Keywords: Praziquantel, Schistosoma, Drug resistance, Miracidia, Schistosomiasis, Helminths
Highlights
-
•
We isolated S. mansoni miracidia from 72 Kenyan adults and children.
-
•
We found no evidence of S. mansoni with reduced praziquantel sensitivity.
-
•
An S. mansoni lab isolate with reduced praziquantel sensitivity was established.
-
•
The potential for the emergence of praziquantel resistance remains.
Abstract
Schistosomiasis is a neglected tropical disease caused by blood-dwelling flukes of the genus Schistosoma. While the disease may affect as many as 249 million people, treatment largely relies on a single drug, praziquantel. The near exclusive use of this drug for such a prevalent disease has led to concerns regarding the potential for drug resistance to arise and the effect this would have on affected populations. In this study, we use an in vitro assay of drug sensitivity to test the effect of praziquantel on miracidia hatched from eggs obtained from fecal samples of Kenyan adult car washers and sand harvesters as well as school children. Whereas in a previous study we found the car washers and sand harvesters to harbor Schistosoma mansoni with reduced praziquantel sensitivity, we found no evidence for the presence of such strains in any of the groups tested here. Using miracidia derived from seven car washers to infect snails, we used the shed cercariae to establish a strain of S. mansoni with significantly reduced praziquantel sensitivity in mice. This was achieved within 5 generations by administering increasing doses of praziquantel to the infected mice until the parasites could withstand a normally lethal dose. This result indicates that while the threat of praziquantel resistance may have diminished in the Kenyan populations tested here, there is a strong likelihood it could return if sufficient praziquantel pressure is applied.
1. Introduction
Schistosomiasis is a water-borne parasitic disease that affects more than 249 million people (World Health Organization, 2014) with a global disease burden calculated at 24–56 million disability-adjusted life-years lost (King, 2010). Of the limited number of drugs available to treat schistosomiasis, praziquantel (PZQ) is the least expensive and easiest to use (Hagan et al., 2004) and, since PZQ is highly effective against all schistosome species that infect humans, its use in mass treatment campaigns has grown significantly. In 2006, approximately 12 million people were treated with PZQ and by 2012 this number reached approximately 42 million (World Health Organization, 2014). While the drug is highly effective against sexually mature forms of the parasite it is often unable to cure infections due to its inability to kill juvenile schistosomes at 2–4 weeks post-infection (Pica-Mattoccia and Cioli, 2004; Aragon et al., 2009). As PZQ is often administered with a significant time lapse measured in months or years between treatments this can leave a significant reservoir of schistosomes infecting people that are unaffected by the drug. This, combined with continuing exposure to the parasite, means the drug can often only provide short-term relief from infection. Despite this drawback, PZQ remains the only readily available treatment for schistosomiasis amid concern that as it becomes more widely dispensed, drug resistance traits may emerge thus removing the most effective, albeit flawed drug from the limited treatment options available.
There have been a number of in vivo and in vitro studies documenting differential sensitivity of Schistosoma mansoni isolates to PZQ. For example, a relatively low cure rate was reported during a study of PZQ efficacy and side effects in Senegal in 1991 (Stelma et al., 1995). A subsequent study of a field isolate derived from snails in the same geographical area suggested that, when compared with two isolates from Puerto Rico and Kenya, the Senegal isolate matured in mice at a significantly slower rate thus likely rendering it less susceptible to the drug at the times tested (Fallon et al., 1997). Ismail et al. (1999) generated 12 S. mansoni isolates from patients who had failed to be cured by 3 doses of PZQ that would normally prove effective. These isolates were maintained in mice and 8 were found to have a significantly higher ED50 than controls as well as a significantly diminished contractile response in vitro. In a related study, 3 of 6 isolates retained their decreased response to PZQ after several passages through the life cycle in the absence of PZQ (William et al., 2001). In addition, these isolates had an associated diminished reproductive fitness suggesting reduced PZQ sensitivity may have come with a significant biological cost. In a study using miracidial assays to determine PZQ sensitivity, Lamberton et al. (2010) noted that S. mansoni hatched from eggs obtained from the feces of children after two or more PZQ treatments were more likely to survive in vitro exposure to the drug compared with those from newly infected children. Although this may point to a variation in susceptibility of adult S. mansoni worm survival to repeated drug treatment, it may also reflect differential susceptibility to PZQ of eggs at different stages of maturity as they pass through the host. While treatment failure has been reported in travellers returning from areas endemic for Schistosoma haematobium (Mendonca da Silva et al., 2005; Alonso et al., 2006), a study of individuals infected with S. haematobium on Pemba Island, Tanzania found no indication of PZQ resistance as determined by egg counts and miracidial viability after 20 years of mass drug administration (Guidi et al., 2010).
