Abstract
Many types of cancer have high antioxidant capacity that effectively scavenges reactive oxygen species and thus protect cancer cells against oxidative damage. The aim of this study was to examine the effect of 20 single nucleotide polymorphisms (SNPs) in 20 oxidative stress-related genes on clinical outcome in 219 patients with advanced non-small cell lung cancer (NSCLC) who were treated with EGFR tyrosine kinase inhibitors (TKIs). We assessed the associations of SNPs with prognosis in all patients as well as stratified by clinical characteristics. Three SNP (rs1695, rs2333227 and rs699512) were significantly associated with overall survival (OS). In a multivariate analysis, rs1695 AA and rs2333227 AG/GG genotypes were identified as independent prognostic factors for poor OS. Stratification analyses revealed that these 3 SNPs remained significantly associated with OS. Furthermore, there was a strong gene-dosage effect of these 3 SNPs on OS that patients with increasing number of unfavorable genotypes had significantly increased death risk. In conclusion, our findings provide the first evidence that genetic variants in oxidative stress-related genes may influence treatment outcome in advanced NSCLC patients receiving EGFR TKIs.
Keywords: Tyrosine kinase inhibitor, lung cancer, glutathione S-transferases P1, myeloperoxidase, biliverdin reductase A, oxidative stress, single nucleotide polymorphism
Introduction
Lung cancer is the leading cause of cancer-related death, accounting for 19.59% of the total cases and 24.87% of the deaths in 2010 in China [1]. Approximately 85% of lung cancers are non-small cell lung cancer (NSCLC) [2]. An activating mutation of epidermal growth factor receptor (EGFR) is present in 40.9% of NSCLC [3]. These mutations are most commonly found in lung adenocarcinomas from East Asian non-smokers. Dysregulated tyrosine kinase activity of EGFR, caused by mutations, result in aberrant EGFR signaling and promote EGFR-mediated pro-survival and anti-apoptotic signals through pathways including RAS/RAF/ERK, PI3K/Akt, and STAT pathways [4]. EGFR mutations predict better response to EGFR tyrosine kinase inhibitors (TKIs) compared with standard platinum-based chemotherapy in patients with advanced NSCLC [5,6].
Reactive oxygen species (ROS) are now appreciated to act as signaling molecules involved in the regulation of various physiological processes, and therefore are essential for maintaining normal cell function. However, overproduction and cumulative production of ROS can cause damage to DNA, proteins, lipids, and other macromolecules, and induce cell death. Accordingly, oxidative stress and oxidative damage have been implicated in the pathogenesis and progression of many human diseases, including cancer [7]. Although the exact mechanism by which ROS promote tumorigenesis remains poorly understood, DNA damage appears to play a key role in cancer development. Cancer cells exhibit greater oxidative stress, which in turn promote cell proliferation, thus contributing to cancer progression [7,8]. In addition, elevated ROS levels in cancer cells lead to enhanced activation of the antioxidant defense system and the upregulation of pro-survival molecules, which promote cell survival upon oxidative stress [7,8]. Reduced intracellular ROS levels through administration of antioxidants impair cell proliferation and survival in some types of cancer, including colorectal cancer (CRC) [9], glioma [10] and lymphomas [11]. Redox pathways may be potential targets for cancer therapy.
Anticancer agents kill cancer cells partly through increased formation of ROS [12], whereas anticancer drug-resistant cancer cells exhibit higher levels of antioxidant enzymes such as glutathione peroxidase 1 (GPX1), and adapt to survive at high ROS levels [13]. For example, the relative effectiveness of paclitaxel is inversely correlated with antioxidant capacity of cancer cells [14,15]. Single nucleotide polymorphisms (SNPs) leading to changes in expression and function of antioxidant enzymes may affect the effectiveness of anticancer drugs. Recent studies found that enhanced antioxidant capacity was involved in mediating EGFR TKI-resistance [16-18]. Therefore, SNPs in antioxidant genes may be associated with survival outcome in cancer patients receiving TKI therapy. The aim of this study was to investigate the effect of 20 SNPs in 20 oxidative stress-related gene on the prognosis of patients with advanced NSCLC who were treated with EGFR TKIs.
