Graphical abstract
Keywords: Protein kinase, Inhibitor, Protein kinase B (PKB), Akt, Schistosoma mansoni, Chemotherapy
Highlights
-
•
A wide range of PK inhibitors affects schistosome viability and reproduction.
-
•
Structure and activity of the Akt/PKB protein are highly conserved in Schistosoma mansoni.
-
•
Commercial Akt inhibitors are active on the recombinant SmAkt protein.
-
•
Akt pathway inhibitors have schistosomicidal activity in vitro.
-
•
SmAkt can be considered as a potential target for the control of schistosomiasis.
Abstract
Protein kinases (PKs) are one of the largest protein families in most eukaryotic organisms. These enzymes are involved in the control of cell proliferation, differentiation and metabolism and a large number of the anticancer drugs currently used are directed against PKs. The structure and function of PKs are well conserved throughout evolution. In schistosome parasites, PKs were shown to be involved in essential functions at every stage of the parasite life cycle, making these enzymes promising anti-parasite drug targets. In this study, we tested a panel of commercial inhibitors for various PKs and analyzed their effects on pairing and egg production by schistosomes as well as their toxicity towards schistosomula larvae. Results obtained confirmed the deleterious effect of PK targeting on Schistosoma mansoni physiology and the important function of different tyrosine and serine/threonine kinases in the biology and reproduction of this parasite. They also indicated for the first time that the Protein kinase B (also called Akt) which is a major downstream target of many receptor tyrosine kinases and a central player at the crossroads of signal transduction pathways activated in response to growth factors and insulin, can constitute a novel target for anti-schistosome chemotherapy. Structural and functional studies have shown that SmAkt is a conserved kinase and that its activity can be inhibited by commercially available Akt inhibitors. In treated adult worms, Akt/PKB kinase pathway inhibitors induced profound alterations in pairing and egg laying and they also greatly affected the viability of schistosomula larvae.
1. Introduction
Schistosomiasis is a disease with major medical and economic importance affecting human and cattle. It is the second major parasitic disease after malaria with more than 230 million individuals infected in the world, and a number of annual deaths estimated to 200 thousands (Colley et al., 2014). Chemotherapy of schistosomiasis relies on a single drug, Praziquantel (PZQ), which efficiently reduces morbidity and mortality due to the disease. However, mass treatment has raised concerns regarding the emergence of resistance to this drug (Doenhoff and Pica-Mattoccia, 2006; Pica-Mattoccia et al., 2009; Melman et al., 2009). This has motivated the search for alternative treatments and intensive efforts have been made during last years to identify novel molecular targets for chemotherapy (Doenhoff et al., 2008; DeMarco and Verjovski-Almeida, 2009).
Protein kinases (PKs) are one of the largest protein families in most eukaryotic organisms. PKs are involved in the control of cell proliferation, differentiation and metabolism and these druggable enzymes are well conserved throughout evolution. Their biochemical characteristics are well studied due to their importance in cancer, and a large variety of small molecules targeting selectively kinase activities is now commercially available and already used for human cancer therapy (Johnson, 2009). In schistosomes, many studies have shown that PKs are implicated in essential functions at every stage of the parasite life cycle, and that these enzymes represent suitable anti-parasite drug targets (Dissous et al., 2007, 2014a; Dissous and Grevelding, 2011; Beckmann et al., 2012).
Tyrosine Kinases (TKs) have been considered as good candidates because of their essential roles in development and metabolism (Dissous et al., 2006, 2007; Dissous and Grevelding, 2011). The tyrosine kinome of Schistosoma mansoni comprises 15 receptor TKs (RTKs) and 19 cytosolic TKs (CTKs) (Andrade et al., 2011; Avelar et al., 2011). Receptor TKs (RTKs) regulate many cellular activities such as proliferation, migration or differentiation, and they are the first actors in TK signaling, being able to integrate perception, response to extracellular signals and propagation by phosphorylation of intracellular targets (Hubbard and Till, 2000). Seven RTK molecules have been studied for their role in the control of parasite development and/or reproduction processes. SER (Schistosoma Epidermal growth factor Receptor) plays potential role in host–parasite interactions, and parasite growth and reproduction (Vicogne et al., 2004). Two insulin receptors (IR) are involved, respectively, in regulating sugar uptake and growth of schistosomes (Khayath et al., 2007; Ahier et al., 2008). Recently, two Venus Kinase Receptors (VKR), that belong to a novel family of RTKs (discovered for the first time in schistosomes) (Vicogne et al., 2003; Vanderstraete et al., 2013a; Dissous et al., 2014b) and that possess a TK domain similar to that of IR (Ahier et al., 2009; Gouignard et al., 2012), were demonstrated to control parasite reproductive activities (Vanderstraete et al., 2014). Also, two proteins of the FGFR (Fibroblast Growth Factor Receptor) family could participate with these RTKs in the development of reproductive organs (Hahnel et al., 2014; Morel et al., 2014). All these schistosome RTKs can be inhibited by commercially available kinase inhibitors as well as the non-receptor CTKs that participate to their downstream signaling. Particularly, three schistosome CTKs-SmTK3 (Src kinase (Kapp et al., 2004)), SmTK4 (Syk kinase (Knobloch et al., 2002)) and SmTK6 (Src/Abl-like kinase (Beckmann et al., 2011))-presumably act in a multikinase complex and their participation to RTK signaling can be inhibited by specific TK inhibitors (Beckmann et al., 2011).
Besides the importance of TK proteins as druggable enzymes, a large number of kinases phosphorylating Serine/Threonine (S/T) residues have been characterized in schistosomes and some of them were described as potential targets against these parasites. Particularly, biochemical approaches and functional screening of activin/TGFβ receptor, Polo, Ste 20, MAPK (Mitogen-Activated Protein Kinase) (ERK, p38 and JNK), MEK (Mitogen/Extracellular signal-regulated Kinase) and AGC (PKC (Ca-dependent protein kinase C) and PKA (cyclic AMP-dependent protein kinase)) kinase families have supported the roles of S/T kinases in cell proliferation, motor activity and reproduction. Evidence has been obtained that TGF-β pathways play a major role in female reproductive development and egg embryogenesis (Knobloch et al., 2006; Loverde et al., 2007; LoVerde et al., 2009; Buro et al., 2013). Inhibition of SmPlk1 (the schistosome Polo-like kinase homolog of the major mitotic kinase overexpressed in many human tumors (Strebhardt, 2010)) by the anticancer compound BI2536 caused profound alterations of the reproductive organs in males and females in vitro, including a reduction of gamete production (Long et al., 2010). Pharmacological modulation of ERK activity in adult worms using the MEK inhibitor, U0126, disturbed significantly the physiology of parasites and the PKC inhibitor, GF109203X, was shown to inhibit worm pairing, egg output, and ventral sucker attachment (Ressurreição et al., 2014). PKC belongs to the AGC group of kinases which are cytoplasmic serine/threonine kinases regulated by secondary messengers such as cyclic nucleotide AMP (for PKA) or GMP (for PKG) or by lipids (for PKC and PKB also called Akt). RNAi experiments in combination with PKA inhibitors H-89 or PKI 14–22 amide have demonstrated the importance of PKA activity for schistosome viability and egg production (Swierczewski and Davies, 2009, 2010) and interference with the PKG activity of the S. mansoni SmcGK by a cGMP analog induced slow motion and reduced egg production by schistosomes (Leutner et al., 2011). However, the role of PKB/Akt proteins in viability and fertility of schistosomes has not been described.
Akt/PKB kinases are attractive targets for drug discovery due to their key role in tumor cell survival or proliferation and to their overexpression or activation in many cancers. Small molecule inhibitors targeting the Akt signaling pathway are very promising therapeutics against cancer (Kumar and Madison, 2005; Pal et al., 2010). In this study, we have tested a new panel of commercial inhibitors for various PKs and we analyzed their effects on pairing and egg production by schistosomes as well as their toxicity towards schistosomula larvae of S. mansoni. Results confirmed the deleterious effect of targeting PKs on schistosome physiology and indicated that signaling of SmAkt, the PKB/Akt homolog of S. mansoni, can constitute a novel potential target for anti-schistosome chemotherapy. Structural and functional studies have shown that SmAkt is a conserved kinase and that its activity can be inhibited by commercially available Akt inhibitors. Three of these inhibitors induced profound alterations in pairing and egg laying in treated worms and they also greatly affected the viability of schistosomula.
2. Materials and methods
2.1. Parasite material
A Puerto-Rican strain of S. mansoni was maintained by passage through albino Biomphalaria glabrata snails and Mesocricetus auratus golden hamsters. Adult schistosomes were collected by portal perfusion from infected hamsters at 42–45 days p.i. Schistosomula were prepared as described previously (Dissous et al., 1981). Experiments with hamsters infected by cercariae were performed in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (ETS No. 123; revised Appendix A) and were approved by the committee for ethics in animal experimentation of the region Nord Pas de Calais France (authorisation No. AF/2009) in the local animal house of the Pasteur Institute of Lille (Agreement No. A59-35009).
2.2. In silico analyzes
Sequences were analyzed using the LASERGENE package (DNAStar, Madison, WI, USA). tBLASTn and BLASTn analyzes were performed using the NCBI databank http://blast.ncbi.nlm.nih.gov.gate2.inist.fr/Blast.cgi. Akt/PKB, PKA and PKC protein sequences were aligned using ClustalW algorithm in the BioEdit v7.1 software, and manually corrected. Neighbor-Joining trees were built using MEGA5 (Tamura et al., 2011) under the JTT model, with 1000 bootstrap repetitions.