Fallon and Doenhoff (1994) were able to induce PZQ resistance in the laboratory by exposing a pool of S. mansoni isolates from Puerto Rico, Brazil, Kenya and Egypt that had been maintained in the laboratory for up to 10 years to increasing sub-curative doses of PZQ. Mice infected with the 6th generation of selected cercariae were then treated with 3 × 300 mg/kg doses at days 28, 35 and 37 after infection, and showed only a 7% reduction in worm burden compared with an 88% reduction in mice infected with non-selected worms. Coeli et al. (2013) infected mice with S. mansoni (LE strain) and treated with PZQ following a protocol based on Fallon and Doenhoff (1994) to generate worms with similarly reduced drug sensitivity. This led to a decrease in genetic heterogeneity suggesting that multi-generational PZQ exposure resulted in reduced population diversity. When schistosomes of the drug-selected strain were bred with those of an unselected strain, the F1 offspring were found to have intermediate PZQ sensitivity suggesting the reduced susceptibility trait was co-dominant (Pica-Mattoccia et al., 2009). Couto et al. (2011) were able to generate S. mansoni with reduced PZQ susceptibility within a single life cycle by feeding PZQ to snails harboring the parasite.
Clearly, while studies of PZQ resistance have to take into account confounding factors such as the rate of schistosome maturity as well as individual variations in drug metabolism and immune competency, the development of reduced susceptibility to PZQ is a real possibility. Here, we examine the in vitro susceptibility of miracidia hatched from eggs derived from fecal samples of patients living in two regions of Kenya endemic for schistosomiasis. In addition, we used some of these miracidia to establish an S. mansoni laboratory isolate with significantly reduced susceptibility to PZQ.
2. Materials and methods
2.1. S. mansoni egg collection from fecal samples of infected individuals to obtain miracidia
Isolates of S. mansoni were recovered from eggs in fecal samples of adults working as car washers or sand harvesters in Kisumu, western Kenya. Both groups spend significant periods of time in the water of Lake Victoria or the surrounding streams as part of their occupation. All adults enrolled for this study came from one of these two groups and all had previously been treated with PZQ with the year of last treatment being between 2006 and 2013.
From 2004 to 2007, collaboration between the Kenya Medical Research Institute (KEMRI) and Japan International Cooperation Agency administered a school-based schistosomiasis and soil-transmitted helminth control project in Mwea, central Kenya. The project dispensed annual doses of dewormers including PZQ to all school-aged children in the region regardless of their infection status. Thereafter, the National Deworming Program took over the control activities, and has continued with annual treatment of children since 2012 to date. In this study, enrolled students were between 5 and 16 years of age and were from three primary schools: Thiba, MbuiNjeru and Mukou. Children were sampled in June 2013 or February 2014 with the date of last treatment within 1 year of sample collection.
Fecal samples were tested for S. mansoni eggs using the modified Kato Katz technique. Miracidia obtained from eggs in positive fecal samples from individual patients were used to estimate their in vitro sensitivity to PZQ and to establish a line of S. mansoni with reduced sensitivity to PZQ.
The KEMRI Scientific and Ethical Committees and the Institutional Review Board of the University of New Mexico approved this study. All adults and the parents or guardians of the children involved provided informed consent.