Materials and methods
Patients
A total of 219 patients with advanced-stage NSCLC were recruited from four hospitals between 2010 and 2014. All patients met the following inclusion criteria: histologically or cytologically proven NSCLC; TNM stage IIIB and IV; Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0, 1 or 2; aged > 18 years; normal liver and renal functions; normal results of completed blood counts and urinalysis. Patients received a standard oral daily dose of 250 mg gefitinib or 150 mg erlotinib until either intolerable toxicity or disease progression occurred. The smoking status was categorized as never, current and former smokers as previously described [19]. All patients gave written informed consent. Three ml of peripheral blood were collected from each patient, according to the protocol approved by the Ethics Committees of Shanghai Chest Hospital, Quanzhou First Hospital, Taizhou Central Hospital, and Taizhou Hospital.
Genotyping
SNPs in oxidative stress-related genes were selected based on the published population-based genetic associations. It is reasonable to assume that SNPs associated with cancer susceptibility, prognosis and treatment outcomes are potentially functional in response to anticancer drugs commonly shared in different types of cancer. For statistical power consideration, only SNPs with a minor allele frequency (MAF) > 0.05 in the populations of Chinese Han were selected for genotyping. As a result, a total of 20 candidate SNPs were selected from 20 oxidative stress-related genes for genotyping (Table S1).
Genomic DNA was extracted from the peripheral leukocytes using TaKaRa MiniBEST Whole Blood Genomic DNA Extraction Kit (TakaRa, Dalian, China) according to manufacturer’s instructions. All SNP were genotyped using a PCR-ligation detection reaction (LDR) method as previously described [19]. In order to validate the genotyping accuracy, 10% of the samples were randomly selected for repeated genotyping by both PCR-LDR and direct sequencing, and the results showed 100% concordant.
Statistical analyses
All statistical analyses were performed using SPSS 19.0 software package (SPSS Inc, Chicago, USA) with a two-sided test. The progression-free survival (PFS) and overall survival (OS) rates were calculated using the Kaplan–Meier method, and the log-rank test was used to compare different survival curves. Univariate and multivariable Cox proportional hazard models were used to calculate the crude and adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs), respectively. A P value < 0.05 was considered statistically significant.
Results
Patient characteristics
The characteristics of the 219 patients with advanced NSCLC were summarized in Table 1. The mean patient age was 57 years for women and 58 years for men. The median follow-up of patients was 19.0 (95% CI 17.1-20.9) months. One hundred sixty two (74.0%) patients died during the follow-up period.
Table 1.
Clinicopathological characteristics of patients
| Characteristics | No. | % |
|---|---|---|
| Age, years | 57 ± 10.3 | |
| Sex | ||
| Male | 107 | 48.9 |
| Female | 112 | 51.1 |
| ECOG PS | ||
| 0-1 | 207 | 94.5 |
| 2 | 11 | 5 |
| TNM | ||
| IIIB | 56 | 25.6 |
| IV | 163 | 74.4 |
| Smoking status | ||
| Never | 151 | 68.9 |
| Current | 47 | 21.5 |
| Former | 17 | 7.8 |
| Unkown | 4 | 1.8 |
| Histology | ||
| Adenocarcinoma | 194 | 88.6 |
| Other | 25 | 11.4 |
Association of SNPs with PDF and OS in NSCLC patients
Two SNPs (rs1801282 and rs1937840) deviated significantly from Hardy-Weinberg equilibrium (P < 0.05, Table S2) and were excluded from further analysis. The associations of oxidative stress-related genetic factors with DFS and OS of patients with advanced NSCLC were presented in Table S2. No SNP showed association with PFS under any genetic model (Table S2). However, we found significant associations of glutathione S-transferases P1 (GSTP1) rs1695, myeloperoxidase (MPO) rs2333227 and biliverdin reductase A (BLVRA) rs699512 with OS. In multivariate analysis, including age, sex, TNM stage, ECOG PS, histology, and smoking status, rs1695 AA genotype (AA vs AG+GG, adjusted HR = 1.458, 95% CI 1.033-2.059, P = 0.032) and rs2333227 AA (AG vs GG, adjusted HR = 1.452, 95% CI 1.014-2.079, P = 0.042) and AG (AA vs GG, adjusted HR = 2.829, 95% CI 1.211-6.607, P = 0.016) genotypes correlated with worse survival and were all identified as independent prognostic factors for poor OS (Table 2, Figure 1). Although rs699512 G allele was associated with poor OS (AG vs AA, HR = 1.452, 95% CI 1.045-2.018, P = 0.026; AG+GG vs AA, HR = 1.450, 95% CI 1.059-1.986, P = 0.021) compared with AA genotype, the difference disappeared after adjustment for age, sex, TNM stage, ECOG PS, histology, smoking status (P > 0.05). We further examined the cumulative effect of rs1695, rs2333227 and rs699512 on OS. The number of unfavorable genotype (rs1695 AA genotype, rs2333227 AA and AG genotypes, and rs699512 AG and GG genotypes) was positively correlated with the risk of mortality. Compared with zero unfavorable genotypes, the effect of 1, 2 and 3 unfavorable genotypes was as follows: adjusted HR = 1.852, 95% CI 1.066-3.218, P = 0.029; adjusted HR = 2.528, 95% CI 1.454-4.396, P = 0.001; adjusted HR = 2.745, 95% CI 1.419-5.309, P = 0.003, respectively (P for trend = 0.001; Figure 1D).
