Abstract
Targeted drug delivery to solid tumors is a very active research area, focusing mainly on improved drug formulation and associated best delivery methods/devices. Drug-targeting has the potential to greatly improve drug-delivery efficacy, reduce side effects, and lower the treatment costs. However, the vast majority of drug-targeting studies assume that the drug-particles are already at the target site or at least in its direct vicinity. In this review, drug-delivery methodologies, drug types and drug-delivery devices are discussed with examples in two major application areas: (1) inhaled drug-aerosol delivery into human lung-airways; and (2) intravascular drug-delivery for solid tumor targeting. The major problem addressed is how to deliver efficiently the drug-particles from the entry/infusion point to the target site. So far, most experimental results are based on animal studies. Concerning pulmonary drug delivery, the focus is on the pros and cons of three inhaler types, i.e., pressurized metered dose inhaler, dry powder inhaler and nebulizer, in addition to drug-aerosol formulations. Computational fluid-particle dynamics techniques and the underlying methodology for a smart inhaler system are discussed as well. Concerning intravascular drug-delivery for solid tumor targeting, passive and active targeting are reviewed as well as direct drug-targeting, using optimal delivery of radioactive microspheres to liver tumors as an example. The review concludes with suggestions for future work, considereing both pulmonary drug targeting and direct drug delivery to solid tumors in the vascular system.
Keywords: Targeted drug delivery, Pulmonary system, Vascular system, Types of drugs and delivery devices, Computational analysis and experimental evidence, Future work
Core tip: Targeted drug delivery to diseased areas or solid tumors has the great potential to significantly improve treatment efficacy, minimize side-effects, and reduce health-care cost. The major problem addressed is how to deliver efficiently the drug-particles from the entry/infusion point to the target site. Past and present developments in drug formulation and associated drug-delivery devices are discussed. Examples of optimal drug delivery to pulmonary target sites as well as targeting solid tumors in the vascular system are reviewed.
INTRODUCTION
In light of the high cost of medicine and potentially devastating side-effects of drug treatment, targeted drug delivery is of great clinical significance. Thus, targeted drug delivery to solid tumors is a very active research area, focusing mainly on improved drug formulation and associated best delivery methods/devices. Drug-targeting has the potential to greatly improve drug-delivery efficacy, reduce side effects, and lower treatment costs. However, the vast majority of drug-targeting studies assume that the drug-particles are already at the target site or at least in its direct vicinity.
In this review, drug-delivery methodologies, drug types and drug-delivery devices are discussed with examples in two major application areas: (1) inhaled drug-aerosol delivery into human lung-airways; and (2) intravascular drug-delivery for solid tumor targeting. The major problem addressed is how to deliver efficiently the drug-particles from the entry/infusion point to the target site. So far, most experimental results are based on animal studies.
Concerning pulmonary drug delivery, the focus is on the advantages and disadvantages of the three inhaler types, i.e., pressurized metered dose, dry powder and nebulizer, in addition to drug-aerosol formulations. Computational fluid-particle dynamics techniques and the underlying methodology for a smart inhaler system (SIS) are discussed as well.
Concerning intravascular drug-delivery for solid tumor targeting, passive and active targeting are reviewed as well as direct drug-targeting, using optimal delivery of radioactive microspheres to liver tumors as an example.
The review concludes with future work for both pulmonary drug targeting and direct drug delivery to solid tumors in the vascular system.
PULMONARY DRUG-TARGETING METHODLOGIES
Targeted drug delivery and controlled release are current challenges in pulmonary drug delivery. Three popular drug delivery ways are per oral (pill swallowing), intravenous (drug injection through the vein), and inhalation (breathing into the human lung).
Pulmonary drug delivery therapies, ranging from the treatment of asthma and chronic obstructive pulmonary diseases (COPD) to lung tumors and systemic diseases, have gained great interest in recent years. Advantages of pulmonary drug delivery, when compared with conventional medical treatments include, improvements in efficiency because of the large surface area of the lung (i.e., 100 m2) and the thin epithelial layer thickness (0.2 to 0.7 μm)[1], reduction of systemic drug levels with a decrease in adverse effects, and higher degree of convenience[2-4]. Specifically, as the drug aerosol directly travels to the designated target area, a much lower dose can be used to produce a therapeutic response with negligible side effects[5].
