Abstract
Adenoid cystic carcinoma (ACC) of the breast is a rare special subtype of breast cancer characterized by the presence of a dual cell population of luminal and basaloid cells arranged in specific growth patterns. Most breast cancers with triple-negative, basal-like breast features (i.e., tumors that are devoid of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 expression, and express basal cell markers) are generally high-grade tumors with an aggressive clinical course. Conversely, while ACCs also display a triple-negative, basal-like phenotype, they are usually low-grade and exhibit an indolent clinical behavior. Many discoveries regarding the molecular and genetic features of the ACC, including a specific chromosomal translocation t(6;9) that results in a MYB-NFIB fusion gene, have been made in recent years. This comprehensive review provides our experience with the ACC of the breast, as well as an overview of clinical, histopathological, and molecular genetic features.
Keywords: Adenoid cystic carcinoma, Breast, Triple-negative and basal-like phenotype, Histology, Molecular genetic features
Core tip: Adenoid cystic carcinoma (ACC) of the breast is a rare, special subtype of breast cancer characterized by the presence of luminal and basaloid cells arranged in specific growth patterns. Although ACCs display a triple-negative, basal-like phenotype, these tumors are usually low-grade and exhibit an indolent clinical behavior. Many discoveries regarding the molecular genetic features of the ACC, including a specific chromosomal translocation t(6;9) that results in a MYB-NFIB fusion gene, have been made in recent years. This review provides our experience with ACCs, as well as an overview of its clinical, histopathological, and molecular genetic features.
INTRODUCTION
Invasive breast carcinoma comprises a heterogeneous group of tumors with various clinical, morphologic, and molecular genetic features[1,2]. According to the 2012 World Health Organization classification, invasive ductal carcinoma of no special type (NST) is the most common histologic type, accounting for up to 75% of all invasive breast carcinomas[3]. The remainder of the invasive cancers represent at least 18 different special and rare histomorphologic subtypes, including adenoid cystic carcinoma (ACC), a salivary gland-type of breast carcinoma[3].
A characteristic histologic pattern of ACC of the breast includes both epithelial and myoepithelial components and resembles a well-known tumor of the salivary gland origin known by the same name. However, patients diagnosed with ACC of the breast have a better prognosis than those who are diagnosed with ACC of the salivary gland[4-6]. ACC of the breast belongs to the basal-like subgroup of breast cancers[7-9]. Based on extensive molecular and genetic profiling studies, basal-like tumors are most often hormone receptor [estrogen receptor (ER) and progesterone receptor (PR)] negative, do not express human epidermal growth factor receptor 2 (Her2), but express one or more basal/myoepithelial cell markers [e.g., cytokeratins (CKs) 5, 5/6, 14 and 17][10]. Unlike other triple-negative breast cancers that are associated with poor prognosis, ACC has an overall excellent prognosis[11]. Because of these distinct clinicopathologic features that set it apart from the other triple-negative breast cancers, an understanding of ACC of the breast is essential for surgical pathologists, breast surgeons, and oncologists. This review will focus on ACC of the breast and will outline important updates in its epidemiology, clinical features, histomorphologic/immunohistochemical characteristics, molecular genetic features, and prognosis/treatment. In addition, we will address our team’s experience with this clinical entity.
EPIDEMIOLOGY
ACC is an uncommon subtype of invasive breast carcinoma and accounts for less than 0.1% of all primary carcinomas of the breast[3,12,13]. Recently, several independent studies based on large patient cohorts have provided more insight into its epidemiology and clinical characteristics[11,14-20]. This information, in the recent studies published in 2010 and after, is summarized in Table 1. The reported age distribution for patients diagnosed with ACC of the breast ranges from 38 to 81 years (with a median age of 60 years; Table 1) and is similar to that seen in other invasive breast cancer cases[3]. Moreover, a previous case series of 338 patients with ACC of the breast conducted over a 30-year period identified its age-adjusted incidence ratio (AAIR) to be 0.92 per 1 million person-years. The AAIR remained constant during the 30-year period and was 39%, lower in African-Americans than in Caucasian-Americans[11]. Most cases are in females, but occasional cases have been reported in male patients[21,22].
Table 1.
Clinical characteristics of adenoid cystic carcinoma of the breast in recently reported cohorts
| Ref. | No. of patients’ | Pathologic T1 or T2 | Lymph node involvement | Distant metastasis | Survival | |
| Cases | Age (yr) | |||||
| Kulkarni et al[14] | 933 | 60 (median) | Not reported | 5.1% | Not reported | 88% (5 yr) |
| Coates et al[15] | 376 | 62 (mean) | 90% | 6.1% | 1.1% (site not specified) | 90% (10 yr) |
| Ghabach et al[11] | 338 | 63 (mean) | 95% | 1.7% | < 1% (site not specified) | 94.9% (10 yr) |
| Thompson et al[16] | 244 | 62 (median) | 92% | 4.9% | 2.9% (site not specified) | 94.9% (10 yr) |
| Khanfir et al[17] | 61 | 59 (median) | 88% | 0% | 6.5% (bone, liver, lung) | 94% (5 yr) |
| Defaud-Hénon et al[18] | 30 | 61 (median) | 95% | 0% | 10% (bone, liver, lung) | Not calculated |
| Vranic et al[19] | 21 | 60.8 (mean) | 85% | 0% | 20% (bone, kidney, lung) | 90% (5 yr) |
CLINICAL FEATURES
ACC of the breast affect the left and right breasts equally and tumors arise irrespective of the breast quadrants. However, in about 50 percent of patients, lesions are found in a subareolar region[23]. Pain or tenderness described in the minority of cases has not been correlated with histologically-confirmed perineural invasion[24]. Mammographically, these tumors may appear as asymmetric densities or irregular masses. Sonographically, they appear as well-defined, irregular, heterogeneous, or hypoechoic masses. Nonetheless, the radiographic findings are non-specific and can be misdiagnosed as benign lesions[13,25]. Subsequently, it could be challenging for a radiologist to make the correct diagnosis of carcinoma without histologic confirmation[25]. Lastly, although most patients present with a solitary tumor, a few cases of multifocal ACC of the breast have also been reported[26,27].
HISTOMORPHOLOGIC/IMMUNOHISTOCHEMICAL CHARACTERISTICS
The mean size of ACC is 3.0 cm (range, 0.7 to 12.0 cm)[28]. Most cases are macroscopically well-circumscribed. Occasionally, pink, tan, or gray microcysts are evident[28]. ACC usually presents as a localized disease of pathologic T1 or T2 (Table 1).
