Graphical abstract
Abbreviations: NTD, Neglected Tropical Diseases; TDR, Special Programme for Research and Training in Tropical Diseases; HTS, high throughput screening; HCS, High Content Screening
Keywords: Leishmania, Phenotypic HTS, Target HTS, Drug discovery
Highlights
-
•
Target-based vs. target-free screenings are on the pipeline of drug discovery.
-
•
High Content Screenings have greater acceptance for drug discovery studies.
-
•
In vivo imaging of transgenic parasites are suitable for pre-clinical trials.
Abstract
Drug discovery programs sponsored by public or private initiatives pursue the same ambitious goal: a crushing defeat of major Neglected Tropical Diseases (NTDs) during this decade. Both target-based and target-free screenings have pros and cons when it comes to finding potential small-molecule leads among chemical libraries consisting of myriads of compounds. Within the target-based strategy, crystals of pathogen recombinant-proteins are being used to obtain three-dimensional (3D) structures in silico for the discovery of structure-based inhibitors. On the other hand, genetically modified parasites expressing easily detectable reporters are in the pipeline of target-free (phenotypic) screenings. Furthermore, lead compounds can be scaled up to in vivo preclinical trials using rodent models of infection monitoring parasite loads by means of cutting-edge bioimaging devices. As such, those preferred are fluorescent and bioluminescent readouts due to their reproducibility and rapidity, which reduces the number of animals used in the trials and allows for an earlier stage detection of the infective process as compared with classical methods. In this review, we focus on the current differences between target-based and phenotypic screenings in Leishmania, as an approach that leads to the discovery of new potential drugs against leishmaniasis.
1. Introduction
Leishmania is a heteroxenous parasite, either as a motile promastigote form developing in sandfly guts or as a non-motile intracellular amastigote form in the definitive host macrophages. The different manifestations of leishmaniasis are always linked to the marginal poorest sectors of the population from low-income countries. Yet no effective vaccine has so far been developed and, moreover, current pharmacological treatments are toxic, expensive or require intravenous administration, which hinders patient treatment adherence. Although recent initiatives have improved the antileishmanial drug vademecum combining current medicines or reprofiling others, there is more than ever an urgent need to discover new antileishmanial drugs that could be translated into clinical practice (Croft and Olliaro, 2011). One of the hot issues of the last 4th Zing Drug Discovery Conference held in Nerja (Spain), hosted by Prof. Peter J. Myler (Seattle Biomedical Research Institute, WA, USA) and Prof. Babu L. Tekwani (National Center for Natural Products Research, Oxford MS, USA), was the limited agreement found between the results obtained from target-based and target-free (phenotypic) screenings in Leishmania. On the one hand, in silico target identification for structure-based drug discovery should be confirmed by further in vitro biochemical studies. On the other hand, in vitro phenotypic screenings allow identifying hit compounds eliminating the parasite regardless of their mechanism of action. These different screening approaches were brought forward and discussed there, highlighting the use of bioimaging readouts to test small-molecule libraries. However, some discrepancies arose when comparing the inhibition of specific targets with their respective antileishmanial effect.
2. Target-based screenings
Small-molecule target-based screenings are designed to disrupt specific proteins that: (i) are essential for parasite survival and (ii) are either absent from, or structurally dissimilar to those occurring in the host. The genomic data availability of Leishmania spp. has enabled the evaluation of several pharmacological targets since complete genomic sequences allow the identification of protein-coding genes (Ivens et al., 2005). In silico high-throughput virtual screening and docking procedures using 3D X-ray structured atomic coordinates and homology models are predictive approaches to design novel drugs against parasite specific targets. In this context, Dr. Myler offered the participants the services of the Seattle Structural Genomics Centre for Infectious Disease (SSGCID) funded by the National Institute of Allergy and Infectious Diseases (NIAID, USA), a facility open to researchers to resolve protein structures of putative targets from emerging microorganisms in order to implement a structure-based drug research strategy (Stacy et al., 2011).