2.3. Protein kinase inhibitors
The InhibitorSelect™ 96-Well Protein Kinase Inhibitor Library I from Calbiochem® consists of 80, well-characterized, cell-permeable, potent and reversible protein kinase inhibitors. Each inhibitor was provided in solution in DMSO at a concentration of 10 mM (except the PI3K inhibitor E10 provided at 5 mM).
2.4. Treatment of cultured adult worms with inhibitors
Ten pairs of adult S. mansoni were incubated for 72 h at 37 °C in a 5% CO2 atmosphere in 6-well plates containing 2 mL of M199 medium (Invitrogen) supplemented with HEPES 10 mM, pH 7.4, penicillin (50 U/mL), streptomycin (50 μg/mL), gentamycin (15 μg/mL), rifampicin (60 μg/mL) and 10% fetal calf serum (Gibco) (hereafter referred as M199 complete medium) in the presence of each compound of the inhibitor library at 10 μM final concentration. Akt inhibitors (A4, A7 and A8) were added at concentrations ranging from 10 nM to 10 μM. Medium containing eggs was harvested everyday and replaced by fresh medium with inhibitors. Medium fractions were pooled and their content in eggs was determined after centrifugation. The number of paired couples was estimated every day by stereomicroscopy.
2.5. Treatment of schistosomula larvae with inhibitors
500 schistosomula were incubated for 72 h in 48-well plates containing 500 μL of M199 complete medium with different concentrations (from 50 nM to 10 μM) of A4, A7 and A8 compounds. Culture medium with inhibitors was refreshed daily. Parasite death was assessed optically under microscope each day using three criteria: absence of motility, tegument defects and granular appearance. A minimum of 300 larvae was observed for each condition, and the ratio dead/total larvae was calculated.
2.6. Molecular cloning of S. mansoni Akt
A putative S. mansoni Akt sequence was identified in Genbank (XM_002578520.1) by tBLASTp analyzes of the S. mansoni genome data bases (Berriman et al., 2009). 5′ and 3′ UTR were determined with the SmAkt encoding ESTs AM042871.1, CF497410.2, AA169931.1 and CF498818.1. The cDNA sequence of SmAkt was obtained by PCR amplification of total cDNA obtained from adult S. mansoni RNA (prepared using TRIzol® reagent, Invitrogen) by reverse transcription using the Superscript III (Invitrogen). The SmAkt sequence was amplified using SmAktFLf (5′-CGGCACGAGGCCAAGTCTTAAATGCTAGT-3′) and SmAktFLr (5′-GTTAAATCATGTTGGTGGCAGTCAATTGAACT-3′) primers, cloned into a pCR2.1 TOPO cloning vector (Invitrogen) and sequenced (EurofinsDNA). A second PCR was performed using as primers SmAktFLRE-f (5′-CCggatccGTTATCGAGATTGCAGATTTTCTGGG-3′) and SmAktFLRE-r (5′-GCctcgagAAAATGTGTCACCAAAACTATAACCAC-3′) containing, respectively, BamHI and XhoI restriction sites and the fragment was inserted in frame in the pcDNA3.1-V5/His expression vector (Invitrogen). Site-directed mutagenesis was performed on the wild-type (WT) SmAkt construct to produce SmAkt E117K and SmAkt L150R active mutants using, respectively, the 5′-GGCTTATGAAACGCGGCAAACATATTAAAAATTGGCGACG-3′ and 5′-TAAAGATGATATGGCGCAACCTCGAAATAATTTTACTGTTCGCG-3′ mutated sequences and their reverse complement as primers.
2.7. Protein expression in Xenopus oocytes
cRNAs encoding SmAkt WT, E117K and L150R were synthesized in vitro using the T7 mMessage mMachine kit (Ambion) and the different SmAkt-pcDNA3.1-V5/His plasmids linearized by SphI as templates. cRNAs were injected in stage VI Xenopus laevis oocytes according to the procedure described previously (Vicogne et al., 2004). Each oocyte was injected with 60 nL (60 ng) of cRNA in the equatorial region and incubated at 19 °C in ND96 medium (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 5 mM Hepes pH 7.4 supplemented with 50 μg/mL Streptomycin/Penicillin, 225 μg/mL sodium pyruvate, 30 μg/mL trypsin inhibitor). For inhibitor assays, oocytes were incubated with compounds (A4–8) from the library I (Calbiochem®) at different concentrations. After 15 h, germinal vesicle breakdown (GVBD) was detected by the appearance of a white spot at the center of the animal pole of the oocyte.
2.8. Immunoprecipitation and Western blot analyzes
Expression and phosphorylation of SmAkt proteins in oocytes were confirmed by immunoprecipitation of oocyte lysates according to the procedure described previously (Vicogne et al., 2004). Following 15 h of expression, oocytes were lysed in buffer A (50 mM Hepes pH 7.4, 500 mM NaCl, 0.05% SDS, 5 mM MgCl2, 1 mg/mL bovine serum albumin, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 10 μg/mL soybean trypsin inhibitor, 10 μg/mL benzamidine, 1 mM PMSF, 1 mM sodium vanadate) containing 0.5% Triton X-100 and centrifuged at 12,000g for 15 min at 4 °C. Lysates were incubated with anti-V5 antibodies (1:100, Invitrogen) for 4 h at 4 °C. Protein A-Sepharose beads (5 mg; Amersham Biosciences) were added for 1 h at 4 °C. Beads were washed three times and resuspended in Laemmli sample buffer. Eluted immune complexes were subjected to a 10% SDS–PAGE, then analyzed by Western blotting using anti-V5 (1:50,000) or anti-phospho-T308 Akt (1:5000; Upstate Biotechnology) antibodies. Mouse or rabbit Trueblot® secondary antibodies (eBioscience) were used as secondary antibodies and chemoluminescence was revealed using the advanced ECL detection system (Amersham Biosciences).
3. Results
3.1. Screening of the library of protein kinase inhibitors on adult worms
The InhibitorSelect™ 96-Well Protein Kinase Inhibitor Library I (Calbiochem) consists of eighty, well-characterized, cell-permeable, potent and reversible protein kinase inhibitors. Most of them are ATP-competitive. S. mansoni adult worm couples, freshly recovered from infected hamsters, were maintained in the culture conditions previously defined (Vanderstraete et al., 2013b), and we analyzed the level of male-female pairing and egg laying following 24, 48 or 72 h of exposure to each inhibitor added at a 10 μM final concentration. Results (Table S1) indicated a highly variable effect of each inhibitor on worms, ranging from a complete (100%) inhibition of pairing and/or egg laying at 24 h to a totally unaffected, as compared to untreated parasites, worm behaviour following 72 h of culture. A selection was made of the molecules that induced more or less profound alterations of pairing and/or egg laying of treated worms during 72 h culture, and for which potential target kinases were already characterized in schistosomes (Table 1). These potential targets include receptor (EGFR, FGFR and IR/IGFR) and cytosolic (Src, Abl, Fyn, Syk) tyrosine kinases as well as serine/threonine kinases (TGF-βR, PKC) known for their major functions in cell signaling and their importance in schistosome development. Indeed, most of these kinases have already been demonstrated to be druggable targets for parasites and many authors have shown the impact of their targeting on the viability and/or the reproduction of schistosomes (see references in Table 1). In this study, we found additionally that three Akt inhibitors contained in the library exerted a potent impairment of pairing and egg-laying and we decided to undertake the study of the S. mansoni Akt protein, in order to analyze its potential as a novel drug target for schistosomes.
Table 1.
List of selected inhibitors that induce a significant effect on adult worm pairing and/or egg laying and their potential kinase targets in S. mansoni.