The histology of ACC of the breast is similar to that of their salivary gland counterparts. A variety of microscopic patterns detected in the ACC of the salivary glands may also be present in the ACC of the breast. A tumor typically consists of a dual-cell population of luminal and myoepithelial-basal cells which may be arranged in one or more of three architectural patterns: tubular-trabecular, cribriform, and solid-basaloid (Figure 1)[3]. There are two types of structures lined by these two different types of cells: true glandular spaces and pseudolumina. Luminal cells, characterized by round nuclei and eosinophilic cytoplasm, surround true gland lumina containing periodic acid-Schiff-positive neutral mucin. Immunohistochemically, the luminal cells are positive for CK7, CK8/18, epithelial membrane antigen, and CD117 (c-Kit)[2,29-31]. On the other hand, the myoepithelial-basal cells exhibit central oval nuclei and scant cytoplasm, and form pseudolumina, which result from intraluminal invaginations of the stroma. The myoepithelial-basal cells are immunoreactive for basal cytokeratins (CK5, CK5/6, CK14, CK17) (Figure 2), myoepithelial markers (p63, actin, calponin, S-100 protein), vimentin, and epidermal growth factor receptor (EGFR)[2,29-32]. Kasami et al[33] reported that the polarity of the different types of cells could be demonstrated by immunohistochemistry: myoepithelial-basal cells usually express laminin, fibronectin, basal lamina related proteins, and type IV collagen, whereas the luminal cells express proteins related to cell polarization and epithelial differentiation, including fodrin, E-cadherin, and β-catenin. The authors suggest that this preserved cell polarity and segregated cell differentiation could explain the lack of metastatic capacity observed in this tumor type. Other reports describe areas of squamous differentiation and even rare sebaceous differentiation in ACC of the breast[34,35].
Figure 1.
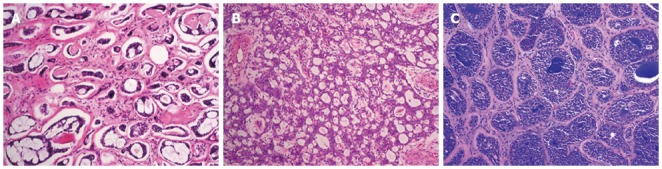
Adenoid cystic carcinoma of the breast. Adenoid cystic carcinomas predominantly showing tubular-trabecular (A), cribriform (B), and solid-basaloid patterns (C). Original magnification × 100.
Figure 2.
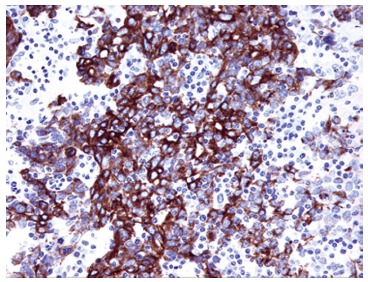
Immunoreactivity of cytokeratin 5/6 in solid pattern of adenoid cystic carcinoma of the breast. The tumor cells are immunoreactive for cytokeratin 5/6, indicating myoepithelial-basal cell origin of tumor cells. Original magnification × 200.
In a way akin to the ACC of the salivary gland, ACCs of the breast are graded according to the proportion of solid growth: tumors with either cribriform or tubular-trabecular pattern and without solid elements are considered grade I, tumors with ≤ 30% of solid growth are classified as grade II, and tumors having more than 30% solid growth are designated grade III[4,36]. Ro et al[4] reported that tumors with a solid pattern (grade II and III) had a tendency to be larger than those without a solid pattern (grade I), and that grade II and III tumors were more likely to develop recurrences. In their series, three patients who developed metastatic ACC had grade II or III lesions. Furthermore, Shin et al[37] reported 9 cases of the solid (basaloid) variant of breast ACC in which the tumor cells tended to be larger, with hyperchromatic nuclei showing moderate to marked atypia, pleomorphism, and increased mitotic activity. This solid variant of ACC was associated with an aggressive clinical course. However, it is important to note that the histological grade defined by this system did not correlate with disease outcomes observed in two other studies[34,38]. The most recent American Joint Committee on Cancer staging manual (7th edition) recommends that Nottingham histologic grading be provided uniformly for all breast carcinomas[39]. Based on this grading scheme, most ACCs would belong to the histologic grade 1 (3 - 1 + 1) or histologic grade 2 (3 + 2 + 1).
Phenotypically, both luminal and myoepithelial-basaloid cells in ACC of the breast are generally negative for ER, PR, and Her2 proteins (Table 2 and Figure 3)[11,14,40-43]. The immunohistochemical profile of ACC of the breast fits well within that of triple-negative breast cancers with basal-like features. In one study, ER and PR expression was detected in 46% and 36% of ACC cases, respectively[5]. Although this cohort was one of the larger series of ACCs reported to date (n = 28), the cases were collected from different institutions and did not undergo a central review of the diagnosis. Consequently, it cannot be ruled out that a substantial number of these cases were actually invasive cribriform carcinomas with ER and PR immunoreactivity. In addition, it should be noted that in the latter study, dextran-coated charcoal assay was used to assess expression for ER and PR instead of the now more widely used immunohistochemistry. Since normal breast lobules and ducts are often entrapped within the bulk of tumor tissues, it may lead to false positive results of the dextran-coated charcoal assay.
Table 2.
Review of data reported on the expression of prognostic and predictive markers of breast adenoid cystic carcinoma (%)
| Ref. | No. of cases | Percentage of cases showing positivity | ||
| ER | PR | Her2 | ||
| Kulkarni et al[14] | 933 | 15 | 13 | NA |
| Ghabach et al[11] | 338 | 12 | 2 | NA |
| Arpino et al[5] | 28 | 46 | 36 | NA |
| Mastropasqua et al[36] | 20 | 15 | 10 | 0 |
| Azouley et al[41] | 18 | 0 | 0 | 0 |
| Crisi et al[42] | 6 | 0 | 0 | 0 |
| Weigelt et al[43] | 4 | 0 | 0 | 0 |
ER: Estrogen receptor; Her2: Human epidermal growth factor receptor 2; NA: Not available; PR: Progesterone receptor.
Figure 3.
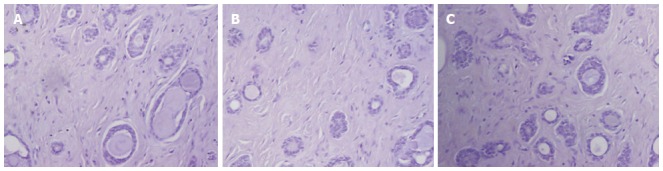
Immunohistochemical findings in adenoid cystic carcinoma of the breast. A: Estrogen receptor; B: Progesterone receptor; C: Human epidermal growth factor receptor 2. All these markers are negative in a case of adenoid cystic carcinoma of the breast. Original magnification × 100.
There have been several case reports suggesting an association between ACC of the breast and various benign lesions including microglandular adenosis, tubular adenosis, adenomyoepithelioma, and fibroadenoma[44-48]. Acs et al[44] suggested that ACC of the breast may develop in a background of and in continuity with microglandular adenosis. Following this hypothesis, their group described a morphological spectrum of lesions with a trend of progression, encompassing microglandular adenosis, “atypical microglandular adenosis” (also described as “ACC in situ”), and invasive ACC[44]. Da Silva et al[45] reported a morphological characterization of tubular adenosis arising concurrently with ACC in the breast, although the comparative genomic hybridization (CGH) analysis performed on these two lesions failed to provide evidence of molecular evolution from tubular adenosis to ACC. Importantly, breast that harbors an ACC can rarely also contain other types of carcinoma, as was shown in a case where the ACC of the breast coexisted with an invasive ductal carcinoma of NST[49,50].
