Abstract
Undifferentiated thyroid carcinoma is one of the most aggressive human cancers. Although genetic changes underlying this aggressive cancer remain to be elucidated, RAS mutations have been frequently identified in it. Mice harboring a mutant thyroid hormone receptor ThrbPV (ThrbPV/PV) spontaneously develop differentiated follicular thyroid carcinoma similar to human thyroid cancer. We recently demonstrated that targeting a RAS mutation (KrasG12D) to the thyroid of ThrbPV/PV mice (ThrbPV/PV KrasG12D mice) promotes initiation and progression of undifferentiated thyroid cancer. To uncover genes destined to drive the aggressive cancer phenotype, we used cDNA microarrays to compare the gene expression profiles of thyroid cells of KrasG12D mice and thyroid tumor lesions of ThrbPV/PV and ThrbPV/PV KrasG12D mice. Analyses of microarray data identified 14 upstream regulators that were significantly altered in thyroid tumors of ThrbPV/PV and ThrbPV/PV KrasG12D mice. Most of these genes with altered expression function as key regulators in growth factor-induced signaling. Further analysis identified gene expression profiles of markedly elevated integrin levels, acting as upstream activators to stimulate ERBB2-mediated downstream signaling in thyroid tumors of ThrbPV/PV KrasG12D mice. The present studies uncovered integrin-activated ERBB2 signaling as one of the mechanisms in synergy between TRβPV and KRASG12D signaling to promote aggressive tumor growth in undifferentiated thyroid cancer.
Keywords: Growth regulation, thyroid cancer, ERBB2, integrins, microarrays, gene expression
Introduction
Thyroid cancer is the most common malignancy of the endocrine organs. There are 4 follicular cell-derived thyroid cancers: well-differentiated papillary and follicular carcinomas, poorly differentiated carcinoma, and undifferentiated carcinoma. Although well-differentiated thyroid cancer often has a favorable outcome, the10-year survival rate of undifferentiated thyroid cancer is less than 10% owing to distant metastasis and lack of effective treatment [1].
The point mutations in RAS genes together with the mutations in TP53 and CTNNB1genes are among prevalent genetic alterations identified in undifferentiated thyroid cancer [1]. Previously we demonstrated that mice with a mutant thyroid hormone receptor ThrbPV (ThrbPV/PV) spontaneously develop well-differentiated follicular thyroid cancer with pathological progression and metastasis frequency similar to human thyroid cancer [2,3]. The PV mutation was originally identified in a patient with resistance to thyroid hormone (RTH) [4]. The PV mutant has completely lost thyroid hormone (T3) binding activity and transcription capacity. It acts to abnormally regulate the expression of the T3 target genes via dominant negative activity.
Recently, we genetically introduced the KrasG12D mutation to express specifically in the thyroids of the ThrbPV/PV mice. We found that double mutant ThrbPV/PV KrasG12D mice have much worse survival than the mice with a single mutation in either the Kras or the Thrb gene as a result of markedly aggressive thyroid tumors [5]. Capsular invasion, vascular invasion, and distant metastases to the lung occur at an earlier age and at a higher frequency than in ThrbPV/PV mice. We identified the occurrence of anaplastic foci with a high frequency in the thyroid of ThrbPV/PV KrasG12D mice [5]. These anaplastic foci have lost normal thyroid follicular morphology as well as the expression of the paired box 8 gene (Pax8). Importantly, the protein level of PAX8 is inversely correlated with MYC in the undifferentiated thyroid tumors of ThrbPV/PV KrasG12D mice. Thus, our recent study established a mouse model of undifferentiated thyroid cancer and identified MYC as a potential target for treatment [5].
Moreover, we showed that synergistic signaling of oncogenic actions of TRβPV and KrasG12D in thyroid tumors of ThrbPV/PV KrasG12D mice led to the aggressive thyroid tumor growth resulting from rapid cell proliferation [5]. However, the mechanisms underlying the increased cell proliferation remain to be elucidated. In the present studies, we used cDNA microarray analysis to compare gene expression profiles of thyroid cells of KrasG12D mice and thyroid tumors of ThrbPV/PV and ThrbPV/PV KrasG12D mice. We uncovered increased integrins-ERBB2 signaling as a novel pathway resulting from the synergistic signaling of oncogenic actions of TRβPV and KrasG12D, thereby promoting the aggressive tumor growth of undifferentiated thyroid cancer.
Materials and methods
Experimental animals
All animal experiments were performed according to the protocols approved by the Animal Care and Use Committee at the National Cancer Institute. The ThrbPV/+, KrasLSL-G12D/+, TPO-Cre (Cre) mice and the mice with four different genotypes were previously described [2,5-7]. Thyroids and other tissues were harvested from the mice and wild-type littermates for weighing, histological analysis, and biochemical studies.
Microarray analysis
Biotinylated-aRNA samples from three individual mice of each group were used in hybridization of the GeneChip Mouse Exon 1.0 ST Array (affymetrix, Santa Clara, CA) and scanned on an Affymetrix GeneChip scanner 3000. Data were collected using Affymetrix GCOS software. Data processing and analysis were done by affy, limma, xps, et al R/Bioconductor packages (http://www.bioconductor.org). Briefly, the robust multichip average (RMA) method was used for computing expression measures, the Benjamini and Hochberg method [8] was used for calculating the adjusted p values. Top differentially expressed genes were selected by the adjusted p values with minimum 1.5 fold change. The differentially expressed genes were further analyzed for enrichment of pathways and functions using the DAVID bioinformatics database [9], Ingenuity Pathway Analysis (IPA, Ingenuity Systems, Inc., Redwood City, CA) and the Gene Set Enrichment Analysis (GSEA) by the Broad Institute [10]. The GEO array data submission is in progress.
