Graphical abstract
Keywords: Eimeria, Anticoccidial drug, Live vaccine, Poultry, Rotation program, Coccidiosis
Highlights
-
•
Resistance has developed to all those drugs used to control coccidiosis in chickens.
-
•
Some vaccines contain strains of Eimeria that are sensitive to drugs.
-
•
Such strains may replace drug resistant strains in commercial poultry houses.
-
•
A production cycle involving chemotherapy followed by vaccination is presented.
-
•
Such a yearly cycle may restore sensitivity to anticoccidial drugs.
Abstract
Drug resistance is a problem wherever livestock are raised under intensive conditions and drugs are used to combat parasitic infections. This is particularly true for the anticoccidial agents used for the prevention of coccidiosis caused by protozoa of the apicomplexan genus Eimeria in poultry. Resistance has been documented for all the dozen or so drugs approved for use in chickens and varying levels of resistance is present for those currently employed. A possible solution may be the introduction of drug-sensitive parasites into the houses where poultry are raised so that they may replace such drug-resistant organisms. This can be achieved by utilizing live vaccines that contain strains of Eimeria that were isolated before most anticoccidial compounds were introduced. Such strains are inherently drug-sensitive. Practical proposals to achieve this objective involve the alternation of vaccination with medication (known as rotation programs) in successive flocks reared in the same poultry house. A proposal for a yearly broiler production cycle involving chemotherapy and vaccination is presented. There are few, if any, examples in veterinary parasitology where it has proved possible to restore sensitivity to drugs used to control a widespread parasite. Further research is necessary to ascertain whether this can result in sustainable and long-term control of Eimeria infections in poultry.
1. Introduction
Modern poultry production frequently involves the raising of large numbers of birds on built-up litter at high stocking densities in houses specifically designed for that purpose (Bell and Weaver, 2002). Thus, a typical broiler house may contain as many as 20–50,000 chickens reared beneath one roof at a stocking density of one bird per 0.08 m2. Production is usually based upon the “all in–all out” principle in which birds are placed on litter upon arrival from the hatchery, remain in the house for the duration of the rearing period, and then are removed either for slaughter (broilers) or transferred to cages (some egg layers). After a brief period of a few weeks, known as the “down-time” a fresh flock is introduced. The litter may be replaced after every flock or after several successive flocks, in the USA it is common for six flocks to be raised each year with no change of litter. Thus, for the period of production, the flock represents a closed population. This has important implications for those organisms that parasitize poultry and the development of preventive control strategies.
Rearing birds under intensive conditions upon litter provides an ideal opportunity for parasite transmission, especially those such as Eimeria that develop in the intestinal tract, and have an oral/faecal life cycle. Without an effective means of control the numbers of parasites present in a house can increase to such an extent that they can cause coccidiosis. Clinical disease is uncommon but poor performance, manifest by reduced feed intake, poor weight gain, and impaired feed conversion results in losses to the poultry industry of many millions of dollars per annum (Williams, 1999).
2. Life cycle and genetics of Eimeria
The life cycle of a typical Eimeria species involves three phases: (1) sporogony or sporulation – resulting in the formation of the infective transmission stage the sporulated oocyst, (2) schizogony – a process of asexual reproduction also known as merogony that results in amplification of parasite numbers in the intestine, and (3) gametogony – the production of male and female gametes which, following fertilization to form a zygote, becomes the unsporulated oocyst. Sporulation involves a meiotic division followed by a mitotic division that results in the formation of four sporocysts; a mitotic division then occurs within each sporocyst to form two genetically identical haploid sporozoites (Canning and Anwar, 1968; Canning and Morgan, 1975). All subsequent stages in the life cycle after the meiotic division in the oocyst are haploid, thus mitotic division occurring within each schizont results in the formation of haploid merozoites which are all genetically identical and therefore represents a clonal population. Genetic homogeneity is also more probable because there is a real possibility of self-fertilization following gametogony, which will result in clonal offspring (Shirley and Millard, 1976; Walker et al., 2013). Furthermore if less than the eight sporozoites in an ingested oocyst fail to infect the chicken, the likelihood of genetically identical gametes increases.