ACC of the breast that exhibits a cribriform/tubular pattern should be distinguished from invasive cribriform/tubular carcinoma or a benign condition termed collagenous spherulosis[51,52]. This is especially important when a pathologist is provided with tiny tissue specimens obtained by core needle biopsies[36]. Invasive cribriform/tubular carcinomas are characterized by the hyper-proliferation of a single type of neoplastic cells (i.e., luminal cell) only, in contrast to the dual cell types observed in ACC. Moreover, cribriform/tubular carcinomas are generally immunoreactive for ER and PR, whereas ACCs are negative for both[53]. In addition, limited evidence exists of c-Kit and/or p63 immunoreactivity in ACCs of the breast (positive for both), compared to the invasive cribriform/tubular carcinomas which are negative for both markers[40]. In collagenous spherulosis, collagenous spherules are irregular, mostly observed at the periphery of the lesions, and no mucosubstance is detected within lumina. Immunohistochemically, ACCs are c-Kit (+), calponin (-), and smooth muscle myosin (-), whereas collagenous spherulosis lesions are c-Kit (-), calponin (+), and smooth muscle myosin (+), which may help to differentiate between these two types of lesions[54]. The differential diagnosis of the solid (basaloid) variant of ACC includes small cell carcinoma (neuroendocrine carcinoma), solid papillary carcinoma, metaplastic carcinoma, and malignant lymphoma[37]. Although an extensive and careful search for a more typical cribriform pattern of ACC should be performed, immunohistochemistry can also be helpful to distinguish these tumors from ACC.
MOLECULAR GENETIC FEATURES
Microarray-based gene expression profiling studies have been performed in common types of breast cancer, such as the invasive ductal and lobular carcinomas[7-9]. However, most of these studies did not focus on special types of breast cancer, and consequently, there is only limited transcriptomic data on the ACC features. A recent molecular subtype analysis using a single sample predictor (i.e., centroid) performed on 4 ACCs revealed that two of the samples were classified as basal-like, while the other two were shown to exhibit the normal breast-like phenotype. Based on this divergence in the results, they could be an artifact of sample representation, perhaps caused by the contamination with normal tissues[55]. In fact, molecular subtype assignment following hierarchical clustering showed that all four ACCs consistently displayed a basal-like phenotype, and all of them clustered with one of the five subgroups of the triple-negative breast cancers. In another study that utilized the immunohistochemical staining analysis and microarray-based gene expression profiling for a series of 113 tumors that belonged to 11 special histologic types of breast cancer (including 4 ACCs), Weigelt et al[43] reported that the ACC, medullary carcinoma, and metaplastic carcinoma were highly similar in their immunohistochemical and gene expression profile. However, ACCs did not intermingle with medullary and metaplastic carcinomas in the hierarchical clustering, but formed a separate group. Another study, an array-based CGH analysis of 59 breast cancers that belonged to 10 special histologic special types established that while medullary and metaplastic carcinomas displayed complex genomes, ACCs consistently exhibited simpler patterns of gene copy number aberrations[56]. In line with these results, a recent CGH analysis study revealed that ACC of the breast manifested significantly lower frequencies of genetic instability and lower copy number alterations than the histologic grade-matched basal-like and invasive ductal carcinomas of NST[29]. At the genomic level, ACC is substantially different from the other basal-like breast cancers. Studies show that it rarely harbors genomic aberrations associated with basal-like invasive ductal carcinomas of NST, such as gains of 1q, 6p, 8q, and 10p, and losses of 4p, 5q, and 10q[29,57,58]. Furthermore, aneuploidy is reported in fewer than 10% of cases with ACC of the breast[5]. Together, these findings illustrate the heterogeneity of triple-negative, basal-like breast cancers. Although the majority of these tumors are high grade cancers with high levels of genetic instability and an aggressive clinical course (e.g., grade 3 invasive ductal carcinoma of NST, medullary carcinoma, and metaplastic carcinoma), there is also a subgroup of low grade tumors with low frequencies of genetic instability and an indolent clinical behavior (e.g., ACC and secretory carcinoma)[10,41,43,59-61]. Thus, we emphasize that based solely on molecular subtyping and without proper histologic classification, ACCs, which have an indolent clinical behavior, would be classified as triple-negative, clinically aggressive tumors. Therefore, information regarding the histologic type of triple-negative breast cancers should be included in histopathology reports and taken into account for clinical decision-making.
Although studies using next-generation sequencing (NGS) for whole exome or microRNA expression profiling for ACC of the salivary gland have been recently reported[62-65], there have been few studies using NGS for ACC of the breast. In one study utilizing microRNA expression profiling for two cases each of ACC of the salivary gland and breast, Kiss et al[65] reported that the let-7b was overexpressed in ACC of the salivary gland, while decreased in ACC of the breast. In addition, the miR-24 was decreased in salivary gland-derived but overexpressed in breast-derived adenoid cystic carcinomas.
Similar to ACCs of the salivary gland, ACCs of the breast are characterized by the t(6;9) (q22-23; p23-24) chromosomal translocation, which generates fusion transcripts involving the oncogene MYB and the transcription factor gene NFIB. Several previous studies reported that this chromosomal translocation is present in over 90% of ACC cases and is a key ACC oncogenic mechanism[29,66,67]. The myeloblastoma (MYB)- nuclear factor I/B (NFIB) fusion protein retains the DNA-binding and transactivation domains of a wild-type MYB, and is therefore expected to activate MYB target genes[29,66]. MYB is a leucine zipper transcription factor that plays an important role in the control of cell proliferation, apoptosis, and differentiation[68,69], while its target genes include BCL2 and GRP78/BIP, which are essential for cell survival[70]. MYB is a direct target of EG signaling and is highly expressed not only in ACCs, but also in cell lines of ER-positive breast cancers[71,72]. Recently, one study reported that 67% (8/12 cases) of dermal cylindroma displayed the t(6;9) and MYB-NFIB fusion transcripts and that the composition of these chimeric transcripts was identical to that seen in ACC[73].
Approximately 7% of breast cancer cases are related to hereditary conditions and caused by mutations in the BRCA1 and BRCA2 genes[3]. Although medullary and metaplastic breast carcinomas, with which ACC shares immunohistochemical and molecular findings, show a frequent promotor methylation of BRCA1 gene, ACC of the breast usually retains normal BRCA1 gene function[2,29]. To our knowledge, BRCA2 gene status has not been investigated in ACCs of the breast.
ACCs of the breast typically do not express the full-length ER-α (ER-α66) and PR[11,14,39-42]. However, several studies have shown that the ACC, apocrine carcinoma, and triple-negative breast cancer of NST exhibited a frequent membranous/cytoplasmic immunoreactivity for ER-α36, a novel ER-α66 splice variant implicated in membrane-initiated estrogen signaling[74-76]. In the experimental cell models of breast cancer, ER-α36 was shown to transduce the membrane-initiated steroid signaling cascade, and served as a dominant-negative modulator of ER-α66 mediated transcription activity[75]. In addition, ER-α36 was reported to be related to non-genomic ER activities, in which activation of the mitogen-activated protein kinase (MAPK/ERK) signaling pathway plays a major role[75]. The MAPK/ERK signaling pathway is activated in response to antiestrogens (e.g., tamoxifen), indicating a subset of ER-α66 (-)/ER-α36 (+) breast carcinomas might still respond to antiestrogen based therapy[74,75]. Finally, ER-α36 protein closely interacts with EGFR protein, which is commonly expressed in ACC and triple-negative breast cancers[75]. Some investigators have reported that ACCs of the breast frequently overexpress EGFR protein in the absence of underlying EGFR gene alterations[19,29].