2.2. PZQ susceptibility assay of miracidia derived from fecal samples
Miracidial sensitivity to PZQ was tested in vitro using a modified version of the technique developed by Liang et al. (2001). Freshly hatched miracidia derived from stools of sand harvesters (n = 24), car washers (n = 14) or school children (n = 34) were placed in each well (4–6 miracidia per well) of a 96-well microtitre plate in 40 μl aged tap water. Each row represented a single group of miracidia and received either 0, 10−6 or 10−5 M PZQ. PZQ was prepared as a stock solution of 10−4 M in 1% DMSO and the final concentration of DMSO was 0.1% in all wells including the control. The mean number of groups of miracidia used per patient per concentration of PZQ was 19.2 (range: 6–42). This was dependent on the number of miracidia obtained from a fecal sample. Miracidia were observed with a dissecting microscope prior to (0 min) and 20 min after the addition of PZQ. An independent observer, who had no knowledge of the miracidial source or PZQ concentration used, recorded the number of dead miracidia. Miracidia were assumed dead if they remained immobile. The percentage of miracidia mortality after treatment for 20 min with 10−6 and 10−5 M PZQ was calculated as follows:
2.3. Snail sampling and parasite propagation in mice
In addition to testing PZQ sensitivity of S. mansoni miracidia from parasite eggs of naturally infected individuals, miracidia derived from cercariae shed from naturally infected, field-collected Biomphalaria snails were also tested after passage through laboratory mice. Biomphalaria spp. were collected from sites where schistosomiasis is known to be endemic including Lake Victoria shores at Kisumu and Asao stream, both in western Kenya as well as Kibwezi stream in southern Kenya and the Mukou and Nice Rice irrigation ditches at Mwea, in central Kenya. Snails were washed and separated into 24-well plates with aged tap water for up to 3 days with intermittent inspection for the appearance of S. mansoni cercariae using a dissecting microscope. Shedding snails were pooled and approximately 100 cercariae were used to infect each of 2–3 outbred mice. At 49 days post-infection, S. mansoni eggs were obtained from the mouse livers and hatched in aged tap water. PZQ sensitivity of hatched miracidia was assessed using the assay outlined above.
All animal experimentation complied with the policies, regulations and guidelines mandated by the Institutional Animal Care and Use Committees of the University of New Mexico and KEMRI.
2.4. Generation of an S. mansoni laboratory strain with reduced PZQ susceptibility
Miracidia were hatched from eggs retrieved from fecal samples obtained from 7 car washers and combined. This pool was used to infect Biomphalaria sudanica and the cercariae shed subsequently used to infect outbred mice. An isolate with reduced sensitivity to PZQ was established after 5 generations with exposure to increasing doses of PZQ essentially as described by Fallon and Doenhoff (1994) with the following differences. Briefly, 10 outbred mice were infected with approximately 100 cercariae and randomly distributed into two groups and treated with either 100 mg/kg/day PZQ in 2.5% Cremophor EL (Sigma, USA) (n = 5) or an equivalent volume of 2.5% Cremophor EL vehicle alone (n = 5) on each of days 28 and 35 post infection. 3 weeks after the final dose of PZQ or vehicle, mice were perfused with RPMI medium and the number of worms counted. Statistical analysis of differences in worm yield from PZQ treated and vehicle treated mice was calculated using an unpaired Student’s t-test assuming equal variance.
For each passage, eggs from the liver that survived PZQ treatment (selected) or vehicle treatment (non-selected) were used to infect B. sudanica and the cercariae subsequently used to infect the next generation of mice. This protocol was repeated for the second passage with the dose of PZQ being increased to 200 and 250 mg/kg/day for the third and fourth passages. Mice in the 5th passage received 300 mg/kg/day on days 28, 35 and 37 post-infection. In addition, mice infected with non-selected S. mansoni received 2 × 200 and 2 × 250 mg/kg PZQ on days 28 and 35 post-infection (passages 3 and 4) and 3 × 300 mg/kg PZQ on days 28, 35 and 37 post-infection (passage 5) to establish lethality of the drug at these concentrations for the isolates being selected upon.
PZQ sensitivity of hatched miracidia obtained after each passage was assessed using the protocol described above. Approximately 6 drug-selected miracidia were placed in each of 48 wells of a 96 well microtitre plate. Twenty four wells were treated with 10−5 M PZQ and an additional 24 with the same volume of PZQ vehicle and observed after 0 (pretreatment control), 10 and 20 min by an independent observer. Non-selected miracidia were treated identically. The percentage of drug selected or non-selected miracidia surviving at each time point after treatment was calculated as follows:
Statistical analysis of miracidial survival was performed using an unpaired Student’s t-test assuming unequal variance.
