Abstract
Lysyl oxidase (LOX) is an oxidative enzyme known to initiate the cross-linking of collagens and elastin, and suggested recently as a tumor suppressor for several tumor types including lung, pancreatic and gastric cancers. Previously we showed that LOX is strongly induced upon hypoxia in nasopharyngeal carcinoma (NPC) cell lines CNE2 and HONE1 but only slightly in HK1 and not in C666-1. Here, we further studied the regulatory mechanism and functions of LOX in NPC. LOX is widely expressed in human normal tissues with variations in expression levels. LOX was expressed in most NPC cell lines except for C666-1, while HK1 and FaDu (laryngeal cancer) only expressed low level of LOX. Methylation analysis showed that the LOX promoter was methylated in C666-1 and partially methylated in HK1. After demethylation with 5-aza-2’-deoxycytidine, LOX expression was reactivated along with increased unmethylated alleles. LOX promoter methylation was detected in 42/49 (85.7%) of NPC primary tumors but only 3/16 (18.75%) of nose swab samples from NPC patients. LOX overexpression reduced the clonogenicity and cell growth of NPC cells, and also inhibited the migration and invasion of the NPC cells. Carbonic anhydrase IX (CA9) mRNA level was obviously decreased in HK1 cells after transfection with LOX. The elevation of CA9 protein upon hypoxia was inhibited in LOX-transfected HK1 cells. The protein levels of an apoptosis marker cPARP were increased in LOX-transfected HK1 cells upon hypoxia treatment. Our data showed that silencing or down-regulation of LOX in NPC was due to its promoter methylation and LOX acts as a tumor suppressor in NPC. LOX silencing would facilitate NPC cells to escape from hypoxia-induced apoptosis and maintains a malignant and metastatic phenotype.
Keywords: Lysyl oxidase, nasopharyngeal carcinoma, epigenetics, growth inhibition, apoptosis, metastasis, hypoxia, carbonic anhydrase IX
Introduction
Nasopharyngeal carcinoma (NPC) is prevalent in Southern Asia and Southern China including Hong Kong, ranked as the sixth common cancers in Hong Kong in men with an incident rate of 19.1 cases /100,000/year in 2011 [Hong Kong Cancer Registry. http://www3.ha.org.hk/cancereg/Summary%20of%20CanStat%202011.pdf]. Major etiological factors for NPC include genetic susceptibility, early-age exposure to chemical carcinogens and latent EBV infection [1]. Methylation of promoter CpG Islands is closely associated with gene silencing [2,3]. Methylation of promoter regions of tumor suppressor genes (TSGs) has been found in multiple types of cancers [4]. Identification of epigenetic alternations including promoter methylation may have potential diagnostic and prognostic value in cancer patients [5,6] and may also help to predict drug response in cancer patients [7,8].
Tumor hypoxia frequently occurs in solid tumor and is associated with a more malignant phenotype, including increased invasiveness, metastases, and poor survival. Cancer cells adapt to hypoxic condition within tumors and show resistance to hypoxia-mediated apoptosis, while underlying mechanisms remain unclear. This adaptation may act through inactivation of several important hypoxia-inducible genes by genetic and epigenetic alterations including promoter methylation, thus resulting in an escape from the hypoxia-induced cell death.
Lysyl oxidase (LOX) as an extracellular copper-containing enzyme catalyzes the crosslinking of collagens and elastin, and plays a critical role in extracellular matrix organization. Previous studies on LOX were focused on its role in connective tissue disorders and fibrotic disorders such as atherosclerosis and liver fibrosis. Increasing evidence suggests that LOX is also involved in tumor progression and metastasis through the deregulation of tumor hypoxia and epithelial-mesenchymal transition (EMT), while its role is still contradictory in tumorigenesis. Both up-regulation and down-regulation of LOX has been reported in different types of cancer cell lines and primary tumors. Of note, LOX has been suggested as a TSG in various cancers including lung, pancreatic and gastric cancers [9,10]. Inactivation of LOX by promoter methylation and loss of heterozygosity has been found in gastric cancer [11]. However, the expression and function of LOX in NPC has not been elucidated.
In this study, we investigated the expression of LOX in NPC cell lines, and further examined its promoter methylation in NPC cell lines and primary tumors. We also unveiled its tumor suppressor function in the context of hypoxia in NPC.
Materials and methods
Cell lines and tumor samples
NPC cell lines (C666-1, CNE1, CNE2, HNE1, HONE1 and HK1), laryngeal cancer cell line (FaDu) and immortalized normal nasopharyngeal epithelial cell line (NP69) were studied. NPC cell lines were routinely maintained in RMPI1640 medium and incubated at 37°C in a humid atmosphere of 5% carbon dioxide in air [12]. Hypoxia was created by culturing cells in a hypoxia chamber (Galaxy R CO2 incubator, RS Biotech Laboratory Equipment Ltd., Ayrshire, Scotland) containing 0.1% O2, 5% CO2 and 94.9% N2 [13]. For treatment combining 5-aza-2’-deoxycytidine (Aza) (Sigma, St. Louis, MO) and trichostatin A (TSA) (Cayman Chemical Co., Ann Arbor, MI), cells were treated with Aza (10 µmol/L) for 3 days and subsequently with TSA (100 ng/ mL) for 24 hours [14,15]. All culture medium and reagents were purchased from GIBCO BRL (GIBCO BRL, Grand Island, NY, USA). Tumor biopsies were taken from treatment naïve NPC patients. All patients have been consented previously for tissue collection for research at the Tumor Bank, Department of Clinical Oncology, the Chinese University of Hong Kong, according to the approved Ethics Approval of Research Protocol.
RNA isolation and reverse transcription (RT)-PCR
Cells were lysed by TRIzol Reagent (Invitrogen, Carlsbad, CA) and total cellular RNA was extracted according to manufacturer’s instruction. RT-PCR was performed as previously described [15]. Briefly, first-strand cDNA was prepared from 1 µg total cellular RNA using random hexamers primers and MuLV reverse transcriptase (Applied Biosystems, Foster City, USA). Primers are shown in Table 1. The PCR reaction was done with AmpliTaq Gold DNA polymerase (Applied Biosystems), using GAPDH as an internal control. The PCR program utilized initial denaturation at 95°C for 10 min, followed by 30 cycles (94°C for 30 s, 55-60°C for 30 s, and 72°C for 30 s), with final extension at 72°C for 10 min. Amplified products was electrophoresed through a 2% agarose gel pre-stained with 1 µg/ml of ethidium bromide and visualized under UV light.
