Abstract
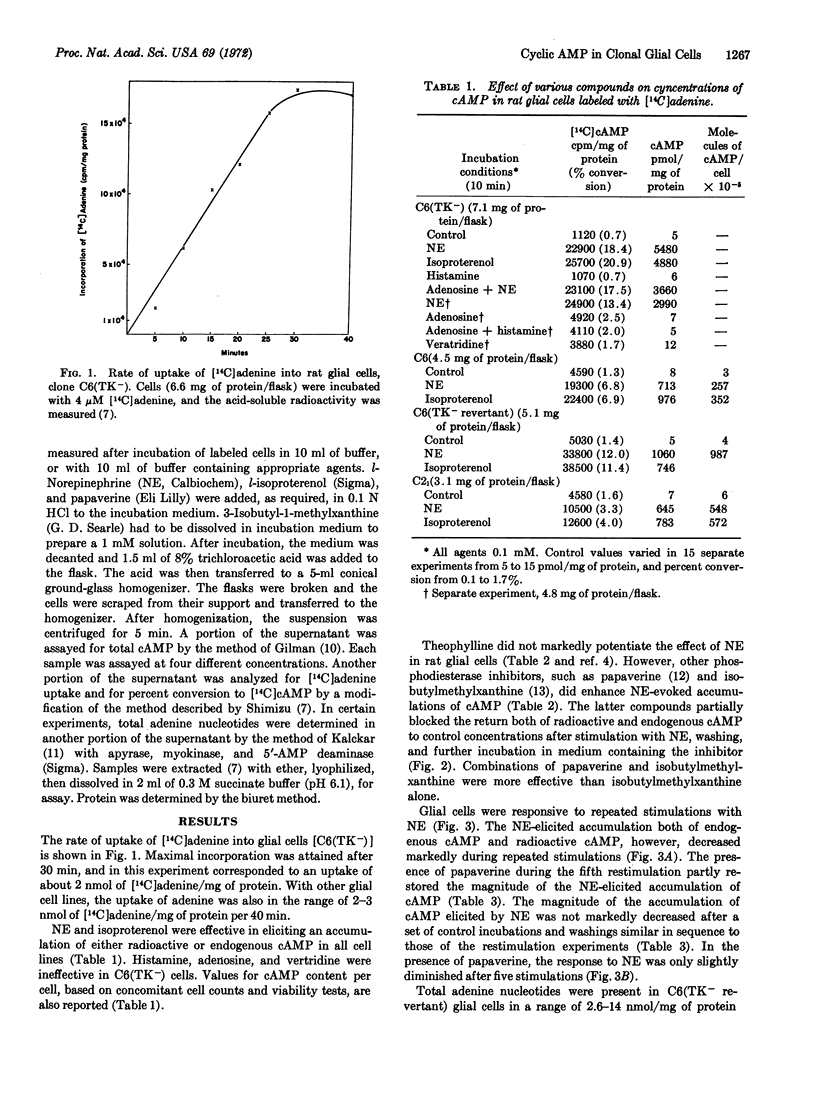
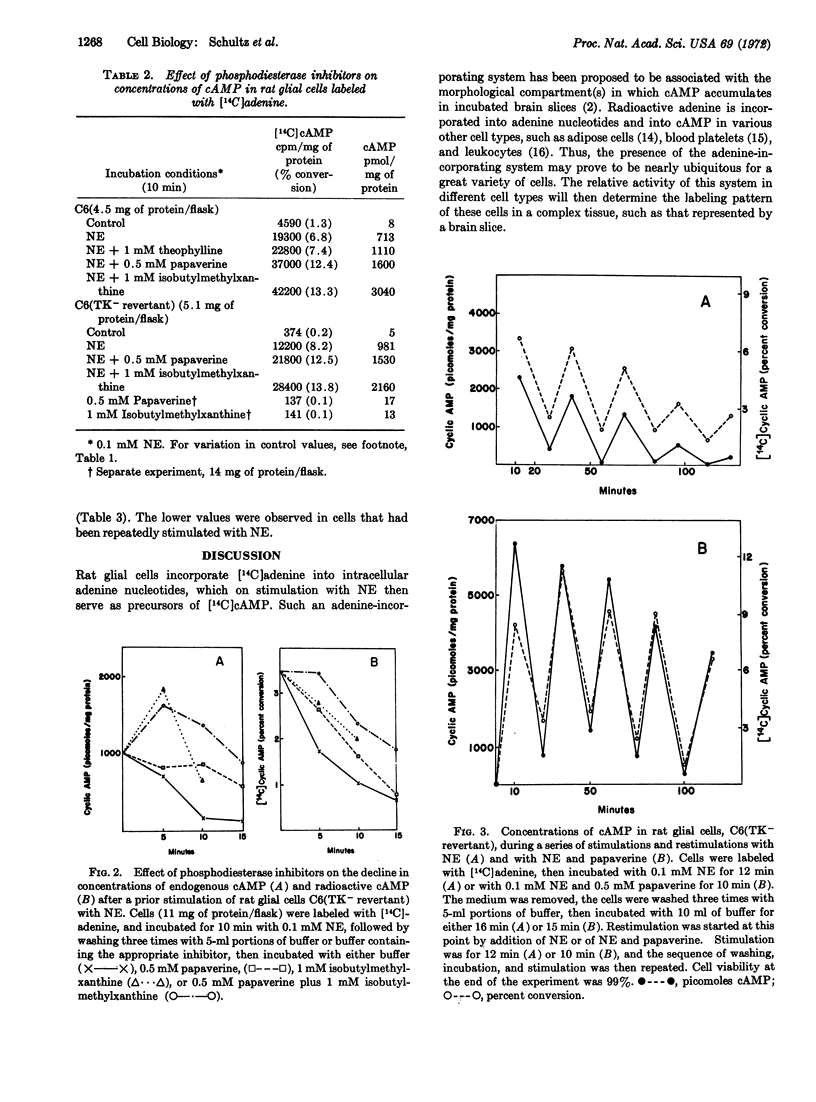
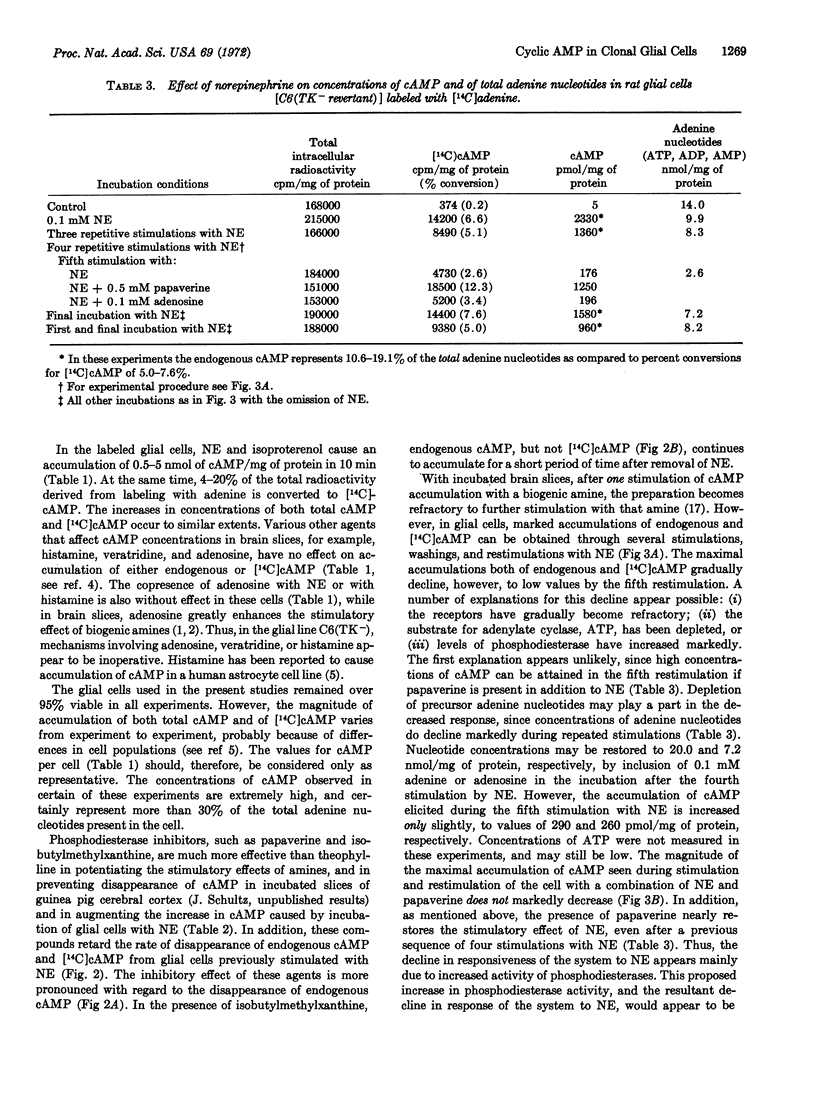
The accumulation of endogenous adenosine 3′:5′-cyclic phosphate (cAMP) and of [14C]cAMP, derived from nucleotides labeled by prior incubation of cells with [14C]adenine, has been investigated in four glial cell lines from rats. The following results are similar to data reported for brain slices: (i) rat glial cells contain a system that readily incorporates [14C]adenine into nucleotides that serve as precursors for cAMP. (ii) norepinephrine stimulates accumulation of both [14C]cAMP and endogenous cAMP, and (iii) the phosphodiesterase inhibitors, papaverine and isobutylmethylxanthine, are effective in enhancing the stimulatory effect of norepinephrine. In contrast to results reported for brain slices, histamine, veratridine, and adenosine, the purine either alone or with a biogenic amine, do not cause an enhanced accumulation of cAMP in rat glial cells, and repetitive accumulations of cAMP can be elicited during a series of restimulations of the cells with norepinephrine. The magnitude of the accumulation of cAMP elicited by the catecholamine decreases markedly by the fifth restimulation in the absence of, but only slightly in the presence of, papaverine. In glial cells, a large portion of the [14C]adenine is incorporated into intracellular compounds that do not serve as precursors of [14C]cAMP.