Table 2.
Associations between rs1695, rs2333227 and rs699512 and OS
| SNP | Genotype | Events/patients | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR (95% CI) | P value | HR (95% CI)a | P value | |||
| rs1695 | ||||||
| AA | 106/136 | 1 | 1 | |||
| AG | 52/74 | 0.719 (0.514-1.005) | 0.053 | 0.729 (0.513-1.035) | 0.077 | |
| GG | 4/9 | 0.361 (0.133-0.982) | 0.046 | 0.367 (0.131-1.023) | 0.055 | |
| AG+GG | 56/83 | 1 | 1 | |||
| AA | 106/136 | 1.488 (1.072-2.064) | 0.017 | 1.458 (1.033-2.059) | 0.032 | |
| rs2333227 | ||||||
| GG | 110/157 | 1 | 1 | |||
| AG | 45/55 | 1.424 (1.006-2.016) | 0.046 | 1.452 (1.014-2.079) | 0.042 | |
| AA | 7/7 | 2.273 (1.056-4.896) | 0.036 | 2.829 (1.211-6.607) | 0.016 | |
| GG | 110/157 | 1 | 1 | |||
| AA+AG | 52/62 | 1.499 (1.076-2.086) | 0.017 | 1.543 (1.093-2.176) | 0.014 | |
| rs699512 | ||||||
| AA | 66/100 | 1 | 1 | |||
| AG | 78/96 | 1.452 (1.045-2.018) | 0.026 | 1.392 (0.984-1.970) | 0.062 | |
| GG | 18/23 | 1.441 (0.855-2.428) | 0.170 | 1.320 (0.772-2.257) | 0.310 | |
| AA | 66/100 | 1 | 1 | |||
| AG+GG | 96/119 | 1.450 (1.059-1.986) | 0.021 | 1.377 (0.989-1.918) | 0.058 | |
| risk genotypesb | ||||||
| 0 | 18/33 | 1 | 1 | |||
| 1 | 58/82 | 1.853 (1.091-3.148) | 0.022 | 1.852 (1.066-3.218) | 0.029 | |
| 2 | 62/77 | 2.439 (1.436-4.141) | 0.001 | 2.528 (1.454-4.396) | 0.001 | |
| 3 | 24/27 | 2.773 (1.499-5.128) | 0.001 | 2.745 (1.419-5.309) | 0.003 | |
aAdjusted for age, sex, ECOG PS, TNM stage, smoking status and histology.
Risk genotype: rs1695 AA, rs2243828 AG/GG, rs699512 AG/GG.
Figure 1.
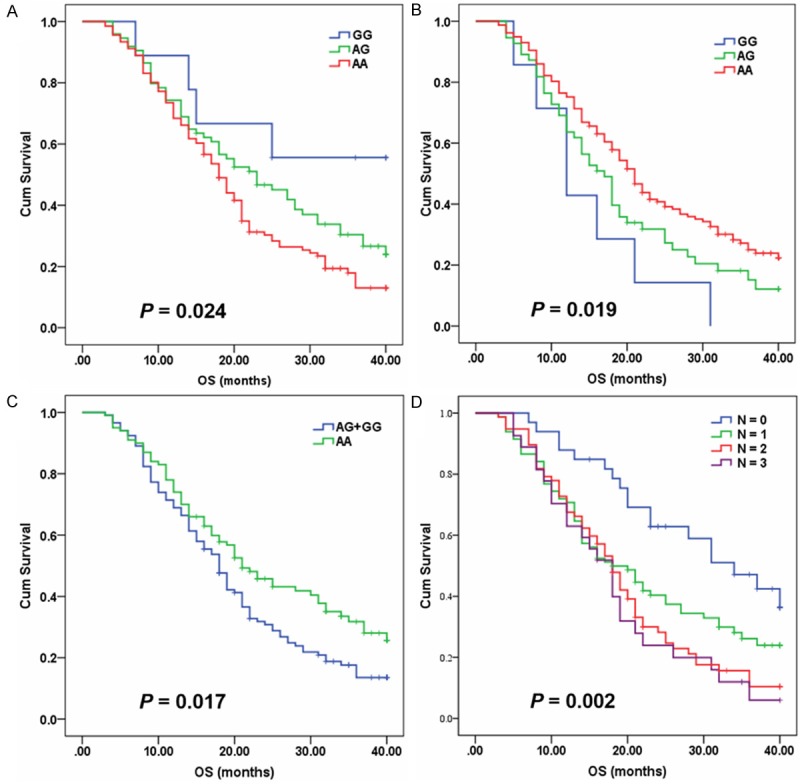
Kaplan-Meier curves of OS for advanced NSCLC patients treated with EGFR TKIs according to genotypes. A. rs1695. B. rs2333227. C. rs699512. D. Cumulative effect of unfavorable genotypes at rs 1695, rs2333227 and rs699512 on OS.