Table 1.
Sequences of LOX primers used in this study
| PCR | Primers | Size (bp) | Annealing temperature (°C) | Cycles |
|---|---|---|---|---|
| RT-PCR for LOX | F2: CGA CCC TTA CAA CCC CTA CA | 234 | 60 | 30 |
| R2: CTG GCC AGA CAG TTT TCC TC | ||||
| MSP for LOX promoter | M11: TTC GTT CGG GAT TGT CGC | 170 | 62 | 40 |
| M2: C CAA AAA AAC GAA CGA AAC ACG | ||||
| U11: TG TTT GTT TTT TGG GAT TGT TGT | 169 | 58 | 40 | |
| U2: CAA C CAA AAA AAC AAA CAA AAC ACA | ||||
| BGS for LOX promoter | BGS1: AGA TTA AGT TAG TGT GTT TT | 541 | 58 | 40 |
| BGS2: TCA CTC CTT TTA CCA AAT TA |
DNA isolation, bisulfite treatment and methylation analysis
Genomic DNA from NPC cell lines and biopsies were extracted by TRIzol Reagent (Invitrogen, Carlsbad, CA) according to manufacturer’s instruction. Bisulfate modification of DNA, methylation specific-PCR (MSP), and bisulfite genomic sequencing (BGS) were carried out as previously described [14,15]. Briefly, the genomic DNA was chemically modified with sodium metabisulfite. The bisulfite modified DNA was then amplified using MSP primer pairs that specifically amplify either methylated (M) or unmethylated (U) sequences of the LOX promoter. The MSP primers were tested for not amplifying any unconverted DNA. For BGS, the bisulfite modified DNA was amplified with BGS primers specific for a region of LOX promoter with 30 CpG sites. Four to six colonies were randomly chosen for analysis.
Plasmid construction
The LOX full-length cDNA was amplified using human larynx cDNA with high-fidelity enzyme. The PCR product was purified with Spin-X column and clone into pCR-BluntII-TOPO vector. The cDNA was further subcloned into pcDNA3.1 (+) vector and was sequenced using ABI Prism 3100 DNA sequencer.
Colony formation assay
Colony formation assays were carried out as described previously [14,16]. Cells were plated in a 12-well plate and transfected with 1 µg LOX plasmid or empty vector using Lipofectamine LTX Reagent (Invitrogen). Cells were trypsinized and plated in a 6-well plate 48 hours post-transfection, and selected for 2 to 3 weeks with 300 µg/ml G418. Surviving colonies (> 50 cells per colony) were counted after Gentian Blue staining. All experiments were done in triplicate wells and repeated three times. The results were shown as values of mean ± SEM. Statistical analysis was carried out with Student’s t-test and P < 0.05 was considered as statistically significant.
Cell proliferation assay
Cell proliferation assay was performed as previously described [14] with little modifications. HK1 cells were transfected with LOX plasmid or vector using Lipofectamine LTX Reagent (Invitrogen) and were then selected by culture medium containing 300 µg/ml G418 after transfection. Cells were plated at 1 × 105 per well in a 6-well plate and cells were counted at indicated time points using a hemocytometer based on trypan blue exclusion. The experiment was done in triplicate wells and repeated three times. Statistical analysis was carried out with Student’s t-test and P < 0.05 was considered as statistically significant.
Migration and invasion assays
HK1 cells were transfected with empty vector or LOX plasmid. Cell migration and invasion were evaluated in vitro using Transwell Migration Chamber with a pore size of 8 µm (SPL Life Sciences, Korea) and MATRIGEL Invasion Chamber with a pore size of 8 µm (BD Biosciences, USA) respectively as previously described [17]. Cells were seeded onto the upper transwell chamber at a density of 1 × 105 cells/chamber and maintained in serum-free medium. The lower chamber contained complete medium. Cells were incubated for 27 h at 37°C in a 5% CO2 incubator or a hypoxia chamber (0.1% O2 and 5% CO2). After incubation, non-migrated cells in the upper chamber were removed with a cotton swab. Cells migrated through the upper transwell chamber were stained with 1% Toluidine Blue O (Sigma, St. Louis, MO). Migrated cells were counted in ten random microscopic fields at 200× magnification. Triplicate experiments were performed. The migration or invasion of HK1-vector under normoxia was expressed as 100%. Statistical analysis was carried out with Student’s t-test and P < 0.05 was considered as statistically significant.
Western blotting
After treatment, cells were lysed in Western lysis buffer (1% Nonidet-P40, 150 mM NaCl, 1 mM EDTA, 10 mM sodium phosphate buffer, pH 7.2, 0.25 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) for 5 min at 4°C. Lysates were then centrifuged at 12,000 rpm, 4°C for 15 min, and supernatants were then collected for protein quantitation. Protein concentrations were measured by a RC DC Protein Assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Fifty µg of cellular proteins were separated by SDS-polyacrylamide gel. The separated proteins were electrophoretically transferred to Hybond ECL nitrocellulose membrane (Amersham Pharmacia Biotech Inc, Piscataway). Membranes were blocked with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween-20 at room temperature for 1 hour and then incubated with primary antibody overnight at 4°C with gentle shaking. After incubation with horseradish peroxide conjugated secondary antibody, the enhanced chemiluminescence detection system ECL (Amersham Biosciences, Buckinghamshire, UK) was used to visualize the appropriate bands. Carbonic anhydrase IX (CA9) antibody and cleaved PARP antibody (human specific) were purchased from Novus Biologicals (Littleton, USA) and Cell Signaling Technology (Beverly, USA) respectively. Actin (Calbiochem, Merck KGaA, Darmstadt, Germany) was used as an internal control to verify equal protein loading in each NPC cell line during experiment. Secondary antibodies were from Invitrogen, CA. The immunoblots were analyzed by densitometry (Bio-Rad Quantity One 1-D Analysis Software Version 4.5.2). The value of band intensity from HK1-vector under normoxia was expressed as 100%. Statistical analysis was carried out with Student’s t-test and P < 0.05 was considered as statistically significant.