3. Results and discussion
In 2009, we (Melman et al., 2009) published a study measuring PZQ sensitivity of S. mansoni miracidia hatched from eggs derived from feces of adult Kenyan car washers and sand harvesters and discovered there was a 2.42-fold increase in the chance that miracidia would survive PZQ exposure if they were from individuals previously treated with PZQ compared to untreated. Miracidia derived from patients who had had between 4 and 20 PZQ treatments showed mortality that ranged from 30 to over 80% when exposed to 10−5 M PZQ in vitro, while the untreated cohort showed mortality ranging from 60% to 100%. For this study we returned to these occupational groups as well as a cohort of Kenyan school children undergoing PZQ therapy to determine if there is significant variability in PZQ sensitivity of the S. mansoni population infecting these individuals. In addition, we examined variation in PZQ sensitivity of S. mansoni miracidia obtained from mice infected with cercariae derived from naturally infected snail populations.
Fecal samples were obtained from a total of 72 individuals with a history of treatment with PZQ. For the purpose of a direct control, it was not possible to identify individuals within our patient groups who had not received PZQ previously. Irrespective of the patient group the mean miracidial mortality of S. mansoni at 10−5 M PZQ was between 82.1% and 84.6% with the lowest observed value of miracidia from a single patient being 72.7% (Table 1). As these data are comparable with the sensitivity of miracidia derived from eggs of the untreated cohort in our 2009 study, it suggests there is no evidence of diminished PZQ sensitivity in S. mansoni infecting these populations. For the car washers and sand harvesters, the last treatment dates were between 1 and 9 years before the current study and thus, it is perhaps not surprising that, without sustained PZQ treatment, a population of S. mansoni with reduced responsiveness to PZQ has failed to materialize. In addition, there was no indication of reduced miracidial susceptibility to PZQ among samples obtained from the 34 school children that previously had between one and five PZQ treatments (one treatment per year) as part of the Kenyan National Deworming Program. Similarly, when miracidia derived from mice infected with cercariae from naturally infected snails collected in Kisumu, Mwea and Kibwezi areas were tested, no evidence of reduced susceptibility to PZQ was found (mortality at 10−5 M PZQ = 82.3 ± 4.9%; range = 75.8–90.3%). Thus, using miracidia as an indicator of S. mansoni sensitivity to PZQ in the definitive host our data implies there is, as yet, no evidence to suggest that resistance or even reduced sensitivity is an immediate threat in the areas surveyed.
Table 1.
In vitro susceptibility to PZQ of S. mansoni miracidia derived from eggs in patient fecal samples.
| Patient group and location | No. of patients sampled | Mean No. of PZQ treatments per patient (range) | Mean% miracidial mortality (range) |
|
|---|---|---|---|---|
| 10−6 M PZQ | 10−5 M PZQ | |||
| Car washers Kisumu |
14 | 8.4 (3–20) |
26.1 ± 5.5 (17.7–33.3) |
83.2 ± 4.2 (74.4–88.0) |
| Sand harvesters Kisumu |
24 | 4.7 (1–11) |
21.4 ± 3.1 (17.2–25.3) |
81.5 ± 4.8 (72.7–88.8) |
| School children Mwea–MbuiNjera |
11 | 3.8 (1–5) |
27.4 ± 5.0 (19.4–32.1) |
84.6 ± 4.8 (76.3–91.6) |
| School children Mwea–Mukou |
18 | 3.3 (1–5) |
27.0 ± 5.4 (19.4–39.8) |
83.8 ± 4.5 (76.5–90.7) |
| School children Mwea–Thiba |
5 | 3.8 (2–5) |
25.4 ± 4.7 (21.0–32.5) |
82.1 ± 3.7 (78.0 ± 82.6) |
Data shown as mean ± 1 standard deviation.