Pulmonary drug delivery therapies are widely used to treat inflammation, asthma, COPD and cystic fibrosis (CF) as well as diabetes and other systemic diseases. For proper treatment the aerodynamic diameters of drug particles/droplets are recommended to be in the range of 0.4 < dp < 7 μm[6,7]. However, due to the sophisticated pharmaceutical aerosol formulations and the complex anatomy and physiology of human lung airways, the optimization of pulmonary drug delivery (e.g., drug-targeting delivery in lung airways) is challenging. The major research concern in pulmonary drug delivery is on the utilization of physical or chemical mechanisms, novel particles or drug carriers, and new drug-delivery device developments with improved performance.
In this section, three major classes of pulmonary drug delivery systems, i.e., pressurized metered-dose inhaler (pMDI), dry powder inhaler (DPI) and nebulizer, are introduced and discussed, focusing on their delivery mechanisms, efficacies, and challenges for future developments. Furthermore, this section is also devoted to the foundation of lung-aerosol dynamics, i.e., the transport and deposition of particulate drug carriers, which include the parameters affecting drug aerosol transport and deposition characteristics. It will be followed by a description of state-of-the-art methodologies for the realization of optimal pulmonary drug delivery.
Inhalers and drug-aerosol transport
Type of inhalers: There is a rich history of the development of pulmonary drug delivery therapy[8]. Pulmonary drug delivery devices or inhalers are classified into three major types: pMDIs, DPI and nebulizers[3].
pMDIs are devices in which the mixture of drugs and propellants is stored in a canister from which accurate amounts can be released when the device is actuated by human power[3]. Thus, pMDIs provide a fast and cost-efficient solution to deliver pulmonary drug aerosols[9]. A pMDI expels the drug aerosol driven by propellants, such as chlorofluorocarbons (CFC) which, however, is being phased out due to environmental concerns. More recently, hydro fluoroalkanes propel the medicine through a nozzle at high velocities (> 30 m/s)[10]. The typical structure of a pMDI is shown in Figure 1, including canister, metering valve, actuator, and propellant. Detailed functions of each part were presented by Newman[11].
Figure 1.
Typical structure of pressurized metered-dose inhaler[23]. (Reprinted with permission from Ref.[23]).
The advantages of pMDIs are: good portability, accurate dosage control, large capacity of medical doses at low cost[12]. Disadvantages of pMDIs include: highly dependent on the coordination of the patient’s inhalation[3,13], limited to certain drugs that are physically and chemically inert in the mixture with the propellant, and not efficient to treat deeper lung conditions due to the strong impaction in the upper respiratory system induced by the high jet velocity[5]. For example, only approximately 10%-20% of the medications emitted from CFC-pMDIs are able to enter and deposit in the lung, while the rest deposits in the oropharynx[14]. Additionally, the deposition of the content of drug formulation inside the canister can result in an incorrect dose of medication delivery (en.wikipedia.org/wiki/Metered-dose_Inhaler).
To replace CFC and find a new propellant, hydrofluoroalkane was introduced[12] and approved. To resolve the synchronization problem between device actuation and patient’s inhalation, breath-actuated MDIs were developed; for example, the Maxair Autohaler[15]. The devices actuate early during inspiration at an inspiratory flow rate of 30 L/min and are well accepted by patients[12,16]. It is announced that using breath-actuated inhalers might improve asthma control and reduce overall cost of asthma therapy compared with conventional pMDIs[12,17].
Furthermore, to improve efficacy of drug delivery and avoid synchronization difficulties between patient’s inhalation and pMDI actuation, spacer devices have been introduced as an alternative method. The basic design of a spacer contains 3 parts: the open tube, the reservoir/holding chamber, and the reverse flow design in which the pMDI is fired in the direction away from the patient. A one-way valve is used to create a holding chamber after pMDI actuation. Holding chambers serve as particle size filter and produce a fine aerosol because of the stronger inertial impaction of drug-aerosol particles with larger size and particle evaporation of propellant within the chamber. Studies indicated that using a larger-volume (> 750 mL) holding chamber (also called spacer) can provide higher fine-particle doses than a pMDI alone[18]. However, spacers may decrease the portability of pMDIs.