Results
Expression of LOX in human normal tissues and NPC cells
We firstly examined the expression of LOX mRNA in human adult tissues and a panel of NPC cell lines. RT-PCR showed that LOX expression varied among different adult tissues. LOX was highly expressed in multiple human adult tissues including larynx and trachea, but lowly expressed in brain, liver, smooth muscle and bone marrow tissues (Figure 1A). We also found that LOX was downregulated or silenced in some NPC cell lines and Fadu cells, compared to the immortalized nasopharyngeal cell line NP69 and normal tissue larynx (Figure 1B). These data suggest that LOX is likely a TSG in NPC.
Figure 1.
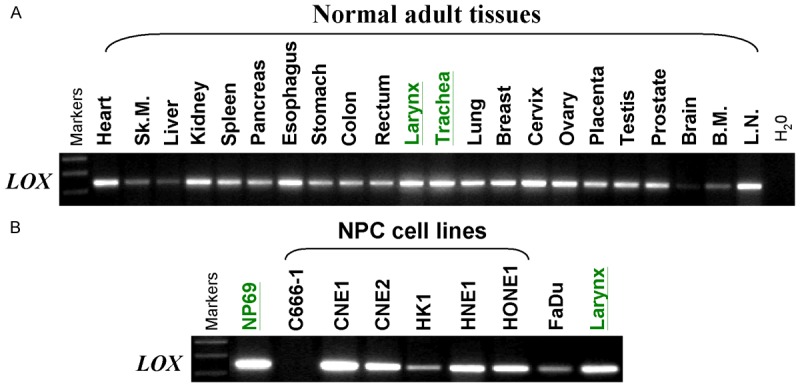
Analysis of LOX mRNA expression by semi-quantitative RT-PCR. A. Broad expression of LOX in human normal adult tissues. Sk.M., skeletal muscle; B.M., bone marrow; L.N., lymph node. B. Basal expression of LOX in NPC cell lines (C666-1, CNE1, CNE2, HK1, HNE1 and HONE1), an immortalized normal epithelial cell line (NP69), a laryngeal cancer cell line (FaDu) and normal adult larynx tissue.
LOX promoter methylation was frequently detected in NPC
One of the mechanisms to silence gene expression is DNA methylation of the promoter region of the gene, thus MSP was performed to examine the promoter methylation status of LOX in NPC cell lines. It has been found that the LOX promoter was methylated in C666-1 and HK1 cell lines (Figure 2A). The LOX promoter methylation was further investigated in 49 NPC primary tumors and 16 nose swab samples from NPC patients. Nose swab samples were non-tumor tissues taken from NPC patients. Results showed that 85.7% (42/49) of NPC primary tumors were methylated and only 18.75% (3/16) of the nose swab samples were methylated (Figure 3A, 3B). High-resolution BGS was performed in C666-1, HK1, HONE1 and NP69 (Figure 2C) and 2 of the NPC primary tumors (Figure 3C). The BGS results showed that C666-1 was heavily methylated and HK1 was partly methylated.
Figure 2.
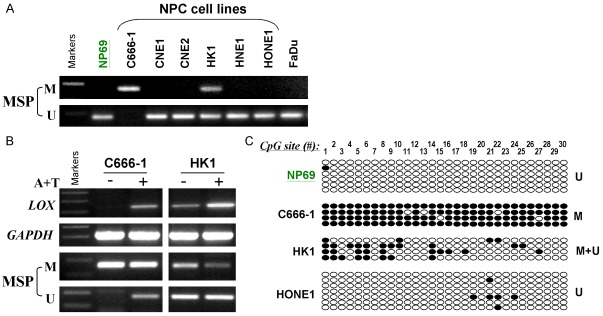
LOX promoter methylation analysis in NPC cell lines. A. Methylation–specific PCR (MSP) analysis of LOX promoter methylation status in NPC cell lines (C666-1, CNE1, CNE2, HK1, HNE1 and HONE1), NP69 and FaDu. Promoter region of LOX in C666-1 cells is methylated and in HK1 cells is partially methylated. M, methylated; U, unmethylated. B. Pharmacologic demethylation and reactivation of LOX with 5-aza-2’-deoxycytidine combined with trichostatin A (A+T) treatment in NPC cell lines. Semiquantitative RT-PCR analysis detected upregulated LOX expression in methylated and silenced NPC cell lines by A+T treatment (upper panel). MSP detected concomitant demethylation of the promoter in A+T-treated NPC cell lines (lower panel). C. High-resolution methylation analysis of the LOX promoter in an immortalized normal epithelial cell line (NP69) and NPC cell lines (C666-1, HK1 and HONE1). Each row indicates a promoter allele analyzed and each circle corresponds to a single CpG site. Dark circles (●) and empty circles (○) represent methylated and unmethylated CpG sites respectively.
Figure 3.
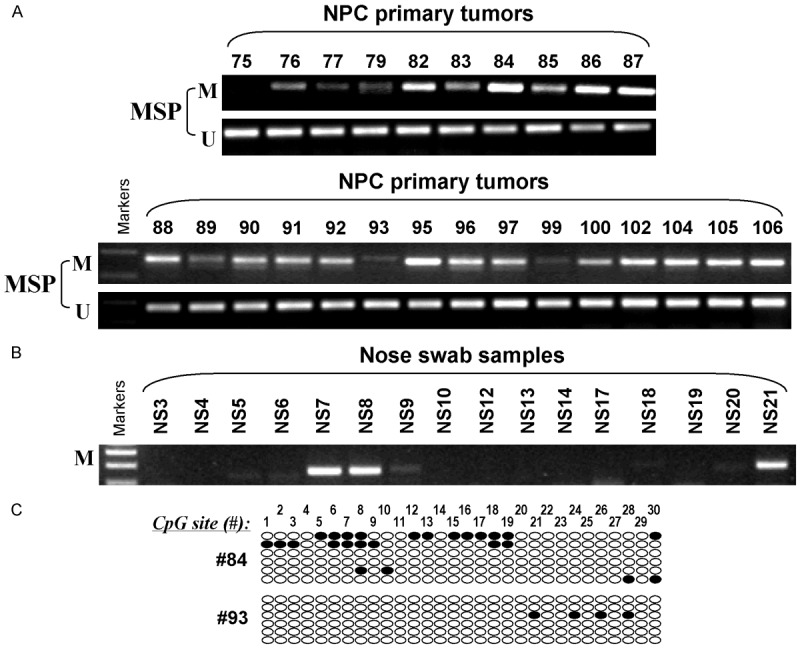
LOX promoter methylation analysis in NPC. Representative MSP results of (A) NPC primary tumors and (B) nose swab samples. (C) High-resolution methylation analysis of the LOX promoter in 2 NPC primary tumors. Each row indicates a promoter allele analyzed and each circle corresponds to a single CpG site. Dark circles (●) and empty circles (○) represent methylated and unmethylated CpG sites respectively.