Administration of one round of PZQ treatment to school children in Tanzania resulted in a significant reduction in genetic diversity of S. mansoni populations within the children (Norton et al., 2010; French et al., 2013). While there are many reasons to account for such genetic ‘bottlenecking’ after PZQ treatment, one concern is it may lead to a greater likelihood of development of PZQ resistant parasite strains. Clearly, at least in the case of S. mansoni infecting the school children who took part in this study this has not happened. A recent analysis of genetic variability of S. mansoni in this cohort suggests there has been no reduction in schistosome burden and genetic diversity actually increased after 4 years of mass drug administration (data not shown). This latter observation would be more in agreement with Huyse et al. (2013) who reported that regular treatment with PZQ did not affect the genetic diversity of S. mansoni in Senegal.
In recent years, PZQ treatment of car washers and sand harvesters in Kisumu has become intermittent with the result that only a small proportion of the infected population is undergoing treatment at any one time. This would leave a significant reservoir of parasites unaffected by the drug and likely allow any S. mansoni strain with reduced susceptibility to be lost from patients, especially if, as has been reported, an ability to withstand PZQ treatment also carries a cost to reproductive fitness (William et al., 2001; Coeli et al., 2013). In order to determine if this population still harbored the potential to generate S. mansoni with reduced PZQ sensitivity, we used a mouse infection model to study the impact of increasing amounts of PZQ on a population of parasites derived from 7 car washers. During the first and second passages, mice were treated with 100 mg/kg PZQ on days 28 and 35 after infection. After the first passage there was a small but significant fall in the number of worms recovered after treatment with 100 mg/kg PZQ compared to vehicle treated controls and an increase in the male to female ratio from 1.8 to 3.0 (Table 2). During passage 5, 3 × 300 mg/kg PZQ was administered to mice on days 28, 35 and 37 after infection with no effect on worm numbers or sex ratio compared with vehicle treated controls. In contrast, treatment of mice infected with non-PZQ selected S. mansoni during passages 3, 4 and 5 with 2 × 200, 2 × 250 and 3 × 300 mg/kg PZQ respectively resulted in 36, 66 and 86% reductions in worm numbers compared to vehicle treated mice. This data suggests that we were able to generate a PZQ isolate with low susceptibility to a normally effective dose of PZQ and is in close accordance with that of Fallon and Doenhoff (1994) who used a number of laboratory strains of S. mansoni from geographically diverse regions as the source of their genetic material. Sexually mature female worms isolated from bisexual infections in mice have been shown to be less sensitive to PZQ in vitro than mature male worms (Pica-Mattoccia et al., 2004) while Delgado et al. (1992) showed a preferential killing of female worms in vivo. Despite some initial selection for males in the first round of PZQ treatment in the experiment reported here we saw no subsequent evidence for the selection of either sex. Interestingly, in a similar experiment using S. mansoni LE strain, Coeli et al. (2013) were also able to generate an isolate that was able to withstand 3 × 300 mg/kg PZQ after 6 generations, but were unable to maintain the strain beyond the 11th generation under PZQ pressure due to a change in the male:female ratio from 2.5 in treated, non-selected worms to 8.7 in treated, PZQ selected worms suggesting that female worms of the LE strain are more susceptible to PZQ after repeated exposure and selection. We will continue to passage our selected S. mansoni strain to determine if heightened female sensitivity to PZQ reported by Coeli et al. (2013) is due to the use of a laboratory strain as the founder population or whether a more genetically diverse founder population leads to a more stable long-term sex ratio.
Table 2.
Worms recovered from infected mice after treatment with PZQ during 5 passages of S. mansoni.
| Passage No. | PZQ treatment mg/kg | No. of worms Mean ± SD | Male:female ratio |
|---|---|---|---|
| 1 | 2 × Vehicle | 48.0 ± 5.9⁎ | 1.8 |
| 2 × 100 | 37.2 ± 5.2 | 3.0 | |
| 2 | 2 × Vehicle | 43.8 ± 7.7 | 2.6 |
| 2 × 100 | 41.0 ± 7.6 | 3.1 | |
| 3 | 2 × Vehicle | 43.2 ± 4.6 | 1.5 |
| 2 × 200 | 36.4 ± 8.6 | 1.4 | |
| 4 | 2 × Vehicle | 53.4 ± 4.2 | 1.5 |
| 2 × 250 | 49.6 ± 4.6 | 1.5 | |
| 5 | 2 × Vehicle | 51.4 ± 1.7 | 1.3 |
| 3 × 300 | 50.2 ± 7.8 | 1.6 | |
SD: 1 standard deviation.
p < 0.05.
