miR-199a-3p in the osteosarcoma cell lines was significantly downregulated compared to the relative expression level set as 1.0 in the normal control cell line NIH3T3 (Figure 1A). To assess the effect of miR-199a-3p expression on the growth of osteosarcoma cell lines, MTT assays were performed and compared with osteosarcoma cells with and without transfected miR-199a-3p mimics. No significant change of the viability of the osteosarcoma cells was observed with or without miR-199a-3p mimics (Figure 1B).
Figure 1.
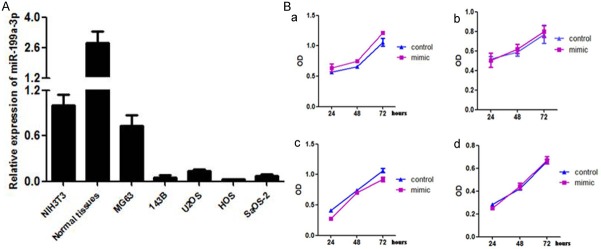
A. The relative expression of miR-199a in osteosarcoma cell lines; P < 0.01 compared to normal control tissues. These data were analyzed by one-way ANOVA. All experiments were repeated three times with triplicates each; B. miR-199a-3p mimics had no effect on the proliferation rate of osteosarcoma cell lines.
miR-199a-3p inhibit cells’ migration and invasion through downregulation of phosphorylated AKT
To assess the function of miR-199a-3p expression on osteosarcoma cells migration and invasion, wound healing assays and migration or invasion assays were performed in Transwell inserts. In all four osteosarcoma cell lines MG63, U2OS, 143B, and HOS, a significant increase of the distance in the wound healing assay was observed with transfection of miR-199a-3p mimics (Figure 2A), and clear decrease of the number of cells in migration and invasion assays (Figure 2B) (t-test, **P < 0.001,***P < 0.0001). Western Blotting showed that the expression of phosphorylated AKT decreased in cells transfected with the miR-199a-3p mimics (Figure 2C).
Figure 2.
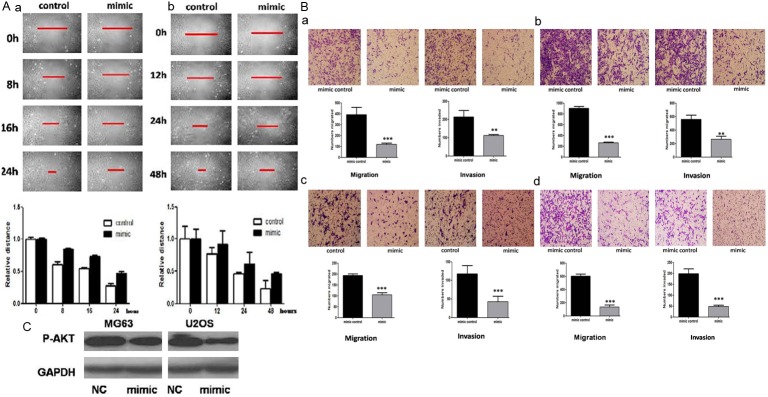
A. miR-199a-3p mimics inhibit the migration rates of MG63 (a) and U2OS cells (b) in a wound healing assay; B. In Transwell assay, miR-199a-3p mimics inhibit the migration and invasion of MG63 (a), U2OS (b), 143B (c), and HOS cells (d); C. Western Blot illustrates that transfection of miR-199a-3p mimics can decrease the expression of phosphorylated AKT.
miR-199a-3p mimics inhibit the expression of AXL mRNA and protein
To further confirm that AXL is regulated by miR-199a-3p in osteosarcoma cells, real-time RT-PCR and flow cytometry were used to detect the expression of AXL mRNA and protein in MG63 and other osteosarcoma cell lines. The expression of AXL mRNA in osteosarcoma cell lines was clearly lower than that in NIH3T3 and the normal control tissues (Figure 3A). After the transfection of miR-199a-3p mimics to these tumor cell lines, the expression of miR-199a-3p significantly increased (Figure 3B) and that of AXL mRNA decreased (Figure 3C). The expression of AXL protein was also significantly downregulated after overexpression of miR-199a-3p by mimics transfection (Figure 3D). Taken together, our results suggested that miR-199a-3p regulated AXL at the transcriptional level, leading to down-regulation of the expression of AXL protein.