| Compound | Pubchem ID | Deleterious effect on |
Potential targets kinases in schistosome | References | |
|---|---|---|---|---|---|
| Pairing | Egg laying | ||||
| Compound 56 | 2857 | + | ++ | EGF-R | Dissous et al. (2014a) |
| EGFR/ErbB-2 inhibitor | 9843206 | +/− | ++ | ||
| EGFR inhibitor | 9549299 | + | ++ | ||
| EGFR/ErbB-2/ErbB-4 inhibitor | 11566580 | − | ++ | ||
| PD 158780 | 4707 | ++ | ++ | ||
| PD 174265 | 4709 | − | + | ||
| AG 1478 | 2051 | − | + | ||
| AG 1024 | 2044 | ++ | ++ | IR/IGFR1-R | Vanderstraete et al. (2013b) |
| IGF-1R inhibitor II | 9549305 | + | ++ | ||
| SU11652 | 5329103 | ++ | ++ | FGF-R | Hahnel et al. (2014) |
| Herbimycin A | 16760502 | ++ | ++ | Src |
Knobloch et al. (2006) Beckmann et al. (2010) Beckmann et al. (2011) |
| Src kinase inhibitor I | 1474853 | − | + | ||
| Flt-3 inhibitor III | 11772958 | + | ++ | Src/Abl | |
| PDGF receptor tyrosine kinase inhibitor IV | 9797370 | − | ++ | Src/Abl/Fyn | |
| PP1 analog II, 1NM-PP1 | 5154691 | + | ++ | Src/Fyn | |
| Syk inhibitor | 6419747 | − | + | Syk | |
| Syk inhibitor III | 672296 | ++ | ++ | Syk/Src | |
| TGF-β RI inhibitor III | 16079009 | ++ | +/− | TGF-βR | Knobloch et al. (2007) |
| Chelerythrine chloride | 72311 | ++ | ++ | PKC |
Ludtmann et al. (2009) Ressurreição et al. (2014) |
| Gö 6976 | 3501 | + | + | ||
| Gö 6983 | 3499 | − | + | ||
| PKCb inhibitor | 6419755 | ++ | ++ | ||
| Staurosporine, N-benzoyl- | 16760627 | ++ | ++ | ||
| Staurosporine, Streptomyces sp. | 451705 | ++ | ++ | ||
| PKCbII/EGFR inhibitor | 6711154 | ++ | +/− | ||
| Akt inhibitor IV | 5719375 | ++ | ++ | Akt | |
| Akt inhibitor X | 16760284 | ++ | ++ | ||
| PDK1/Akt/Flt Dual Pathway inhibitor | 5113385 | ++ | ++ | ||
| Akt inhibitor V (=Triciribine) | 290486 | − | − | ||
| Akt inhibitor VIII (=Akti1/2) | 10196499 | +/− | +/− | ||
Inhibitors with a significant influence on adult worm physiology were selected and classified in this list according to their specific targets, and their putative kinases already identified in S. mansoni (see references). A scale of intensity of the action of each inhibitor on pairing and egg laying was defined as follows. For pairing, ++ indicates that <50% pairing is maintained at 24 h, + indicates <50% at 48 h and +/− indicates <50% of pairing at 72 h. For egg laying, ++ indicates a total number of eggs <10% of the eggs laid in controls during 72 h of culture, + indicates a number comprised between 10% and 30% and +/− a number comprised between 30% and 50%.
3.2. Molecular cloning and structural characterization of SmAkt
Using tBLASTp analyzes, a putative nucleotide coding sequence for S. mansoni Akt was identified in GenBank (XM_002578520.1). 5′ and 3′ UTR were determined with SmAkt encoding ESTs (AM042871.1, CF497410.2, AA169931.1 and CF498818.1). The complete nucleotide sequence of SmAkt was amplified by PCR from total cDNA of adult worms. Its open reading frame encodes a 586 amino acid protein with a predicted molecular weight of 67.8 kDa. Screening of S. mansoni genomic databases with the SmAkt cDNA sequence indicated the presence of a single Akt gene in the parasite. A single Akt gene has also been identified in other platyhelminth parasites (Echinococcus, Hymenolepis) (Tsai et al., 2013; Hemer et al., 2014) and in various insects (Drosophila melanogaster, Anopheles gambiae, Aedes aegypti) (Andjelkovic et al., 1995; Riehle and Brown, 2003). However, multiple copies are present in vertebrate genomes and three different genes encode the Akt1, Akt2 and Akt3 proteins which are highly similar in sequence but have different functions in mammals (Hanada et al., 2004; Cohen, 2013). Phylogenetic analysis confirmed that SmAkt belongs to the Akt/PKB family, which is distinct from the two other PKA and PKC families of AGC kinases (Fig. S1). SmAkt groups with trematode (Clonorchis sinensis) and cestode (Echinococcus, Hymenolepis) Akt proteins on a branch separate from that of vertebrate and insect Akt kinases. Sequence alignment of SmAkt with Akt proteins from various species illustrates the important conservation of these kinases (Fig. 1). Global identities between SmAkt and other Akt proteins range from 45% to 56%. SmAkt was found to be more similar to Akt1 than to Akt2 and Akt3 vertebrate proteins. As with the other members of the Akt family, SmAkt possesses a conserved (55–80% identity with others) N-terminal pleckstrin homology (PH) domain (AA 106–208) involved in the binding of 3-phosphoinositides and the recruitment of the protein to the membrane. The central S/T-type protein kinase domain (AA 244–501) is also highly conserved (68–75% identity with others) and contains the consensus D385FG387 triplet corresponding to the ATP-binding domain as well as the conserved residue T401 in the activation loop. In Akt proteins, phosphorylation of this residue by PDK1 (phosphoinositide-dependent kinase-1) stimulates enzymatic activity whereas additional phosphorylation by mTORC2 (mTOR complex 2) of a conserved serine residue in the C-terminal hydrophobic motif (HM) of the regulatory domain, increases the Akt activity (Sarbassov et al., 2005). This serine residue is present in SmAkt at the position S565 inside of the conserved (F-X-X-F/Y-S/T-Y/F) HM sequence F561EQFSF566. In SmAkt, as in cestode and insect Akt proteins, an N-terminal extension precedes the PH domain. This poorly conserved sequence does not contain any known motifs; it is absent from vertebrate Akt proteins and its role is still unknown.
Fig. 1.

Protein sequence alignment of SmAkt with Akt proteins from other species. The SmAkt amino acid sequence was aligned using CLUSTAL W algorithm with Akt sequences from Echinococcus multilocularis (EmAkt: CCW28045.1), Drosophila melanogaster (DmAkt1: Q8INB9.3), Anopheles gambiae (AgAkt: XP_003436044.1), Xenopus laevis (XlAkt: AAG59601.1) and Homo sapiens (HsAkt1: AAL55732.1). Pleckstrin Homology (PH) domain is indicated in green, kinase domain (KD) in red and regulatory region in blue. Stars indicate conserved residues (DFG motif of the ATP binding site, threonine and serine phosphorylation sites) implicated in phosphorylating activity. Arrows indicated the PH domain residues (E117 and L150) involved in PH–KD interactions which maintain Akt kinases in inactive state. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Production of constitutively active mutants of SmAkt and kinase inhibition
Molecular modeling and structure-based studies have indicated that interactions between the PH and KD domains maintain the Akt protein in a closed inactive conformation (Calleja et al., 2007, 2009) (Fig. 2). In this closed conformation, PDK1 is unable to access and phosphorylate T401 of SmAkt, until an upstream signal and PIP3 binding modifies this conformation and allows Akt phosphorylation and activation (Fig. 2). It has been shown that constitutive activation of Akt can result from mutations of residues at the interface between PH and KD domains. Particularly, the E17K and L52R mutations have been described in human cancers and such Akt1 somatic mutants are constitutively active, leading to oncogenic signaling (Parikh et al., 2012).
Fig. 2.
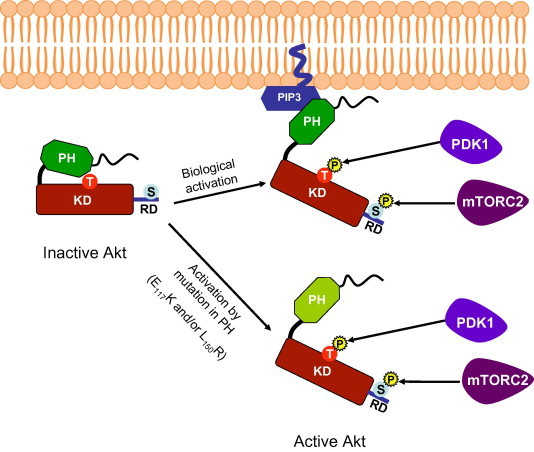
Schematic representation of Akt activation. Intramolecular interactions between PH and KD domains maintain Akt in an inactive conformation. Biological activation of Akt occurs by its targeting at the membrane and the binding of PH domain with PIP3 phospholipids which disrupts PH-KD interactions, unmasking the threonine (T) residue and allowing its phosphorylation by PDK1. Activation of Akt is reinforced by the phosphorylation of a second serine residue present in the C-terminal regulatory domain by mTORC2. Mutations of residues in the PH domain (E117 and L150 in the SmAkt protein) disrupt interactions between PH and KD and lead to Akt activation. (PH: Pleckstrin Homology domain, KD: Kinase Domain, RD: Regulatory domain).