Miracidia hatched from livers of PZQ and vehicle treated mice during each passage were assayed for their ability to survive PZQ treatment in vitro (Fig. 1). Our data suggests that miracidia produced by drug-selected S. mansoni are also less susceptible to the drug, especially during the 4th and 5th passage suggesting that acquired resistance may be a heritable trait.
Fig. 1.
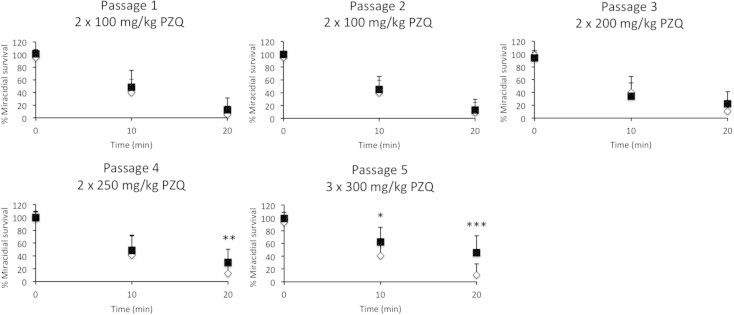
In vitro efficacy of 10−5 M PZQ in killing PZQ selected and non-selected S. mansoni miracidia. Mice infected with S. mansoni were treated with the indicated doses of PZQ over 5 passages (selected) or PZQ vehicle alone (non-selected). The survival of the selected (■) and non-selected (♢) miracidia treated with 10−5 M PZQ was calculated as a percentage of vehicle treated controls at each time point. Data shown as mean + 1 SD (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001).
We have shown that a cohort of 72 adults and children living in endemic areas of western and central Kenya who have undergone recent or historical treatment with PZQ do not harbor S. mansoni with reduced PZQ susceptibility. Nonetheless, while schistosomes with a ‘resistant’ phenotype may not be problematic within these populations, we have also shown there is a significant potential for the emergence of such a phenotype should sufficient PZQ pressure be applied. It is fortuitous that with perhaps only approximately 15% of people with schistosomiasis being treated with PZQ together with the intermittent nature of much of that treatment, a large refugium for drug sensitive parasites will continue to exist. This, together with apparent fitness costs associated with PZQ resistance (William et al., 2001; Coeli et al., 2013), may well prevent the near-term establishment of drug resistant strains in the human population. The far-term prospects for keeping resistance at bay are more worrisome. In 2012, the WHO announced a ‘roadmap’ for the elimination of 17 neglected tropical diseases (NTD) (World Health Organization, 2012), one of which was schistosomiasis. It was proposed that the disease could be eliminated as a public health problem in multiple African countries by 2020 and globally by 2025. This in turn inspired a global alliance of 22 partners including the WHO, The Bill and Melinda Gates Foundation, World Bank and major pharmaceutical companies to announce through the 2012 ‘London Declaration’ a sustained program to ‘control’ schistosomiasis by 2020 (http://unitingtocombatntds.org). While 42 million PZQ tablets were dispensed in 2012 (World Health Organization, 2014) this number is likely to increase greatly in the near future to meet the immediate goal of disease control. Merck KgaA will make 250 million PZQ tablets per year freely available in the medium-term and, with other manufacturers expected to contribute tablets to help bridge the expected shortfall in supply, there are significant grounds for concern that drug pressure will increase significantly in the years ahead. Clearly, close monitoring of drug efficacy should have an important role to play as control efforts are ramped up in the coming years.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgments
The authors would like to acknowledge Joseph Kinuthia, Geoffrey Maina, Martin Mutuku and Stephen Kamau of the Center for Biotechnology Research and Development, KEMRI, and Drs. Diana Karanja and Pauline Mwinzi of the Center for Global Health, KEMRI, Kisian, Kisumu, and their staff for their assistance in field collections. This work was supported through NIH NIAID Grants, R56AI087807 and 1R01AI087807-01A1 and is published with the approval of the Director, KEMRI.