Several genetic traits have been identified within species of avian coccidia, including developmental rate, enzyme variation, antigenicity, and drug sensitivity (Jeffers, 1978). Drug resistance in field populations of Eimeria is widespread and generally reflects the previous use of anticoccidials in the respective poultry facility (Jeffers, 1974a,b,c). Genetic recombination between strains resistant to drugs with different modes of action has been demonstrated (e.g. Joyner and Norton, 1975; Chapman, 1984) and it has also proved possible to obtain recombination between drug-sensitive vaccine strains and drug-resistant strains (Jeffers, 1976b; Shirley and Harvey, 2000). This may contribute to a reduction in drug resistance (Williams, 1998, 2002a). However, the impact of coccidiosis vaccines in restoring drug sensitivity to be described is largely due to replacement of the resident population of coccidia by vaccine-derived drug-sensitive coccidia, because the rate of genetic recombination in avian coccidia is likely quite low. For example, recombinant coccidia constituted no more than 0.04% of the total oocysts produced in a cross between drug-sensitive and drug-resistant strains of Eimeria tenella (Jeffers, 1974d). Although a greater degree of hybridization was found in a cross of two drug-resistant strains of E. tenella, the recombinant coccidia still only comprised 1.5% of the total oocysts produced. Other studies support the notion that the rate of genetic recombination in avian coccidia is apparently quite low relative to the number of parental type coccidia produced in crosses of strains expressing different genetic traits (see review by Chapman et al. (2013)).
3. Chemotherapy and drug resistance
Anticoccidial drugs have been used in broilers for the prevention of coccidiosis for many years, and today, in many countries with large poultry industries (e.g. Brazil, China, and the USA) they remain the mainstay of poultry production and are likely to continue to do so in the foreseeable future. Thus in a survey conducted in the USA from 1995 to 1999 it was found that the use of drugs was almost universal, anticoccidials being employed by 99% of commercial broiler operations (Chapman, 2001). An analysis for 2013/2014 indicated that drug use in the USA varied from 60 to 99% depending upon the time of year (Chapman, unpublished observations). A consequence of this extensive drug use has been the development of resistance, which has been documented for all drugs and wherever poultry are reared under intensive conditions (Chapman, 1997).
Anticoccidial drugs can be broadly divided into two categories: the ionophores that are produced by fermentation and the synthetic drugs that are produced by chemical synthesis. Ionophores include lasalocid, monensin, narasin, salinomycin, and semduramicin and are thought to have a common mode of action involving disruption of ion gradients across the parasite cell membrane (Chapman, 1997). Synthetic drugs include a diverse range of compounds with various modes of action including inhibition of parasite mitochondrial respiration (decoquinate, clopidol) inhibition of the folic acid pathway (sulphonamides), and competitive inhibition of thiamine uptake (amprolium). In many cases (e.g. diclazuril, halofuginone, nicarbazin, and robenidine) the mode of action is not known. A combination of a synthetic drug (nicarbazin) and an ionophore (narasin) is also widely used in poultry production and in the past a combination of methyl benzoquate and clopidol was employed.
One approach adopted by the poultry industry to ameliorate resistance has been to alternate the use of compounds with different modes of action. This can take several forms such as the so-called “shuttle” program in which different drugs are incorporated in different feeds provided to birds. Often a synthetic drug such as nicarbazin will be incorporated in the first (starter) feed followed by an ionophore in the second (grower) feed. Another approach is the so called “rotation” program in which drugs with different modes of action are employed in successive flocks (McDougald, 1982). An underlying philosophy behind these programs is that if resistance is selected during use of the first drug then it will be lost during use of the second but this remains unproven. Although shuttle and rotation programs may have slowed the acquisition of resistance, most field isolates of Eimeria show varying levels of resistance to more than one drug (Chapman, 1997; Peek and Landman, 2003).