RNA extraction and real time RT-PCR validation of microarray data
Total RNA from thyroids was isolated using TRIzol (Invitrogen, Carlsbad, CA) as indicated by the protocol of the manufacturer. Selected genes from microarray data were chosen for real time RT-PCR validation. A total 50-200 ng of RNA extracted from thyroids of wild-type, KrasG12D, ThrbPV/PV, and ThrbPV/PV KrasG12D mice was used in the real-time RT-PCR. The reactions were performed with the QuantiTect SYBR RT-PCR kit (Qiagen, Germantown, MD) on an ABI 7900HT system. In each group, four to six samples with triplicates were tested on the target genes. Data were analyzed using Prism V5 (GraphPad Software, Inc., La Jolla, CA). Primers were as follows: for the endogenous control gene mouse glyceraldehyde-3-phosphate dehydrogenase (Gapdh), forward, 5’-cgtcccgtagacaaaatggt-3’; reverse, 5’-gaatttgccgtgagtggagt-3’. For Itga6, forward, 5’-CAGGTTGTGGAACAGCACAT, reverse, 5’-AAGAACAGCCAGGAGGATGA-3’. For Itgb4, forward, 5’-GAGGGGCCCTATAGCTCACT-3’; reverse, 5’-GTTGTCCACGAGCACCTTCT3’. For Itgb1, forward, 5’-TCGTGCATGTTGTGGAGACT-3’; reverse, 5’-CACAGTTGTCACGGCACTCT-3’. For Itgb3, forward, 5’-TGACATCGAGCAGGTGAAAG-3’; reverse, 5’-GAGTAGCAAGGCCAATGAGC-3’. For Itgav, forward, 5’-GATAGAGGCAAGAGCGCAAT-3’; reverse, 5’-AATGCCCCAGGTGATGTTAG-3’. For Itga5, forward, 5’-GCCAAGAGAGCCGTAGTCTG-3’; reverse, 5’-CCTTCTGCCTTGGTCCACT-3’. For Fibronectin, forward, 5’-GATCGGCAGGGAGAAAATG-3’; reverse, 5’-CAGGTCTACGGCAGTTGTCA-3’.
Western blot analysis
The Western blot analysis was carried out as described by Furumoto et al. [11]. Primary antibody for GAPDH (#2118) was purchased from Cell Signaling Technology (Danvers, MA). Primary antibodies for Integrin β1 (sc-8978), Integrin β3 (sc-14009), Integrin α5 (sc-10729), Integrin αV (sc-10719), Fibronectin (sc-6952), Integrin β4 (sc-9090), and Integrin α6 (sc-10730) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies were used at the concentration recommended by the manufacturers. For control of protein loading, the blot was probed with the antibody against GAPDH.
Statistical analysis
All data are expressed as means ± standard errors. Statistical analyses were preformed performed and p < 0.05 was considered significant unless otherwise specified. GraphPad Prism version 5.0 for Mac OS X was used to perform analysis of variances.
Results
Differential gene expression profiles in the thyroid of KrasG12D mice and thyroid tumors of ThrbPV/PV and ThrbPV/PV KrasG12D mice
In our previous studies, we demonstrated that KrasG12D, ThrbPV/PV, and ThrbPV/PV KrasG12D mice had different outcomes of tumorigenesis as the mice aged. KrasG12D mice have normal morphology up to 10 months old. During the same observation period, ThrbPV/PV mice develop follicular thyroid carcinoma. ThrbPV/PV KrasG12D mice develop aggressive undifferentiated thyroid carcinoma.
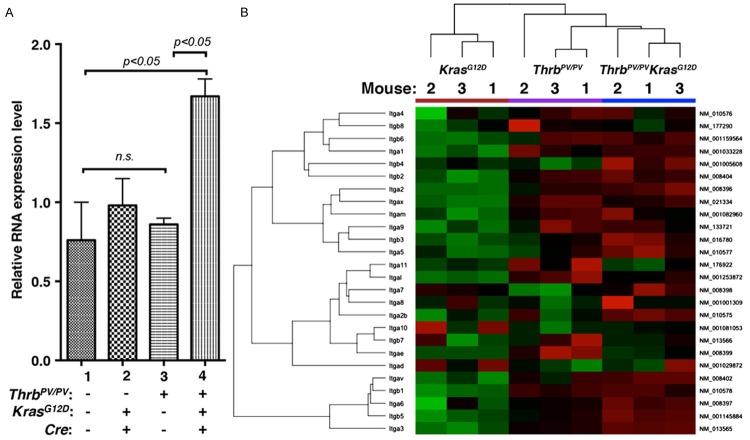
Here we obtained array data from thyroid samples of age-matched KrasG12D, ThrbPV/PV, and ThrbPV/PV KrasG12D mice (n=3 for each type of mice). Figure 1A shows principal component analysis (PCA) of the gene expression profiles from the mice with 3 different genotypes. The 3-dimensional projection of the top 3 principal components of PCA, capturing 89.5% of total variance, shows clear separation of the 3 groups.
Figure 1.
Principal component analysis (PCA) and heat-map of hierarchical clustering analysis of the top 2% of genes selected by variance in gene expression of follicular cells of KrasG12D mice and thyroid tumor cells of ThrbPV/PV and ThrbPV/PV KrasG12D mice. A. Three-dimensional projection of the top 3 principal components of PCA in the figure, which captures 89.5% of total variance, shows clear separation of the 3 mouse groups. B. A heat-map (cropped) presentation of hierarchical clustering analysis (average of Euclidean distance) of the top 2% of genes selected by variance in gene expression of the 3 groups of mice. The clustering in the figure shows the 3 groups of mice form 3 clusters, but at the top level, the KrasG12D mice remain 1 cluster and the ThrbPV/PV and ThrbPV/PV KrasG12D mice form another cluster, which indicates the ThrbPV/PV mice might closer to ThrbPV/PV KrasG12D mice in the gene expression profiling.