Several attempts have been made to identify essential targets against Leishmania spp. The Special Programme for Research and Training in Tropical Diseases (TDR) database, hosted at the World Health Organization (WHO), provides a bank of potential targets obtained from biochemical, genetic and pharmacological data of several well-known pathogens. At the top rank of this list can be found those targets needed for parasite survival such as trypanothione reductase, an enzyme involved in the maintenance of the parasite’s redox equilibrium (Eberle et al., 2011); dihydrofolate reductase, required for the de novo synthesis of purines; cysteine protease B, a stage-regulated virulence factor that is secreted by the amastigote form to the phagolysosome (Caffrey et al., 2011); or DNA topoisomerases, whose structure radically differs from that of the host and which are involved in DNA replication, transcription and recombination (Prada et al., 2013). Finally, it is worth mentioning the LeishDrug network (a FP7 consortium supported by EU funds), an interdisciplinary approach ready to reveal the role of protein kinases associated with amastigote virulence in visceral leishmaniasis (Palmeri et al., 2011).
Nonetheless, target-based screenings are not exempt from drawbacks. The intracellular amastigote is localized inside the macrophage parasitophorous vacuole, where the compound must enter and remain active. Consequently, in order to retain full drug efficacy, the putative hit should be permeable enough to pass across several membranes as well as remain stable in an acidic environment. In addition, it should not be substrate of xenobiotic-metabolizing enzymes for both the host and the parasite. Moreover, many compounds designed against specific parasite targets usually present low selectivity. This fact shows that target exclusiveness in the parasite might be a necessary but not sufficient condition to validation. Therefore, phenotypic screening stands out as the keystone assay for those studies preceding the preclinical drug discovery phase.
3. Phenotypic screenings (HTS)
There was general agreement at the Conference that there is an urgent need for a more reliable model of phenotypic in vitro screening that might mimic as much as possible the real environment within the definitive host. Inside the mature parasitophorous vacuole within the infected macrophage, the pathogen must withstand low pH and enhanced oxidative stress. These conditions could be responsible for the degradation of many drugs. On the one hand, monoxenous parasites, such as the procyclic bloodstream forms of Trypanosoma brucei, modified or not to express fluorescent or bioluminescent reporters, can serve by themselves as models for in vitro target-free screening. On the other hand, stage-specific drug susceptibilities are commonly described by researchers in heteroxenous Trypanosoma cruzi and Leishmania spp. parasites. In vitro Leishmania free-living promastigotes are easy to culture and handle, but they represent the insect stage of the parasite life cycle. It has been suggested that only 4% of the hits identified by using Leishmania promastigotes have some effect on intracellular amastigote forms (Freitas-Junior et al., 2012). Therefore, an amastigote-based phenotypic screening is reinforced as the most suitable approach to be developed. According to this assumption, thousands of compounds were tested against Leishmania spp. and T. cruzi on axenic amastigotes, due to their easy handling and cultivation in the absence of host cells (Bustamante et al., 2011). Nevertheless, axenic amastigotes present markedly different gene-expression profiles as compared with lesion-derived amastigotes (Holzer et al., 2006). To this end, phenotypic HTS for antileishmanial drug discovery should be carried out in intracellular amastigotes only. In this context, the use of monocytic cell lines appears to be as an interesting although technically challenging alternative to perform phenotypic HTS in Leishmania. The human acute monocytic leukemia cell line (THP-1) has permitted to screen up to 300,000 compounds from different libraries, obtaining 350 hits that kill the parasite at concentrations below 10 μM using a High Content Screening (HCS) image-based readout (Siqueira-Neto et al., 2012).