Contributor Information
Ibrahim N. Mwangi, Email: indungu@kemri.org.
Melissa C. Sanchez, Email: melissa3@unm.edu.
Gerald M. Mkoji, Email: gmkoji@kemri.org.
Lelo E. Agola, Email: elelo@kemri.org.
Steven M. Runo, Email: smruno@gmail.com.
Pauline M. Cupit, Email: cupitcu@unm.edu.
Charles Cunningham, Email: ccunnin@unm.edu.
References
- Alonso D., Munoz J., Gascon J., Valls M.E., Corachan M. Failure of standard treatment with praziquantel in two returned travellers with Schistosoma haematobium infection. Am. J. Trop. Med. Hyg. 2006;74:342–344. [PubMed] [Google Scholar]
- Aragon A.D., Imani R.A., Blackburn V.R., Cupit P.M., Melman S.D., Goronga T., Webb T., Loker E.S., Cunningham C. Towards an understanding of the mechanism of action of praziquantel. Mol. Biochem. Parasitol. 2009;164:57–65. doi: 10.1016/j.molbiopara.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coeli R., Baba E.H., Araujo N., Coelho P.M.Z., Oliveira G. Praziquantel treatment decreases Schistosoma mansoni genetic diversity in experimental infections. PLoS Negl. Trop. Dis. 2013;7:e2596. doi: 10.1371/journal.pntd.0002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto F.F., Coelho P.M., Araujo N., Kusel J.R., Jatz N., Jannotti-Passos L.K., Mattos A.C. Schistosoma mansoni: a method for inducing resistance to praziquantel using infected Biomphalaria glabrata snails. Mem. Inst. Oswaldo Cruz. 2011;106:153–157. doi: 10.1590/s0074-02762011000200006. [DOI] [PubMed] [Google Scholar]
- Delgado V.S., Suarez D.P., Cesari I.M., Incani R.N. Experimental chemotherapy of Schistosoma mansoni with praziquantel and oxamniquine: differential effect of single or combined formulations of drugs on various strains and on both sexes of the parasite. Parasitol. Res. 1992;78:648–654. doi: 10.1007/BF00931515. [DOI] [PubMed] [Google Scholar]
- Fallon P.G., Doenhoff M.J. Drug-resistant schistosomiasis: resistance to praziquantel and oxamniquine induced in Schistosoma mansoni in mice is drug specific. Am. J. Trop. Med. Hyg. 1994;53:83–88. doi: 10.4269/ajtmh.1994.51.83. [DOI] [PubMed] [Google Scholar]
- Fallon P.G., Mubarak J.S., Fookes R.E., Niang M., Butterworth A.E., Sturrock R.F., Doenhoff M.J. Schistosoma mansoni: maturation rate and drug susceptibility of different geographical isolates. Exp. Parasitol. 1997;86:29–36. doi: 10.1006/expr.1997.4149. [DOI] [PubMed] [Google Scholar]
- French D.M., Churcher T.S., Basanez M.-G., Norton A.J., Lwambo N.J.S., Webster J.P. Reductions in genetic diversity of Schistosoma mansoni populations under chemotherapeutic pressure: the effect of sampling approach and parasite population definition. Acta Trop. 2013;128:196–205. doi: 10.1016/j.actatropica.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Guidi A., Andolina C., Makame Ame S., Albonico M., Cioli D., Juma Haji H. Praziquantel efficacy and long-term appraisal of schistosomiasis control in Pemba Island. Trop. Med. Int. Health. 2010;15:614–618. doi: 10.1111/j.1365-3156.2010.02488.x. [DOI] [PubMed] [Google Scholar]
- Hagan P., Appleton C.C., Coles G.C., Kusel J.R., Tchuem-Tchuente L.-A. Schistosomiasis control: keep taking the tablets. Trends Parasitol. 2004;20:92–97. doi: 10.1016/j.pt.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Huyse T., Van den Broeke F., Jombart T., Webster B.L., Diaw O., Volckaert F.A.M., Balloux F., Rollinson D., Polman K. Regular treatments of praziquantel do not impact on the genetic make-up of Schistosoma mansoni in Northern Senegal. Infect. Genet. Evol. 2013;18:100–105. doi: 10.1016/j.meegid.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Ismail M., Botros S., Metwally A., William S., Farghally A., Tao L.-F., Day T.A., Bennett J.L. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am. J. Trop. Med. Hyg. 1999;60:932–935. doi: 10.4269/ajtmh.1999.60.932. [DOI] [PubMed] [Google Scholar]