A hierarchical clustering analysis shows that the expression of ThrbPV/PV KrasG12D mice was closer to that of ThrbPV/PV mice than that of KrasG12D mice (Figure 1B). However, it is also clear that the gene expression profile in ThrbPV/PV KrasG12D mice was distinct from that in ThrbPV/PV mice. Comparison analysis of the array data between ThrbPV/PV KrasG12D and ThrbPV/PV mice showed that 311 genes were differentially expressed (>1.5-fold change, adjusted p < 0.1). Of those 311 genes, 150 were upregulated and 161 were downregulated. Comparison between ThrbPV/PV KrasG12D and KrasG12D mice displayed 2492 genes (>1.5-fold change, adjusted p < 0.1); among them, 1436 genes were upregulated and 1056 were downregulated. Comparison between ThrbPV/PV and KrasG12D mice identified 1952 differentially expressed genes (>1.5-fold change, adjusted p < 0.1), of which 1143 were upregulated and 809 were downregulated.
Growth factor and growth factor receptor signaling profiles of thyroid tumors in ThrbPV/PV KrasG12D mice
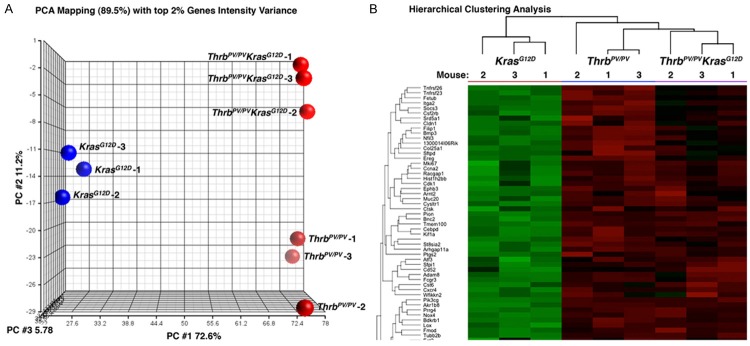
The distinct clusters of data derived from KrasG12D, ThrbPV/PV, and ThrbPV/PV KrasG12D mice enabled us to compare the changes in gene expression due to either activated TRβPV signaling (compare ThrbPV/PV KrasG12D mice with KrasG12D mice), activated KasG12D signaling (compare ThrbPV/PV KrasG12D mice with ThrbPV/PV mice) or synergistic signaling of both TRβPV and KrasG12D signaling in ThrbPV/PV KrasG12D mice. Accordingly, we used a Venn diagram to analyze the relationship of gene expression profiles among the 3 comparisons. Interestingly, only 53 genes were unique to the comparison of ThrbPV/PV vs ThrbPV/PV KrasG12D. Furthermore, 204 genes were common between the comparison of ThrbPV/PV vs ThrbPV/PV KrasG12D and KrasG12D vs ThrbPV/PV KrasG12D. We found 906 genes were specific to the comparisons of KrasG12D vs ThrbPV/PV KrasG12D and 458 were specific to the comparison of KrasG12D vs ThrbPV/PV. In addition, 1440 genes were common between comparisons of ThrbPV/PV KrasG12D vs ThrbPV/PV and ThrbPV/PV vs KrasG12D mice. Interestingly, only 58 genes were common to all in the 3 comparisons (Figure 2A).
Figure 2.
A. Differential and common expression genes among KrasG12D, ThrbPV/PV, and ThrbPV/PV KrasG12D mice. B. Top 14 upstream regulators derived from common genes by comparison of ThrbPV/PV vs ThrbPV/PV KrasG12D mice and KrasG12D vs ThrbPV/PV KrasG12D mice.
To identify the pathways specifically involved in the aggressive thyroid tumor growth of ThrbPV/PV KrasG12D mice, we examined the upstream regulators present in the differential gene expression profiles. To do this, we uploaded common differentially expressed genes between 2 comparisons—ThrbPV/PV vs ThrbPV/PV KrasG12D and KrasG12D vs ThrbPV/PV KrasG12D—into Ingenuity Pathway Analysis (IPA, http://www.ingenuity.com). The 14 top potential upstream regulators thus retrieved are listed with p-values in Table 1. These 14 upstream regulators could potentially drive differential expression of genes between ThrbPV/PVKrasG12D and ThrbPV/PV mice (Figure 2B). Among the factors analyzed, 9 of 14 genes were related signaling mediated by growth factors and their receptors (Table 1 and Figure 2B). Among these factors, five were in the category of cytokines (transforming growth factor beta 1, interleukin-1 beta, tumor necrosis factors, interferon gamma, and oncostatin M). In our previous studies, we identified transforming growth factor beta 1 (TGFβ) as a critical factor driving thyroid carcinogenesis [12]. Another 8 factors [epidermal growth factor (EGF), epidermal growth factor receptor erbB2 (ERBB2), vascular endothelial growth factor A (VEGFA), insulin receptor (INSR), basic fibroblast growth factor 2 (FGF2), hepatogrowth factor/scatter factor (HGF), epidermal growth factor receptor (EGFR) and V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS)] are related to growth factor- and growth factor receptor-promoted cell signaling.
Table 1.
Upstream regulators of the common genes
| Upstream Regulator | Molecule type | p-value of overlap |
|---|---|---|
| EGF | Growth factor | 7.48E-12 |
| TGFB1 | Growth factor | 1.90E-10 |
| ERBB2 | Kinase receptor | 2.55E-08 |
| IL1B | Cytokine | 1.73E-07 |
| VEGFA | Growth factor | 1.82E-07 |
| INSR | Kinase receptor | 4.18E-07 |
| TNF | Cytokine | 1.04E-06 |
| IFNG | Cytokine | 1.58E-06 |
| FGF2 | Growth factor | 2.72E-06 |
| HGF | Growth factor | 3.88E-06 |
| EGFR | Kinase receptor | 1.13E-05 |
| KRAS | Growth factor | 1.33E-05 |
| OSM | Cytokine | 1.39E-05 |
| ESR | Nuclear receptor | 1.75E-05 |
Because of the overwhelming enrichment of growth factors and growth factor receptors, we explored the mechanisms by which these upstream regulators could contribute to the aggressive growth of thyroid tumors in ThrbPV/PV KrasG12D mice. Among 4 upregulated growth factor receptors (ERBB2, INSR, EGFR, and estrogen receptor), 2 of them (ERBB2 and EGFR) belong to the EGFR family. EGF is a ligand for EGFR family members. As shown in Figure 2B, ERBB2 was the most upregulated growth factor receptor in ThrbPV/PV KrasG12D mice. Therefore, we further explored whether ERBB2 could play a major role in driving aggressive thyroid tumor growth in ThrbPV/PV KrasG12D mice.