Cancer stem cells have been reported to be associated with tumor initiation, progression, survival, and resistance to therapy[77]. However, the cancer stem cell field is still fairly controversial and stem cell markers have not been fully elucidated. In the majority of studies, breast cancer cells with a CD44 (+)/CD24 (-) phenotype have been proposed to have tumor-initiating properties with stem cell-like features[78], and Defaud-Hénon et al[18] recently reported that a characteristic CD44 (+)/CD24 (-) phenotype is commonly observed in the ACC of the breast. On the other hand, frequent overexpression of c-Kit and EGFR proteins was observed in undifferentiated carcinomas with stem cell-like features[79]. Although several studies illustrated that a consistent c-Kit protein expression was detected in most ACCs[29,40-43], underlying KIT gene alterations, such as gene mutations, have not been previously detected[80]. Finally, SOX10 transcription factor appears to support stem-like properties in normal tissues and cancer cells[81]. Recently, Ivanov et al[82] described SOX10 as a novel diagnostic marker for ACCs of the salivary gland and breast basal-like carcinomas, indicating that SOX10 expression might be worth examining in ACCs of the breast.
Although triple-negative NST breast cancers usually have high proliferative activity, ACC of the breast exhibits a low proliferation rate using standard Ki-67 labeling index[29,83]. Interestingly, their typical proliferation rate is even lower than that of low-grade conventional breast carcinomas[84]. Mastropasqua et al[40] suggested that proliferative indices showed grater values in high-grade ACCs when compared to low-grade lesions. However, another study reported that the proliferative activity is not associated with the outcome of ACC patients with ACC[38]. In addition to low Ki-67 labeling index, ACCs of the breast, including high-grade solid-basaloid lesions, also show low p53 protein expression[29,39,83]. Trendell-Smith et al[53] described a slightly higher p53 protein expression in ACC than that in invasive cribriform carcinoma.
Finally there are several recent studies that identified potential breast ACC biomarkers. Insulin-like growth factor-II mRNA-binding protein 3 (IMP3) is an oncofetal protein and a component of the insulin-like growth factor-II pathway. Studies indicate that it could serve as a biomarker for basal-like breast carcinomas[84-87], and a recent report showed that the IMP3 is commonly overexpressed in ACCs of the breast[88]. In another report, the molecular genetic analysis of a primary ACC of the breast and its renal metastasis revealed PTEN and PIK3CA gene mutations[89].
PROGNOSIS AND TREATMENT
A striking feature of ACC of the breast, which is in stark contrast with other triple-negative, basal-like breast cancers and the ACC of the salivary gland, is its excellent prognosis. As shown in Table 1, the 10-year survival rate is 90%-100%, and lymph node metastasis is rare, as well as distant metastases, which affect mainly visceral organs[11,14-19,90]. Based on its indolent clinical course and favorable outcome, the ACC of the breast is generally cured by breast-conserving surgery, such as wide excision or quadrantectomy with or without radiotherapy[11,17,91]. Mastectomy is recommended for invasive lesions when a cosmetically satisfactory excision is not possible, especially when the tumor has a high-grade pattern[4,36,92]. A recent study of a large patient cohort reported a considerable benefit of adjuvant radiotherapy on overall and disease-specific survival in patients with ACC[15]. Moreover, because a high rate of positive surgical margins has been detected following breast conserving surgery, adjuvant radiotherapy may be beneficial[93]. Furthermore, while some clinicians recommend systemic adjuvant chemotherapy for patients with high-grade lesions or axillary lymph node/distant metastasis[36], its role in breast ACC patients remains controversial.
When patients with ACC demonstrate local recurrence or distant metastases, a prolonged and indolent clinical course is still likely[94-97]. However, long-term follow-up is recommended, since their long clinical course carries a risk of secondary malignancies[98,99], and the risk of distant metastases increases with time[100].
As treatment of cancer enters a new stage with the development of targeted therapies, the common MYB-NFIB fusion gene may provide new therapeutic avenues for the management of advanced ACC of the breast. Consequently, further functional studies investigating the biological consequences of the MYB gene of function due to the MYB-NFIB fusion are needed. Gene silencing experiments are also necessary to demonstrate that MYB expression is required for the survival of cancer cells with genetically activated MYB. Finally, the functional role of the ER-α36 variant in ACC merits further research as experimental evidence in triple-negative breast cancer cell lines suggests that breast cancer cells with ER-α66 (-)/ER-α36 (+) phenotype might still be responsive to antiestrogens[72,73].
HOUSTON METHODIST EXPERIENCE OF ACC OF THE BREAST
A search of the electronic data base at Houston Methodist Hospital from 2004 to 2010 yielded five cases of ACC of the breast. The clinicopathologic and follow-up status of these five patients are summarized in Table 3. The five female patients ranged from 48 to 76 years in age, with a mean age of 60 years. All tumors had distinct morphologic features of classic ACC: histologic grade 1 with cribriform, trabecular or glandular architectural patterns, and basement membrane deposition. No cases of grade II and III tumors were identified. Perineural invasion was identified in one case. Lymphovascular invasion was not seen in any of the cases. An associated adenomyoepithelioma was observed in one case. All patients received lumpectomy and two of these patients had axillary lymph node dissections, with no nodal metastasis found. No patients received adjuvant chemotherapy or radiotherapy. Pulmonary metastasis developed in one case (case 2) seven years after the initial diagnosis. All of the tumors, including the pulmonary metastatic lesion in case 2, were ER/PR negative and did not express Her2. No synchronous/metachronous in-situ carcinoma, invasive ductal/lobular carcinoma, or microglandular adenosis was reported in any of the cases. Four patients without metastasis were alive and showed no evidence of disease for an average (follow-up) of 45.3 mo (range 12-90 mo). The last patient (case 2) who was diagnosed with pulmonary metastasis is alive with disease at 85 mo (one month after metastasis was detected).
Table 3.
Houston Methodist experience of adenoid cystic carcinoma of the breast (2004 to 2010)
| Case No. | Age (yr) | Laterality | Tumor size (cm) | Perineural invasion | Lymph node metastasis | Distant metastasis | TMN stage (AJCC) | Follow-up (mo) |
| 1 | 61 | Left | 1.6 | - | pN0 | - | IA | 14 |
| 2 | 83 | Right | 3.0 | - | pN0 | Lungs, multiple | IV | 85 |
| 3 | 51 | Right | 2.2 | - | cN0 | - | IIA | 12 |
| 4 | 57 | Left | 4.5 | + | cN0 | - | IIA | 65 |
| 5 | 48 | Left | 2.0 | - | cN0 | - | IIA | 90 |
AJCC: American Joint Committee on Cancer.
CONCLUSION
The correct classification of the histological special types of breast cancer is not just an academic exercise, as it has both prognostic and predictive implications. Although the majority of triple-negative, basal-like breast carcinomas are high-grade tumors, ACC is a subgroup of low-grade tumors with an indolent clinical behavior that also displays a triple-negative, basal-like phenotype. Because of its low incidence, there have been only few comprehensive studies of ACC of the breast, which is one of the major limitations of this review. However, this review of recent updates, including certain molecular genetic features in breast ACC, herein will hopefully serve as a prognostic and treatment guide for surgical pathologists, breast surgeons, and oncologists, and lead to the development of more specific, personalized therapies for this rare tumor subtype.