4. Vaccination
Most commercially available anticoccidial vaccines contain live oocysts of attenuated or non-attenuated strains of coccidia (Shirley et al., 2007) but until recently their use was limited to birds reared for egg production. They may be administered in the drinking water or sprayed on the feed at the farm but this is labour intensive and expensive, improved methods of administration, such as spray cabinets in the hatchery, have facilitated their use in broiler chickens (Chapman, 2000; Williams, 2002a). Low numbers of oocysts are included in live vaccines and it may seem surprising that they could have a significant impact on the population of coccidia already present in a poultry production facility. However, experiments have shown that as few as 10 oocysts (of E. tenella) ingested by a chicken may give rise to more than 500,000 oocysts as progeny (Chapman, 1978). Although administration of coccidiosis vaccines by a spray cabinet in the hatchery does not assure that every chick actually ingests oocysts, most birds probably do ingest them either by preening, or by receiving oocysts in the eye, whereby it is thought that they pass through the lacrimal duct into the nasal cavity and thereby reach the intestine through the pharynx (Chapman et al., 2002). Therefore, if it is conservatively assumed that only 50% of the chicks ingest 10 or more oocysts as a result of spray cabinet administration, 50,000 vaccinated birds could produce more than one billion new drug-sensitive vaccine-derived oocysts when placed in a broiler production facility.
Some vaccines (e.g. Coccivac® and Paracox®) contain species that were isolated before most anticoccidial drugs were introduced and are inherently sensitive to them. Their seed stocks have subsequently been maintained in the laboratory for many years free of exposure to medication. Thus, the coccidia in these vaccines are thought to be genetically sensitive to all anticoccidials and there is a high probability that their progeny will be drug-sensitive as well.
Freshly passed vaccine derived oocysts are likely more infective than older resident coccidia, thus producing many more vaccine-derived drug-sensitive parasites following their ingestion from the litter. This will increase the drug sensitivity of the population of coccidia within the poultry facility. This supposition is supported by the observation that in a mixed population consisting of drug-resistant and drug-sensitive coccidia, the drug-sensitive parasites dominate in the absence of medication needed to confer a selective advantage to the drug-resistant population (Long et al., 1985). Therefore, in attempting to restore sensitivity to a particular drug by the use of coccidiosis vaccines, it is important that medication be avoided for a period sufficient to allow recycling of vaccine-derived oocysts from the litter. As birds continue to consume albeit small numbers of vaccine derived oocysts their immunity to subsequent coccidial infection increases, further negating the build-up of the resident drug-resistant population of coccidia.
5. Restoration of drug sensitivity
The pattern of drug sensitivity in a population of coccidia can be greatly altered by the introduction of drug-sensitive coccidia, which can be accomplished through the use of some coccidiosis vaccines, or by the use of drug-sensitive laboratory maintained lines (Jeffers, 1976a). In a laboratory experiment, Ball (1966) showed that when a small number of oocysts (of E. tenella) from a drug-resistant population were included in a larger number of drug-sensitive parasites and propagated in unmedicated chickens, the number of drug-resistant parasites decreased. A similar loss of resistance to amprolium by admixture of sensitive and resistant strains was shown by McLoughlin and Chute (1979). Restoration of sensitivity to monensin through the use of a non-attenuated coccidiosis vaccine was demonstrated in commercial broiler production (Chapman, 1994) the vaccine employed (Coccivac-B®) was first introduced in 1952 and the component strains have been propagated in the laboratory ever since (Williams, 2002b). An increase in the sensitivity of field strains of Eimeria to diclazuril and salinomycin was shown following use of the same vaccine (Mathis and Broussard, 2006; Jenkins et al., 2010). It might be thought that use of a vaccine containing attenuated parasites was less likely to result in replacement of drug-resistant strains since the parasites in such vaccines have an inherently poor reproductive potential (Jeffers, 1975; Shirley, 1989). However, partial restoration of sensitivity to diclazuril and monensin was observed following use of the attenuated vaccine Paracox® (Peek and Landman, 2006). Restoration of sensitivity by introduction of drug-sensitive coccidia is not confined to parasites of the chicken but also been shown in the turkey (Mathis and McDougald, 1989).