Integrin-ERBB2 signaling in thyroid tumors of ThrbPV/PV KrasG12D mice
As 1 member of the EGFR family, ERBB2 is a receptor tyrosine kinase whose activity depends on dimerization with another ligand-binding ERBB receptor [13]. Binding of ligands such as EGF or neuregulins to the extracellular domain of ERBB receptors induces formation of dimerization and activation of the intrinsic receptor kinase activity, ultimately leading to stimulation of intracellular signaling cascades [14,15]. The ERBB2 signaling pathway has been shown to increase several other signaling pathways including mitogen-activated protein kinase (MAPK). Overexpression of the ERBB2 gene occurs in 15-30% of breast cancers [16]. It is strongly associated with increased breast cancer recurrence and a poor prognosis [17,18]. Overexpression is also detected in several cancers including some differentiated and undifferentiated thyroid cancer [19-21].
To confirm the role of ERBB2 in thyroid carcinogenesis, we examined whether its expression was upregulated in thyroid tumors of ThrbPV/PVKrasG12D mice (Table 2). As shown in Figure 3A, real-time quantitative mRNA analysis indicated that the mRNA level of the Erbb2 gene in the thyroid of KrasG12D mice (bar 2) and ThrbPV/PV mice (bar 3) was not significantly different from that in wild-type mice (bar 1). Strikingly, the mRNA level in the thyroid tumors of ThrbPV/PV KrasG12D mice (bar 4) was significantly higher than that in the thyroid of wild-type, KrasG12D mice, and thyroid tumors of ThrbPV/PV mice. These increases in the Erbb2 mRNA levels indicated that the Erbb2 gene might be critical for the aggressive thyroid tumor growth in ThrbPV/PVKrasG12D mice.
Table 2.
Expression of Integrins
| Symbol | Accession | Probeset_id | Total_probes | Mean | Percentile of Mean |
|---|---|---|---|---|---|
| Itga1 | NM_001033228 | 10412298 | 29 | 8.911 | 87.482 |
| Itga10 | NM_001081053 | 10494467 | 32 | 5.909 | 44.463 |
| Itga11 | NM_176922 | 10586079 | 30 | 6.289 | 50.023 |
| Itga2 | NM_008396 | 10412267 | 28 | 7.617 | 69.311 |
| Itga2b | NM_010575 | 10391697 | 34 | 6.758 | 56.42 |
| Itga3 | NM_013565 | 10390117 | 29 | 10.078 | 96.342 |
| Itga4 | NM_010576 | 10473125 | 32 | 9.349 | 91.7 |
| Itga5 | NM_010577 | 10433114 | 30 | 7.775 | 71.786 |
| Itga6 | NM_008397 | 10472820 | 25 | 10.54 | 98.019 |
| Itga7 | NM_008398 | 10367440 | 30 | 6.347 | 50.821 |
| Itga8 | NM_001001309 | 10480090 | 30 | 6.198 | 48.755 |
| Itga9 | NM_133721 | 10590031 | 28 | 7.456 | 66.802 |
| Itgad | NM_001029872 | 10557928 | 22 | 4.84 | 26.113 |
| Itgae | NM_008399 | 10378286 | 32 | 5.551 | 39.004 |
| Itgal | NM_001253872 | 10557591 | 32 | 6.267 | 49.634 |
| Itgam | NM_001082960 | 10557862 | 32 | 7.458 | 66.844 |
| Itgav | NM_008402 | 10473281 | 30 | 10.566 | 98.082 |
| Itgax | NM_021334 | 10557895 | 29 | 7.388 | 65.942 |
| Itgb1 | NM_010578 | 10576661 | 36 | 10.542 | 98.023 |
| Itgb2 | NM_008404 | 10364262 | 34 | 8.343 | 80.074 |
| Itgb3 | NM_016780 | 10381809 | 31 | 7.172 | 62.677 |
| Itgb4 | NM_001005608 | 10382713 | 42 | 8.375 | 80.49 |
| Itgb5 | NM_001145884 | 10435305 | 30 | 10.401 | 97.529 |
| Itgb6 | NM_001159564 | 10483000 | 44 | 9.102 | 89.444 |
| Itgb7 | NM_013566 | 10432957 | 28 | 5.833 | 43.315 |
| Itgb8 | NM_177290 | 10403229 | 28 | 9.188 | 90.233 |
Figure 3.
A. An increased mRNA expression of the Erbb2 gene in the thyroid of ThrbPV/PVKrasG12D mice. Total RNA was extracted from thyroids of wild-type, KrasG12D, ThrbPV/PV, and ThrbPV/PV KrasG12D mice and analyzed by quantitative real-time RT-PCR. B. A heat-map presentation of hierarchical clustering (average of Euclidean distance) analysis of the integrins gene expression in mice with the genotypes indicated.
To further validate the role of ERBB2 in thyroid carcinogenesis, we looked into other factors contributing to ERBB2 signaling. Integrins are a large family of heterodimeric transmembrane receptors that mediate cell binding to the extracellular matrix and link the extracellular environment to the intracellular cytoskeleton [22]. The different combination of 18 alpha subunits and 8 beta subunits gives rise to 24 distinct heterodimers [23]. Integrins belonging to the β1, αv, β7, and β4 subfamilies have been shown to potentiate signaling pathways in response to many growth factors [24,25], cytokines [26], and TGFβ [22,27]. Overexpression of integrin αvβ3 is associated with the progression and metastasis of several cancers including glioblastoma, carcinomas of the breast, prostate, pancreas, and lung [28]. Increased expression of αvβ6 is associated with greater invasive potential in oral squamous carcinoma, pancreatic, ovarian, breast, and lung cancer [28]. Integrin β4 forms a complex with ERBB2 and enhances activation of the transcription factors. In mice absent an integrin β4 signaling domain, both initiation and metastatic progression of mammary tumors induced by ERRB2 are significantly delayed, suggesting integrins act as cooperating oncogenes [29].