Footnotes
P- Reviewer: Cho W S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
References
- 1.Schnitt SJ, Collins LC. Biopsy interpretation of the breast. Philadelphia: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 2.Badve S, Dabbs DJ, Schnitt SJ, Baehner FL, Decker T, Eusebi V, Fox SB, Ichihara S, Jacquemier J, Lakhani SR, et al. Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol. 2011;24:157–167. doi: 10.1038/modpathol.2010.200. [DOI] [PubMed] [Google Scholar]
- 3.Lakhani SR, Ellis LO, Schnitt SJ, Tan PH, van de Vijver MJ. World Health Organization Classification of tumours of the breast, 4th ed. Lyon: IARC Press; 2012. [Google Scholar]
- 4.Ro JY, Silva EG, Gallager HS. Adenoid cystic carcinoma of the breast. Hum Pathol. 1987;18:1276–1281. doi: 10.1016/s0046-8177(87)80413-6. [DOI] [PubMed] [Google Scholar]
- 5.Arpino G, Clark GM, Mohsin S, Bardou VJ, Elledge RM. Adenoid cystic carcinoma of the breast: molecular markers, treatment, and clinical outcome. Cancer. 2002;94:2119–2127. doi: 10.1002/cncr.10455. [DOI] [PubMed] [Google Scholar]
- 6.Wells CA, Nicoll S, Ferguson DJ. Adenoid cystic carcinoma of the breast: a case with axillary lymph node metastasis. Histopathology. 1986;10:415–424. doi: 10.1111/j.1365-2559.1986.tb02494.x. [DOI] [PubMed] [Google Scholar]
- 7.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 8.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakha EA, Elsheikh SE, Aleskandarany MA, Habashi HO, Green AR, Powe DG, El-Sayed ME, Benhasouna A, Brunet JS, Akslen LA, et al. Triple-negative breast cancer: distinguishing between basal and nonbasal subtypes. Clin Cancer Res. 2009;15:2302–2310. doi: 10.1158/1078-0432.CCR-08-2132. [DOI] [PubMed] [Google Scholar]
- 11.Ghabach B, Anderson WF, Curtis RE, Huycke MM, Lavigne JA, Dores GM. Adenoid cystic carcinoma of the breast in the United States (1977 to 2006): a population-based cohort study. Breast Cancer Res. 2010;12:R54. doi: 10.1186/bcr2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen PP. Adenoid cystic carcinoma of the breast. A morphologically heterogeneous neoplasm. Pathol Annu. 1989;24 Pt 2:237–254. [PubMed] [Google Scholar]
- 13.Glazebrook KN, Reynolds C, Smith RL, Gimenez EI, Boughey JC. Adenoid cystic carcinoma of the breast. AJR Am J Roentgenol. 2010;194:1391–1396. doi: 10.2214/AJR.09.3545. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni N, Pezzi CM, Greif JM, Suzanne Klimberg V, Bailey L, Korourian S, Zuraek M. Rare breast cancer: 933 adenoid cystic carcinomas from the National Cancer Data Base. Ann Surg Oncol. 2013;20:2236–2241. doi: 10.1245/s10434-013-2911-z. [DOI] [PubMed] [Google Scholar]
- 15.Coates JM, Martinez SR, Bold RJ, Chen SL. Adjuvant radiation therapy is associated with improved survival for adenoid cystic carcinoma of the breast. J Surg Oncol. 2010;102:342–347. doi: 10.1002/jso.21638. [DOI] [PubMed] [Google Scholar]
- 16.Thompson K, Grabowski J, Saltzstein SL, Sadler GR, Blair SL. Adenoid cystic breast carcinoma: is axillary staging necessary in all cases? Results from the California Cancer Registry. Breast J. 2011;17:485–489. doi: 10.1111/j.1524-4741.2011.01117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khanfir K, Kallel A, Villette S, Belkacémi Y, Vautravers C, Nguyen T, Miller R, Li YX, Taghian AG, Boersma L, et al. Management of adenoid cystic carcinoma of the breast: a Rare Cancer Network study. Int J Radiat Oncol Biol Phys. 2012;82:2118–2124. doi: 10.1016/j.ijrobp.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Defaud-Hénon F, Tunon-de-Lara C, Fournier M, Marty M, Velasco V, de Mascarel I, MacGrogan G. Adenoid cystic carcinoma of the breast: clinical, histological and immunohistochemical characterization. Ann Pathol. 2010;30:7–16. doi: 10.1016/j.annpat.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Vranic S, Frkovic-Grazio S, Lamovec J, Serdarevic F, Gurjeva O, Palazzo J, Bilalovic N, Lee LM, Gatalica Z. Adenoid cystic carcinomas of the breast have low Topo IIα expression but frequently overexpress EGFR protein without EGFR gene amplification. Hum Pathol. 2010;41:1617–1623. doi: 10.1016/j.humpath.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Kim M, Lee DW, Im J, Suh KJ, Keam B, Moon HG, Im SA, Han W, Park IA, Noh DY. Adenoid cystic carcinoma of the breast: a case series of six patients and literature review. Cancer Res Treat. 2014;46:93–97. doi: 10.4143/crt.2014.46.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miliauskas JR, Leong AS. Adenoid cystic carcinoma in a juvenile male breast. Pathology. 1991;23:298–301. doi: 10.3109/00313029109063592. [DOI] [PubMed] [Google Scholar]
- 22.Yoo SJ, Lee DS, Oh HS, Kim HJ, Kim MH, Ahn YC, Kang GH, Eom DW, Cha CH, Ahn HJ. Male breast adenoid cystic carcinoma. Case Rep Oncol. 2013;6:514–519. doi: 10.1159/000356062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azzopardi JG. Problems in breast pathology. London: Saunders Company; 1979. [PubMed] [Google Scholar]
- 24.McClenathan JH, de la Roza G. Adenoid cystic breast cancer. Am J Surg. 2002;183:646–649. doi: 10.1016/s0002-9610(02)00858-9. [DOI] [PubMed] [Google Scholar]
- 25.Sheen-Chen SM, Eng HL, Chen WJ, Cheng YF, Ko SF. Adenoid cystic carcinoma of the breast: truly uncommon or easily overlooked? Anticancer Res. 2005;25:455–458. [PubMed] [Google Scholar]
- 26.Alis H, Yigitbas H, Kapan S, Kalayci M, Kilic G, Aygun E. Multifocal adenoid cystic carcinoma of the breast: an unusual presentation. Can J Surg. 2008;51:E36–E37. [PMC free article] [PubMed] [Google Scholar]
- 27.Franceschini G, Terribile D, Scafetta I, Magno S, Fabbri C, Chiesa F, Di Leone A, Moschella F, Scaldaferri A, Fragomeni S, et al. Conservative treatment of a rare case of multifocal adenoid cystic carcinoma of the breast: case report and literature review. Med Sci Monit. 2010;16:CS33–CS39. [PubMed] [Google Scholar]
- 28.Tavassoli FA, Eusebi V. Carcinomas of low-grade malignancy. Adenoid cystic carcinoma. In: Tavassoli FA, Eusebi V, editors. Tumors of the mammary gland. AFIP atlas of tumor pathology. 4th series; fasc. 10. Washington, D. C, USA: American Registry of Pathology in collaboration with the Armed Forces Institute of Pathology; 2009. pp. 183–187. [Google Scholar]