4. Ex vivo murine explants as a model of phenotypic screening
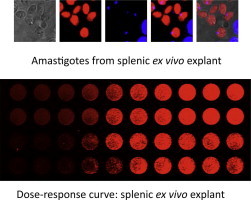
An exciting approach that is midway between in vitro infections with human THP-1 cells and experimental infections in mice consists in the use of ex vivo explants of Leishmania-infected organs. Rodents are experimentally infected with fluorescent or bioluminescent Leishmania strains. Once the infection is established, target-infected organs are harvested in order to develop the ex vivo explant culture. It is of paramount importance that genetic manipulation does not alter the virulence of transgenic strains, so that they are able to develop the disease in the same conditions as wild-type parasites. Rodent visceral leishmaniasis results in a marked splenomegaly, whilst lymphadenopathy prevails in cutaneous infections. BALB/c mice are susceptible to reproduce acute visceral and cutaneous leishmaniasis by Leishmania infantum and Leishmania major, respectively, developing an adaptative Th2 immune response. For its part, the hamster is the preferred animal to develop a chronic infection model of visceral leishmaniasis by Leishmania donovani. Splenic or lymph node ex vivo infected explants show advantages over in vitro systems, because they include the whole cellular population involved in the host-parasite interaction: macrophages, CD3+ and CD4+ T cells, B lymphocytes and granulocytes; which could affect the therapeutic effect of the tested compound. In addition, the number of animals used in these trials can be drastically reduced, since a single infected spleen can yield up to four 96-well plates, thus enabling the evaluation of several collections of small molecules at a single dose.
This approach was successfully validated by Dr. Melby’s group in the University of Texas Medical Branch at Galveston (TX, USA). They performed a single-dose concentration screening of four small-molecule libraries containing 4035 compounds. The spleens from hamsters infected with a luciferase-transfected L. donovani strain served to detect more than two hundred hits under a 96-well plate format (Osorio et al., 2011). The validation of a lymph node ex vivo explant model has been recently published; in this case, a L. major strain was transfected with the same bioluminescent reporter (Peniche et al., 2014). However, one of the main disadvantages of bioluminescence is the need for cell lysis prior to the addition of a light-emitting substrate and subsequent plate readout. This time-consuming step can be avoided by using fluorescent proteins that significantly reduce time and cost of the screening (Calvo-Álvarez et al., 2012).
5. Towards preclinical in vivo models
Fluorescent or bioluminescent transfected parasites are indeed a step forward towards identifying lead compounds in in vivo models; thus reducing the number of animals to be used. In vivo real-time imaging systems allow the acquisition of fluorescent or bioluminescent images at infection sites and, in some cases, in internal organs. Both bioluminescent parasites, which require the parenteral administration of light-emitting substrates, and fluorescent ones permit the appraisal of the infection by recording fluorescent or bioluminescent signals prior to the visual appearance of the clinical symptoms.
In regard to visceral models, bioluminescence has advantages over fluorescence due to the poor penetration of excitation light by fluorescent proteins and tissue autofluorescence. Even so, transgenic parasites overexpressing fluorescent proteins would be more appropriate models in order to develop cutaneous or mucocutaneous infections in BALB/c mice without the need for a light-emitting substrate injection. Alternatively, infrared fluorescent proteins with excitation/emission wavelengths between 650 and 900 nm could be suitable for whole-body imaging given that such wavelengths penetrate tissue well and minimize the absorbance by haemoglobin, water and lipids as well as light scattering, as previously reported for viral infections (Shu et al., 2009). Finally, the use of humanized murine models, which are being developed nowadays against other diseases (Rochford et al., 2013), could become an area of rising interest to explore cutaneous leishmaniasis in the near future.
6. Summary
Public and private initiatives are supporting the discovery of novel chemotherapeutic treatments to eliminate NTDs by this decade. This ambitious proposal has permitted researchers to access a vast collection of compounds that can be tested and screened. The main objective of this first drug-screening phase is to find small-molecules with optimal selective indexes for a given pathogen that guarantee good results in a preclinical model of the disease. However, there is a lack of predictive in vitro models that permit achieving potency, specificity and safety goals, as well as a good compliance with Lipinski’s rules for further in vivo studies. Researchers often complain about great hit molecules selected in target-based screenings that fail in target binding because of poor permeability, degradation into inactive metabolites by microsomal enzymes or inadequate host immune response.