Our analyses of the gene expression profiles showed a global upregulation of integrins in ThrbPV/PV KrasG12D mice (Figure 3B). A hierarchical clustering heat map shows that the expression of integrins in ThrbPV/PV KrasG12D mice was closer to that of ThrbPV/PV mice than that of KrasG12D mice (Figure 3B). However, it was also clear that the gene expression profiles in ThrbPV/PV KrasG12D mice were distinct from those in ThrbPV/PV mice.
We next used qRT–PCR to examine 3 integrin α genes and 3 integrin β genes for the validation of the gene expression profiles obtained by array analysis. Genes selected for validation were integrin α6, integrin β4, integrin αV, integrin β1, integrin β3, and integrin α5 as shown in Figure 4A-F. Consistent with array data, qRT–PCR showed that the expression of the 6 integrins (Figure 4A-F) was significantly increased in thyroid tumors of ThrbPV/PVKrasG12D mice. The expression patterns of integrins in ThrbPV/PV mice also had an upward trend to be in line with the findings in the array analysis. But the expression of integrins in ThrbPV/PV mice was lower than in ThrbPV/PV KrasG12D mice.
Figure 4.
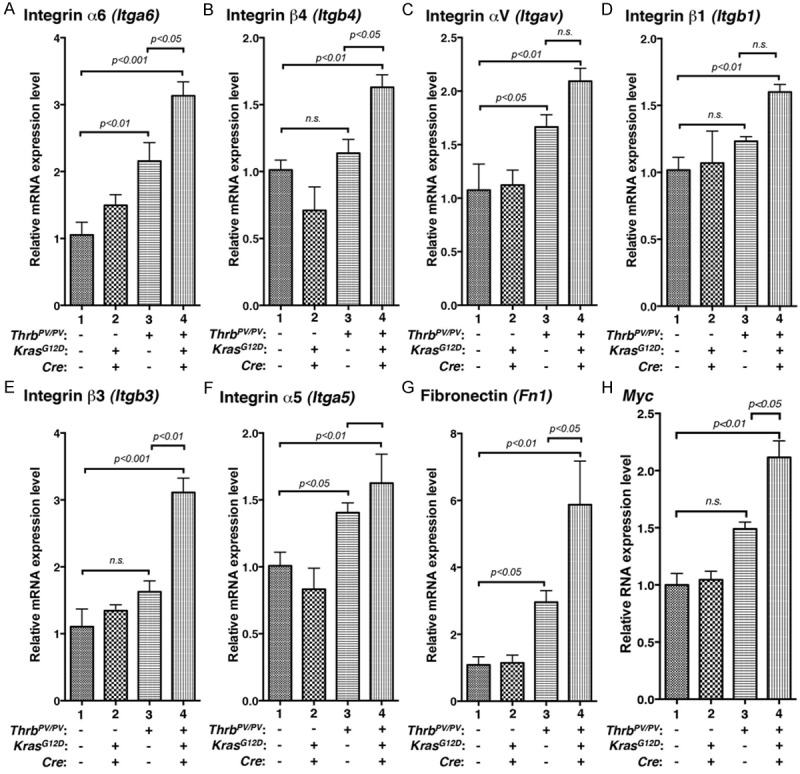
Validation of the extent in altered gene expression from arrays by quantitative real-time RT-PCR. Total RNA was extracted from thyroids of wild-type, KrasG12D, ThrbPV/PV, and ThrbPV/PV KrasG12D mice. Eight representative genes: (A) Integrin α6, (B) Integrin β4, (C) Integrin αV, (D) Integrin β1, (E) Integrin β3, (F) Integrin α5, (G) Fibronectin, and (H) Myc.
It is known that integrins bind to ligands such as fibronectin to transmit the signaling from the extracellular matrix into cytokeratin inside cells. Fibronectin has been implicated in the development of multiple types of human cancer [30,31], and it has been associated with cell migration and invasion in several metastatic models [30,32]. qRT–PCR determined that fibronectin (Figure 4G) was significantly increased with a pattern similar to integrins in ThrbPV/PVKrasG12D mice (Figure 4A-F).
In our previous studies, we found that upregulated MYC is crucial for the manifestation of aggressive tumor phenotypes [5]. We examined whether the Myc mRNA had an expression pattern similar to integrins. We found that the expression level of the Myc gene determined by qRT/PCR was significantly increased (Figure 4H). The expression changes of the Myc gene in 4 genotypes of mice were similar to that of integrins. Therefore, we identified increased Myc expression as one of the downstream effector of the integrin-ERBB2 signaling to promote tumor growth.
To confirm that ERBB2 had a crucial role in aggressive thyroid tumor growth, we further compared protein abundance of ERBB2 in thyroids of wild-type and KrasG12D mice and in thyroid tumors of ThrbPV/PV and ThrbPV/PV KrasG12D mice. As shown in Figure 5A, there was no significant difference between ERBB2 protein levels in wild-type, KrasG12D, and ThrbPV/PV mice. The level of ERBB2 protein was significantly increased only in ThrbPV/PKrasG12D mice. ERBB2 signaling has been shown to be regulated by integrins [28].
Figure 5.