LOX was silenced by methylation in NPC cells and could be activated by pharmacologic demethylation
To further confirm the silence of LOX gene was due to promoter methylation, cells were treated with 3-day Aza and 1-day TSA and then MSP and RT-PCR for LOX were performed. Results showed that the promoter of LOX gene in C666-1 was de-methylated and the LOX gene was re-expressed after the Aza/TSA treatment (Figure 2B). In HK1 cells, band intensity from the LOX MSP primers was decreased after de-methylation and the LOX mRNA level was also increased (Figure 2B).
Overexpression of LOX suppressed the growth and colony formation of NPC cells
Plasmid expressing full-length LOX cDNA (pcDNA3.1-LOX) was transfected to NPC cell lines HK1 and C666-1. HK1 cells with overexpression of LOX grew significantly lower than the empty vector-transfected cells (Figure 4A, 39.89% growth inhibition at day 7), indicating that LOX inhibited cell proliferation. The colony formation efficiency of HK1 cells and C666-1 cells were evaluated by monolayer culture with G418 selection. Overexpression of LOX in HK1 cells and C666-1 cells greatly reduced the number of colonies formed (Figure 4B, 30.3 ± 7.5% and 28.3 ± 4.9% compared to empty vector transfection respectively). This reduction of colony formation ability of the NPC cells suggesting LOX has a tumor suppressor function in these NPC cells.
Figure 4.

Overexpression of LOX suppressed growth and clonogenicity of NPC cells. A. Growth curves of HK1 cells after transfection with LOX plasmid or empty vector. HK1 cells were transfected with LOX plasmid (LOX) or empty vector (vector) and selected by culture medium containing G418. Cells were then plated at 1 × 105 per well in a 6-well plate. At day 2, 4 and 7, cell numbers were counted and plotted as values (% of cell number relative to day 0) of mean ± SEM of three independent experiments. B. Clonogenicity of NPC cells was analyzed by colony formation assays. Upper panels: HK1 or C666-1 cells were transfected with empty vector (vector) or LOX-plasmid (LOX) and selected with G418. Untransfected cells without vector or LOX plasmid would not survive G418 selection. Surviving colonies (≥ 50 cells per colony) were counted after Gentian Blue staining. Photos were taken from one of the representative experiments. Lower panels are quantitative analyses of colony numbers as values of mean ± SEM of three independent experiments. P-values were calculated using the Student’s t test. The asterisk indicates statistical significant (P < 0.05). Double asterisk indicates statistical significant (P < 0.01).
Overexpression of LOX reduced migration and invasion of NPC cells under normoxia and hypoxia condition
The effect of LOX overexpression on the invasiveness of NPC cells was examined in HK1 cells. Cell migration and invasion were evaluated in vitro using Transwell Migration Chamber and MATRIGEL Invasion Chamber respectively. The results showed that LOX overexpression exhibited potent inhibition of in vitro migration and invasion in HK1 cells especially under hypoxia condition. As shown in Figure 5A, the migration of HK1 cells was reduced by LOX overexpression by 55.72% and 69.53% under normoxia and hypoxia condition respectively, when compared with vector control. Similarly, the invasion of HK1 was reduced by LOX overexpression by 78.01% and 81.36% under normoxia and hypoxia condition respectively (Figure 5B).
Figure 5.
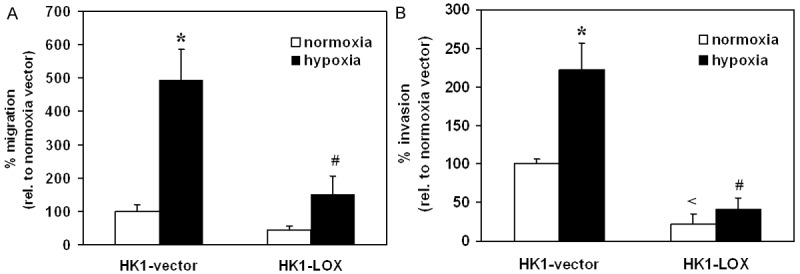
Overexpression of LOX inhibited (A) migration and (B) invasion of HK1 cells. Cells were seeded in serum-free medium in the inserts of Transwell chambers (A) or Matrigel invasion chambers (B). Medium with 10% FBS was added in the lower chambers. After 27 h, cells migrating or invading through the inserts were fixed, stained and counted (at 200× magnification). The migration or invasion of HK1-vector under normoxia was expressed as 100%. Results were depicted as means ± SEM of three independent experiments. The * indicates statistical significant when compared to same cells under normoxia condition. The # indicated statistical significant when compared to vector control during hypoxia conditions. The < indicates statistical significant when compared to vector control during normoxia conditions.
LOX overexpression affected carbonic anhydrase IX (CA9) and cPARP expression in NPC
The mRNA levels of LOX, CA9 and vascular endothelial growth factor (VEGF) were examined in vector- or LOX-transfected HK1 cells. The LOX mRNA increased in LOX-transfected cells indicating that the transfections were successful. The levels of CA9 mRNA in HK1 cells were decreased after LOX overexpression while VEGF mRNA levels were not affected (Figure 6A). CA9 is known to be upregulated by hypoxia and involved in tumor growth and progression. Here we examined the effect of hypoxia on CA9 protein levels in LOX-overexpressed HK1 cells. Upon hypoxia, the protein levels of CA9 were increased in both vector- and LOX-transfected HK1 cells but the induction was significantly lower in LOX-transfected HK1 cells (Figure 6B, 6C). The protein levels of cleaved poly (ADP-ribose) polymerase (cPARP), an apoptosis marker, were increased after hypoxia and the increase was significantly potentiated in LOX-transfected HK1 cells (Figure 6B, 6C). However, the cPARP protein levels were not significantly changed by LOX overexpression alone without hypoxia treatment.