Figure 3.
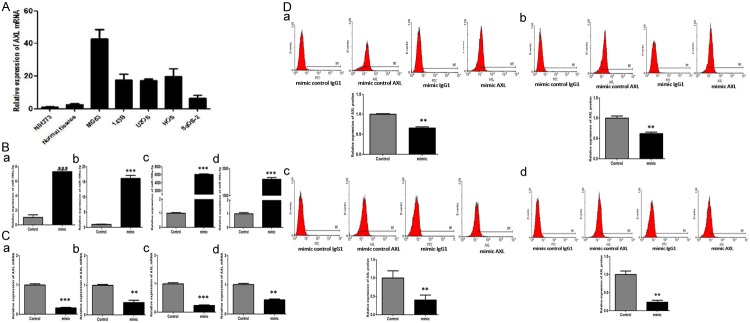
A. The relative expression of AXL mRNA in osteosarcoma cell lines are clearly increased compared to normal control tissues; P < 0.01. These data were analyzed by one-way ANOVA. All experiments were repeated three times with triplicates each; B. The expression of miR-199a-3p after transfection of miR-199a-3p mimics (a, MG63; b, U2OS; c, 143B; d, HOS); C. The expression of AXL mRNA after transfection of miR-199a-3p mimics (a, MG63; b, U2OS; c, 143B; d, HOS); D. The expression of AXL protein in a flow cytometry assay after transfection of miR-199a-3p mimics. (a, MG63; b, U2OS; c, 143B; d, HOS).
miR-199a-3p targets AXL gene in osteosarcoma cells
Complementarities of the AXL 3’-UTR with miR-199a-3p were predicted by bioinformatics software. Sequential replacement of 7 base pair region of the primers from 265 bp to 272 bp of AXL 3’-UTR was performed to produce the mutant vector (Figure 4A) and the amplified product measured up to 1863 bp. To further investigate whether the predicted binding site of miR-199a-3p to the 3’-UTR of AXL was responsible for this regulation, we cloned the 3’-UTR of AXL downstream of a luciferase reporter gene (wt-AXL); its mutant variant (mut-AXL) was also constructed by the binding site mutagenesis. We cotransfected the wt-AXL vector and miR-199a-3p mimics into 293T cells (Figure 4B) and the osteosarcoma cell lines 143B and MG63 (Figure 4C and 4D). The relative luciferase activities of the AXL wild-type (WT) and mutant (Mut) 3’-prime UTR regions were measured by cotransfection of control miRNA or miR-199a-3p mimics, and pmiR-RB-REPORT™ plasmid, calculated as the ratio of firefly/renilla activities in the cells and normalized to those of the control. The results were presented as the mean±SD from three independent experiments with each experiment in triplicate. Moreover, miR-199a-3p-mediated suppression of luciferase activity was reversed by the mutant putative binding site.