In the present study, we have analyzed the impact of the five Akt inhibitors contained in the InhibitorSelect™ library on the physiology of S. mansoni adult worms (see Table S1). Three of them were shown to considerably affect pairing and egg-laying of adult worms (Table 1). In order to correlate these effects to an inhibition of the kinase activity of SmAkt, we tested the five compounds (A4: Akt inhibitor IV, A5: Triciribine, A6: Akt inhibitor VIII, A7: Akt inhibitor X and A8: PDK1/Akt/Flt dual pathway inhibitor; see formulas in Table S2) on constitutively active mutants of SmAkt created by mutations of the conserved residues E117 and L150 of SmAkt, exactly as in human Akt1 oncogenic active mutants. SmAkt E117K and SmAkt L150R mutants were obtained by site-directed mutagenesis of the SmAkt wild-type sequence contained in the V5-pcDNA expression vector and their activity was monitored in the Xenopus oocyte model. We have already demonstrated that the Xenopus oocyte is a suitable model for expressing S. mansoni proteins and for studying phosphorylating activity of protein kinases (Vicogne et al., 2004). In stage VI oocytes, which are large cells blocked in prophase I of meiosis I, the expression of any active kinase triggers resumption of meiosis and induces Germinal Vesicle BreakDown (GVBD), a process easily detected by eye. Following injection of cRNAs and protein expression in oocytes, V5-tagged SmAktWT, SmAkt E117K and SmAkt L150R proteins were immunoprecipitated by anti-V5 antibodies and detected in Western blots at a molecular weight of about 65 kDa (Fig. 3A). Results in Fig. 3A also indicated that E117K and L150R mutations provoked the constitutive activation of SmAkt and its phosphorylation on the conserved T401 residue, which is recognized by anti-phospho T308 human Akt antibodies. As a result of their activity, expression of SmAkt E117K and SmAkt L150R proteins triggered meiosis resumption in oocytes monitored by GVBD (Fig. 3A and B). In these experimental conditions, we analyzed the capacity of the five Akt inhibitors (A4–8), tested previously on adult worms, to inhibit resumption of meiosis in SmAkt E117K or SmAkt L150R-expressing oocytes. Results in Fig. 3B show that all the inhibitors when used at 10 μM final concentration are able to completely inhibit the phosphorylation of SmAkt mutants and their potential to induce GVBD. Levels of inhibition were further shown to be dose-dependent and to vary between the two Akt mutants. Globally, SmAkt L150R appeared to be more sensitive than SmAkt E117K to each inhibitor. All inhibitors, with the exception of A8, were already active on SmAkt L150R at 1 μM decreasing GVBD levels to more than 60% and they could induce 100% inhibition at 5 μM, whereas the kinase activity of SmAkt E117K was partially (30–50%) inhibited at 5 μM, with the exception of A4 where a total inhibition was obtained. These data confirmed the ability of A4, A5, A6, A7 and A8 molecules to inhibit the recombinant SmAkt kinase whereas both A5 and A6 molecules had no visible effects on adult worms.
Fig. 3.
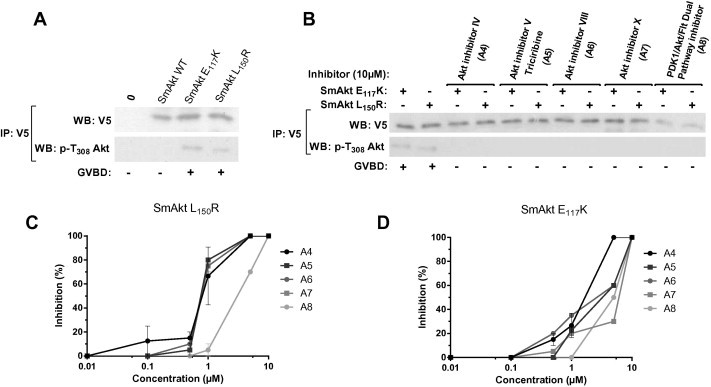
Inhibition of constitutively active mutants of SmAkt expressed in Xenopus oocytes by Akt inhibitors. (A) Western blot analysis of wild type, E117K and L150R V5-tagged SmAkt proteins expressed in Xenopus oocytes. Anti V5-immunoprecipitates were analyzed in Western blot with anti-V5 or with anti-pT308 active Akt antibodies. SmAkt E117K and L150R active kinases are recognized by anti-pT308 serum and are able to trigger meiosis resumption in oocytes (monitored by GVBD). (B) Oocytes expressing SmAkt E117K or SmAkt L150R were incubated with 10 μM of each Akt inhibitor (A4–8). Anti V5-immunoprecipitates were analyzed as in (A). The five compounds inhibit phosphorylation and activity of SmAkt mutants. (C, D) Oocytes expressing SmAkt L150R (C) or E117K (D) were incubated with variable concentrations of Akt inhibitors (A4–8). For each condition, percentages of oocytes with GVBD were calculated and the results expressed in % of inhibition of maturation using as reference oocytes without inhibitor (mean ± SEM of three independent experiments).
3.4. Dose-dependent effect of Akt inhibitors on adult worm pairing in vitro
Results in Table S1 have shown that Triciribine (A5) and Akt inhibitor VIII (A6) had a very low impact on pairing and egg-laying by adult worms. At the opposite, A4, A7 and A8 compounds exerted a potent deleterious effect on adult worms when added at 10 μM in the culture medium, inhibiting totally worm pairing after 24 h of contact with drugs. A kinetic study was performed to measure the effect on schistosomes of these three inhibitors when used at concentrations varying from 10 nM to 10 μM. Results (Fig. 4) confirmed the complete (A4 and A8) and almost complete (A7) inhibition of pairing after 24 h at the maximal dose of 10 μM. Moreover they indicated a stronger effect of the A4 compound which still significantly (90%) inhibits worm pairing at 50 nM after 48 h whereas the two other compounds A7 and A8 did not have any effect at this dose even after 72 h. Results of egg counting were correlated with pairing data. Within 72 h of incubation no egg was laid by worms treated with 100 nM of A4 while 5 μM and 1 μM of A7 and A8 compounds were respectively needed to block 100% egg laying (data not shown).
Fig. 4.
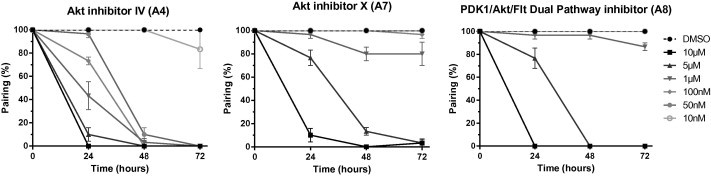
Kinetic study of the influence of Akt inhibitors on adult worm pairing. Freshly perfused paired worms (10 couples) were incubated for 72 h with increasing concentrations (10 nM–10 μM) of Akt inhibitor IV (A4), Akt inhibitor X (A7) and PDK1/Akt/Flt Dual Pathway inhibitor (A8). Controls were incubated with the same aliquot of DMSO solvent. The number of paired worms was determined at 24 h, 48 h and 72 h and results expressed in % of total worms (mean ± SEM of three independent experiments).
3.5. Dose-dependent effect of Akt inhibitors on the viability of schistosomula larvae
Further experiments were performed to measure the impact of Akt inhibitors on the viability of S. mansoni larvae in vitro. One-day old schistosomula were cultured for 3 days with different concentrations of the Akt inhibitors and parasite death was assessed by loss of motility, tegument alterations and granular aspect, as in previous studies (Vanderstraete et al., 2013b). At 10 μM concentration, we observed that A4, A7 and A8 compounds induced 100% mortality in schistosomula cultures after 2 days whereas A6 was less efficient at this period and A5 without any effect on schistosomula viability at 72 h (Fig. S2). In a kinetic study, similar to that performed on adult worms, we analyzed the effect of A4, A7 and A8 on schistosomula survival, using 50 nM to 10 μM doses. Results in Fig. 5 indicated that A8 was the most efficient compound towards schistosomula. At 1 μM, 100% of schistosomula were killed at 24 h with A8 whereas only 50% and 30% parasites were killed after 72 h with this dose of A7 and A4, respectively. The compound A8 induced 50% mortality at 72 h at 50 nM, a concentration which is for A4 and A7 almost inactive at this time period on schistosomula viability.
Fig. 5.
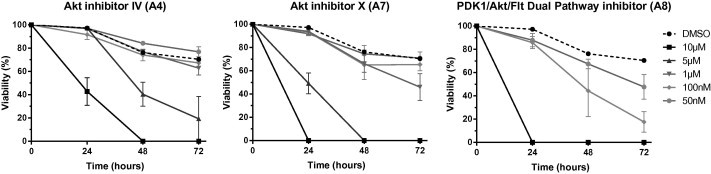
Kinetic study of the influence of Akt inhibitors on schistosomula viability. S. mansoni schistosomula were incubated with different concentrations (50 nM–10 μM) of Akt inhibitor IV (A4), Akt inhibitor X (A7) and PDK1/Akt/Flt Dual Pathway inhibitor (A8). Percentages of alive schistosomula were determined under microscope at 24 h, 48 h and 72 h. Results are expressed as the mean ± SEM of three independent experiments.
4. Discussion and conclusion
Protein kinases are highly conserved molecules that play key roles in the activation of many signaling pathways initiated by external signals. They are involved in many biological processes such as cell growth, proliferation and differentiation. In schistosomes, PKs have been shown to play major roles in development and reproduction processes (Beckmann et al., 2012; Dissous et al., 2014a). Targeting of diverse PKs was shown to affect reproductive biology of parasites, as well as survival of adult worm and/or schistosomula in vitro, supporting the hypothesis that PK inhibitors are potential chemotherapeutics against schistosomiasis (Dissous and Grevelding, 2011).
In this context, we have extended the research of novel anti-schistosome drugs by screening the InhibitorSelect™ 96-Well Protein Kinase Inhibitor Library I (Calbiochem) and by analysing their impact on S. mansoni worm pairing and egg laying in vitro. Most of these PK inhibitors are ATP-competitive and are directed against a large variety of kinases including receptor and soluble STKs or TKs. First results indicated a highly variable effect of these molecules on the parasite, some of them causing a total inhibition of pairing and egg laying while others had no obvious effect on the general worm physiology. Decrease of the level of pairing was in most cases associated with inhibition of egg laying, but in some cases (particularly with EGFR but also with GSK3 (Glycogen synthase kinase 3) and Aurora inhibitors), egg laying was profoundly affected while worm pairing was maintained, suggesting that the targeted kinases were preferentially involved in gamete production. While the role of EGFR in oogenesis and fertilization are well-known (Sundaram, 2006; Cheung et al., 2011), the functions of GSK3 in these processes are not clearly established. However, different studies have reported the role of GSK3 in oocyte maturation dependent on insulin signaling (Acevedo et al., 2007; Uzbekova et al., 2009) and the demonstration that HyGSK3 of Hydra vulgaris is overexpressed during ovogenesis and spermatogenesis and that the inhibition of its kinase activity stops sexual reproduction in Hydra (Rentzsch et al., 2005), supports the hypothesis that GSK3 is important for reproduction of schistosomes. Similarly, the function of Aurora kinases is essential in mitosis and cell proliferation (Marumoto et al., 2003) and this could explain the important effect of Aurora inhibitors on schistosome egg laying.