Stratified analyses of rs1695, rs2333227 and rs699512 on OS in NSCLC patients
The associations between rs1695, rs2333227 and rs699512, and OS were further investigated by stratification of age, sex, TNM stage, histology and smoking status. As shown in Table 3, there was a significant toward to a high risk of mortality associated with rs1695 AA genotypes in the following subjects: subjects aged ≤ 60 years; those with TNM stage IV; those with adenocarcinoma; smokers; and males (Table 3). For rs2333227, an increased risk of mortality was observed in the following subjects: subjects aged ≤ 60 years; those with TNM stage IV; those with adenocarcinoma; and smokers. For rs699512, an increased risk of mortality was observed in the following subjects: females; never-smokers; and subjects with adenocarcinoma.
Table 3.
Stratification analysis of rs1695, rs2333227 and rs699512 associated with survival of NSCLC patients
| Variablesa | rs1695b | rs2333227b | rs699512b | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| HR (95% CI)c | P value | HR (95% CI)c | P value | HR (95% CI)c | P value | |
| Age (years) | ||||||
| ≤ 60 | 1.665 (1.047-2.648) | 0.031 | 1.722 (1.081-2.742) | 0.022 | 1.276 (0.810-2.011) | 0.293 |
| > 60 | 1.338 (0.773-2.316) | 0.298 | 1.693 (0.977-2.933) | 0.061 | 1.622 (0.962-2.734) | 0.069 |
| Sex | ||||||
| Male | 4.622 (2.206-9.685) | < 0.001 | 1.654 (0.996-2.745) | 0.052 | 0.946 (0.594-1.509) | 0.817 |
| Female | 0.779 (0.497-1.221) | 0.276 | 1.691 (1.026-2.785) | 0.039 | 1.945 (1.224-3.090) | 0.005 |
| TNM | ||||||
| IIIB | 1.272 (0.557-2.908) | 0.568 | 1.140 (0.558-2.332) | 0.719 | 1.887 (0.904-3.938) | 0.091 |
| IV | 1.521 (1.034-2.239) | 0.033 | 1.918 (1.279-2.876) | 0.002 | 1.210 (0.824-1.777) | 0.332 |
| Smoking status | ||||||
| Never-smoker | 0.980 (0.663-1.450) | 0.920 | 1.421 (0.937-2.154) | 0.098 | 1.521 (1.026-2.255) | 0.037 |
| Smoker | 6.527 (2.357-18.076) | < 0.001 | 2.193 (1.156-4.161) | 0.016 | 0.865 (0.452-1.656) | 0.662 |
| Histology (%) | ||||||
| Adenocarcinoma | 1.692 (1.162-1.464) | 0.006 | 1.796 (1.229-2.624) | 0.003 | 1.487 (1.033-2.142) | 0.033 |
| Others | 0.601 (0.148-2.437) | 0.476 | 1.422 (0.418-4.836) | 0.573 | 0.832 (0.278-2.489) | 0.742 |
ECOG PS was excluded for stratification analysis due to only 11 cases of NSCLC patients with ECOG PS 2.
Rs1695: AA vs AG+GG; rs2333227: AA+AG vs GG; rs699512: AG+GG vs AA.
Adjusted for age, sex, ECOG PS, TNM stage, smoking status and histology, as appropriate.