In order to avoid a discussion going round in circles, target-free HTS appears as the most promising method to discover drugs under a “hit to lead” strategy, although there is still a long way to go. For this purpose, it is necessary to find a unified screening model that uses the parasite forms that actually interact with the definitive host cells, so as to substitute others that use artificially-created axenic parasites under laboratory conditions.
In this context, optimized phenotypic assays resembling the pathophysiological environment of infected spleens and lymph nodes, combined with cutting-edge bioimaging devices, could become a promising ex vivo system to screen libraries of small-molecules against Leishmania.
Acknowledgements
Work in this area in the authors’ laboratory was supported by MINECO (Grants AGL2010 16078/GAN), Instituto de Salud Carlos III (PI12/00104) and Junta de Castilla y León (LE182U13).
References
- Bustamante J.M., Park H.J., Tarleton R.L. Report of the 2nd chagas drug discovery consortium meeting, held on 3 November 2010; Atlanta GA, USA. Expert Opin. Drug Discov. 2011;6:965–973. doi: 10.1517/17460441.2011.602063. [DOI] [PubMed] [Google Scholar]
- Caffrey C.R., Lima A.P., Steverding D. Cysteine peptidases of kinetoplastid parasites. Adv. Exp. Med. Biol. 2011;712:84–99. doi: 10.1007/978-1-4419-8414-2_6. [DOI] [PubMed] [Google Scholar]
- Calvo-Álvarez E., Guerrero N.A., Alvarez-Velilla R., Prada C.F., Requena J.M., Punzón C., Llamas M.Á., Arévalo F.J., Rivas L., Fresno M., Pérez-Pertejo Y., Balaña-Fouce R., Reguera R.M. Appraisal of a Leishmania major strain stably expressing mCherry fluorescent protein for both in vitro and in vivo studies of potential drugs and vaccine against cutaneous leishmaniasis. PLoS Negl. Trop. Dis. 2012;6:e1927. doi: 10.1371/journal.pntd.0001927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft S.L., Olliaro P. Leishmaniasis chemotherapy-challenges and opportunities. Clin. Microbiol. Infect. 2011;17:1478–1483. doi: 10.1111/j.1469-0691.2011.03630.x. [DOI] [PubMed] [Google Scholar]
- Eberle C., Lauber B.S., Fankhauser D., Kaiser M., Brun R., Krauth-Siegel R.L., Diederich F. Improved inhibitors of trypanothione reductase by combination of motifs: synthesis, inhibitory potency, binding mode, and antiprotozoal activities. ChemMedChem. 2011;6:292–301. doi: 10.1002/cmdc.201000420. [DOI] [PubMed] [Google Scholar]
- Freitas-Junior L.H., Chatelain E., Kim H.A., Siqueira-Neto J.L. Visceral leishmaniasis treatment: what do we have, what do we need and how to deliver it? Int. J. Parasitol. Drugs Drug Resist. 2012;2:11–19. doi: 10.1016/j.ijpddr.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer T.R., McMaster W.R., Forney J.D. Expression profiling by whole-genome interspecies microarray hybridization reveals differential gene expression in procyclic promastigotes, lesion-derived amastigotes, and axenic amastigotes in Leishmania mexicana. Mol. Biochem. Parasitol. 2006;146:198–218. doi: 10.1016/j.molbiopara.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Ivens A.C., Peacock C.S., Worthey E.A., Murphy L., Aggarwal G., Berriman M., Sisk E., Rajandream M.A., Adlem E., Aert R., Anupama A., Apostolou Z., Attipoe P., Bason N., Bauser C., Beck A., Beverley S.M., Bianchettin G., Borzym K., Bothe G., Bruschi C.V., Collins M., Cadag E., Ciarloni L., Clayton C., Coulson R.M., Cronin A., Cruz A.K., Davies R.M., De Gaudenzi J., Dobson D.E., Duesterhoeft A., Fazelina G., Fosker N., Frasch A.C., Fraser A., Fuchs M., Gabel C., Goble A., Goffeau