miR-199a-3p is downregulated in osteosarcoma and associated with lung recurrence and survival of the patients
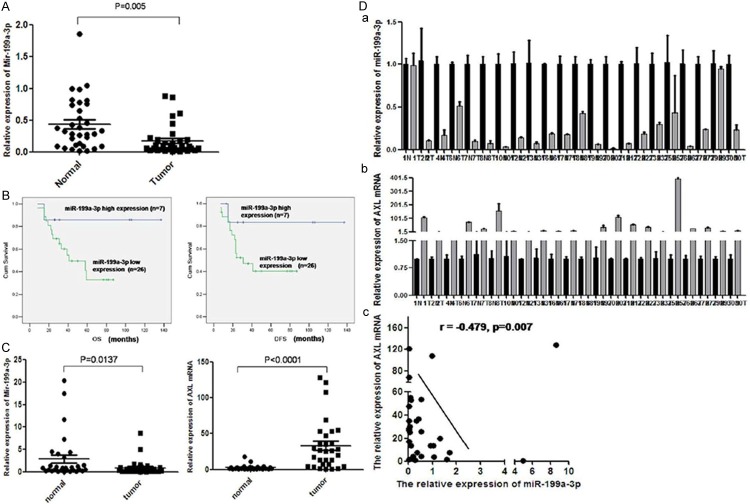
To determine the expression of miR-199a-3p in osteosarcoma tissues, we measured the levels of miR-199a-3p expression in 33 cases of paraffin-embedded osteosarcoma tissues by TaqMan real-time PCR. miR-199a-3p was expressed at low levels in 26 of 33 cases of paraffin-embedded osteosarcoma tissues. Comparing to the paired normal soft tissue of the same patient, the expression level of miR-199a-3p decreased significantly in osteosarcoma (Figure 5A). These results were consistent with the expression of miR-199a-3p in epithelial tumors as liver, bladder, and ovarian cancer [15,16]. Clinicopathologically, the recurrence or lung metastasis rate with low miR-199a-3p expression was 61.54% (16/26), comparing to 14.29% (1/7) in high group (P=0.026). The survival rate with low miR-199a-3p expression was 34.62% (9/26), comparing to 85.71% (6/7) in high group (P=0.016) (Table 1). DFS of low group was 23.73±11.35, comparing to 61.57±21.37 months in high group. Similarly, OS of low group was 30.73±13.16 months, comparing to 63.51±23.12 months in high group (Figure 5B). These data showed that miR-199a-3p expression predicted the recurrence or metastasis and survival rates of osteosarcoma patients (P < 0.05).
Figure 5.
A. The relative expression of miR-199a-3p was detected by qRT-PCR in 33 cases of paraffin-embedded osteosarcoma tissues and paired normal muscle tissues. The expression of miR-199a-3p was clearly lower in tumors than in paired normal tissues; B. In those 33 cases studied, DFS and OS in the group with low miR-199a-3p expression were significantly lower than in group with high miR-199a-3p expression (P < 0.001); C. The relative expression of miR-199a-3p and AXL mRNAs were detected in 30 cases of fresh frozen osteosarcoma tissues and paired normal tissues. Consistent with the expression level in paraffin-embedded tissues, the expression of miR-199a-3p is clearly lower in tumor than in normal tissues, while the expression of AXL showed the opposite; D. A statistically significant negative correlation was found between miR-199a-3p and AXL in those 30 osteosarcoma cases by Pearson statistical analysis, r=-0.479, P < 0.01).
Table 1.
Associations between miR-199a-3p and the clinicopathological characteristics of patients with osteosarcoma
| Clinic-pathologic characteristics | Mir-199a-3p | expression | X2 | P |
|---|---|---|---|---|
|
| ||||
| Low (n=26) | High (n=7) | |||
| Gender | 2.266 | 0.132 | ||
| Male | 19 | 3 | ||
| Female | 7 | 4 | ||
| Age | 0.024 | 0.876 | ||
| < 18 | 14 | 4 | ||
| ≥ 18 | 12 | 3 | ||
| Primary location | 0.005 | 0.943 | ||
| Humerus | 4 | 1 | ||
| Femur | 15 | 3 | ||
| Tibia | 5 | 2 | ||
| Fibula | 1 | 1 | ||
| Others | 1 | 0 | ||
| Histological type | 0.024 | 0.876 | ||
| Osteoblastic | 12 | 3 | ||
| Chondroblastic | 2 | 0 | ||
| Fibroblastic | 1 | 0 | ||
| Dilated blood vessels | 2 | 0 | ||
| Others | 9 | 4 | ||
| Tumor size | 0.698 | 0.403 | ||
| < 6 cm | 14 | 5 | ||
| ≥ 6 cm | 12 | 2 | ||
| Local recurrence/ Lung metastasis | 4.930 | 0.026 | ||
| Yes | 16 | 1 | ||
| No | 10 | 6 | ||
| Survival status | 5.808 | 0.016 | ||
| Alive | 9 | 6 | ||
| Dead | 17 | 1 | ||
miR-199a-3p is negatively associated with the expression of AXL in osteosarcoma tissues
In the same frozen fresh osteosarcoma samples, AXL mRNA levels increased contrasting to miR-199a-3p expression (Figure 5C). And there was significantly inverse correlation between them with two-tailed Pearson’s correlation analysis (r=-0.479; P=0.007) (Figure 5D). The clinicopathological analysis was consistent with the finding of the osteosarcoma cell lines. Univariate Cox regression analysis showed miR-199a-3p was one of the impactors of the patients survival (Table 2). Multivariate Cox regression analysis also demonstrated that low miR-199a-3p expression in osteosarcoma tumors was an independent factor to predict DFS (P=0.025) and OS (P=0.011) (Table 3).