Discussion
Despite the initial beneficial effect of EGFR TKIs in NSCLC patients with EGFR activating mutations, most patients eventually develop acquired resistance to EGFR-TKIs. Therefore, improving treatment outcomes for this group of NSCLC patients remains an area of high unmet clinical need. Although somatic EGFR mutations are the major determinants of response to EGFR TKI therapy, recent studies have found that genetic variants affect the clinical outcome of NSCLC patients treated with EGFR TKIs [20-22]. Increased antioxidant capacity is mechanisms that has been widely implicated in anticancer drug resistance [15-17,23]. In the present study, we found that rs1695, rs2333227 and rs699512 were associated with poor OS in advanced NSCLC patients who received EGFR TKIs. To the best of our knowledge, this is the first study evaluating the impact of genetic polymorphisms in oxidative stress-related genes on treatment outcome in advanced NSCLC patients.
The oxidative stress-related pathways have been widely studied for their roles in cancer development and treatment. For example, ROS is shown to mediate EGFR TKI-resistance through maintenance of EGFR tyrosine phosphorylation, whereas inhibiting ROS production restored gefitinib sensitivity in resistant breast cancer cells [18]. Increased glutamine metabolism was observed in erlotinib-resistant lung cancer cell lines [16,17]. Glutathione S-transferases play important role in detoxification through the conjugation of glutathione to electrophilic xenobiotics. GSTP1 isoenzyme is upregulated in various types of cancer, such as NSCLC [24-26] and CRC [27], and might influence response to platinum-based chemotherapy [28]. GSTP1 rs1695, leading to the substitution of isoleucine to valine at 105 amino acid position (Ile105Val), exhibits a significant influence on enzymatic activity [29], and is related to clinical outcome of patients receiving chemotherapy [30-32]. In a cohort of 115 advanced NSCLC patients treated with platinum-based chemotherapy, those with rs1695 G allele had a higher response rate and better prognosis [31]. Jun et al. reported the association of rs1695 GG genotype with better clinical outcome in advanced CRC patients who were treated with 5-FU-oxaliplatin-based chemotherapy [30]. These results are in agreement with our findings that G allele was associated with better survival time. However, there are inconsistent associations between rs1695 and prognosis of cancer patients. For example, Liu et al. [32] reported that rs1695 GG genotype was linked to worse prognosis in osteosarcoma patients treated with doxorubicin based chemotherapy. Genetic heterogeneity may partly explain these inconsistent results despite different type of cancer and therapeutic regimen. Furthermore, the frequencies of the G allele and GG genotype of rs1695 were remarkable higher, and rs1695 deviated significantly from Hardy-Weinberg equilibrium in Liu study [32].
MPO is an endogenous oxidant lysosomal enzyme secreted by neutrophils that catalyze formation of numerous reactive oxidant species. Rymaszewski et al. [33] reported that administration of MPO inhibitor reduced butylated hydroxytoluene-promotion of MCA-induced lung carcinogenesis by 50% in BALB mice. MPO may play an important role in inflammation promoted lung carcinogenesis. Rs2333227 is located in the strong stimulatory protein 1 (SP1) transcription factors binding site in the promoter region of the MPO gene, and thus affects MPO expression [34]. The A allele is associated with reduced MPO mRNA and protein levels attributable to the disruption of a SP1-binding site. Many studies have shown that rs2333227 is associated with risk of many human diseases, including lung cancer [35]. In this study, we found that rs2333227 A allele conferred a high risk of death for NSCLC patients. In a more recent study, rs2243828, located in the promoter region of the MPO gene, was reported as a prognostic marker for patients with aggressive B-cell non-Hodgkin lymphoma [36]. Rs2243828 is 122 bp away from rs2333227, and rs2243828 G allele is in linkage disequilibrium with rs2333227 A allele. Therefore, rs2243828 G allele may be linked to lower MPO expression. Together with these findings, decreased MPO expression may be beneficial for cancer cells to survive under anticancer treatment. However, the lung cancer graft is slower growing in an MPO-knockout mouse, indicating that a non-enzymatic function of MPO is required for cancer growth in the later phases of tumor progression [33]. These imply that cancer cell is more susceptible to oxidative stress under extreme conditions. Further studies are warranted to elucidate the role of MPO in NSCLC.
BLVRA plays an important role in the maintenance of intracellular redox homeostasis through its production of bilirubin, a major natural and potent antioxidant [37,38]. Recent data show that BLVRA can act as a dual-specificity protein kinase and transcription factor, involved in various cellular functions [38]. BLVRA is upregulated in chemoresistant cancer cell lines, and inhibition of BLVRA reverses drug resistance in cancer cells [39,40]. Meanwhile, the present study found that the BLVRA rs699512 G allele, resulting in the substitution of threonine to alanine at 3 amino acid position (Thr3Ala), was significantly associated with worse survival in univariate Cox analysis. In a previous association study for patients with essential hypertension, rs699512 G allele was related to reduced risk of hypertension, and G allele carriers had lower systolic and diastolic blood pressures [41]. Rs699512 is possible functional SNP that could impair BLVRA function.