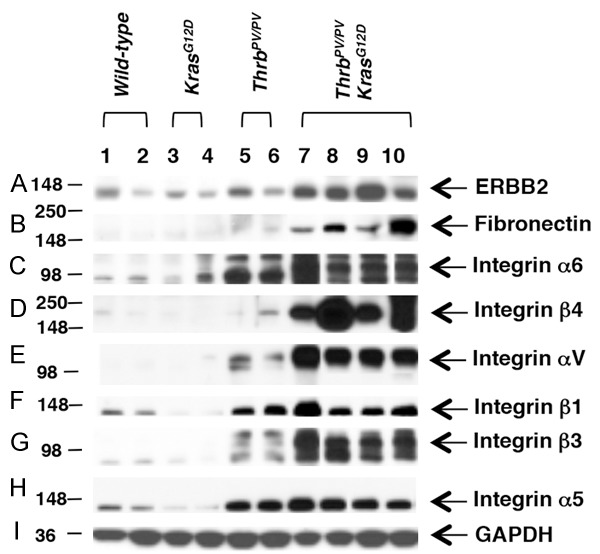
Protein abundance of integrin-ERBB2 signaling pathway in wild-type, KrasG12D, ThrbPV/PV, and ThrbPV/PV KrasG12D mice thyroids. Protein levels were measured using antibodies against (A) ERBB2, (B) Fibronectin, (C) Integrin α6, (D) Integrin β4, (E) Integrin αV, (F) Integrin β1, (G) Integrin β3, (H) Integrin α5, and (I) loading control GAPDH. Total protein extracts were prepared from thyroids of wild-type and KrasG12D mice and thyroid tumors of ThrbPV/PV and ThrbPV/PV KrasG12D mice, and the Western blot analysis was carried out as described in Materials and Methods.
Because fibronectin is one of the ligands for integrins, we also examined its protein level. We found that the abundance of fibronectin was significantly higher in thyroid tumors of ThrbPV/PV KrasG12D mice than in WT, KrasG12D, and ThrbPV/PV mice (compare lanes 7-10 to lanes 1-6, Figure 5B). Co-upregulation of ERBB2 and fibronectin supported the idea that interaction of 2 activated signaling pathways could be important for the tumor phenotypes observed in ThrbPV/PKrasG12D mice.
We also validated the protein abundance of integrins. Consistent with the highly elevated the mRNA expression (Figure 4A-F), Western blot analysis further showed that the protein abundance of integrins αV, β1, β3, and α5 was significantly elevated in thyroid tumors of ThrbPV/PV KrasG12D mice as compared with WT, KrasG12D, and ThrbPV/PV mice (lanes compare lanes 7-10 to lanes 1-6, Figure 5C-H). ERBB2 has been shown to promote the formation of a multimeric complex that includes ERBB2 and α6β4 integrins. Cooperative signaling of ERBB2 and α6β4 integrins results in the activation of downstream factors that regulate carcinogenesis [29]. As shown in Figure 5C and 5D, both integrin α6 and β4 were significantly increased in thyroid tumors of ThrbPV/PV KrasG12D mice. The enrichment of integrins/fibronectin and ERBB2 proteins indicated that their signaling is critical for the aggressive thyroid tumor growth.
Discussion
Recently, we genetically introduced the KrasG12D mutation to express specifically in the thyroids of the ThrbPV/PV mice. Double mutant ThrbPV/PVKrasG12D mice have much worse survival than the mice with a single mutation in either the Kras or Thrb gene. The more aggressive thyroid tumor phenotypes result from a synergy of the oncogenic actions of 2 mutants in thyroids, leading to increased proliferation for rapid tumor expansion. However, the molecular events leading to the synergy of 2 mutants were not well characterized. In our current studies, through microarray gene profiling, the top upstream regulators identified are main growth factors or growth factor receptors, which promoted the aggressive phenotypes of undifferentiated thyroid cancer. We found that ERBB2 was increased only in the aggressive undifferentiated thyroid tumors of ThrbPV/PVKrasG12D mice, not in ThrbPV/PV mice with differentiated tumors. We further identified markedly increased integrins, such as β1, β3, β4, αV, α5 and α6, as activators to stimulate ERBB2-mediated downstream signaling in thyroid tumors of ThrbPV/PV KrasG12D mice. The present studies have uncovered the collaborative signaling by integrins and ERBB2 as one of the mechanisms in synergy mediated by the oncogenic actions of KrasG12D and ThrbPV/PV mutants to promote the aggressive tumor growth.
The critical role of ERBB2 in cancer development and progression has been well documented. Overexpression of the ERBB2 gene occurs in 15-30% of breast cancers [16]. Increased levels of ERBB2 are strongly associated with increased breast cancer recurrence and a poor prognosis [17,18]. The expression of ERBB2 was detected in differentiated and undifferentiated thyroid cancer [19-21]. Binding of ligands, such as EGF or neuroregulins, to the extracellular domain of ERBB receptors results in the formation of receptor dimerization and activation of ERBB2. However, integrins such as β4 and α6 are known to amplify ERBB2 signaling [29,33]. Importantly, it has been demonstrated that ERBB2 makes integrins collaborators in the initiation and progression of carcinogenesis. In mice without an integrin β4 signaling domain, the initiation and metastatic progression of mammary tumors induced by ERRB2 are significantly delayed [29]. In the presence of integrins, increased ERBB2 induces the intrinsic receptor kinase activity, ultimately leading to stimulation of multiple intracellular signaling cascades including the ERK pathway [14,15].
In line with these reports, our present studies showed that there was a global upregulation of integrins, including α6 and β4, in thyroid tumors of ThrbPV/PV KrasG12D mice (Figure 3B). Abundance of ERBB2 was also markedly increased in the thyroid tumors of ThrbPV/PV KrasG12D mice (Figure 5A). Moreover, we have previously shown activated ERK signaling and elevated MYC and cyclin D1 protein abundance in thyroid tumors ThrbPV/PV KrasG12D mice [5]. Taken together, the present findings support the molecular pathways proposed in Figure 6. The multiple upstream regulators (i.e., integrins and ERBB2) are activated via synergistic oncogenic actions of TRβPV and KRASG12D, converging through ERK to increase MYC and cyclin D1 activation. These 2 critical growth signals downstream of ERK activation drive the aggressive thyroid tumor growth of ThrbPV/PV KrasG12D mice.
Figure 6.