Figure 6.
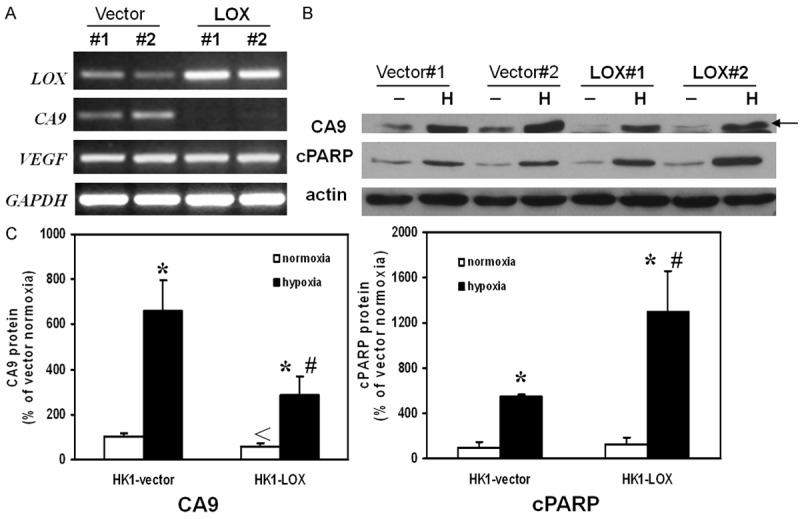
RT-PCR and Western Immunoblotting analysis. A. Expression of LOX in HK1 cells transfected with LOX plasmid was confirmed. Expression of CA9 was down-regulated in LOX plasmid transfected HK1 cells while the expression of VEGF was not altered. B. Overexpression of LOX altered protein levels of CA9 and cPARP under hypoxia treatment. Representative photos were taken from three independent experiments. C. Quantitative analysis of CA9 protein and cPARP protein by densitometry. The value of band intensity from HK1-vector under normoxia was expressed as 100%. Results were depicted as means ± SEM of three independent experiments. The * indicates statistical significant when compared to same cells under same treatment condition. The # indicated statistical significant when compared to vector control during hypoxia conditions. The < indicates statistical significant when compared to vector control during normoxia conditions.
Discussion
In the present study, we demonstrated that LOX gene was epigenetically silenced by promoter methylation in some of the NPC cell lines and large numbers of primary tumors of NPC patients. Overexpression of LOX gene in NPC cell lines could significantly reduce the clonogenicity, the growth and the invasion of the cells indicating LOX gene may be a candidate tumor suppressor gene in NPC. Furthermore, we showed that LOX gene down-regulated the expression of carbonic anhydrase IX (CA9) in which this CA9 down-regulation may contribute to the anti-tumor effects of LOX in NPC.
LOX is a hypoxia-inducible protein [18] and the LOX promoter contains a hypoxia-responsive element [19], so LOX is a transcriptional target of hypoxia-inducible factor (HIF). It is well-known that HIF-1α is the principal transcriptional factor involved in the regulation of the hypoxia-related responses in the level of transcription. Elevation of HIF-1α protein level is one of the major pathways that up-regulate the hypoxia-inducible genes upon hypoxia. Previously we have shown that upon hypoxia, all of the four NPC cell lines studied including CNE-2, HONE-1, HK1 and C666-1 demonstrated an increase in HIF-1α protein level and either VEGF or CA9 expression indicating that all these NPC cell lines have intact HIF-1-responsive machinery [13]. Upon hypoxia, the LOX mRNA levels were dramatically up-regulated in CNE2 and HONE1 cells, but only a low level of up-regulation in HK1 cells and no up-regulation in C666-1 cells were found [13]. The unresponsiveness of LOX induction in C666-1 cells is not due to the failure of HIF-1α protein function. It may be caused by epigenetic gene silencing of LOX in C666-1 cells.
Promoter methylation is one of the well-known mechanisms of gene silencing. Silencing of tumor suppressor gene by promoter methylation is one of the important factors for carcinogenesis in NPC and a number of tumor suppressor genes have been found to be methylated in NPC [1]. By the use of methylation-specific primers, the promoter of LOX gene has been shown to be methylated in C666-1 cells and HK1 cells (Figure 2A). The LOX gene is possibly silenced epigenetically by promoter methylation; therefore it cannot be up-regulated upon hypoxia in C666-1 cells. In HK1 cells, the promoter is partly methylated, so there is a weak elevation of LOX expression upon hypoxia. After pharmacological demethylation by 3-day Aza and 1-day TSA treatment, the LOX gene in C666-1 and HK1 was re-expressed, this result further confirmed that the gene silencing was due to promoter methylation (Figure 2B). After showing that the LOX gene is silenced by methylation in NPC cell lines, we elucidated the LOX methylation status in NPC patient samples. We found that promoter of LOX gene was methylated in 85.7% of primary tumors but only 18.75% in nose swab samples in NPC patients (Figure 3) suggesting that this gene is clinical relevant, not limited in NPC cell lines.
Next, the functional effects of LOX in NPC cell lines were investigated. The growth assay results showed that LOX overexpression could reduce cell growth in HK cells (Figure 4A). In addition, results from the colony formation assay suggested that overexpression of LOX in HK1 and C666-1 cells significantly reduced the clonogenicity in these cell lines (Figure 4B). Furthermore, the results from the migration assay and invasion assay demonstrated the LOX overexpression could reduce the invasiveness of the HK1 cells (Figure 5). It is probable that LOX may exert its tumor suppressor effects on NPC through inhibition of growth and metastasis.