DPIs are an alternative to pMDIs, delivering pulmonary drugs into the respiratory system in the form of dry solid particles[3]. The dispersion of a dry powder aerosol is conducted from a static powder bed. When the patient takes a breath, air is introduced into the powder bed which creates turbulent flow and leads to fluidization of a static powder blend, entering the patient’s airways. DPIs contain medicine in a variety of types, e.g., single-dose capsule-based designs, multi-dose units containing the drug in bulk, and multi-dose units containing individual blister packages[19,20]. DPIs can also be divided into two types in terms of its drive force, i.e., a passive DPI dependent on patients’ inhalation and an active DPI dependent on external forces[21,22]. Active DPIs have become a preferred method to uniformly distribute drugs independent of the inspiration flow. A typical structure of a unit-dose DPI is shown in Figure 2.
Figure 2.
Typical structure of unit-dose dry powder inhaler.
DPIs are small, portable and easy to use. Several advantages were discussed by Sahane et al[3]. For example, there is no need for coordination of actuation and inhalation, because the patient’s inspiratory force de-aggregates the powder and generates the aerosol. Furthermore, DPIs are able to deliver higher drug payloads to the airways[20].
However, there are several disadvantages of DPIs. For example, if the therapeutic dose of a drug is high, the patient needs to manually reload several individual units per dose, as delivery is limited to a capsule-based or blister-based unit[19]. Also, DPI medications must be stored in a dry place at a temperature of not more than 25 °C and humidity between 40%-50% in sealed packages (en.wikipedia.org/wiki/Dry_Powder_Inhaler). Specifically, exposing the powder in a high-humidity environment destroy the medication dispersion ability of the device, implying that the efficacy of a DPI depends mainly on the flow properties of the powder suspension.
Nebulizers are breathing devices that generate droplets in small scales from a liquid in solution/suspension[12], which are used to treat lung diseases. The inhaled drug is in the form of mist aerosols. Nebulizers are often used in situations in which a conventional inhaler is ineffective. In simple targeting cases, nebulizers may limit side effects of certain medication, say, steroids, by delivering the drug directly to the desired site. Several conventional designs of nebulizers are shown in Figure 3.
Figure 3.
Typical structures of different nebulizer categories. (Reprinted with permission from Ref.[117]. A: Atomizer (jet nebulizer); B: Ultrasonic wave nebulizer; C: Vibrating mesh nebulizer.
Atomizers (or jet nebulizers)[23] are the most common ones (Figure 3A). They use compressed gas, or a compressor, to generate high-velocity air streams through a tight opening and across the fluid medication suspension to create particulate/droplet aerosols. The fluid is split up by the airstream and divided into droplets inside the nebulizing chamber. The primary advantage of jet nebulizers is the low operational expense. However, there is always the lack of portability because of the need of compressed gas[12].
Ultrasonic wave nebulizers generate aerosols via the vibration of a piezoelectric crystal at a high frequency (> 1 MHz) through the drug liquid (Figure 3B). Contrasted with jet nebulizers, ultrasonic nebulizers work with lower noise and provide faster delivery of pharmaceuticals; although due to the complimentary heat generated during the operation, there are several medication restrictions for the ultrasonic wave nebulizers. With both types of nebulizers, the patient inhales vapor through a mouthpiece or face mask. Electronic nebulizers form a subcategory of ultrasonic nebulizers.
Based on the vibrating mesh technology (VMT), smaller portable devices have been developed as advanced nebulizers, i.e., vibrating mesh nebulizers[24]. With the VMT, a mesh/membrane with multiple apertures vibrates at the top of the liquid reservoir (Figure 3C), generating drug aerosols consisting of small-scale droplets. Vibrating mesh nebulizers are claimed to have higher output efficiency, minimal residual volume, and high percentage of fine particulate drugs in the emitting stream[24].
Another type of new-generation nebulizers is called human-powered nebulizers which are breath-enhanced[25]. Their improved designs avoid exhaled loss and apparatus loss of aerosols. Ambient air is entrained through a one-way valve along with the power gas during inspiration, while during exhalation the one-way plastic flapper valve is closed.