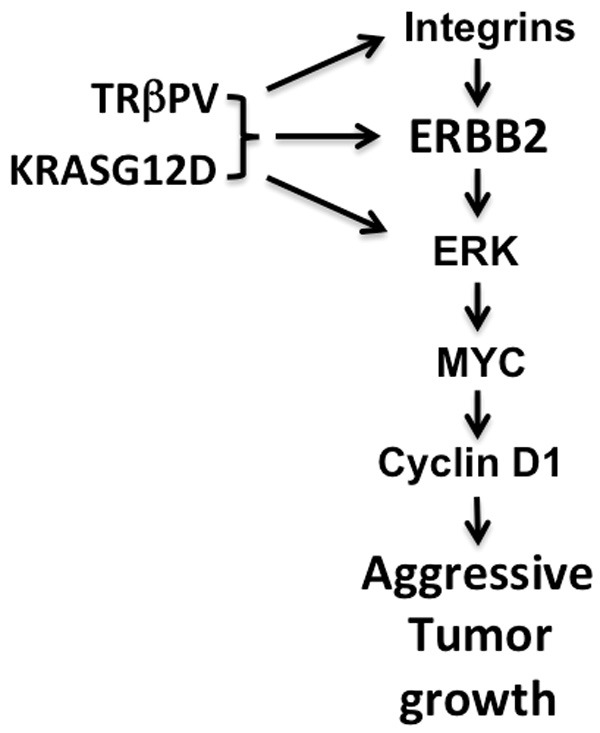
Proposed molecular pathways for integrin-ERBB2 signaling to stimulate proliferation of thyroid tumor cells in ThrbPV/PV KrasG12D mice. Synergistic oncogenic actions of TRβPV and KrasG12D activate signaling mediated by integrins, ERBB2, and ERK, leading to increased expression of MYC. Elevated MYC stimulates downstream targets such as cyclin D1 to promote aggressive tumor growth in ThrbPV/PV KrasG12D mice.
The finding uncovered in the present studies that integrins-ERBB2 signaling contributes to aggressive tumor growth of undifferentiated thyroid cancer has important clinical implications. Currently, the modalities for the treatment of undifferentiated thyroid cancer are limited. ERBB2 has been shown to express in some differentiated and undifferentiated thyroid cancers [19-21]. Clinical trials are underway using anti-ERBB2 agents at various phases in several solid tumors [34]. Moreover, drugs against integrins are being developed to treat cancers [35]. Thus, integrins and ERBB2 could be tested as potential targets for the treatment of thyroid tumors with elevated integrins and ERBB2 expression and/or with resistance to therapy [36].
The prevalent genetic alterations identified in undifferentiated thyroid cancer are point mutations of the RAS genes together with the mutations of the TP53 and CTNNB1 genes. These observations suggest that mutations of the RAS genes alone might not be sufficient to bring out the aggressive dedifferentiated thyroid cancer. In our studies, we found mice harboring the single mutation of the KrasG12D gene did not develop thyroid tumors [5]. ThrbPV/PV mice with the mutation of the 2 alleles of the Thrb gene develop differentiated thyroid cancer. However, ThrbPV/PV KrasG12D mice exhibit aggressive dedifferentiated thyroid cancer with large and rapid tumor growth [5]. The present studies show that synergistic oncogenic actions of TRβPV and KRASG12D brought out the aggressive tumor phenotypes. Thus findings from the studies using the mouse models support clinical observations that other oncogenic alterations in addition to a single mutation of the RAS gene are necessary to bring out the dedifferentiated thyroid cancer. At present, the homozygous mutations of the THRB gene have yet to be discovered in human dedifferentiated thyroid cancer. However, it is known that in ThrbPV/PV mice, altered signaling pathways such as the activation of the CTNNB1 gene have been demonstrated. Thus, it is likely that synergistic oncogenic actions of TRβPV and KRASG12D could be, at least, the result of cross talk of KRASG12D- and TRβPV-mediated activated β-catenin signaling, which is consistent with observations in human patients [37]. Thus, the ThrbPV/PV KrasG12D mouse is an excellent thyroid cancer model to further elucidate other altered signaling pathways during development of undifferentiated thyroid cancer.
Acknowledgements
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH.
Disclosure of conflict of interest
The authors declare no conflicts of interest.
References
- 1.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7:569–580. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 2.Kaneshige M, Kaneshige K, Zhu X, Dace A, Garrett L, Carter TA, Kazlauskaite R, Pankratz DG, Wynshaw-Boris A, Refetoff S, Weintraub B, Willingham MC, Barlow C, Cheng S. Mice with a targeted mutation in the thyroid hormone beta receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc Natl Acad Sci U S A. 2000;97:13209–13214. doi: 10.1073/pnas.230285997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki H, Willingham MC, Cheng SY. Mice with a mutation in the thyroid hormone receptor beta gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid. 2002;12:963–969. doi: 10.1089/105072502320908295. [DOI] [PubMed] [Google Scholar]
- 4.Parrilla R, Mixson AJ, McPherson JA, McClaskey JH, Weintraub BD. Characterization of seven novel mutations of the c-erbA beta gene in unrelated kindreds with generalized thyroid hormone resistance. Evidence for two “hot spot” regions of the ligand binding domain. J Clin Invest. 1991;88:2123–2130. doi: 10.1172/JCI115542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu X, Zhao L, Park JW, Willingham MC, Cheng SY. Synergistic signaling of KRAS and thyroid hormone receptor β mutants promotes undifferentiated thyroid cancer through MYC up-regulation. Neoplasia. 2014;16:757–69. doi: 10.1016/j.neo.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusakabe T, Kawaguchi A, Kawaguchi R, Feigenbaum L, Kimura S. Thyrocyte-specific expression of Cre recombinase in transgenic mice. Genesis. 2004;39:212–216. doi: 10.1002/gene.20043. [DOI] [PubMed] [Google Scholar]
- 8.Klipper-Aurbach Y, Wasserman M, Braunspiegel-Weintrob N, Borstein D, Peleg S, Assa S, Karp M, Benjamini Y, Hochberg Y, Laron Z. Mathematical formulae for the prediction of the residual beta cell function during the first two years of disease in children and adolescents with insulin-dependent diabetes mellitus. Med Hypotheses. 1995;45:486–490. doi: 10.1016/0306-9877(95)90228-7. [DOI] [PubMed] [Google Scholar]