Of the PK inhibitors identified in this work for their activity on adult parasites, many have been shown to target specific TK molecules, and particularly TKs for which schistosome homologs were already highlighted for their role in development and reproduction. Indeed, we confirmed here the efficacy of tyrphostin AG1024, a potent inhibitory compound towards S. mansoni insulin and VKR receptors which provoked alterations of reproductive organs, and killed adult worms within 48 h at 10 μM (Vanderstraete et al., 2013b). Tyrphostin AG1478, already shown to inhibit SER but also SmVKR1 (Vanderstraete et al., 2013b), reduced considerably the number of laid eggs (16% of control) but as mentioned above it did not affect significantly worm pairing. Recently, SmFGFR-A/B were described for their potential roles in gonad differentiation and reproduction. BIBF1120, a potent triple inhibitor for FGFR, VEGFR and PDGFR affected the viability of adult worms and provoked changes in gonad morphology and a drastic decline of mitotically active cells in testes (Hahnel et al., 2014). In this study, the ATP competitive inhibitor SU11652, which targets FGFR, VEGFR and PDGFR similarly to BIBF1120, also had a drastic effect on pairing and egg laying. Three soluble TKs have been shown to contribute to RTK signaling in schistosomes, SmTK3 (Src-like), SmTK4 (Syk-like) and SmTK6 (Src/Abl-like). The potential of Herbimycin, a Src-specific inhibitor, able to reduce mitotic activity and egg production in female worms (Knobloch et al., 2006) was confirmed in this work by its effect on pairing and egg laying, supporting the role of SmTK3 in the control of fecundity (Kapp et al., 2004). Inhibition of SmTK4 by Syk inhibitors provoked a decrease of egg laying, similar to that observed previously with another Syk inhibitor, Piceatannol (Beckmann et al., 2010). The Syk inhibitor III, which is active both on Syk and Src, was the most efficient on inhibition of pairing and egg laying, probably by its dual effect on both kinases. Other compounds targeting more than one CTKs exerted also a strong inhibitory effect on egg laying, supporting the importance of TK signaling in reproductive activities of schistosomes. Besides these TK enzymes, S/T kinases are fundamental for survival and reproductive function in schistosomes. The strong effect of the TGFβR1 inhibitor III on pairing here confirms the major role of TGFβ signaling in worm pairing and fertility already observed by the use of the TβRI serine/threonine kinase inhibitor (TRIKI) and the demonstration of its deleterious effect on vitelline cell mitotic activity and egg production in female worms (Knobloch et al., 2007; Buro et al., 2013). The PKC inhibitor GF109203X was recently shown to inhibit worm pairing and egg output (Ressurreição et al., 2014). Several other PKC inhibitory compounds were tested in this study and their deleterious effect on adult worms confirmed the importance of PKC in S. mansoni physiology and reproduction.
Additionally, anti-Akt compounds were identified for their potent inhibitory action on pairing and egg laying, and this prompted us to investigate on SmAkt and to study its sensitivity to these inhibitors. Akt (or PKB) proteins belong to the PKA, PKG and PKC (AGC) superfamily of S/T kinases. Akt mediates a variety of physiological responses, including promotion of cell survival and inhibition of apoptosis (Brazil et al., 2004). In mammals, the Akt family consists of three members Akt1, Akt2 and Akt3. Akt1 plays a crucial role in tumorigenesis and is involved in cellular survival pathways, by inhibiting apoptosis and inducing protein synthesis. Akt2 is an important signaling molecule in the insulin signaling pathway, involved in glucose transport and Akt3 is preferentially active in brain (Hanada et al., 2004). In most invertebrates, a single Akt molecule is present, and a single gene encoding a protein homologous to Akt was found in S. mansoni genome databases (Berriman et al., 2009). Phylogenetic analysis confirmed that the protein SmAkt groups with other vertebrate and invertebrate Akt/PKB proteins on a branch distinct from those of PKA and PKC members. SmAkt is close to Akt proteins from other platyhelminths and its protein structure is highly conserved. SmAkt shares with others the domains which are essential for its kinase activity, ie the pleckstrin homology (PH) domain for binding PI(3,4,5)P in membranes and the conserved kinase domain containing an ATP binding motif (D385FG387) and the two conserved phosphorylation sites T401 and S565 required for its activation by upstream kinases. Similarly to other invertebrate proteins, SmAkt contains a divergent N-terminal extension (of about 100 AA) located upstream from the PH domain and which is not found in vertebrate Akt proteins. In D. melanogaster, splicing variants of the single DmAkt gene can generate two proteins differing by the presence or not of the first 81 residues composing this extension sequence (Andjelkovic et al., 1995). It is conceivable that similar variants of Akt could exist in other species, and particularly in S. mansoni. To our knowledge, a possible incidence of this extra sequence in differential activities of DmAkt isoforms was not shown.
The PH domain of Akt plays a significant role in recognition by upstream kinases since it acts as an inhibitor of phosphorylation of T308 of human Akt by masking this residue from PDK1. Intramolecular interactions between PH domain-kinase domain (KD) maintain Akt in an inactive conformation and it has been shown that destabilizing interdomain contacts results in constitutive activation of Akt in human cancers. E17K and L52R mutations in the PH domain of Akt found in human cancers generate constitutively active Akt mutants. Two-hybrid assays using mutated Akt PH and wild type KD constructs have confirmed that PH mutants were deficient in PH–KD interaction (Parikh et al., 2012). As human E17 and L52 residues are conserved, respectively, at positions 117 and 150 in SmAkt, we generated by site-directed mutagenesis E117K and L150R SmAkt mutants. Effectively, both mutated proteins (but not wild-type Akt) were shown to be spontaneously active when expressed in Xenopus oocyte and they were further used in inhibition assays to test anti-Akt compounds on their activity. The five Akt inhibitors contained in the library were shown to inhibit (when used at 10 μM) 100% of the capacity of E117K and L150R SmAkt mutants to be phosphorylated and to induce oocyte maturation. However, as the mode of action on Akt differs significantly for each Akt inhibitor, and specially that some of them like A4 and A8 have also an activity on the upstream kinase PDK1, these results only indicated that the five Akt inhibitors were active on the Akt pathway, by direct or indirect inhibition of the Akt enzyme. Indeed, if A5, A6 and A7 are selective for Akt (Yang et al., 2004; Barnett et al., 2005; Thimmaiah et al., 2005), A4 and A8 are known to also target Akt upstream kinases such as PDK1 (Kau et al., 2003; Zeng et al., 2006) and could as well inhibit Akt activity by this way. A putative Ser/Thr kinase similar to PDK1 is present in the S. mansoni genome (CAZ 36594) (Protasio et al., 2012) susceptible to participate to the PI3K/Akt pathway in schistosomes. Thus, these data confirmed the high conservation of SmAkt with other Akt proteins and the possibility to target SmAkt pathway with Akt inhibitors in living parasites.
During initial screening of the library on adult worms, we noticed that only three out of the five Akt inhibitors reduced at 10 μM worm pairing and egg laying. The five anti-Akt molecules have been classified as cell permeable by the manufacturer, and it is difficult to know whether the inefficiency of Triciribine (A5) and Akt inhibitor VIII (A6) results from the limited permeability or from the instability of the molecules in culture medium. Kinetic studies made to compare the efficacy of A4, A7 and A8 on adult schistosomes confirmed complete (for A4 and A8) and almost complete (for A7) inhibition of worm pairing as soon as 24 h at 10 μM. The A4 compound appeared to be the most active on adult worms, since it remained highly efficient at 50 nM after 48 h whereas the two other compounds A7 and A8 had no more activity at this dose. As on adult parasites, A4, A7 and A8 were the most toxic compounds on schistosomula, able to kill 100% of larvae within 2 days at 10 μM whereas the toxicity of A6 was limited and A5 was totally inefficient on this parasite stage. Interestingly, the most active compound on schistosomula was A8 and not A4 as on adult worms. A8 was able to kill 50% of schistosomula within 3 days at 50 nM whereas A4 and A7 had no activity at this dose. Again, it is difficult to explain the stronger effect of A8 compared to A4 and A7 on larvae, but its PDK1/Akt/Flt dual pathway inhibitory action (Zeng et al., 2006) might reinforce its lethal activity towards parasite larvae.