Table 2.
Univariate Cox regression analysis of the association between miR-199a and the clinicopathological features of patients with osteosarcoma
| Clinic-pathologic Characteristics | DFS | OS | ||
|---|---|---|---|---|
|
| ||||
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| Gender | ||||
| (Male vs Female) | 0.448 (0.126-1.592) | 0.215 | 1.039 (0.220-4.869) | 0.444 |
| Age | ||||
| (< 18 years vs ≥ 18 years) | 0.359 (0.114-1.133) | 0.081 | 0.328 (0.103-1.039) | 0.058 |
| Tumor size | ||||
| (< 6cm vs ≥ 6cm) | 2.263 (0.803-6.374) | 0.122 | 4.362 (1.332-14.286) | 0.015 |
| Primary Location1 | 1.159 (0.638-2.108) | 0.628 | 1.162 (0.657-2.053) | 0.606 |
| Surgery type | ||||
| (Limb sparing vs Amputation) | ||||
| 1.174 (0.400-3.445) | 0.770 | 1.143 (0.386-3.385) | 0.809 | |
| Histological type2 | ||||
| 0.967 (0.741-1.262) | 0.803 | 1.006 (0.767-1.320) | 0.965 | |
| Local recurrence/ Lung metastasis | ||||
| (Yes vs No) | 3.007 (1.233-7.333) | 0.017 | 4.007 (1.513-10.612) | 0.024 |
| miR-199a-3p Expression | ||||
| (Low vs High) | 0.374 (0.121-1.154) | 0.016 | 0.740 (0.251-2.179) | 0.005 |
Primary location include: Humerus, Radius, Femur, Tibia, Fibula, Others;
Histological type include: Osteoblastic, Chondroblastic, Fibroblastic, Dilated blood vessels, Others.
Table 3.
Multivariate Cox regression analysis of the association between miR-199a and the clinicopathological features of patients with osteosarcoma
| Clinic-pathologic Characteristics) | DFS | OS | ||
|---|---|---|---|---|
|
| ||||
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| Tumor size | ||||
| (< 6 cm vs ≥ 6 cm) | 1.562 (0.479-5.095) | 0.460 | 3.185 (0.846-11.995) | 0.350 |
| Local recurrence/Lung metastasis | ||||
| (Yes vs No) | 7.535 (1.830-31.030) | 0.029 | 6.554 (1.783-24.097) | |
| miR-199a-3p Expression | 0.038 | |||
| (Low vs High) | 0.390 (0.116-1.317) | 0.025 | 0.724 (0.219-2.394) | 0.011 |
AXL siRNA transfection correlates inversely with miR-199a expression
To explore whether feedback regulation of AXL to miR-199a-3p exists, we transfected AXL siRNA to the four osteosarcoma cell lines MG63, U2OS, 143B, and HOS. Interestingly, with reduced expression of AXL mRNA, the expression of miR-199a-3p clearly increased (Figure 6A and 6B). To date, there has been no report of such observation, which may depend on methylation regulation.
Figure 6.