In conclusion, the current findings provide evidence that genetic variants in oxidative stress-related genes may modify prognosis in advanced NSCLC patients treated with EGFR TKIs. Cellular redox state is associated with the treatment efficacy of EGFR TKIs in NSCLC patients with activating EGFR mutations. Further prospective studies with large NSCLC populations receiving EGFR TKIs are required to confirm the predictive value of SNPs in oxidative stress-related genes for treatment outcome.
Acknowledgements
This work was supported by grants from the Taizhou Science and Technology Agency, China (131KY14-04), and the Department of Science and Technology of Zhejiang Province, China (2014C33154 and 2011C13039-2).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Chen W, Zheng R, Zhang S, Zhao P, Zeng H, Zou X, He J. Annual report on status of cancer in China, 2010. Chin J Cancer Res. 2014;26:48–58. doi: 10.3978/j.issn.1000-9604.2014.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramalingam S, Belani C. Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. Oncologist. 2008;13(Suppl 1):5–13. doi: 10.1634/theoncologist.13-S1-5. [DOI] [PubMed] [Google Scholar]
- 3.Kimura H, Ohira T, Uchida O, Matsubayashi J, Shimizu S, Nagao T, Ikeda N, Nishio K. Analytical performance of the cobas EGFR mutation assay for Japanese non-small-cell lung cancer. Lung Cancer. 2014;83:329–333. doi: 10.1016/j.lungcan.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Z, Stiegler AL, Boggon TJ, Kobayashi S, Halmos B. EGFR-mutated lung cancer: a paradigm of molecular oncology. Oncotarget. 2010;1:497–514. doi: 10.18632/oncotarget.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linardou H, Dahabreh IJ, Bafaloukos D, Kosmidis P, Murray S. Somatic EGFR mutations and efficacy of tyrosine kinase inhibitors in NSCLC. Nat Rev Clin Oncol. 2009;6:352–366. doi: 10.1038/nrclinonc.2009.62. [DOI] [PubMed] [Google Scholar]
- 6.Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer. 2010;10:760–774. doi: 10.1038/nrc2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmstrom KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 8.Gupta SC, Hevia D, Patchva S, Park B, Koh W, Aggarwal BB. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal. 2012;16:1295–1322. doi: 10.1089/ars.2011.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chinery R, Beauchamp RD, Shyr Y, Kirkland SC, Coffey RJ, Morrow JD. Antioxidants reduce cyclooxygenase-2 expression, prostaglandin production, and proliferation in colorectal cancer cells. Cancer Res. 1998;58:2323–2327. [PubMed] [Google Scholar]
- 10.Martin V, Herrera F, Garcia-Santos G, Antolin I, Rodriguez-Blanco J, Rodriguez C. Signaling pathways involved in antioxidant control of glioma cell proliferation. Free Radic Biol Med. 2007;42:1715–1722. doi: 10.1016/j.freeradbiomed.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 11.Sharma R, Vinayak M. Antioxidant alpha-tocopherol checks lymphoma promotion via regulation of expression of protein kinase C-alpha and c-Myc genes and glycolytic metabolism. Leuk Lymphoma. 2012;53:1203–1210. doi: 10.3109/10428194.2011.637213. [DOI] [PubMed] [Google Scholar]
- 12.Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 13.Schulz R, Emmrich T, Lemmerhirt H, Leffler U, Sydow K, Hirt C, Kiefer T, Link A, Bednarski PJ. Identification of a glutathione peroxidase inhibitor that reverses resistance to anticancer drugs in human B-cell lymphoma cell lines. Bioorg Med Chem Lett. 2012;22:6712–6715. doi: 10.1016/j.bmcl.2012.08.091. [DOI] [PubMed] [Google Scholar]
- 14.Alexandre J, Batteux F, Nicco C, Chereau C, Laurent A, Guillevin L, Weill B, Goldwasser F. Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. Int J Cancer. 2006;119:41–48. doi: 10.1002/ijc.21685. [DOI] [PubMed] [Google Scholar]