As part of the developments of human-powered nebulizers, a dosimetric nebulizer is defined as one that releases aerosols only during inhalation, being the most efficient nebulizer generating aerosols. Comparisons of different types of nebulizers are presented in Table 1[12]. A variety of nebulizer products on the market are summarized in Table 2.
Table 1.
Comparisons of conventional categories of nebulizers
| Jet | Ultrasonic | Vibrating mesh | |
| Features | |||
| Power source | Compressed gas or electrical mains | Electrical mains | Batteries or electrical mains |
| Portability | Restricted | Restricted | Portable |
| Treatment time | Long | Intermediate | Short |
| Output rate | Low | Higher | Highest |
| Residual volume | 0.8-2.0 mL | Variable but low | < 0.2 mL |
| Environmental contamination | |||
| Continuous use | High | High | High |
| Breath-activated | Low | Low | Low |
| Performance variability | High | Intermediate | Low |
| Formulation characteristics | |||
| Temperature | Decreases | Increases | Minimum change |
| Concentration | Increases | Variables | Minimum change |
| Suspensions | Low efficiency | Poor efficiency | Variable efficiency |
| Denaturation | Possible | Probable | Possible |
| Cleaning | Required, after single use | Required, after multiple use | Required, after single use |
| Cost | Very low | High | High |
Table 2.
Advantages and disadvantages of different nebulizer products
| Product name | Type | Pros | Cons |
| PARI Vios® | Jet nebulizer | Low operational cost, robust in structure, effective in nebulizing suspensions | Relatively bigger in size and heavier in weight; more noisy |
| Omron MicroAir® | Electronic nebulizer | Does not heat the medicine, quiet and fast drug delivery | Comparatively expensive; |
| Omron NE-U17®, Beurer Nebulizer IH30® | Ultrasonic nebulizer | Silent and portable | replaced much more frequently |
| Omron NE-U22V®, | Vibrating mesh nebulizer | Higher output efficiency, minimal residual volume, and High percentage of particles in the emission, small in size | Less efficient in nebulizing aerosols |
| Pari E-Flow® | |||
| Pari LCD® | Breathe-enhanced nebulizers | Higher output efficiency avoiding apparatus loss and exhaled loss | N/A |
| AeroEclipse® | Dosimetric nebulizer | Higher output efficiency avoiding apparatus loss and exhaled loss | N/A |
In addition to the three major classes of inhalers, new types of inhalers were designed in order to improve the efficacy. “Soft mist inhaler” is another type of drug delivery device[5,26]. It was developed in order to overcome the limitations of traditional inhaler devices and to meet the need for a convenient propellant-free inhaler[5]. For example, the Respimat® Soft Mist™ inhaler (SMI) utilizes the mechanical force from a spring instead of a fluid-gas propellant to produce a drug aerosol which is suitable for inhalation. The spring system inside the inhaler can guarantee that the aerosol is produced by a reliable and reproducible energy source. Thus, dosage and size distribution of the drug aerosols are insensitive to the inspiratory characteristics of the patient. Medicine delivered by the SMI is stored in a collapsible bag in a sealed plastic container inside the cartridge. With each actuation, the correct dose is drawn from the reservoir and the flexible bag contracts, which is launched by the spring[27]. A recent papers[28] also claimed that SMI can avoid the coordination difficulty between inhalation and actuation. Also, the emitted stream velocity of the aerosol is much slower than that of a pMDI. Theoretically, with the SMI aerosol transport to deeper lung airways is easier due to lower inertial impaction in the upper respiratory system, so that the resulting deposition fraction can reach 40% for adults[19]. However, besides the advantages of SMI, such a device is relatively expensive. Other SMI designs include AERx, and AERx Essence platform (Aradigm, Hayward, California)[29].
MDIs and DPIs are both portable and fast delivering devices for low medication dosages. Another characteristics of both device types is that the aerosol generator and the medication are not detachable. In contrast, nebulizers are able to deliver high medication dosages, and a single device can be used with different drugs[30].