- King C.H. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberton P.H.L., Hogan S.C., Kabatereine N.B., Fenwick A., Webster J.P. In vitro praziquantel test capable of detecting reduced in vivo efficacy in Schistosoma mansoni human infections. Am. J. Trop. Med. Hyg. 2010;83:1340–1347. doi: 10.4269/ajtmh.2010.10-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y.-S., Coles G.C., Doenhoff M.J., Southgate V.R. In vitro responses of praziquantel-resistant and -susceptible Schistosoma mansoni to praziquantel. Int. J. Parasitol. 2001;11:1227–1235. doi: 10.1016/s0020-7519(01)00246-6. [DOI] [PubMed] [Google Scholar]
- Melman S.D., Steinauer M.L., Cunningham C., Kubato L.S., Mwangi I.N., Wynn N.B., Mutuku M.W., Karanja D.M., Colley D.G., Black C.L., Secor W.E., Mkoji G.M., Loker E.S. Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Negl. Trop. Dis. 2009;18:e504. doi: 10.1371/journal.pntd.0000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonca da Silva I., Thiengo R., Conceicao M.J., Rey L., Lenzi H.L., Filho E.P., Ribeiro P.C. Therapeutic failure of praziquantel in the treatment of Schistosoma haematobium infection in Brazilians returning from Africa. Mem. Inst. Oswaldo Cruz. 2005;100:445–449. doi: 10.1590/s0074-02762005000400018. [DOI] [PubMed] [Google Scholar]
- Norton A.J., Gower C.M., Lamberton P.H.L., Webster B.L., Lwambo N.J.S., Blair L., Fenwick A., Webster J.P. Genetic consequences of mass human chemotherapy for Schistosoma mansoni: population structure pre- and post-praziquantel treatment in Tanzania. Am. J. Trop. Med. Hyg. 2010;83:951–957. doi: 10.4269/ajtmh.2010.10-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pica-Mattoccia L., Cioli D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int. J. Parasitol. 2004;34:527–533. doi: 10.1016/j.ijpara.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Pica-Mattoccia L., Doenhoff M.J., Valle C., Basso A., Troiani A.-R., Liberti P., Festucci A., Guidi A., Cioli D. Genetic analysis of decreased praziquantel sensitivity in a laboratory strain of Schistosoma mansoni. Acta Trop. 2009;111:82–85. doi: 10.1016/j.actatropica.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Stelma F.F., Talla I., Sow S., Kongs A., Niang M., Polman K., Deedler A.M., Gryseels Efficacy and side effects of praziquantel in an epidemic focus of Schistosoma mansoni. Am. J. Trop. Med. Hyg. 1995;53:167–170. doi: 10.4269/ajtmh.1995.53.167. [DOI] [PubMed] [Google Scholar]
- William S., Sabra A., Ramzy F., Mousa M., Demerdash Z., Bennett J.L., Day T.A., Botros S. Stability and reproductive fitness of Schistosoma mansoni isolates with decreased sensitivity to praziquantel. Int. J. Parasitol. 2001;31:1093–1100. doi: 10.1016/s0020-7519(01)00215-6. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2012. Accelerating work to overcome the global impact of neglected tropical diseases – a roadmap for implementation executive summary. WHO/HTM/NTD/2012.1. World Heath Organization, Switzerland, Geneva.
- World Health Organization Schistosomiasis: number of people receiving preventative chemotherapy in 2012. Wkly. Epidemiol. Rec. 2014;89:21–28. [PubMed] [Google Scholar]