- 15.Ramanathan B, Jan KY, Chen CH, Hour TC, Yu HJ, Pu YS. Resistance to paclitaxel is proportional to cellular total antioxidant capacity. Cancer Res. 2005;65:8455–8460. doi: 10.1158/0008-5472.CAN-05-1162. [DOI] [PubMed] [Google Scholar]
- 16.Serizawa M, Kusuhara M, Zangiacomi V, Urakami K, Watanabe M, Takahashi T, Yamaguchi K, Yamamoto N, Koh Y. Identification of metabolic signatures associated with erlotinib resistance of non-small cell lung cancer cells. Anticancer Res. 2014;34:2779–2787. [PubMed] [Google Scholar]
- 17.Weaver Z, Difilippantonio S, Carretero J, Martin PL, El Meskini R, Iacovelli AJ, Gumprecht M, Kulaga A, Guerin T, Schlomer J, Baran M, Kozlov S, McCann T, Mena S, Al-Shahrour F, Alexander D, Wong KK, Van Dyke T. Temporal molecular and biological assessment of an erlotinib-resistant lung adenocarcinoma model reveals markers of tumor progression and treatment response. Cancer Res. 2012;72:5921–5933. doi: 10.1158/0008-5472.CAN-12-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giordano CR, Mueller KL, Terlecky LJ, Krentz KA, Bollig-Fischer A, Terlecky SR, Boerner JL. A targeted enzyme approach to sensitization of tyrosine kinase inhibitor-resistant breast cancer cells. Exp Cell Res. 2012;318:2014–2021. doi: 10.1016/j.yexcr.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Chen Q, He C, Mao W, Zhang L, Xu X, Zhu J, Chen B. Polymorphisms on 8q24 are associated with lung cancer risk and survival in Han Chinese. PLoS One. 2012;7:e41930. doi: 10.1371/journal.pone.0041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao M, Zhang Y, Cai W, Li J, Zhou F, Cheng N, Ren R, Zhao C, Li X, Ren S, Zhou C, Hirsch FR. The Bim deletion polymorphism clinical profile and its relation with tyrosine kinase inhibitor resistance in Chinese patients with non-small cell lung cancer. Cancer. 2014;120:2299–2307. doi: 10.1002/cncr.28725. [DOI] [PubMed] [Google Scholar]
- 21.Ng KP, Hillmer AM, Chuah CT, Juan WC, Ko TK, Teo AS, Ariyaratne PN, Takahashi N, Sawada K, Fei Y, Soh S, Lee WH, Huang JW, Allen JC Jr, Woo XY, Nagarajan N, Kumar V, Thalamuthu A, Poh WT, Ang AL, Mya HT, How GF, Yang LY, Koh LP, Chowbay B, Chang CT, Nadarajan VS, Chng WJ, Than H, Lim LC, Goh YT, Zhang S, Poh D, Tan P, Seet JE, Ang MK, Chau NM, Ng QS, Tan DS, Soda M, Isobe K, Nothen MM, Wong TY, Shahab A, Ruan X, Cacheux-Rataboul V, Sung WK, Tan EH, Yatabe Y, Mano H, Soo RA, Chin TM, Lim WT, Ruan Y, Ong ST. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med. 2012;18:521–528. doi: 10.1038/nm.2713. [DOI] [PubMed] [Google Scholar]
- 22.Winther Larsen A, Nissen PH, Meldgaard P, Weber B, Sorensen BS. EGFR CA repeat polymorphism predict clinical outcome in EGFR mutation positive NSCLC patients treated with erlotinib. Lung Cancer. 2014;85:435–41. doi: 10.1016/j.lungcan.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Corti A, Franzini M, Paolicchi A, Pompella A. Gamma-glutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting. Anticancer Res. 2010;30:1169–1181. [PubMed] [Google Scholar]
- 24.Oguztuzun S, Aydin M, Demirag F, Yazici U, Ozhavzali M, Kilic M, Iscan M. The expression of GST isoenzymes and p53 in non-small cell lung cancer. Folia Histochem Cytobiol. 2010;48:122–127. doi: 10.2478/v10042-008-0084-6. [DOI] [PubMed] [Google Scholar]
- 25.Xu C, Feng D, Li L, Yu P, Hu X, Liu Y. [The expression and prognostic significance of ERCC1 and GST-pi in lung cancer] . Zhongguo Fei Ai Za Zhi. 2010;13:195–200. doi: 10.3779/j.issn.1009-3419.2010.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimminger PP, Maus MK, Schneider PM, Metzger R, Holscher AH, Sugita H, Danenberg PV, Alakus H, Brabender J. Glutathione S-transferase PI (GST-PI) mRNA expression and DNA methylation is involved in the pathogenesis and prognosis of NSCLC. Lung Cancer. 2012;78:87–91. doi: 10.1016/j.lungcan.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Zhang R, Kang KA, Piao MJ, Kim KC, Zheng J, Yao CW, Cha JW, Maeng YH, Chang WY, Moon PG, Baek MC, Hyun JW. Epigenetic alterations are involved in the overexpression of glutathione S-transferase pi-1 in human colorectal cancers. Int J Oncol. 2014;45:1275–1283. doi: 10.3892/ijo.2014.2522. [DOI] [PubMed] [Google Scholar]