- 29.Wetterskog D, Lopez-Garcia MA, Lambros MB, A’Hern R, Geyer FC, Milanezi F, Cabral MC, Natrajan R, Gauthier A, Shiu KK, et al. Adenoid cystic carcinomas constitute a genomically distinct subgroup of triple-negative and basal-like breast cancers. J Pathol. 2012;226:84–96. doi: 10.1002/path.2974. [DOI] [PubMed] [Google Scholar]
- 30.Marchiò C, Weigelt B, Reis-Filho JS. Adenoid cystic carcinomas of the breast and salivary glands (or ‘The strange case of Dr Jekyll and Mr Hyde’ of exocrine gland carcinomas) J Clin Pathol. 2010;63:220–228. doi: 10.1136/jcp.2009.073908. [DOI] [PubMed] [Google Scholar]
- 31.Franzese C, Zei G, Masoni T, Cecchini S, Monteleone E, Livi L, Biti G. Adenoid cystic carcinoma of the breast. The double face of an exocrine gland carcinoma. Strahlenther Onkol. 2013;189:1049–1050. doi: 10.1007/s00066-013-0461-8. [DOI] [PubMed] [Google Scholar]
- 32.Reyes C, Jorda M, Gomez-Fernández C. Salivary gland-like tumors of the breast express basal-type immunohistochemical markers. Appl Immunohistochem Mol Morphol. 2013;21:283–286. doi: 10.1097/PAI.0b013e31826a277e. [DOI] [PubMed] [Google Scholar]
- 33.Kasami M, Olson SJ, Simpson JF, Page DL. Maintenance of polarity and a dual cell population in adenoid cystic carcinoma of the breast: an immunohistochemical study. Histopathology. 1998;32:232–238. doi: 10.1046/j.1365-2559.1998.00383.x. [DOI] [PubMed] [Google Scholar]
- 34.Lamovec J, Us-Krasovec M, Zidar A, Kljun A. Adenoid cystic carcinoma of the breast: a histologic, cytologic, and immunohistochemical study. Semin Diagn Pathol. 1989;6:153–164. [PubMed] [Google Scholar]
- 35.Tavassoli FA, Norris HJ. Mammary adenoid cystic carcinoma with sebaceous differentiation. A morphologic study of the cell types. Arch Pathol Lab Med. 1986;110:1045–1053. [PubMed] [Google Scholar]
- 36.Rosen PP. Adenoid cystic carcinoma. In: Rosen PP, editor. Rosen’s breast pathology, third edition. Philadelphia: Lippincott Williams & Wilkins; 2009. pp. 590–604. [Google Scholar]
- 37.Shin SJ, Rosen PP. Solid variant of mammary adenoid cystic carcinoma with basaloid features: a study of nine cases. Am J Surg Pathol. 2002;26:413–420. doi: 10.1097/00000478-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Kleer CG, Oberman HA. Adenoid cystic carcinoma of the breast: value of histologic grading and proliferative activity. Am J Surg Pathol. 1998;22:569–575. doi: 10.1097/00000478-199805000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Edge SB, Byrd DR. AJCC cancer staging manual. 7th ed. Berlin: Springer; 2010. [Google Scholar]
- 40.Mastropasqua MG, Maiorano E, Pruneri G, Orvieto E, Mazzarol G, Vento AR, Viale G. Immunoreactivity for c-kit and p63 as an adjunct in the diagnosis of adenoid cystic carcinoma of the breast. Mod Pathol. 2005;18:1277–1282. doi: 10.1038/modpathol.3800423. [DOI] [PubMed] [Google Scholar]
- 41.Azoulay S, Laé M, Fréneaux P, Merle S, Al Ghuzlan A, Chnecker C, Rosty C, Klijanienko J, Sigal-Zafrani B, Salmon R, et al. KIT is highly expressed in adenoid cystic carcinoma of the breast, a basal-like carcinoma associated with a favorable outcome. Mod Pathol. 2005;18:1623–1631. doi: 10.1038/modpathol.3800483. [DOI] [PubMed] [Google Scholar]
- 42.Crisi GM, Marconi SA, Makari-Judson G, Goulart RA. Expression of c-kit in adenoid cystic carcinoma of the breast. Am J Clin Pathol. 2005;124:733–739. doi: 10.1309/61MV-ENEK-5EJ7-JKGF. [DOI] [PubMed] [Google Scholar]
- 43.Weigelt B, Horlings HM, Kreike B, Hayes MM, Hauptmann M, Wessels LF, de Jong D, Van de Vijver MJ, Van’t Veer LJ, Peterse JL. Refinement of breast cancer classification by molecular characterization of histological special types. J Pathol. 2008;216:141–150. doi: 10.1002/path.2407. [DOI] [PubMed] [Google Scholar]
- 44.Acs G, Simpson JF, Bleiweiss IJ, Hugh J, Reynolds C, Olson S, Page DL. Microglandular adenosis with transition into adenoid cystic carcinoma of the breast. Am J Surg Pathol. 2003;27:1052–1060. doi: 10.1097/00000478-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Da Silva L, Buck L, Simpson PT, Reid L, McCallum N, Madigan BJ, Lakhani SR. Molecular and morphological analysis of adenoid cystic carcinoma of the breast with synchronous tubular adenosis. Virchows Arch. 2009;454:107–114. doi: 10.1007/s00428-008-0700-z. [DOI] [PubMed] [Google Scholar]
- 46.Van Dorpe J, De Pauw A, Moerman P. Adenoid cystic carcinoma arising in an adenomyoepithelioma of the breast. Virchows Arch. 1998;432:119–122. doi: 10.1007/s004280050144. [DOI] [PubMed] [Google Scholar]
- 47.Canyilmaz E, Uslu GH, Memış Y, Bahat Z, Yildiz K, Yoney A. Adenoid cystic carcinoma of the breast: A case report and literature review. Oncol Lett. 2014;7:1599–1601. doi: 10.3892/ol.2014.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blanco M, Egozi L, Lubin D, Poppiti R. Adenoid cystic carcinoma arising in a fibroadenoma. Ann Diagn Pathol. 2005;9:157–159. doi: 10.1016/j.anndiagpath.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Righi A, Lenzi M, Morandi L, Flamminio F, De Biase D, Farnedi A, Foschini MP. Adenoid cystic carcinoma of the breast associated with invasive duct carcinoma: a case report. Int J Surg Pathol. 2011;19:230–234. doi: 10.1177/1066896909332321. [DOI] [PubMed] [Google Scholar]
- 50.Kontos M, Karles D, Petrou A, Alexandrou PT. Adenoid cystic carcinoma intermingled with ductal carcinoma of the breast: a case report and review of the literature. J Med Case Rep. 2011;5:437. doi: 10.1186/1752-1947-5-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Resetkova E, Albarracin C, Sneige N. Collagenous spherulosis of breast: morphologic study of 59 cases and review of the literature. Am J Surg Pathol. 2006;30:20–27. doi: 10.1097/01.pas.0000179237.91515.81. [DOI] [PubMed] [Google Scholar]
- 52.Ogata K, Sakamoto G, Sakurai T. Adenoid cystic carcinoma with collagenous spherulosis-like structures in the breast: report of a case. Pathol Int. 2004;54:332–336. doi: 10.1111/j.1440-1827.2004.01627.x. [DOI] [PubMed] [Google Scholar]
- 53.Trendell-Smith NJ, Peston D, Shousha S. Adenoid cystic carcinoma of the breast: a tumour commonly devoid of oestrogen receptors and related proteins. Histopathology. 1999;35:241–248. doi: 10.1046/j.1365-2559.1999.00722.x. [DOI] [PubMed] [Google Scholar]