Akt/PKB is a downstream target of many RTKs and a central player at the crossroads of many metabolic pathways activated in response to growth factors and primarily to insulin. We have previously demonstrated the importance of VKR and insulin-like receptor signaling in the reproductive activities of schistosomes and the capacity of VKR to activate the PI3K/Akt pathway has been confirmed in the model of Xenopus oocytes (Vanderstraete et al., 2014). Preliminary experiments (results not shown) have indicated that the targeting of SmAkt by RNA interference in adult worms induced phenotypes similar to those observed in parasites interfered for SmVKR, showing important morphological changes in female worms, with a disorganization of the ovary and a defect of egg formation (Vanderstraete et al., 2014). In insects, insulin pathway and particularly its most positive regulator the Akt kinase, have been shown to directly participate in reproductive processes in dipterans (Riehle and Brown, 2003) and in hymenopterans (Okada et al., 2010). In this study, we have shown that bisindolylmaleimide I, which targets and inhibits GSK3, the major substrate of Akt demonstrated to contribute to gametogenesis (Rentzsch et al., 2005), also stopped egg laying in schistosomes. Altogether, these data strongly suggest that SmAkt, situated at the junction of diverse essential signaling pathways, acts as a key regulator of reproduction processes in schistosomes, and that it represents a novel interesting drug target for schistosomiasis control. Further structure-based activity studies are now needed to improve our knowledge of SmAkt and to design parasite-specific molecules that could be used for the development of novel chemotherapies.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgments
This research was supported by the Institut de la Sante et de la Recherche Medicale, University Lille 2, France. M.M. and M.V. fellowships were from the Ministere de l’Education Nationale et de la Recherche, France. We declare that no conflict of interest exists.
Appendix A. Supplementary data
Phylogenetic analysis of SmAkt. The tree was generated with MEGA5 using the Neighbor-Joining method under the JTT matrix-based method with 1000 bootstrap repetitions. Phylogeny of SmAkt was analysed using Akt/PKB, PKA and PKC protein kinases of the following species: Aedes aegypti (AaAkt: AAP37655.1), Clonorchis sinensis (CsAkt: GAA49945.1), Drosophila melanogaster (DmAkt1-b: Q8INB9.3), Echinococcus granulosus (EgAkt: CDJ17771.1, EgPKC: CDJ19239.1), Echinococcus multilocularis (EmAkt: CCW28045.1), Homo sapiens (HsAkt1: AAL55732.1, HsAkt2: AAI20996.1, HsAkt3: AAH20479.1, HsPKA Ca: P17612.2, HsPKA Cb: P22694.2, HsPKCa: P17252.4), Hymenolepis microstoma (HmAkt: CDJ07747.1, HmPKC: CDJ12337.1), Mus musculus (MmAkt1: NP_033782.1, MmAkt2: AAH40377.1, MmAkt3: Q9WUA6.1, MmPKA Ca: P05132.3, MmPKA Cb: P68181.2, MmPKC: CAA36907.1), Schistosoma mansoni (SmPKA: ACT63838.1, SmPKC1: AAR00731.1) and Xenopus laevis (XlAkt: AAG59601.1, XlAkt2A: NP_001080091.1, XlAkt2B: NP_001085101.1).
Influence of A5 and A6 Akt inhibitors on schistosomula viability. S. mansoni schistosomula were incubated with 10 μM of A5 (Akt inhibitor V) or A6 (Akt inhibitor VIII). Results are expressed as % of surviving larvae at 24 h, 48 h and 72 h (mean ± SEM of three independent experiments).
Results of the protein kinase inhibitor library screening on adult worms of Schistosoma mansoni. Freshly perfused worms (10 couples) were incubated for 72 h with 10 μM of each inhibitor from the InhibitorSelect™ 96-Well Protein Kinase Inhibitor Library I (Calbiochem). The percentage of paired worms at 24 h, 48 h and 72 h (mean ± SEM of three independent experiments) and the total number of eggs laid during 72 h were determined. Results of egg laying are expressed in % of the number of eggs laid in DMSO control worms during 72 h (mean ± SEM of three independent experiments).
Characteristics of Akt inhibitors.
References
- Acevedo N., Ding J., Smith G.D. Insulin signaling in mouse oocytes. Biol. Reprod. 2007;77:872–879. doi: 10.1095/biolreprod.107.060152. [DOI] [PubMed] [Google Scholar]
- Ahier A., Khayath N., Vicogne J., Dissous C. Insulin receptors and glucose uptake in the human parasite Schistosoma mansoni. Parasite. 2008;15:573–579. doi: 10.1051/parasite/2008154573. [DOI] [PubMed] [Google Scholar]
- Ahier A., Rondard P., Gouignard N., Khayath N., Huang S., Trolet J., Donoghue D.J., Gauthier M., Pin J.P., Dissous C. A new family of receptor tyrosine kinases with a Venus Flytrap binding domain in insects and other invertebrates activated by aminoacids. PLoS One. 2009;4:e5651. doi: 10.1371/journal.pone.0005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andjelkovic M., Jones P.F., Grossniklaus U., Cron P., Schier A.F., Dick M., Bilbe G., Hemmings B.A. Developmental regulation of expression and activity of multiple forms of the Drosophila RAC protein kinase. J. Biol. Chem. 1995;270:4066–4075. doi: 10.1074/jbc.270.8.4066. [DOI] [PubMed] [Google Scholar]
- Andrade L., Nahum L.A., Avelar L.G.A., Silva L.L., Zerlotini A., Ruiz J.C., Oliveira G. Eukaryotic protein kinases (ePKs) of the helminth parasite Schistosoma mansoni. BMC Genomics. 2011;12:215–234. doi: 10.1186/1471-2164-12-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avelar L.G.A., Nahum L.A., Andrade L.F., Oliveira G. Functional diversity of the Schistosoma mansoni tyrosine kinases. J. Signal Transduct. 2011;603290 doi: 10.1155/2011/603290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett S.F., Defeo-Jones D., Fu S., Hancock P.J., Haskell K.M., Jones R.E., Kahana J.A., Kral A.M., Leander K., Lee L.L., Malinowski J., McAvoy E.M., Nahas D.D., Robinson R.G., Huber H.E. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochem. J. 2005;385:399–408. doi: 10.1042/BJ20041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann S., Buro C., Dissous C., Hirzmann J., Grevelding C.G. The Syk kinase SmTK4 of Schistosoma mansoni is involved in the regulation of spermatogenesis and oogenesis. PLoS Pathog. 2010;6:e1000769. doi: 10.1371/journal.ppat.1000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann S., Hahnel S., Cailliau K., Vanderstraete M., Browaeys E., Dissous C., Grevelding C.G. Characterization of the Src/Abl hybrid kinase SmTK6 of Schistosoma mansoni. J. Biol. Chem. 2011;286:42325–42336. doi: 10.1074/jbc.M110.210336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann S., Leutner S., Gouignard N., Dissous C., Grevelding C.G. Protein kinases as potential targets for novel anti-schistosomal strategies. Curr. Pharm. Des. 2012;18:3579–3594. [PubMed] [Google Scholar]
- Berriman M., Haas B.J., LoVerde P.T., Wilson R.A., Dillon G.P., Cerqueira G.C., Mashiyama S.T., Al Lazikani B., Andrade L.F., Ashton P.D., Aslett M.A., Bartholomeu D.C., Blandin G., Caffrey C.R., Coghlan A., Coulson R., Day T.A., Delcher A., DeMarco R., Djikeng A., Eyre T., Gamble J.A., Ghedin E., Gu Y., Hertz-Fowler C., Hirai H., Hirai Y., Houston R., Ivens A., Johnston D.A., Lacerda D., Macedo C.D., McVeigh P., Ning Z., Oliveira G., Overington J.P., Parkhill J., Pertea M., Pierce R.J., Protasio A.V., Quail M.A., Rajandream M.A., Rogers J., Sajid M., Salzberg S.L., Stanke M., Tivey A.R., White O., Williams D.L., Wortman J., Wu W., Zamanian M., Zerlotini A., Fraser-Liggett C.M., Barrell B.G., El Sayed N.M. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460:352–358. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazil D.P., Yang Z.Z., Hemmings B.A. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem. Sci. 2004;29:233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Buro C., Oliveira K.C., Lu Z., Leutner S., Beckmann S., Dissous C., Cailliau K., Verjovski-Almeida S., Grevelding C.G. Transcriptome analyses of inhibitor-treated schistosome females provide evidence for cooperating Src-kinase and TGFβ receptor pathways controlling mitosis and eggshell formation. PLoS Pathog. 2013;9:e1003448. doi: 10.1371/journal.ppat.1003448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calleja V., Alcor D., Laguerre M., Park J., Vojnovic B., Hemmings B.A., Downward J., Parker P.J., Larijani B. Intramolecular and intermolecular interactions of protein kinase B define its activation in vivo. PLoS Biol. 2007;5:e95. doi: 10.1371/journal.pbio.0050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calleja V., Laguerre M., Parker P.J., Larijani B. Role of a novel PH-kinase domain interface in PKB/Akt regulation: structural mechanism for allosteric inhibition. PLoS Biol. 2009;7:e17. doi: 10.1371/journal.pbio.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung L.S., Schüpbach T., Shvartsman S.Y. Pattern formation by receptor tyrosine kinases: analysis of the Gurken gradient in Drosophila oogenesis. Curr. Opin. Genet. Dev. 2011;21:719–725. doi: 10.1016/j.gde.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.M., Jr. The AKT genes and their roles in various disorders. Am. J. Med. Genet. A. 2013;161A:2931–2937. doi: 10.1002/ajmg.a.36101. [DOI] [PubMed] [Google Scholar]