- 28.Sawers L, Ferguson MJ, Ihrig BR, Young HC, Chakravarty P, Wolf CR, Smith G. Glutathione S-transferase P1 (GSTP1) directly influences platinum drug chemosensitivity in ovarian tumour cell lines. Br J Cancer. 2014;111:1150–8. doi: 10.1038/bjc.2014.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong SL, Zhou SF, Chen X, Chan SY, Chan E, Ng KY, Duan W, Huang M. Relationship between genotype and enzyme activity of glutathione S-transferases M1 and P1 in Chinese. Eur J Pharm Sci. 2006;28:77–85. doi: 10.1016/j.ejps.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Jun L, Haiping Z, Beibei Y. Genetic polymorphisms of GSTP1 related to response to 5-FU-oxaliplatin-based chemotherapy and clinical outcome in advanced colorectal cancer patients. Swiss Med Wkly. 2009;139:724–728. doi: 10.4414/smw.2009.12754. [DOI] [PubMed] [Google Scholar]
- 31.Sun N, Sun X, Chen B, Cheng H, Feng J, Cheng L, Lu Z. MRP2 and GSTP1 polymorphisms and chemotherapy response in advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2010;65:437–446. doi: 10.1007/s00280-009-1046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu S, Yi Z, Ling M, Shi J, Qiu Y, Yang S. Predictive potential of ABCB1, ABCC3, and GSTP1 gene polymorphisms on osteosarcoma survival after chemotherapy. Tumour Biol. 2014;35:9897–904. doi: 10.1007/s13277-014-1917-x. [DOI] [PubMed] [Google Scholar]
- 33.Rymaszewski AL, Tate E, Yimbesalu JP, Gelman AE, Jarzembowski JA, Zhang H, Pritchard KA Jr, Vikis HG. The role of neutrophil myeloperoxidase in models of lung tumor development. Cancers (Basel) 2014;6:1111–1127. doi: 10.3390/cancers6021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piedrafita FJ, Molander RB, Vansant G, Orlova EA, Pfahl M, Reynolds WF. An Alu element in the myeloperoxidase promoter contains a composite SP1-thyroid hormone-retinoic acid response element. J Biol Chem. 1996;271:14412–14420. doi: 10.1074/jbc.271.24.14412. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Fu Y, Zhao B, Xiao Y, Chen R. Myeloperoxidase G463A polymorphism and risk of lung cancer. Tumour Biol. 2014;35:821–829. doi: 10.1007/s13277-013-1113-4. [DOI] [PubMed] [Google Scholar]
- 36.Gustafson HL, Yao S, Goldman BH, Lee K, Spier CM, LeBlanc ML, Rimsza LM, Cerhan JR, Habermann TM, Link BK, Maurer MJ, Slager SL, Persky DO, Miller TP, Fisher RI, Ambrosone CB, Briehl MM. Genetic polymorphisms in oxidative stress-related genes are associated with outcomes following treatment for aggressive B-cell non-Hodgkin lymphoma. Am J Hematol. 2014;89:639–645. doi: 10.1002/ajh.23709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sedlak TW, Saleh M, Higginson DS, Paul BD, Juluri KR, Snyder SH. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci U S A. 2009;106:5171–5176. doi: 10.1073/pnas.0813132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kapitulnik J, Maines MD. Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol Sci. 2009;30:129–137. doi: 10.1016/j.tips.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Kim SS, Seong S, Lim SH, Kim SY. Targeting biliverdin reductase overcomes multidrug resistance in leukemia HL60 cells. Anticancer Res. 2013;33:4913–4919. [PubMed] [Google Scholar]
- 40.Kim SS, Seong S, Lim SH, Kim SY. Biliverdin reductase plays a crucial role in hypoxia-induced chemoresistance in human glioblastoma. Biochem Biophys Res Commun. 2013;440:658–663. doi: 10.1016/j.bbrc.2013.09.120. [DOI] [PubMed] [Google Scholar]
- 41.Lin R, Wang X, Zhou W, Fu W, Wang Y, Huang W, Jin L. Association of a BLVRA common polymorphism with essential hypertension and blood pressure in Kazaks. Clin Exp Hypertens. 2011;33:294–298. doi: 10.3109/10641963.2010.531854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