Based upon these findings, a yearly rotation program for chickens involving the use of both anticoccidial chemotherapy and vaccination was proposed (Chapman et al., 2002, 2010). A variation of such a program based upon a yearly cycle involving six successive flocks of broilers is shown in Fig. 1. For the first two flocks (January to April), an anticoccidial chemotherapy program is employed. This could involve a full ionophore program (ionophore in both starter and grower feed), a shuttle program involving either a synthetic drug or ionophore in the starter feed and a different ionophore in the grower feed, or the use of a potentiated ionophore such as a combination of nicarbazin and narasin. This is followed by a clean-out, or in-house composting of litter, in order to greatly reduce the resident population of coccidia prior to placement of the next flock of birds. A vaccine based upon sensitive strains may then be used for two flocks (May to August) to repopulate the house with the drug-sensitive strains. It is then proposed that for the final two flocks (September to December) a different anticoccidial program be employed with the hope that the efficacy of the latter will be improved. Programs such as this are employed by approximately 35–40% of broiler companies in the USA (Broussard, personal communication). The rotation program described is designed for the broiler industry in the USA but many variations may be appropriate for different production systems in other parts of the world.
Fig. 1.
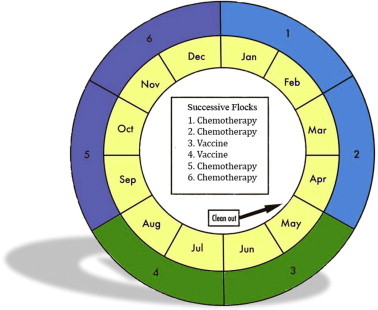
Proposal for a yearly chicken production cycle involving six successive flocks of broilers. Chemotherapy comprises an anticoccidial program involving synthetic drugs, ionophores, or a potentiated ionophore (see text). Litter is removed (clean-out) after the second flock. The Figure is based upon an illustration by Chapman, H.D. in Poultry Health Today.com, 2014.
6. Conclusions
The control of avian coccidiosis is one of the major success stories of veterinary parasitology. However, as long as birds are reared in contact with their faeces, as when they are raised on built-up litter, then diseases such as coccidiosis will continue to occur. Alternative production systems might limit such contact, such as the raising of birds on wire floors, but such systems have proved to be uneconomic in broiler production. Attempts to eradicate the parasite by quarantine, disinfection, and sanitation have not been successful (McDougald, 2008). Vaccination is often promoted as an alternative to chemotherapy for the control of coccidiosis and indeed is the method of choice for the prevention of the disease in egg-laying stock. However, most current commercial vaccines comprise live oocysts of several species of Eimeria that are expensive and difficult to produce and it is unlikely that they could be generated in sufficient quantity to satisfy the needs of the much larger broiler industry. Coccidiosis vaccines may have a significant impact on the resident drug-resistant population of coccidia in poultry facilities and play an important role in integrated control programs involving chemotherapy and vaccination. By this means, long-term, sustainable control of coccidiosis may be achieved.
Conflict of interest
The authors declared that there is no conflict of interest.