- 54.Rabban JT, Swain RS, Zaloudek CJ, Chase DR, Chen YY. Immunophenotypic overlap between adenoid cystic carcinoma and collagenous spherulosis of the breast: potential diagnostic pitfalls using myoepithelial markers. Mod Pathol. 2006;19:1351–1357. doi: 10.1038/modpathol.3800658. [DOI] [PubMed] [Google Scholar]
- 55.Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, van de Vijver MJ. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9:R65. doi: 10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horlings HM, Weigelt B, Anderson EM, Lambros MB, Mackay A, Natrajan R, Ng CK, Geyer FC, van de Vijver MJ, Reis-Filho JS. Genomic profiling of histological special types of breast cancer. Breast Cancer Res Treat. 2013;142:257–269. doi: 10.1007/s10549-013-2740-6. [DOI] [PubMed] [Google Scholar]
- 57.Natrajan R, Lambros MB, Rodríguez-Pinilla SM, Moreno-Bueno G, Tan DS, Marchió C, Vatcheva R, Rayter S, Mahler-Araujo B, Fulford LG, et al. Tiling path genomic profiling of grade 3 invasive ductal breast cancers. Clin Cancer Res. 2009;15:2711–2722. doi: 10.1158/1078-0432.CCR-08-1878. [DOI] [PubMed] [Google Scholar]
- 58.Natrajan R, Lambros MB, Geyer FC, Marchio C, Tan DS, Vatcheva R, Shiu KK, Hungermann D, Rodriguez-Pinilla SM, Palacios J, et al. Loss of 16q in high grade breast cancer is associated with estrogen receptor status: Evidence for progression in tumors with a luminal phenotype? Genes Chromosomes Cancer. 2009;48:351–365. doi: 10.1002/gcc.20646. [DOI] [PubMed] [Google Scholar]
- 59.Reis-Filho JS, Milanezi F, Steele D, Savage K, Simpson PT, Nesland JM, Pereira EM, Lakhani SR, Schmitt FC. Metaplastic breast carcinomas are basal-like tumours. Histopathology. 2006;49:10–21. doi: 10.1111/j.1365-2559.2006.02467.x. [DOI] [PubMed] [Google Scholar]
- 60.Laé M, Fréneaux P, Sastre-Garau X, Chouchane O, Sigal-Zafrani B, Vincent-Salomon A. Secretory breast carcinomas with ETV6-NTRK3 fusion gene belong to the basal-like carcinoma spectrum. Mod Pathol. 2009;22:291–298. doi: 10.1038/modpathol.2008.184. [DOI] [PubMed] [Google Scholar]
- 61.Weigelt B, Kreike B, Reis-Filho JS. Metaplastic breast carcinomas are basal-like breast cancers: a genomic profiling analysis. Breast Cancer Res Treat. 2009;117:273–280. doi: 10.1007/s10549-008-0197-9. [DOI] [PubMed] [Google Scholar]
- 62.Ross JS, Wang K, Rand JV, Sheehan CE, Jennings TA, Al-Rohil RN, Otto GA, Curran JC, Palmer G, Downing SR, et al. Comprehensive genomic profiling of relapsed and metastatic adenoid cystic carcinomas by next-generation sequencing reveals potential new routes to targeted therapies. Am J Surg Pathol. 2014;38:235–238. doi: 10.1097/PAS.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 63.Stephens PJ, Davies HR, Mitani Y, Van Loo P, Shlien A, Tarpey PS, Papaemmanuil E, Cheverton A, Bignell GR, Butler AP, et al. Whole exome sequencing of adenoid cystic carcinoma. J Clin Invest. 2013;123:2965–2968. doi: 10.1172/JCI67201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cho WC. MicroRNAs: potential biomarkers for cancer diagnosis, prognosis and targets for therapy. Int J Biochem Cell Biol. 2010;42:1273–1281. doi: 10.1016/j.biocel.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 65.Kiss O, Tőkés AM, Spisák S, Szilágyi A, Lippai N, Szász AM, Kulka J. MicroRNA-profiling in breast- and salivary gland-derived adenoid cystic carcinomas. Orv Hetil. 2013;154:963–968. doi: 10.1556/OH.2013.29643. [DOI] [PubMed] [Google Scholar]
- 66.Brill LB, Kanner WA, Fehr A, Andrén Y, Moskaluk CA, Löning T, Stenman G, Frierson HF. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod Pathol. 2011;24:1169–1176. doi: 10.1038/modpathol.2011.86. [DOI] [PubMed] [Google Scholar]
- 67.Persson M, Andrén Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci USA. 2009;106:18740–18744. doi: 10.1073/pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stenman G, Andersson MK, Andrén Y. New tricks from an old oncogene: gene fusion and copy number alterations of MYB in human cancer. Cell Cycle. 2010;9:2986–2995. doi: 10.4161/cc.9.15.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramsay RG, Gonda TJ. MYB function in normal and cancer cells. Nat Rev Cancer. 2008;8:523–534. doi: 10.1038/nrc2439. [DOI] [PubMed] [Google Scholar]
- 70.Miao RY, Drabsch Y, Cross RS, Cheasley D, Carpinteri S, Pereira L, Malaterre J, Gonda TJ, Anderson RL, Ramsay RG. MYB is essential for mammary tumorigenesis. Cancer Res. 2011;71:7029–7037. doi: 10.1158/0008-5472.CAN-11-1015. [DOI] [PubMed] [Google Scholar]
- 71.Gonda TJ, Leo P, Ramsay RG. Estrogen and MYB in breast cancer: potential for new therapies. Expert Opin Biol Ther. 2008;8:713–717. doi: 10.1517/14712598.8.6.713. [DOI] [PubMed] [Google Scholar]
- 72.Nicolau M, Levine AJ, Carlsson G. Topology based data analysis identifies a subgroup of breast cancers with a unique mutational profile and excellent survival. Proc Natl Acad Sci USA. 2011;108:7265–7270. doi: 10.1073/pnas.1102826108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fehr A, Kovács A, Löning T, Frierson H, van den Oord J, Stenman G. The MYB-NFIB gene fusion-a novel genetic link between adenoid cystic carcinoma and dermal cylindroma. J Pathol. 2011;224:322–327. doi: 10.1002/path.2909. [DOI] [PubMed] [Google Scholar]
- 74.Vranic S, Gatalica Z, Deng H, Frkovic-Grazio S, Lee LM, Gurjeva O, Wang ZY. ER-α36, a novel isoform of ER-α66, is commonly over-expressed in apocrine and adenoid cystic carcinomas of the breast. J Clin Pathol. 2011;64:54–57. doi: 10.1136/jcp.2010.082776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. A variant of estrogen receptor-{alpha}, hER-{alpha}36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci USA. 2006;103:9063–9068. doi: 10.1073/pnas.0603339103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang XT, Kang LG, Ding L, Vranic S, Gatalica Z, Wang ZY. A positive feedback loop of ER-α36/EGFR promotes malignant growth of ER-negative breast cancer cells. Oncogene. 2011;30:770–780. doi: 10.1038/onc.2010.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weinberg RA. The biology of cancer. New York, USA: Garland Science, Taylor & Francis Group, LLC; 2007. [Google Scholar]