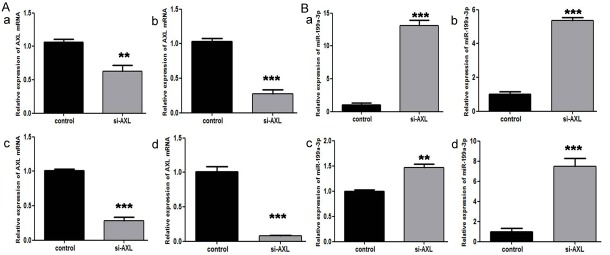
A. The expression of AXL mRNA decreased after transfection of AXL siRNA; B. The expression of miR-199a-3p increased as a result of silencing AXL expression by siRNA transfection. (a, MG63; b, U2OS; c, 143B; d, HOS).
Discussion
Many studies have demonstrated that miRNAs play an important role in tumor progression due to their involvement in tumor cell survival and growth, increased migration and angiogenesis [3-6]. miR-199a is one of the micro-RNAs dysregulated in different tumors [14-17]. However, little is known about the expression and function of miR-199a in osteosarcoma. Only limited research has shown that miRNA-199a-3p is downregulated in human osteosarcoma [18,23]. Nevertheless, the precise role of miR-199a in the progression and prognosis of osteosarcoma and the underlying mechanism regulating the invasion and metastasis of osteosarcoma are still largely unknown.
Using the online miRBase target database research tool, we have identified that the receptor tyrosine kinase (RTK) AXL may be one of the putative target genes of miR-199a. Interestingly, we have found that activated AXL is significantly correlated with the recurrence and lung metastasis of osteosarcoma, which is also an independent predictor for worse prognosis [22]. Limited research had shown the regulatory function of microRNAs on AXL: one report from the Mudduluru group [16] had shown that miR-34a and miR-199a/b suppressed Axl protein expression in a panel of non-small cell lung cancer (NSCLC), colorectal cancer (CRC) and breast cancer (BRC) cell lines. To date, no reports elucidating the exact role of miR-199a on tumor progression and the regulatory mechanisms of miR-199a on the potential target gene AXL in osteosarcoma have appeared. Therefore, it is very interesting that miR-199a regulates AXL directly in osteosarcoma. And how does the association between miR-199a and AXL affect the clinicopathological characteristics in osteosarcoma?
In this study, we demonstrated that miR-199a-3p was decreased in osteosarcoma cell lines and that miR-199a-3p overexpression by transfected mimics decreased tumor cells’ migration and invasion in vitro. Meanwhile, the overexpression of miR-199a-3p had no effect on the viability of the osteosarcoma cell lines. On the contrary, the expression of AXL mRNA in osteosarcoma cell lines increased dramatically compared with normal control cells, which was consistent with our earlier results in osteosarcoma tissues [22]. With the transfection of miR-199a-3p mimics, the expression of AXL mRNA and proteins clearly decreased in all four osteosarcoma cell lines, indicating that miR-199a-3p may downregulate the expression of AXL in osteosarcoma cells. Using the luciferase activity assay, we confirmed that the 3’-UTR of AXL is a functional target for miR-199a-3p; in other words, miR-199a-3p could regulate the progression of osteosarcoma cells through direct downregulation of the AXL gene.
In this study, we demonstrated that miR-199a-3p was significantly decreased in osteosarcoma patient samples. The level of down-expression of miR-199a-3p is statistically correlated with the recurrence of tumor, lung metastasis, and the survival status of the patients. Furthermore, we also demonstrated that the downregulation of miR-199a-3p expression in osteosarcoma is an independent factor that correlates with patient prognosis. All these studies showed that activated miR-199a-3p participates in the progression of osteosarcoma. Interestingly, in the same groups of osteosarcoma and paired normal tissues samples, the expression of miR-199a-3p was statistically inversely associated with the expression of AXL mRNA. This observation is consistent with the results from the osteosarcoma cells, and confirm the hypothesis that miR-199a-3p regulates the progression of osteosarcoma by downregulating the tyrosine kinase AXL.