Compared to pMDIs and DPIs, another advantage of using nebulizers for drug inhalation is that no special inhalation techniques are required[12]. However, due to the need of compressed gas or a compressor to operate, conventional nebulizers are generally not portable. Additionally, the drug-delivery efficacy and treatment time using conventional nebulizers are much lower and longer than those for pMDIs and DPIs[31]. However, presently there appears to be a tendency among physicians to prefer to prescribe a pMDI rather than a jet nebulizer which produces more noise and is less portability.
Comparative efficacy of different pulmonary drug delivery devices has been performed by different research groups. Chou et al[32] concluded that MDIs can give a faster and more economical approach to deliver bronchodilator drug aerosols for asthma treatment in elder children and adults. A similar conclusion was reported by Batra et al[33] for the aerosolized salbutamol in an acute exacerbation of asthma in children. Delgado et al[9] investigated nebulizers vs MDI with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 mo. They drew the conclusion that although younger patients are often unable to coordinate inspiration with activation of the MDI, thereby limiting the effective amount of drug inhaled, MDI with spacers may be as efficacious as nebulizers for the emergency department treatment of wheezing in children aged two years or younger. Dhuper et al[34] claimed that using a MDI with a spacer may result in a marked reduction in time and effort, thereby saving the total cost for treatment compared with conventional nebulizers.
While the optimization of treatment using MDIs requires coordination between inspiration and actuation, which is difficult to achieve by patients[13], most DPIs generate and deliver drug aerosols depending on subject-specific inhalation efforts. Indeed, the dose delivered by DPIs is more related to the inspiratory flow rate than by the device[35]. Specifically, MDIs are able to generate more consistent aerosol sizes across a range of inspiratory flow rates. In contrast, the aerosol-size distributions produced by DPIs are reported to be highly dependent on the inhalation patterns, with some being showing more sensitive than others[36].
Not surprising, all drug delivery devices have advantages and limitations. Device selection must consider portability, convenience level for users, therapeutic efficacy as well as low cost and high safety[12]. As mentioned, the therapeutic efficacy is determined by many factors, i.e., the kind of medication used, the aerosol’s physical and chemical properties, as well as the patient’s inhalation patterns and the physiology of their respiratory systems[30].
The efficacy enhancement of drug delivery devices can be achieved through two major strategies: (1) improved drug formulation; and (2) improved device structure design. The primary goal is to control the agglomeration of drug aerosols inside devices to reduce drug residues in the device, thereby enhancing the efficacy.
Drug formulation: The physical and chemical properties (e.g., particle size distribution, shape, surface charge, hygroscopicity, etc.) of drug-particles are most important. Usually, drug formulations, including drug-carrier selections, are able to lower inhaler retention and improve transport, the proportion of drugs that reach the desired lung site, and the stability of the drug in vivo. Sufficiently strong attractions between carrier and drug must be guaranteed for maintaining the stability of the medication mixture. However, the attractions between carrier and drug must also be sufficiently weak so that the release of the medication from the carrier will not be impeded inside the human respiratory system[3]. Sahane et al[3] also discussed the formulation of drugs for DPIs. For example, DPIs are formulated using four types of formulation strategies: Carrier Free, Drug Carrier, Drug Additive, Drug Carrier Additive. Specifically, therapeutic dry powders for inhalation are often composed of fine drug particles and inert coarse carrier particles such as lactose[37]. Sufficient detachment of the drug from its carrier must be guaranteed to improve the delivery efficiency. Improved formulation should reduce the particle-particle interacting forces which are the major cause of the agglomeration between drugs and carriers. Furthermore, adding preservatives and mixing it with other drugs will also influence device output and aerosol characteristics in a good way[38]. Formulations for pMDIs are also necessary. For example, mast cell stabilizers such as cromoglicate or nedocromil are used to extend the duration of the device[11]. In the recent decade, non-hygroscopic mannitol exhibits particularly great promise as an attractive carrier in DPI systems to replace lactose[37,39]. Additionally, it is necessary to minimize the adhesion intensity between the drug particle and the inner wall of the inhalers by using surface treatment to reduce inhaler retention and enhance the drug delivery efficacy[40].