- Colley D.G., Bustinduy A.L., Secor W.E., King C.H. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarco R., Verjovski-Almeida S. Schistosomes–proteomics studies for potential novel vaccines and drug targets. Drug Discov. Today. 2009;14:472–478. doi: 10.1016/j.drudis.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Dissous C., Grevelding C.G. Piggy-backing the concept of cancer drugs for schistosomiasis treatment: a tangible perspective? Trends Parasitol. 2011;27:59–66. doi: 10.1016/j.pt.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Dissous C., Dissous C., Capron A. Isolation and characterization of surface antigens from Schistosoma mansoni schistosomula. Mol. Biochem. Parasitol. 1981;3:215–225. doi: 10.1016/0166-6851(81)90053-0. [DOI] [PubMed] [Google Scholar]
- Dissous C., Khayath N., Vicogne J., Capron M. Growth factor receptors in helminth parasites: signalling and host–parasite relationships. Febs Lett. 2006;580:2968–2975. doi: 10.1016/j.febslet.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Dissous C., Ahier A., Khayath N. Protein tyrosine kinases as new potential targets against human schistosomiasis. Bioessays. 2007;29:1281–1288. doi: 10.1002/bies.20662. [DOI] [PubMed] [Google Scholar]
- Dissous C., Vanderstraete M., Beckmann S., Gouignard N., Leutner S., Buro C., Grevelding C.G. Receptor tyrosine kinase signaling and drug targeting in schistosomes. In: Doerig C., Späth G., Wiese M., editors. Protein Phosphorylation in Parasites: Novel Targets for Antiparasitic Intervention. Wyley-VCH Verlag GmbH&Co.KgaA; Weinheim: 2014. pp. 337–356. [Google Scholar]
- Dissous C., Morel M., Vanderstraete M. Venus Kinase Receptors: prospects in signaling and biological functions of these invertebrate receptors. Front. Endocrinol. 2014;5:72. doi: 10.3389/fendo.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doenhoff M.J., Pica-Mattoccia L. Praziquantel for the treatment of schistosomiasis: its use for control in areas with endemic disease and prospects for drug resistance. Exp. Rev. Anti-Infect. Ther. 2006;4:199–210. doi: 10.1586/14787210.4.2.199. [DOI] [PubMed] [Google Scholar]
- Doenhoff M.J., Cioli D., Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis. 2008;21:659–667. doi: 10.1097/QCO.0b013e328318978f. [DOI] [PubMed] [Google Scholar]
- Gouignard N., Vanderstraete M., Cailliau K., Lescuyer A., Browaeys E., Dissous C. Schistosoma mansoni: structural and biochemical characterization of two distinct Venus Kinase Receptors. Exp. Parasitol. 2012;132:32–39. doi: 10.1016/j.exppara.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Hahnel S., Quack T., Parker-Manuel S.J., Lu Z., Vanderstraete M., Morel M., Dissous C., Cailliau K., Grevelding C.G. Gonad RNA-specific qRT-PCR analyses identify genes with potential functions in schistosome reproduction such as SmFz1 and SmFGFRs. Front. Genet. 2014;5:170. doi: 10.3389/fgene.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada M., Feng J., Hemmings B.A. Structure, regulation and function of PKB/AKT- a major therapeutic target. Biochim. Biophys. Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Hemer S., Konrad C., Spiliotis M., Koziol U., Schaack D., Förster S., Gelmedin V., Stadelmann B., Dandekar T., Hemphill A., Brehm K. Host insulin stimulates Echinococcus multilocularis insulin signalling pathways and larval development. BMC Biol. 2014;12:5. doi: 10.1186/1741-7007-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S.R., Till J.F. Protein tyrosine kinase structure and function. Annu. Rev. Biochem. 2000;69:373–398. doi: 10.1146/annurev.biochem.69.1.373. [DOI] [PubMed] [Google Scholar]
- Johnson L.N. Protein kinase inhibitors: contributions from structure to clinical compounds. Q. Rev. Biophys. 2009;42:1–40. doi: 10.1017/S0033583508004745. [DOI] [PubMed] [Google Scholar]
- Kapp K., Knobloch J., Schussler P., Sroka S., Lammers R., Kunz W., Grevelding C.G. The Schistosoma mansoni Src kinase TK3 is expressed in the gonads and likely involved in cytoskeletal organization. Mol. Biochem. Parasitol. 2004;138:171–182. doi: 10.1016/j.molbiopara.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Kau T.R., Schroeder F., Ramaswamy S., Wojciechowski C.L., Zhao J.J., Roberts T.M., Clardy J., Sellers W.R., Silver P.A. A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell. 2003;4:463–476. doi: 10.1016/s1535-6108(03)00303-9. [DOI] [PubMed] [Google Scholar]
- Khayath N., Vicogne J., Ahier A., Ben Younes A., Konrad C., Trolet J., Viscogliosi E., Brehm K., Dissous C. Diversification of the insulin receptor family in the helminth parasite Schistosoma mansoni. Febs J. 2007;274:659–676. doi: 10.1111/j.1742-4658.2006.05610.x. [DOI] [PubMed] [Google Scholar]
- Knobloch J., Winnen R., Quack M., Kunz W., Grevelding C.G. A novel Syk-family tyrosine kinase from Schistosoma mansoni which is preferentially transcribed in reproductive organs. Gene. 2002;294:87–97. doi: 10.1016/s0378-1119(02)00760-6. [DOI] [PubMed] [Google Scholar]
- Knobloch J., Kunz W., Grevelding C.G. Herbimycin A suppresses mitotic activity and egg production of female Schistosoma mansoni. Int. J. Parasitol. 2006;36:1261–1272. doi: 10.1016/j.ijpara.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Knobloch J., Beckmann S., Burmeister C., Quack T., Grevelding C.G. Tyrosine kinase and cooperative TGFbeta signaling in the reproductive organs of Schistosoma mansoni. Exp. Parasitol. 2007;117:318–336. doi: 10.1016/j.exppara.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Kumar C.C., Madison V. Akt crystal structure and Akt-specific inhibitors. Oncogene. 2005;24:7493–7501. doi: 10.1038/sj.onc.1209087. [DOI] [PubMed] [Google Scholar]
- Leutner S., Beckmann S., Grevelding C.G. Characterization of the cGMP-dependent protein kinase SmcGK1 of Schistosoma mansoni. An. Acad. Bras. Cienc. 2011;83:637–648. doi: 10.1590/s0001-37652011000200023. [DOI] [PubMed] [Google Scholar]
- Long T., Cailliau K., Beckmann S., Browaeys E., Trolet J., Grevelding C.G., Dissous C. Schistosoma mansoni Polo-like kinase 1: a mitotic kinase with key functions in parasite reproduction. Int. J. Parasitol. 2010;40:1075–1086. doi: 10.1016/j.ijpara.2010.03.002. [DOI] [PubMed] [Google Scholar]
- LoVerde P.T., Osman A., Hinck A.P. Schistosoma mansoni: TGF-b signaling pathways. Exp. Parasitol. 2007;117:304–317. doi: 10.1016/j.exppara.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoVerde P.T., Andrade L.F., Oliveira G. Signal transduction regulates schistosome reproductive biology. Curr. Opin. Microbiol. 2009;12:422–428. doi: 10.1016/j.mib.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtmann M.H., Rollinson D., Emery A.M., Walker A.J. Protein kinase C signalling during miracidium to mother sporocyst development in the helminth parasite, Schistosoma mansoni. Int. J. Parasitol. 2009;39:1223–1233. doi: 10.1016/j.ijpara.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Marumoto T., Honda S., Hara T., Nitta M., Hirota T., Kohmura E., Saya H. Aurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cells. J. Biol. Chem. 2003;278:51786–51795. doi: 10.1074/jbc.M306275200. [DOI] [PubMed] [Google Scholar]