In order to elucidate the possible mechanism of LOX-inhibited cell growth and invasion, the mRNA and protein levels were investigated in LOX-overexpressed cells. Carbonic anhydrase IX (CA9) is a hypoxia-inducible protein and has been suggested as an endogenous marker for tumor hypoxia [20,21]. CA9 expression is associated with the prognosis of multiple cancers including carcinomas of breast [22], head and neck [23], cervix [21], etc. CA9 has been suggested to be associated with tumor progression [24,25] and metastasis [26,27] and is a potential target for antitumor therapy [28,29]. CA9 is frequently overexpressed in NPC and its expression as well as that of HIF-1α is associated with a worse progression-free survival in NPC patients [30]. It has been shown that inhibition of CA9 by selective inhibitors decreases cell proliferation and induces apoptosis in human renal carcinoma cell lines and cervical cancer cell lines [31]. The exact mechanisms of the oncogenic effects of CA9 are not fully resolved. As CA9 is a transmembrane glycoprotein involved in physiologic acid/base regulation, one of the possible mechanisms is that during hypoxia CA9 contributes to a more acidic extracellular environment while helps to maintain a more alkaline intracellular pH [32,33]. These changes in extra- and intra-cellular pH may facilitate the invasive properties and growth of tumor cells. In the present study, basal mRNA levels of CA9 in LOX-overexpressed cells were decreased while those of another hypoxia-inducible gene VEGF were unchanged (Figure 6A). Since both LOX and CA9 are hypoxia-inducible genes, the changes of CA9 protein levels in LOX-overexpressed cells and empty vector-transfected cells were further investigated in hypoxia condition. Upon hypoxia treatment, CA9 protein levels were up-regulated in both LOX-overexpressed cells and empty vector-transfected cells. However, the induction was greatly inhibited in LOX-overexpressed cells (Figure 6B, 6C). In addition, an apoptosis marker, cPARP, was also investigated under hypoxia condition in these cell lines. The cPARP protein levels were greatly up-regulated in LOX-overexpressed cells upon hypoxia (Figure 6B, 6C), implying that LOX can enhance apoptosis of NPC during hypoxia condition.
LOX gene has recently been suggested as a tumor suppressor gene inactivated in various cancers including lung, pancreatic and gastric cancers [9,10]. On the other hand, Giaccia’s group has shown that LOX expression was negatively correlated with distant metastasis-free survival and overall survival in breast cancer patients with ER-negative tumors as well as in head and neck cancer patients [19]. LOX is also proposed to be oncogenic in several types of squamous cell carcinoma [34-36]. The functions of the LOX gene on tumors may depend on the tumor type and its functions on NPC remain unknown. The gene product of LOX gene is a 46 kDa preprotein. After removal of the signal peptide and glycosylation, a 50 kDa inactive proenzyme, i.e. proLOX, is produced. The proLOX is further processed to become an active LOX enzyme and a LOX propeptide (LOX-PP). LOX is well-known as its classic role as a collagen or elastin cross-linking enzyme. However, the functions of LOX-PP are largely unknown. Previously, LOX-PP has been shown to be not essential for the proper folding of the LOX protein as well as the generation of catalytic function and the secretion of the LOX protein [37]. Recently Trackman’s group has demonstrated that the tumor suppressing effect of the LOX gene is mapped to the LOX-PP [38]. They also suggested that LOX enzyme alone may promote transformed phenotype of breast cancer cells but LOX-PP can reduce the transforming ability of LOX enzyme [39]. LOX-PP has recently been shown to suppressed Akt and ERK in Her-2/neu-driven breast cancer cells [40], H1299 lung cancer cells and PANC-1 pancreatic cancer cells [10]. It seems that the effects of LOX gene on specific cell types may be affected by the balance of the LOX enzyme and LOX-PP. Although not much information on the metabolism and cellular distribution of LOX-PP in different cell types is available from literature, data from our current study has strongly suggested that LOX gene has tumor suppressive effects on NPC.
In conclusion, our study suggests that LOX is a candidate TSG epigenetically silenced in NPC. Overexpression of LOX gene in NPC can reduce clonogenicity, cell growth as well as cell migration and invasion. LOX may also exert its tumor suppressive effects during hypoxia, a common condition in solid tumors including NPC, through reducing the CA9 induction and increasing apoptosis, leading to reduction in metastasis and growth of the tumor. This study helps to improve our understanding of the tumorigenesis of NPC, which may help to identify new therapeutic targets and provide new insights on treatment.
Acknowledgements
This project was supported by Hong Kong RGC grants CUHK #4443/05M and TBRS#T12-401/13R, a CUHK direct grant and the CUHK Group Research Scheme. We thank Prof. George Tsao, (Dolly Huang), Guiyuan Li, Kaitai Yao for some cell lines.
Disclosure of conflict of interest
The authors declare no conflict of interest.