In the osteosarcoma cells MG63 and U2OS, the expression of phosphorylated AKT decreased with the transfection of miR-199a-3p mimics. This finding indicates that miR-199a-3p may inhibit osteosarcoma cell invasion through the inhibition of the phosphorylation of the AKT pathway. On the basis of our previous research [22], we hypothesize that a miR-199a-3p-AXL regulatory axis may exist in osteosarcoma cells that regulate tumor invasion and metastasis through the AKT pathway. This axis may be complex and require much more work to demonstrate its existence conclusively. Further investigation is also needed to evaluate whether this pathway and miR-199a-3p may be a potential therapeutic target.
Acknowledgements
This manuscript was supported by the National Natural Science Foundation of China (NSFC) (Grant No.81302348) and the project sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Ministry of Education. The manuscript was edited by Nature Publishing Group Language Editing.
Disclosure of conflict of interest
None to declare.
References
- 1.Letourneau PA, Xiao L, Harting MT, Lally KP, Cox CS Jr, Andrassy RJ, Hayes-Jordan AA. Location of pulmonary metastasis in pediatric osteosarcoma is predictive of outcome. J Pediatr Surg. 2011;46:1333–1337. doi: 10.1016/j.jpedsurg.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian Q, Jia J, Ling S, Liu Y, Yang S, Shao Z. A causal role for circulating miR-34b in osteosarcoma. Eur J Surg Oncol. 2014;40:67–72. doi: 10.1016/j.ejso.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Tang M, Lin L, Cai H, Tang J, Zhou Z. MicroRNA-145 downregulation associates with advanced tumor progression and poor prognosis in patients suffering osteosarcoma. Onco Targets Ther. 2013;6:833–838. doi: 10.2147/OTT.S40080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Zhao W, Fu Q. miR-335 suppresses migration and invasion by targeting ROCK1 in osteosarcoma cells. Mol Cell Biochem. 2013;384:105–111. doi: 10.1007/s11010-013-1786-4. [DOI] [PubMed] [Google Scholar]
- 6.Jin J, Cai L, Liu ZM, Zhou XS. miRNA-218 inhibits osteosarcoma cell migration and invasion by down-regulating of TIAM1, MMP2 and MMP9. Asian Pac J Cancer Prev. 2013;14:3681–3684. doi: 10.7314/apjcp.2013.14.6.3681. [DOI] [PubMed] [Google Scholar]
- 7.Xu JQ, Liu P, Si MJ, Ding XY. MicroRNA-126 inhibits osteosarcoma cells proliferation by targeting Sirt1. Tumour Biol. 2013;34:3871–3877. doi: 10.1007/s13277-013-0974-x. [DOI] [PubMed] [Google Scholar]
- 8.Shen L, Chen XD, Zhang YH. MicroRNA-128 promotes proliferation in osteosarcoma cells by downregulating PTEN. Tumour Biol. 2014;35:2069–2074. doi: 10.1007/s13277-013-1274-1. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Cai H, Lin L, Tang M, Cai H. Upregulated expression of microRNA-214 is linked to tumor progression and adverse prognosis in pediatric osteosarcoma. Pediatr Blood Cancer. 2014;61:206–210. doi: 10.1002/pbc.24763. [DOI] [PubMed] [Google Scholar]
- 10.Jianwei Z, Fan L, Xiancheng L, Enzhong B, Shuai L, Can L. MicroRNA 181a improves proliferation and invasion, suppresses apoptosis of osteosarcoma cell. Tumour Biol. 2013;34:3331–3337. doi: 10.1007/s13277-013-0902-0. [DOI] [PubMed] [Google Scholar]