References
- Ball S.J. The development of resistance to glycarbylamide and 2-chloro-4-nitrobenzamide in Eimeria tenella in chicks. Parasitology. 1966;56:25–37. doi: 10.1017/s0031182000071067. [DOI] [PubMed] [Google Scholar]
- Bell D.D., Weaver W.D. 5th ed. Kluwer Academic Publishers; 2002. Commercial Chicken Meat and Egg Production. [Google Scholar]
- Canning E.U., Anwar M. Studies on meiotic division in coccidial and malarial parasites. J. Protozool. 1968;15:290–298. doi: 10.1111/j.1550-7408.1968.tb02125.x. [DOI] [PubMed] [Google Scholar]
- Canning E.U., Morgan K. DNA synthesis, reduction and elimination during life cycles of Eimeriine coccidian, Eimeria tenella and the Haemogregarine, Hepatozoon domerguei. Exp. Parasitol. 1975;38:217–277. doi: 10.1016/0014-4894(75)90024-7. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. The effect of monensin on the immunity arising from repeated low-level infections with Eimeria maxima, E. brunetti and E. tenella. Avian Pathol. 1978;7:269–277. doi: 10.1080/03079457808418278. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Development by genetic recombination of a line of Eimeria tenella resistant to robenidine, decoquinate and amprolium. Z. Parasitenkd. 1984;70:437–441. doi: 10.1007/BF00926683. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Sensitivity of field isolates of Eimeria to monensin following the use of a coccidiosis vaccine in broiler chickens. Poult. Sci. 1994;73:476–478. doi: 10.3382/ps.0730476. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian Pathol. 1997;26:221–244. doi: 10.1080/03079459708419208. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Practical use of vaccines for the control of coccidiosis in the chicken. Wld’s Poult. Sci. J. 2000;56:7–20. [Google Scholar]
- Chapman H.D. Use of anticoccidial drugs in broiler chickens in the USA: analysis for the years 1995–1999. Poult. Sci. 2001;80:572–580. doi: 10.1093/ps/80.5.572. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Jeffers T.K., Williams R.B. Forty years of monensin for the control of coccidiosis in poultry. Poult. Sci. 2010;89:1788–1801. doi: 10.3382/ps.2010-00931. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Barta J.R., Blake D.P., Gruber A., Jenkins M., Smith N.C., Suo X., Tomley F.M. A selective review of advances in coccidiosis research. Adv. Parasitol. 2013;83:107–116. doi: 10.1016/B978-0-12-407705-8.00002-1. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Cherry T.E., Danforth H.D., Richards G., Shirley M.W., Williams R.B. Sustainable coccidiosis control in poultry production: the role of live vaccines. Int. J. Parasitol. 2002;32:617–629. doi: 10.1016/s0020-7519(01)00362-9. [DOI] [PubMed] [Google Scholar]
- Jeffers T.K. Eimeria tenella: incidence, distribution, and anticoccidial drug resistance of isolants in major broiler-producing areas. Avian Dis. 1974;18:74–84. [PubMed] [Google Scholar]
- Jeffers T.K. Eimeria acervulina and E. maxima: incidence and anticoccidial drug resistance of isolants in major broiler-producing areas. Avian Dis. 1974;18:331–342. [PubMed] [Google Scholar]
- Jeffers T.K. Anticoccidial drug resistance: differences between Eimeria acervulina and E. tenella strains within broiler houses. Poult. Sci. 1974;53:1009–1013. doi: 10.3382/ps.0531009. [DOI] [PubMed] [Google Scholar]
- Jeffers T.K. Genetic transfer of anticoccidial drug resistance in Eimeria tenella. J. Parasitol. 1974;60:900–904. [PubMed] [Google Scholar]
- Jeffers T.K. Attenuation of Eimeria tenella through selection for precociousness. J. Parasitol. 1975;61:1083–1090. [PubMed] [Google Scholar]
- Jeffers T.K. Reduction of anticoccidial drug resistance by massive introduction of drug sensitive coccidia. Avian Dis. 1976;20:649–653. [PubMed] [Google Scholar]
- Jeffers T.K. Genetic recombination of precociousness and anticoccidial drug resistance in Eimeria tenella. Z. Parasitenkd. 1976;50:251–255. doi: 10.1007/BF02462970. [DOI] [PubMed] [Google Scholar]
- Jeffers T.K. Genetics of coccidia and the host response. In: Long P.L., Boorman K.N., Freeman B.M., editors. Avian Coccidiosis. Brit. Poult. Sci. Ltd.; Edinburgh, UK: 1978. pp. 50–125. [Google Scholar]