References
- 1.Tao Q, Chan AT. Nasopharyngeal carcinoma: molecular pathogenesis and therapeutic developments. Expert Rev Mol Med. 2007;9:1–24. doi: 10.1017/S1462399407000312. [DOI] [PubMed] [Google Scholar]
- 2.Singal R, Ginder GD. DNA methylation. Blood. 1999;93:4059–70. [PubMed] [Google Scholar]
- 3.Robertson KD, Wolffe AP. DNA methylation in health and disease. Nat Rev Genet. 2000;1:11–9. doi: 10.1038/35049533. [DOI] [PubMed] [Google Scholar]
- 4.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–28. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 5.Chiang JW, Karlan BY, Cass L, Baldwin RL. BRCA1 promoter methylation predicts adverse ovarian cancer prognosis. Gynecol Oncol. 2006;101:403–10. doi: 10.1016/j.ygyno.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Kikuchi S, Yamada D, Fukami T, Maruyama T, Ito A, Asamura H, Matsuno Y, Onizuka M, Murakami Y. Hypermethylation of the TSLC1/IGSF4 promoter is associated with tobacco smoking and a poor prognosis in primary nonsmall cell lung carcinoma. Cancer. 2006;106:1751–8. doi: 10.1002/cncr.21800. [DOI] [PubMed] [Google Scholar]
- 7.Widschwendter M, Siegmund KD, Muller HM, Fiegl H, Marth C, Muller-Holzner E, Jones PA, Laird PW. Association of breast cancer DNA methylation profiles with hormone receptor status and response to tamoxifen. Cancer Res. 2004;64:3807–13. doi: 10.1158/0008-5472.CAN-03-3852. [DOI] [PubMed] [Google Scholar]
- 8.Paz MF, Yaya-Tur R, Rojas-Marcos I, Reynes G, Pollan M, Aguirre-Cruz L, Garcia-Lopez JL, Piquer J, Safont MJ, Balaña C, Sanchez-Cespedes M, García-Villanueva M, Arribas L, Esteller M. CpG island hypermethylation of the DNA repair enzyme methyltransferase predicts response to temozolomide in primary gliomas. Clin Cancer Res. 2004;10:4933–8. doi: 10.1158/1078-0432.CCR-04-0392. [DOI] [PubMed] [Google Scholar]
- 9.Woznick AR, Braddock AL, Dulai M, Seymour ML, Callahan RE, Welsh RJ, Chmielewski GW, Zelenock GB, Shanley CJ. Lysyl oxidase expression in bronchogenic carcinoma. Am J Surg. 2005;189:297–301. doi: 10.1016/j.amjsurg.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 10.Wu M, Min C, Wang X, Yu Z, Kirsch KH, Trackman PC, Sonenshein GE. Repression of BCL2 by the tumor suppressor activity of the lysyl oxidase propeptide inhibits transformed phenotype of lung and pancreatic cancer cells. Cancer Res. 2007;67:6278–85. doi: 10.1158/0008-5472.CAN-07-0776. [DOI] [PubMed] [Google Scholar]
- 11.Kaneda A, Wakazono K, Tsukamoto T, Watanabe N, Yagi Y, Tatematsu M, Kaminishi M, Sugumura T, Ushijima T. Lysyl oxidase is a tumor suppressor gene inactivated by methylation and loss of heterozygosity in human gastric cancers. Cancer Res. 2004;64:6410–5. doi: 10.1158/0008-5472.CAN-04-1543. [DOI] [PubMed] [Google Scholar]
- 12.Sung FL, Poon TC, Hui EP, Ma BB, Liong E, To KF, Huang DP, Chan AT. Antitumor effect and enhancement of cytotoxic drug activity by cetuximab in nasopharyngeal carcinoma cells. In Vivo. 2005;19:237–45. [PubMed] [Google Scholar]
- 13.Sung FL, Hui EP, Tao Q, Li H, Tsui NB, Dennis Lo YM, Ma BB, To KF, Harris AL, Chan AT. Genome-wide expression analysis using microarray identified complex signaling pathways modulated by hypoxia in nasopharyngeal carcinoma. Cancer Lett. 2007;253:74–88. doi: 10.1016/j.canlet.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Ying J, Srivastava G, Hsieh WS, Gao Z, Murray P, Liao SK, Ambinder R, Tao Q. The stress-responsive gene GADD45G is a functional tumor suppressor, with its response to environmental stresses frequently disrupted epigenetically in multiple tumors. Clin Cancer Res. 2005;11:6442–9. doi: 10.1158/1078-0432.CCR-05-0267. [DOI] [PubMed] [Google Scholar]
- 15.Chan SL, Cui Y, van Hasselt A, Li H, Srivastava G, Jin H, Ng KM, Wang Y, Lee KY, Tsao GS, Zhong S, Robertson KD, Rha SY, Chan AT, Tao Q. The tumor suppressor Wnt inhibitory factor 1 is frequently methylated in nasopharyngeal and esophageal carcinomas. Lab Invest. 2007;87:644–50. doi: 10.1038/labinvest.3700547. [DOI] [PubMed] [Google Scholar]
- 16.Jin H, Wang X, Ying J, Wong AH, Cui Y, Srivastava G, Shen ZY, Li EM, Zhang Q, Jin J, Kupzig S, Chan AT, Cullen PJ, Tao Q. Epigenetic silencing of a Ca(2+)-regulated Ras GTPase-activating protein RASAL defines a new mechanism of Ras activation in human cancers. Proc Natl Acad Sci U S A. 2007;104:12353–8. doi: 10.1073/pnas.0700153104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lui VW, Yau DM, Cheung CS, Wong SC, Chan AK, Zhou Q, Wong EY, Lau CP, Lam EK, Hui EP, Hong B, Hui CW, Chan AS, Ng PK, Ng YK, Lo KW, Tsang CM, Tsui SK, Tsao SW, Chan AT. FGF8b oncogene mediates proliferation and invasion of Epstein-Barr virus-associated nasopharyngeal carcinoma cells: implication for viral-mediated FGF8b upregulation. Oncogene. 2011;30:1518–30. doi: 10.1038/onc.2010.529. [DOI] [PubMed] [Google Scholar]
- 18.Brody JS, Kagan H, Manalo A. Lung lysyl oxidase activity: relation to lung growth. Am Rev Respir Dis. 1979;120:1289–95. doi: 10.1164/arrd.1979.120.6.1289. [DOI] [PubMed] [Google Scholar]
- 19.Erler JT, Bennewith KL, Nicolau M, Dornhöfer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–6. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 20.Beasley NJ, Wykoff CC, Watson PH, Leek R, Turley H, Gatter K, Pastorek J, Cox GJ, Ratcliffe P, Harris AL. Carbonic anhydrase IX, an endogenous hypoxia marker, expression in head and neck squamous cell carcinoma and its relationship to hypoxia, necrosis, and microvessel density. Cancer Res. 2001;61:5262–7. [PubMed] [Google Scholar]
- 21.Loncaster JA, Harris AL, Davidson SE, Logue JP, Hunter RD, Wycoff CC, Pastorek J, Ratcliffe PJ, Stratford IJ, West CM. Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001;61:6394–9. [PubMed] [Google Scholar]