- Melman S.D., Steinauer M.L., Cunningham C., Kubatko L.S., Mwangi I.N., Wynn N.B., Mutuku M.W., Karanja D.M., Colley D.G., Black C.L., Secor W.E., Mkoji G.M., Loker E.S. Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Negl. Trop. Dis. 2009;3:e504. doi: 10.1371/journal.pntd.0000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel M., Vanderstraete M., Hahnel S., Grevelding C.G., Dissous C. Receptor tyrosine kinases and schistosome reproduction: new targets for chemotherapy. Front. Genet. 2014;5:238. doi: 10.3389/fgene.2014.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Miyazaki S., Miyakawa H., Ishikawa A., Tsuji K., Miura T. Ovarian development and insulin-signaling pathways during reproductive differentiation in the queenless ponerine ant Diacamma sp. J. Insect. Physiol. 2010;56:288–295. doi: 10.1016/j.jinsphys.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Pal S.K., Reckamp K., Yu H., Figlin R.A. Akt inhibitors in clinical development for the treatment of cancer. Expert Opin. Invest. Drugs. 2010;19:1355–1366. doi: 10.1517/13543784.2010.520701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh C., Janakiraman V., Wu W.I., Foo C.K., Kljavin N.M., Chaudhuri S., Stawiski E., Lee B., Lin J., Li H., Lorenzo M.N., Yuan W., Guillory J., Jackson M., Rondon J., Franke Y., Bowman K.K., Sagolla M., Stinson J., Wu T.D., Wu J., Stokoe D., Stern H.M., Brandhuber B.J., Lin K., Skelton N.J., Seshagiri S. Disruption of PH-kinase domain interactions leads to oncogenic activation of AKT in human cancers. Proc. Natl. Acad. Sci. USA. 2012;109:19368–19373. doi: 10.1073/pnas.1204384109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pica-Mattoccia L., Doenhoff M.J., Valle C., Basso A., Troiani A.R., Liberti P., Festucci A., Guidi A., Cioli D. Genetic analysis of decreased praziquantel sensitivity in a laboratory strain of Schistosoma mansoni. Acta Trop. 2009;111:82–85. doi: 10.1016/j.actatropica.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Protasio A.V., Tsai I.J., Babbage A., Nichol S., Hunt M., Aslett M.A., De Silva N., Velarde G.S., Anderson T.J., Clark R.C., Davidson C., Dillon G.P., Holroyd N.E., Loverde P.T., Lloyd C., McQuillan J., Oliveira G., Otto T.D., Parker-Manuel S.J., Quail M.A., Wilson R.A., Zerlotini A., Dunne D.W., Berriman M. A systematically improved high quality genome and transcriptome of the human blood fluke Schistosoma mansoni. PLoS Negl. Trop. Dis. 2012;6:e1455. doi: 10.1371/journal.pntd.0001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzsch F., Hobmayer B., Holstein T.W. Glycogen synthase kinase 3 has a proapoptotic function in Hydra gametogenesis. Dev. Biol. 2005;278:1–12. doi: 10.1016/j.ydbio.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Ressurreição M., De Saram P., Kirk R.S., Rollinson D., Emery A.M., Page N.M., Davies A.J., Walker A.J. Protein kinase C and extracellular signal-regulated kinase regulate movement, attachment, pairing and egg release in Schistosoma mansoni. PLoS Negl. Trop. Dis. 2014;8(6):e2924. doi: 10.1371/journal.pntd.0002924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehle M.A., Brown M.R. Molecular analysis of the serine/threonine kinase Akt and its expression in the mosquito Aedes aegypti. Insect Mol. Biol. 2003;12:225–232. doi: 10.1046/j.1365-2583.2003.00405.x. [DOI] [PubMed] [Google Scholar]
- Sarbassov D.D., Guertin D.A., Ali S.M., Sabatini D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Strebhardt K. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat. Rev. Drug Discov. 2010;9:643–660. doi: 10.1038/nrd3184. [DOI] [PubMed] [Google Scholar]
- Sundaram M.V. RK/Ras/MAPK signaling. Wormbook. 2006;11:1–19. doi: 10.1895/wormbook.1.80.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swierczewski B.E., Davies S.J. A schistosome cAMP-dependent protein kinase catalytic subunit is essential for parasite viability. PLoS Negl. Trop. Dis. 2009;3:e505. doi: 10.1371/journal.pntd.0000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swierczewski B.E., Davies S.J. Developmental regulation of protein kinase A expression and activity in Schistosoma mansoni. Int. J. Parasitol. 2010;40:929–935. doi: 10.1016/j.ijpara.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmaiah K.N., Easton J.B., Germain G.S., Morton C.L., Kamath S., Buolamwini J.K., Houghton P.J. Identification of N10-substituted phenoxazines as potent and specific inhibitors of Akt signaling. J. Biol. Chem. 2005;280:31924–31935. doi: 10.1074/jbc.M507057200. [DOI] [PubMed] [Google Scholar]
- Tsai I.J., Zarowiecki M., Holroyd N., Garciarrubio A., Sanchez-Flores A., Brooks K.L., Tracey A., Bobes R.J., Fragoso G., Sciutto E., Aslett M., Beasley H., Bennett H.M., Cai J., Camicia F., Clark R., Cucher M., De Silva N., Day T.A., Deplazes P., Estrada K., Fernández C., Holland P.W., Hou J., Hu S., Huckvale T., Hung S.S., Kamenetzky L., Keane J.A., Kiss F., Koziol U., Lambert O., Liu K., Luo X., Luo Y., Macchiaroli N., Nichol S., Paps J., Parkinson J., Pouchkina-Stantcheva N., Riddiford N., Rosenzvit M., Salinas G., Wasmuth J.D., Zamanian M., Zheng Y., Taenia solium Genome Consortium, Cai X., Soberón X., Olson P.D., Lacletten J.P., Brehm K., Berriman M. The genomes of four tapeworm species reveal adaptations to parasitism. Nature. 2013;496:57–63. doi: 10.1038/nature12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzbekova S., Salhab M., Perreau C., Mermillod P., Dupont J. Glycogen synthase kinase 3B in bovine oocytes and granulosa cells: possible involvement in meiosis during in vitro maturation. Reproduction. 2009;138:235–246. doi: 10.1530/REP-09-0136. [DOI] [PubMed] [Google Scholar]
- Vanderstraete M., Gouignard N., Ahier A., Morel M., Vicogne J., Dissous C. The venus kinase receptor (VKR) family: structure and evolution. BMC Genomics. 2013;14:361. doi: 10.1186/1471-2164-14-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderstraete M., Gouignard N., Cailliau K., Morel M., Lancelot J., Bodart J.F., Dissous C. Dual targeting of insulin and venus kinase receptors of Schistosoma mansoni for novel anti-schistosome therapy. PLoS Negl. Trop. Dis. 2013;7:e2226. doi: 10.1371/journal.pntd.0002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderstraete M., Gouignard N., Cailliau K., Morel M., Hahnel S., Leutner S., Beckmann S., Grevelding C.G., Dissous C. Venus Kinase Receptors control reproduction in the platyhelminth parasite Schistosoma mansoni. PloS Pathog. 2014;10:e1004138. doi: 10.1371/journal.ppat.1004138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicogne J., Pin J.P., Lardans V., Capron M., Noël C., Dissous C. An unusual receptor tyrosine kinase of Schistosoma mansoni contains a Venus Flytrap module. Mol. Biochem. Parasitol. 2003;126:51–62. doi: 10.1016/s0166-6851(02)00249-9. [DOI] [PubMed] [Google Scholar]
- Vicogne J., Cailliau K., Tulasne D., Browaeys E., Yan Y., Fafeur V., Vilain J.P., Legrand D., Trolet J., Dissous C. Conservation of epidermal growth factor receptor function in the human parasitic helminth Schistosoma mansoni. J. Biol. Chem. 2004;279:37407–37414. doi: 10.1074/jbc.M313738200. [DOI] [PubMed] [Google Scholar]
- Yang L., Dan H.C., Sun M., Liu Q., Sun X.M., Feldman R.I., Hamilton A.D., Polokoff M., Nicosia S.V., Herlyn M., Sebti S.M., Cheng J.Q. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–4399. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- Zeng Z., Samudio I.J., Zhang W., Estrov Z., Pelicano H., Harris D., Frolova O., Hail N., Jr., Chen W., Kornblau S.M., Huang P., Lu Y., Mills G.B., Andreeff M., Konopleva M. Simultaneous inhibition of PDK1/AKT and Fms-like tyrosine kinase 3 signaling by a small-molecule KP372-1 induces mitochondrial dysfunction and apoptosis in acute myelogenous leukemia. Cancer Res. 2006;66:3737–3746. doi: 10.1158/0008-5472.CAN-05-1278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic analysis of SmAkt. The tree was generated with MEGA5 using the Neighbor-Joining method under the JTT matrix-based method with 1000 bootstrap repetitions. Phylogeny of SmAkt was analysed using Akt/PKB, PKA and PKC protein kinases of the following species: Aedes aegypti (AaAkt: AAP37655.1), Clonorchis sinensis (CsAkt: GAA49945.1), Drosophila melanogaster (DmAkt1-b: Q8INB9.3), Echinococcus granulosus (EgAkt: CDJ17771.1, EgPKC: CDJ19239.1), Echinococcus multilocularis (EmAkt: CCW28045.1), Homo sapiens (HsAkt1: AAL55732.1, HsAkt2: AAI20996.1, HsAkt3: AAH20479.1, HsPKA Ca: P17612.2, HsPKA Cb: P22694.2, HsPKCa: P17252.4), Hymenolepis microstoma (HmAkt: CDJ07747.1, HmPKC: CDJ12337.1), Mus musculus (MmAkt1: NP_033782.1, MmAkt2: AAH40377.1, MmAkt3: Q9WUA6.1, MmPKA Ca: P05132.3, MmPKA Cb: P68181.2, MmPKC: CAA36907.1), Schistosoma mansoni (SmPKA: ACT63838.1, SmPKC1: AAR00731.1) and Xenopus laevis (XlAkt: AAG59601.1, XlAkt2A: NP_001080091.1, XlAkt2B: NP_001085101.1).
Influence of A5 and A6 Akt inhibitors on schistosomula viability. S. mansoni schistosomula were incubated with 10 μM of A5 (Akt inhibitor V) or A6 (Akt inhibitor VIII). Results are expressed as % of surviving larvae at 24 h, 48 h and 72 h (mean ± SEM of three independent experiments).
Results of the protein kinase inhibitor library screening on adult worms of Schistosoma mansoni. Freshly perfused worms (10 couples) were incubated for 72 h with 10 μM of each inhibitor from the InhibitorSelect™ 96-Well Protein Kinase Inhibitor Library I (Calbiochem). The percentage of paired worms at 24 h, 48 h and 72 h (mean ± SEM of three independent experiments) and the total number of eggs laid during 72 h were determined. Results of egg laying are expressed in % of the number of eggs laid in DMSO control worms during 72 h (mean ± SEM of three independent experiments).
Characteristics of Akt inhibitors.