- Jenkins M., Klopp S., Ritter D., Miska K., Fetterer R. Comparison of Eimeria species distribution and salinomycin resistance in commercial broiler operations utilizing different coccidiosis control strategies. Avian Dis. 2010;54:1002–1006. doi: 10.1637/9137-111109-Reg.1. [DOI] [PubMed] [Google Scholar]
- Joyner L.P., Norton C.C. Transferred drug resistance in Eimeria maxima. Parasitology. 1975;71:385–392. doi: 10.1017/s0031182000047168. [DOI] [PubMed] [Google Scholar]
- Long P.L., Johnson J., Baxter S. Eimeria tenella: relative survival of drug-resistant and drug-sensitive populations in floor pen chickens. Poult. Sci. 1985;64:2403–2405. doi: 10.3382/ps.0642403. [DOI] [PubMed] [Google Scholar]
- Mathis G.F., Broussard C. Increased level of Eimeria sensitivity to diclazuril after using a live coccidial vaccine. Avian Dis. 2006;50:321–324. doi: 10.1637/7455-101305R1.1. [DOI] [PubMed] [Google Scholar]
- Mathis G.F., McDougald L.R. Restoration of drug sensitivity on turkey farms after introduction of sensitive coccidia during controlled-exposure immunization. In: Yvoré P., editor. Coccidia and Intestinal Coccidiomorphs. Proceedings of the Vth International Coccidiosis Conference. INRA; Tours, France: 1989. pp. 339–343. [Google Scholar]
- McDougald L.R. Chemotherapy of coccidiosis. In: Long P.L., editor. The Biology of the Coccidia. University Park Press; Baltimore, MD: 1982. pp. 373–427. [Google Scholar]
- McDougald L.R. Protozoal infections. In: Saif Y.M., Fadly A.M., Glisson J.R., McDougald L.R., Nolan L.K., Swayne D.E., editors. 12th ed. Blackwell Publishing; 2008. pp. 1067–1085. (Diseases of Poultry). [Google Scholar]
- McLoughlin D.K., Chute M.B. Loss of amprolium resistance in Eimeria tenella by admixture of sensitive and resistant strains. Proc. Helm. Soc. Wash. 1979;46:138–141. [Google Scholar]
- Peek H.W., Landman W.J.M. Resistance to anticoccidial drugs of Dutch avian Eimeria spp. field isolates originating from 1996, 1999 and 2001. Avian Pathol. 2003;32:391–401. doi: 10.1080/0307945031000121149. [DOI] [PubMed] [Google Scholar]
- Peek H.W., Landman W.J.M. Higher incidence of Eimeria spp. field isolates sensitive to diclazuril and monensin associated with the use of live coccidiosis vaccination with paracox-5 in broiler farms. Avian Dis. 2006;50:434–439. doi: 10.1637/7486-121205R.1. [DOI] [PubMed] [Google Scholar]
- Shirley M.W. Development of a live attenuated vaccine against coccidiosis of poultry. Parasite Immunol. 1989;11:117–124. doi: 10.1111/j.1365-3024.1989.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Shirley M.W., Harvey D. A genetic linkage map of the apicomplexan protozoan parasite Eimeria tenella. Genome Res. 2000;10:1587–1593. doi: 10.1101/gr.149200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley M.W., Millard B.J. Some observations on the sexual differentiation of Eimeria tenella using single sporozoites in vitro. J. Parasitol. 1976;69:666–670. doi: 10.1017/s0031182000047016. [DOI] [PubMed] [Google Scholar]
- Shirley M.W., Smith A.L., Blake D.P. Challenges in the successful control of the avian coccidia. Vaccine. 2007;25:5540–5547. doi: 10.1016/j.vaccine.2006.12.030. [DOI] [PubMed] [Google Scholar]
- Walker R.A., Ferguson D.J.P., Miller C.M.D., Smith N.C. Sex and Eimeria: a molecular perspective. Parasitology. 2013;140:1701–1717. doi: 10.1017/S0031182013000838. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Epidemiological aspects of the use of live anticoccidial vaccines for chickens. Int. J. Parasitol. 1998;28:1089–1098. doi: 10.1016/s0020-7519(98)00066-6. [DOI] [PubMed] [Google Scholar]
- Williams R.B. A compartmentalised model for the estimation of the cost of coccidiosis to the world’s chicken production industry. Int. J. Parasitol. 1999;29:1209–1229. doi: 10.1016/s0020-7519(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Anticoccidial vaccines for broiler chickens: pathways to success. Avian Pathol. 2002;31:317–353. doi: 10.1080/03079450220148988. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Fifty years of anticoccidial vaccines for poultry (1952–2002) Avian Dis. 2002;46:775–802. doi: 10.1637/0005-2086(2002)046[0775:FYOAVF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]