- 22.Beketic-Oreskovic L, Ozretic P, Rabbani ZN, Jackson IL, Sarcevic B, Levanat S, Maric P, Babic I, Vujaskovic Z. Prognostic significance of carbonic anhydrase IX (CA-IX), endoglin (CD105) and 8-hydroxy-2’-deoxyguanosine (8-OHdG) in breast cancer patients. Pathol Oncol Res. 2011;17:593–603. doi: 10.1007/s12253-010-9355-6. [DOI] [PubMed] [Google Scholar]
- 23.Peridis S, Pilgrim G, Athanasopoulos I, Parpounas K. Carbonic anhydrase-9 expression in head and neck cancer: a meta-analysis. Eur Arch Otorhinolaryngol. 2011;268:661–70. doi: 10.1007/s00405-011-1488-z. [DOI] [PubMed] [Google Scholar]
- 24.Robertson N, Potter C, Harris AL. Role of carbonic anhydrase IX in human tumor cell growth, survival, and invasion. Cancer Res. 2004;64:6160–5. doi: 10.1158/0008-5472.CAN-03-2224. [DOI] [PubMed] [Google Scholar]
- 25.Kon-no H, Ishii G, Nagai K, Yoshida J, Nishimura M, Nara M, Fujii T, Murata Y, Miyamoto H, Ochiai A. Carbonic anhydrase IX expression is associated with tumor progression and a poor prognosis of lung adenocarcinoma. Lung Cancer. 2006;54:409–18. doi: 10.1016/j.lungcan.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Svastova E, Witarski W, Csaderova L, Kosik I, Skvarkova L, Hulikova A, Zatovicova M, Barathova M, Kopacek J, Pastorek J, Pastorekova S. Carbonic anhydrase IX interacts with bicarbonate transporters in lamellipodia and Increases cell migration via Its catalytic domain. J Biol Chem. 2012;287:3392–402. doi: 10.1074/jbc.M111.286062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sansone P, Piazzi G, Paterini P, Strillacci A, Ceccarelli C, Minni F, Biasco G, Chieco P, Bonafè M. Cyclooxygenase-2/carbonic anhydrase-IX up-regulation promotes invasive potential and hypoxia survival in colorectal cancer cells. J Cell Mol Med. 2009;13:3876–87. doi: 10.1111/j.1582-4934.2008.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poulsen SA. Carbonic anhydrase inhibition as a cancer therapy: a review of patent literature, 2007 - 2009. Expert Opin Ther Pat. 2010;20:795–806. doi: 10.1517/13543776.2010.484803. [DOI] [PubMed] [Google Scholar]
- 29.Winum JY, Scozzafava A, Montero JL, Supuran CT. Inhibition of carbonic anhydrase IX: a new strategy against cancer. Anticancer Agents Med Chem. 2009;9:693–702. doi: 10.2174/187152009788680028. [DOI] [PubMed] [Google Scholar]
- 30.Hui EP, Chan AT, Pezzella F, Turley H, To KF, Poon TC, Zee B, Mo F, Teo PM, Huang DP, Gatter KC, Johnson PJ, Harris AL. Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res. 2002;8:2595–604. [PubMed] [Google Scholar]
- 31.Cianchi F, Vinci MC, Supuran CT, Peruzzi B, De Giuli P, Fasolis G, Perigli G, Pastorekova S, Papucci L, Pini A, Masini E, Puccetti L. Selective inhibition of carbonic anhydrase IX decreases cell proliferation and induces ceramide-mediated apoptosis in human cancer cells. J Pharmacol Exp Ther. 2010;334:710–9. doi: 10.1124/jpet.110.167270. [DOI] [PubMed] [Google Scholar]
- 32.Svastová E, Hulíková A, Rafajová M, Zat’ovicová M, Gibadulinová A, Casini A, Cecchi A, Scozzafava A, Supuran CT, Pastorek J, Pastoreková S. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004;577:439–45. doi: 10.1016/j.febslet.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 33.Chiche J, Ilc K, Laferrière J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouysségur J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69:358–68. doi: 10.1158/0008-5472.CAN-08-2470. [DOI] [PubMed] [Google Scholar]
- 34.Albinger-Hegyi A, Stoeckli SJ, Schmid S, Storz M, Iotzova G, Probst-Hensch NM, Rehrauer H, Tinguely M, Moch H, Hegyi I. Lysyl oxidase expression is an independent marker of prognosis and a predictor of lymph node metastasis in oral and oropharyngeal squamous cell carcinoma (OSCC) Int J Cancer. 2010;126:2653–62. doi: 10.1002/ijc.24948. [DOI] [PubMed] [Google Scholar]
- 35.Le QT, Harris J, Magliocco AM, Kong CS, Diaz R, Shin B, Cao H, Trotti A, Erler JT, Chung CH, Dicker A, Pajak TF, Giaccia AJ, Ang KK. Validation of lysyl oxidase as a prognostic marker for metastasis and survival in head and neck squamous cell carcinoma: Radiation Therapy Oncology Group trial 90-03. J. Clin. Oncol. 2009;27:4281–6. doi: 10.1200/JCO.2008.20.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakai M, Kato H, Sano A, Tanaka N, Inose T, Kimura H, Sohda M, Nakajima M, Kuwano H. Expression of lysyl oxidase is correlated with lymph node metastasis and poor prognosis in esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;16:2494–501. doi: 10.1245/s10434-009-0559-5. [DOI] [PubMed] [Google Scholar]
- 37.Kagan HM, Reddy VB, Panchenko MV, Nagan N, Boak AM, Gacheru SN, Thomas KM. Expression of lysyl oxidase from cDNA constructs in mammalian cells: the propeptide region is not essential to the folding and secretion of the functional enzyme. J Cell Biochem. 1995;59:329–38. doi: 10.1002/jcb.240590305. [DOI] [PubMed] [Google Scholar]
- 38.Palamakumbura AH, Jeay S, Guo Y, Pischon N, Sommer P, Sonenshein GE, Trackman PC. The propeptide domain of lysyl oxidase induces phenotypic reversion of ras-transformed cells. J Biol Chem. 2004;279:40593–600. doi: 10.1074/jbc.M406639200. [DOI] [PubMed] [Google Scholar]
- 39.Min C, Yu Z, Kirsch KH, Zhao Y, Vora SR, Trackman PC, Spicer DB, Rosenberg L, Palmer JR, Sonenshein GE. A loss-of-function polymorphism in the propeptide domain of the LOX gene and breast cancer. Cancer Res. 2009;69:6685–93. doi: 10.1158/0008-5472.CAN-08-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Min C, Kirsch KH, Zhao Y, Jeay S, Palamakumbura AH, Trackman PC, Sonenshein GE. The tumor suppressor activity of the lysyl oxidase propeptide reverses the invasive phenotype of Her-2/neu-driven breast cancer. Cancer Res. 2007;67:1105–12. doi: 10.1158/0008-5472.CAN-06-3867. [DOI] [PubMed] [Google Scholar]