Keywords: catecholamines, restimulation, phosphodiesterase inhibitors, rat
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavo J. A., Rogers N. L., Crofford O. B., Hardman J. G., Sutherland E. W., Newman E. V. Effects of xanthine derivatives on lipolysis and on adenosine 3',5'-monophosphate phosphodiesterase activity. Mol Pharmacol. 1970 Nov;6(6):597–603. [PubMed] [Google Scholar]
- Benda P., Lightbody J., Sato G., Levine L., Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968 Jul 26;161(3839):370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- Blume A., Gilbert F., Wilson S., Farber J., Rosenberg R., Nirenberg M. Regulation of acetylcholinesterase in neuroblastoma cells. Proc Natl Acad Sci U S A. 1970 Oct;67(2):786–792. doi: 10.1073/pnas.67.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Lehrer R. I., Cline M. J., Melmon K. L. Cyclic 3',5'-adenosine monophosphate in the human lukocyte: synthesis, degradation, andeffects n neutrophil candidacidal activity. J Clin Invest. 1971 Apr;50(4):920–929. doi: 10.1172/JCI106564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. B., Perkins J. P. Regulation of adenosine 3':5'-cyclic monophosphate concentration in cultured human astrocytoma cells by catecholamines and histamine. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2757–2760. doi: 10.1073/pnas.68.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Armiento M., Johnson G. S., Pastan I. Regulation of adenosine 3',5'-cyclic monophosphate phosphodiesterase activity in fibroblasts by intracellular concentrations of cyclic adenosine monophosphate (3T3-dibutyryl cyclic AMP-SV40-transformed cells-michaelis constants-L cells-prostaglandin E 1 ). Proc Natl Acad Sci U S A. 1972 Feb;69(2):459–462. doi: 10.1073/pnas.69.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forn J., Schonhofer P. S., Skidmore I. F., Krishna G. Effect of aging on the adenyl cyclase and phosphodiesterase activity of isolated fat cells of rat. Biochim Biophys Acta. 1970 May 12;208(2):304–309. doi: 10.1016/0304-4165(70)90249-7. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G., Nirenberg M. Effect of catecholamines on the adenosine 3':5'-cyclic monophosphate concentrations of clonal satellite cells of neurons. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2165–2168. doi: 10.1073/pnas.68.9.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G., Nirenberg M. Regulation of adenosine 3',5'-cyclic monophosphate metabolism in cultured neuroblastoma cells. Nature. 1971 Dec 10;234(5328):356–358. doi: 10.1038/234356a0. [DOI] [PubMed] [Google Scholar]
- Haslam R. J., Taylor A. Effects of catecholamines on the formation of adenosine 3':5'-cyclic monophosphate in human blood platelets. Biochem J. 1971 Nov;125(1):377–379. doi: 10.1042/bj1250377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiuchi S., Rall T. W. The influence of chemical agents on the accumulation of adenosine 3',5'-Phosphate in slices of rabbit cerebellum. Mol Pharmacol. 1968 Jul;4(4):367–378. [PubMed] [Google Scholar]
- Maganiello V., Vaughan M. Prostaglandin E 1 effects on adenosine 3':5'-cyclic monophosphate concentration and phosphodiesterase activity in fibroblasts (mouse L cells-tissue culture-enzyme kinetics-prostaglandin homologues). Proc Natl Acad Sci U S A. 1972 Jan;69(1):269–273. doi: 10.1073/pnas.69.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall T. W., Sattin A. Factors influencing the accumulation of cyclic AMP in brain tissue. Adv Biochem Psychopharmacol. 1970;3:113–133. [PubMed] [Google Scholar]
- Sheppard H., Wiggan G. Different sensitivities of the phosphodiesterases (adenosine-3',5'-cyclic phosphate 3'-phosphohydrolase) of dog cerebral cortex and erythrocytes to inhibition by synthetic agents and cold. Biochem Pharmacol. 1971 Aug;20(8):2128–2130. doi: 10.1016/0006-2952(71)90426-6. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Creveling C. R., Daly J. W. Effect of membrane depolarization and biogenic amines on the formation of cyclic AMP in incubated brain slices. Adv Biochem Psychopharmacol. 1970;3:135–154. [PubMed] [Google Scholar]
- Shimizu H., Daly J. W., Creveling C. R. A radioisotopic method for measuring the formation of adenosine 3',5'-cyclic monophosphate in incubated slices of brain. J Neurochem. 1969 Dec;16(12):1609–1619. doi: 10.1111/j.1471-4159.1969.tb10360.x. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Daly J. Formation of cyclic adenosine 3',5'-monophosphate from adenosine in brain slices. Biochim Biophys Acta. 1970 Nov 24;222(2):465–473. doi: 10.1016/0304-4165(70)90137-6. [DOI] [PubMed] [Google Scholar]