- 9.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 10.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furumoto H, Ying H, Chandramouli GV, Zhao L, Walker RL, Meltzer PS, Willingham MC, Cheng SY. An unliganded thyroid hormone beta receptor activates the cyclin D1/cyclin-dependent kinase/retinoblastoma/E2F pathway and induces pituitary tumorigenesis. Mol Cell Biol. 2005;25:124–135. doi: 10.1128/MCB.25.1.124-135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DW, Walker RL, Meltzer PS, Cheng SY. Complex temporal changes in TGFβ oncogenic signaling drive thyroid carcinogenesis in a mouse model. Carcinogenesis. 2013;34:2389–2400. doi: 10.1093/carcin/bgt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100:8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 16.Burstein HJ. The distinctive nature of HER2-positive breast cancers. N Engl J Med. 2005;353:1652–1654. doi: 10.1056/NEJMp058197. [DOI] [PubMed] [Google Scholar]
- 17.Tan M, Yu D. Molecular mechanisms of erbB2-mediated breast cancer chemoresistance. Adv Exp Med Biol. 2007;608:119–129. doi: 10.1007/978-0-387-74039-3_9. [DOI] [PubMed] [Google Scholar]
- 18.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 19.Bieche I, Franc B, Vidaud D, Vidaud M, Lidereau R. Analyses of MYC, ERBB2 and CCND1 genes in benign and malignant thyroid follicular cell tumors by real-time polymerase chain reaction. Thyroid. 2001;11:147–152. doi: 10.1089/105072501300042802. [DOI] [PubMed] [Google Scholar]
- 20.Ensinger C, Prommegger R, Kendler D, Gabriel M, Spizzo G, Mikuz G, Kremser R. Her2/neu expression in poorly-differentiated and anaplastic thyroid carcinomas. Anticancer Res. 2003;23:2349–2353. [PubMed] [Google Scholar]
- 21.Kremser R, Obrist P, Spizzo G, Erler H, Kendler D, Kemmler G, Mikuz G, Ensinger C. Her2/neu overexpression in differentiated thyroid carcinomas predicts metastatic disease. Virchows Arch. 2003;442:322–328. doi: 10.1007/s00428-003-0769-3. [DOI] [PubMed] [Google Scholar]
- 22.Brizzi MF, Tarone G, Defilippi P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr Opin Cell Biol. 2012;24:645–651. doi: 10.1016/j.ceb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 24.Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- 25.Ivaska J, Heino J. Cooperation between integrins and growth factor receptors in signaling and endocytosis. Annu Rev Cell Dev Biol. 2011;27:291–320. doi: 10.1146/annurev-cellbio-092910-154017. [DOI] [PubMed] [Google Scholar]
- 26.Defilippi P, Rosso A, Dentelli P, Calvi C, Garbarino G, Tarone G, Pegoraro L, Brizzi MF. {beta}1 Integrin and IL-3R coordinately regulate STAT5 activation and anchorage-dependent proliferation. J Cell Biol. 2005;168:1099–1108. doi: 10.1083/jcb.200405116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11:97–105. doi: 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun CC, Qu XJ, Gao ZH. Integrins: players in cancer progression and targets in cancer therapy. Anticancer Drugs. 2014;25:1107–21. doi: 10.1097/CAD.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 29.Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, Giancotti FG. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126:489–502. doi: 10.1016/j.cell.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 30.Waalkes S, Atschekzei F, Kramer MW, Hennenlotter J, Vetter G, Becker JU, Stenzl A, Merseburger AS, Schrader AJ, Kuczyk MA, Serth J. Fibronectin 1 mRNA expression correlates with advanced disease in renal cancer. BMC Cancer. 2010;10:503. doi: 10.1186/1471-2407-10-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu M, Carles-Kinch KL, Zelinski DP, Kinch MS. EphA2 induction of fibronectin creates a permissive microenvironment for malignant cells. Mol Cancer Res. 2004;2:533–540. [PubMed] [Google Scholar]
- 32.Jia D, Yan M, Wang X, Hao X, Liang L, Liu L, Kong H, He X, Li J, Yao M. Development of a highly metastatic model that reveals a crucial role of fibronectin in lung cancer cell migration and invasion. BMC Cancer. 2010;10:364. doi: 10.1186/1471-2407-10-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon SO, Shin S, Lipscomb EA. A novel mechanism for integrin-mediated ras activation in breast carcinoma cells: the alpha6beta4 integrin regulates ErbB2 translation and transactivates epidermal growth factor receptor/ErbB2 signaling. Cancer Res. 2006;66:2732–2739. doi: 10.1158/0008-5472.CAN-05-2941. [DOI] [PubMed] [Google Scholar]
- 34.Tolaney S. New HER2-positive targeting agents in clinical practice. Curr Oncol Rep. 2014;16:359. doi: 10.1007/s11912-013-0359-8. [DOI] [PubMed] [Google Scholar]
- 35.Sun CC, Qu XJ, Gao ZH. Arginine-Glycine-Aspartate-Binding Integrins as Therapeutic and Diagnostic Targets. Am J Ther. 2014 doi: 10.1097/MJT.0000000000000053. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Moore KM, Thomas GJ, Duffy SW, Warwick J, Gabe R, Chou P, Ellis IO, Green AR, Haider S, Brouilette K, Saha A, Vallath S, Bowen R, Chelala C, Eccles D, Tapper WJ, Thompson AM, Quinlan P, Jordan L, Gillett C, Brentnall A, Violette S, Weinreb PH, Kendrew J, Barry ST, Hart IR, Jones JL, Marshall JF. Therapeutic targeting of integrin alphavbeta6 in breast cancer. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castellone MD, De Falco V, Rao DM, Bellelli R, Muthu M, Basolo F, Fusco A, Gutkind JS, Santoro M. The beta-catenin axis integrates multiple signals downstream from RET/papillary thyroid carcinoma leading to cell proliferation. Cancer Res. 2009;69:1867–1876. doi: 10.1158/0008-5472.CAN-08-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]