A., Harris D., Hertz-Fowler C., Hilbert H., Horn D., Huang Y., Klages S., Knights A., Kube M., Larke N., Litvin L., Lord A., Louie T., Marra M., Masuy D., Matthews K., Michaeli S., Mottram J.C., Müller-Auer S., Munden H., Nelson S., Norbertczak H., Oliver K., O’neil S., Pentony M., Pohl T.M., Price C., Purnelle B., Quail M.A., Rabbinowitsch E., Reinhardt R., Rieger M., Rinta J., Robben J., Robertson L., Ruiz J.C., Rutter S., Saunders D., Schäfer M., Schein J., Schwartz D.C., Seeger K., Seyler A., Sharp S., Shin H., Sivam D., Squares R., Squares S., Tosato V., Vogt C., Volckaert G., Wambutt R., Warren T., Wedler H., Woodward J., Zhou S., Zimmermann W., Smith D.F., Blackwell J.M., Stuart K.D., Barrell B., Myler P.J. The genome of the kinetoplastid parasite Leishmania major. Science. 2005;309:436–442. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio Y., Travi B.L., Renslo A.R., Peniche A.G., Melby P.C. Identification of small molecule lead compounds for visceral leishmaniasis using a novel ex vivo splenic explant model system. PLoS Negl. Trop. Dis. 2011;5:e962. doi: 10.1371/journal.pntd.0000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmeri A., Gherardini P.F., Tsigankov P., Ausiello G., Späth G.F., Zilberstein D., Helmer-Citterich M. PhosTryp: a phosphorylation site predictor specific for parasitic protozoa of the family trypanosomatidae. BMC Genomics. 2011;12:614. doi: 10.1186/1471-2164-12-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peniche A.G., Osorio Y., Renslo A.R., Frantz D.E., Melby P.C., Travi B.L. Development of an ex vivo lymph node explant model for identification of novel molecules active against Leishmania major. Antimicrob. Agents Chemother. 2014;58:78–87. doi: 10.1128/AAC.00887-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada C.F., Alvarez-Velilla R., Balaña-Fouce R., Prieto C., Calvo-Álvarez E., Escudero-Martínez J.M., Requena J.M., Ordóñez C., Desideri A., Pérez-Pertejo Y., Reguera R.M. Gimatecan and other camptothecin derivatives poison Leishmania DNA-topoisomerase IB leading to a strong leishmanicidal effect. Biochem. Pharmacol. 2013;85:1433–1440. doi: 10.1016/j.bcp.2013.02.024. [DOI] [PubMed] [Google Scholar]
- Rochford R., Ohrt C., Baresel P.C., Campo B., Sampath A., Magill A.J., Tekwani B.L., Walker L.A. Humanized mouse model of glucose 6-phosphate dehydrogenase deficiency for in vivo assessment of hemolytic toxicity. Proc. Natl. Acad. Sci. U. S. A. 2013;110:17486–17491. doi: 10.1073/pnas.1310402110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu X., Royant A., Lin M.Z., Aguilera T.A., Lev-Ram V., Steinbach P.A., Tsien R.Y. Mammalian expression of infrared fluorescent proteins engineered from a bacterial phytochrome. Science. 2009;32:804–807. doi: 10.1126/science.1168683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira-Neto J.L., Moon S., Jang J., Yang G., Lee C., Moon H.K., Chatelain E., Genovesio A., Cechetto J., Freitas-Junior L.H. An image-based high-content screening assay for compounds targeting intracellular Leishmania donovani amastigotes in human macrophages. PLoS Negl. Trop. Dis. 2012;6:e1671. doi: 10.1371/journal.pntd.0001671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy R., Begley D.W., Phan I., Staker B.L., Van Voorhis W.C., Varani G., Buchko G.W., Stewart L.J., Myler P.J. Structural genomics of infectious disease drug targets: the SSGCID. Acta Crystallogr., Sect. F: Struct. Biol. Cryst. Commun. 2011;67:979–984. doi: 10.1107/S1744309111029204. [DOI] [PMC free article] [PubMed] [Google Scholar]