- 11.Won KY, Kim YW, Kim HS, Lee SK, Jung WW, Park YK. MicroRNA-199b-5p is involved in the Notch signaling pathway in osteosarcoma. Hum Pathol. 2013;44:1648–55. doi: 10.1016/j.humpath.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong Q, Qin L, Wu X, Zheng Y, Yang Y, Tian W, Zhang Q, Wang C, Zhang Q, Zhuang SM, Zheng L, Liang A, Tao W, Cao X. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell. 2011;19:232–243. doi: 10.1016/j.ccr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Fan KJ, Sun Q, Chen AZ, Shen WL, Zhao ZH, Zheng XF, Yang X. Functional screening for miRNAs targeting Smad4 identified miR-199a as a negative regulator of TGF-β signalling pathway. Nucleic Acids Res. 2012;40:9286–9297. doi: 10.1093/nar/gks667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichimi T, Enokida H, Okuno Y, Kunimoto R, Chiyomaru T, Kawamoto K, Kawahara K, Toki K, Kawakami K, Nishiyama K, Tsujimoto G, Nakagawa M, Seki N. Identification of novel microRNA targets based on microRNA signatures in bladder cancer. Int J Cancer. 2009;125:345–352. doi: 10.1002/ijc.24390. [DOI] [PubMed] [Google Scholar]
- 15.Cheng W, Liu T, Wan X, Gao Y, Wang H. MicroRNA-199a targets CD44 to suppress the tumorigenicity and multidrug resistance of ovarian cancer-initiating cells. FEBS J. 2012;279:2047–2059. doi: 10.1111/j.1742-4658.2012.08589.x. [DOI] [PubMed] [Google Scholar]
- 16.Mudduluru G, Ceppi P, Kumarswamy R, Scagliotti GV, Papotti M, Allgayer H. Regulation of Axl receptor tyrosine kinase expression by miR-34a and miR-199a/b in solid cancer. Oncogene. 2011;30:2888–2899. doi: 10.1038/onc.2011.13. [DOI] [PubMed] [Google Scholar]
- 17.Garzon R, Volinia S, Liu CG, Fernandez-Cymering C, Palumbo T, Pichiorri F, Fabbri M, Coombes K, Alder H, Nakamura T, Flomenberg N, Marcucci G, Calin GA, Kornblau SM, Kantarjian H, Bloomfield CD, Andreeff M, Croce CM. MicroRNA signatures associated with cytogenetics and prognosis in acute myeloid leukemia. Blood. 2008;111:3183–3189. doi: 10.1182/blood-2007-07-098749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan Z, Choy E, Harmon D, Liu X, Susa M, Mankin H, Hornicek F. MicroRNA-199a-3p is downregulated in human osteosarcoma and regulates cell proliferation and migration. Mol Cancer Ther. 2011;10:1337–1345. doi: 10.1158/1535-7163.MCT-11-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng W, Liu T, Jiang F, Liu C, Zhao X, Gao Y, Wang H, Liu Z. microRNA-155 regulates angiotensin II type 1 receptor expression in umbilical vein endothelial cells from severely pre-eclamptic pregnant women. Int J Mol Med. 2011;27:393–399. doi: 10.3892/ijmm.2011.598. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Liu T, Huang Y, Liu J. microRNA-182 inhibits the proliferation and invasion of human lung adenocarcinoma cells through its effect on human cortical actin-associated protein. Int J Mol Med. 2011;28:381–388. doi: 10.3892/ijmm.2011.679. [DOI] [PubMed] [Google Scholar]
- 21.Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S. Targeting Axl and Mer kinases in cancer. Mol Cancer Ther. 2011;10:1763–1773. doi: 10.1158/1535-7163.MCT-11-0116. [DOI] [PubMed] [Google Scholar]
- 22.Han J, Tian R, Yong B, Luo C, Tan P, Shen J, Peng T. Gas6/Axl mediates tumor cell apoptosis, migration and invasion and predicts the clinical outcome of osteosarcoma patients. Biochem Biophys Res Commun. 2013;435:493–500. doi: 10.1016/j.bbrc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 23.Ouyang L, Liu P, Yang S, Ye S, Xu W, Liu X. A three-plasma miRNA signature serves as novel biomarkers for osteosarcoma. Med Oncol. 2013;30:340–347. doi: 10.1007/s12032-012-0340-7. [DOI] [PubMed] [Google Scholar]