- 78.Honeth G, Bendahl PO, Ringnér M, Saal LH, Gruvberger-Saal SK, Lövgren K, Grabau D, Fernö M, Borg A, Hegardt C. The CD44+/CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10:R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsuda H, Tani Y, Weisenberger J, Kitada S, Hasegawa T, Murata T, Tamai S, Hirohashi S, Matsubara O, Natori T. Frequent KIT and epidermal growth factor receptor overexpressions in undifferentiated-type breast carcinomas with ‘stem-cell-like’ features. Cancer Sci. 2005;96:333–339. doi: 10.1111/j.1349-7006.2005.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wetterskog D, Wilkerson PM, Rodrigues DN, Lambros MB, Fritchie K, Andersson MK, Natrajan R, Gauthier A, Di Palma S, Shousha S, et al. Mutation profiling of adenoid cystic carcinomas from multiple anatomical sites identifies mutations in the RAS pathway, but no KIT mutations. Histopathology. 2013;62:543–550. doi: 10.1111/his.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ivanov SV, Panaccione A, Brown B, Guo Y, Moskaluk CA, Wick MJ, Brown JL, Ivanova AV, Issaeva N, El-Naggar AK, et al. TrkC signaling is activated in adenoid cystic carcinoma and requires NT-3 to stimulate invasive behavior. Oncogene. 2013;32:3698–3710. doi: 10.1038/onc.2012.377. [DOI] [PubMed] [Google Scholar]
- 82.Ivanov SV, Panaccione A, Nonaka D, Prasad ML, Boyd KL, Brown B, Guo Y, Sewell A, Yarbrough WG. Diagnostic SOX10 gene signatures in salivary adenoid cystic and breast basal-like carcinomas. Br J Cancer. 2013;109:444–451. doi: 10.1038/bjc.2013.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pastolero G, Hanna W, Zbieranowski I, Kahn HJ. Proliferative activity and p53 expression in adenoid cystic carcinoma of the breast. Mod Pathol. 1996;9:215–219. [PubMed] [Google Scholar]
- 84.Lerma E, Peiro G, Ramón T, Fernandez S, Martinez D, Pons C, Muñoz F, Sabate JM, Alonso C, Ojeda B, et al. Immunohistochemical heterogeneity of breast carcinomas negative for estrogen receptors, progesterone receptors and Her2/neu (basal-like breast carcinomas) Mod Pathol. 2007;20:1200–1207. doi: 10.1038/modpathol.3800961. [DOI] [PubMed] [Google Scholar]
- 85.Nielsen J, Christiansen J, Lykke-Andersen J, Johnsen AH, Wewer UM, Nielsen FC. A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol Cell Biol. 1999;19:1262–1270. doi: 10.1128/mcb.19.2.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walter O, Prasad M, Lu S, Quinlan RM, Edmiston KL, Khan A. IMP3 is a novel biomarker for triple negative invasive mammary carcinoma associated with a more aggressive phenotype. Hum Pathol. 2009;40:1528–1533. doi: 10.1016/j.humpath.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 87.Sidoni A, Cartaginese F. IMP3 expression in triple-negative breast carcinoma. Hum Pathol. 2010;41:1355–1356; author reply 1356-1357. doi: 10.1016/j.humpath.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 88.Vranic S, Gurjeva O, Frkovic-Grazio S, Palazzo J, Tawfik O, Gatalica Z. IMP3, a proposed novel basal phenotype marker, is commonly overexpressed in adenoid cystic carcinomas but not in apocrine carcinomas of the breast. Appl Immunohistochem Mol Morphol. 2011;19:413–416. doi: 10.1097/PAI.0b013e3182143399. [DOI] [PubMed] [Google Scholar]
- 89.Vranić S, Bilalović N, Lee LM, Kruslin B, Lilleberg SL, Gatalica Z. PIK3CA and PTEN mutations in adenoid cystic carcinoma of the breast metastatic to kidney. Hum Pathol. 2007;38:1425–1431. doi: 10.1016/j.humpath.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 90.Silva I, Tome V, Oliveira J. Adenoid cystic carcinoma of the breast with cerebral metastisation: a clinical novelty. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.08.2011.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xue Y, Liu X, Poplack S, Memoli VA. Adenoid cystic carcinoma of the breast in reduction mammoplasty. Breast J. 2012;18:611–613. doi: 10.1111/tbj.12029. [DOI] [PubMed] [Google Scholar]
- 92.Youk JH, Kim MJ, Kim EK, Lee JY, Oh KK, Park BW. Recurrence of adenoid cystic carcinoma in the breast after lumpectomy and adjuvant therapy. J Ultrasound Med. 2006;25:921–924. doi: 10.7863/jum.2006.25.7.921. [DOI] [PubMed] [Google Scholar]
- 93.Hodgson NC, Lytwyn A, Bacopulos S, Elavathil L. Adenoid cystic breast carcinoma: high rates of margin positivity after breast conserving surgery. Am J Clin Oncol. 2010;33:28–31. doi: 10.1097/COC.0b013e31819fdfc8. [DOI] [PubMed] [Google Scholar]
- 94.Wilson WB, Spell JP. Adenoid cystic carcinoma of breast: a case with recurrence and regional metastasis. Ann Surg. 1967;166:861–864. doi: 10.1097/00000658-196711000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Herzberg AJ, Bossen EH, Walther PJ. Adenoid cystic carcinoma of the breast metastatic to the kidney. A clinically symptomatic lesion requiring surgical management. Cancer. 1991;68:1015–1020. doi: 10.1002/1097-0142(19910901)68:5<1015::aid-cncr2820680518>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 96.Koller M, Ram Z, Findler G, Lipshitz M. Brain metastasis: a rare manifestation of adenoid cystic carcinoma of the breast. Surg Neurol. 1986;26:470–472. doi: 10.1016/0090-3019(86)90260-0. [DOI] [PubMed] [Google Scholar]
- 97.Lim SK, Kovi J, Warner OG. Adenoid cystic carcinoma of breast with metastasis: a case report and review of the literature. J Natl Med Assoc. 1979;71:329–330. [PMC free article] [PubMed] [Google Scholar]
- 98.Millar BA, Kerba M, Youngson B, Lockwood GA, Liu FF. The potential role of breast conservation surgery and adjuvant breast radiation for adenoid cystic carcinoma of the breast. Breast Cancer Res Treat. 2004;87:225–232. doi: 10.1007/s10549-004-8693-z. [DOI] [PubMed] [Google Scholar]
- 99.Page DL. Adenoid cystic carcinoma of breast, a special histopathologic type with excellent prognosis. Breast Cancer Res Treat. 2005;93:189–190. doi: 10.1007/s10549-005-5198-3. [DOI] [PubMed] [Google Scholar]
- 100.Sinn HP, Lehnert T, Otto HF. Adenoid cystic cancer of the breast. Case report and meta-analysis of the literature. Chirurg. 1993;64:198–202. [PubMed] [Google Scholar]