Device structure: Device structures can have a strong impact on the velocity field inside the device, thereby influencing the drug suspension characteristics. For example, the design of the actuator plays an important role in the delivered spray characteristics generated by pMDIs[11]. Specifically, the design variables of the actuator are expansion chamber size and shape, nozzle diameter, nozzle path length, mouthpiece length and shape, breath-actuation, spray velocity modification, and spacer attachment[19,41]. For nebulizers, the chamber design using a one-way valve is important to decrease aerosol waste during exhalation[27]. Also, the mouthpiece design is essential to lower the percentage of drugs remaining in the device. For example, increasing the cross-sectional area of the mouthpiece section will decrease the emitting velocity of the drug aerosol thereby lowering the extent of drug deposition in the oral cavity due to the inertial impaction effect[42]. Other factors related to structure which will influence the device performance are flow path design and manufacturing design[7].
The patient’s inhalation behavior, in light of the correct utilization of a drug delivery device, is likewise essential for successful therapy. Specifically, it needs to be guaranteed that a patient can easily use the device correctly and properly thereby optimizing the therapeutic efficacy[27]. Therefore, the ideal design of drug delivery devices should include the following characteristics[11,22]: (1) attractive in appearance, easy to use, and easy to carry; (2) accurate and uniform delivery of medication over a variety of patient’s inhalation intensities; (3) reliable qualitative drug delivery control throughout the life of the inhaler; and (4) low cost with high efficacy.
Traditionally, the biotechnology and pharmaceutical industries preferred jet nebulizers for novel medication development, considering their capabilities to deliver higher doses of drugs and the lower research and development expenses than other types of devices[19]. However, recently many companies have recognized the long time duration at each treatment experienced by patients. Therefore, to handle that issue they have been starting preclinical development with novel devices based on more efficient and advanced drug delivery technologies.
In the previous two decades, a few innovative inhaler designs have been developed, providing more efficient drug aerosol delivery[12]. New designs and new improvements were presented in several papers[11,12,19,20,43-46]. For example, the improvement of pMDIs is mainly focusing on providing more precise targeting, dose metering, and easy actuation. Related new products are listed by Dolovich et al[12]. For new designs of the major three classes of inhalers, Zhang et al[20] proposed novel active and multi-dose dry powder inhalers which are able to utilize the compressed air to deliver a small quantity of extra fine particles with high delivery efficacy. A new design for a nebulizer with flow meter function was proposed by Addington et al[46]. By changing DPI device structure and drug formulation, Behara et al[47] proposed a design option of a DPI which controls the aerosol diameters and increases the emitted drug dose.
Drug-aerosol dynamics: Liquid and solid micro/nano- particulate matter (i.e., solid particles and droplets) and vapors are generated by drug delivery devices for therapeutic purposes. The objective of targeting is to guide and deliver drugs from their releasing position to expected deposition regions in the human respiratory system to optimize the medical effectiveness[48]. The fluid-particle dynamics during drug-aerosol transport, deposition, absorption, and clearance are essential for guiding the optimization technique of drug delivery. Specifically, “targeting” can be categorized into three levels[1]: (1) delivery to a specific lung region, i.e., central or peripheral, right or left; (2) delivery to the site of disease; and (3) delivery to distinct cell types with biological barrier transport, e.g., epithelial cells, or cells of the lung associated lymphatic tissue. Traditional targeting activities can be also grouped into passive and active targeting[49].
As discussed in the previous sections, great progress has been made in drug aerosol formulation for better drug-aerosol delivery efficiency, as well as controlling drug aerosol transport before deposition in lung airways. However, it is also necessary to understand the absorption and systemic transport of drug aerosols after the deposition at the air-blood barriers, which has been rarely investigated. In this section, underlying principles for drug-aerosol transport and deposition in human respiratory systems are discussed to provide valuable insight into certain aspects for optimal pulmonary drug-targeting.
Physical and chemical factors which can affect the transport and deposition of drug-aerosols include: initial particle size, shape, density, concentration, as well as release position and velocity; furthermore, the drug-aerosol formulation (i.e., hygroscopicity, charge and surfactant) as well as the geometric characteristics of the patient-specific respiratory system are important[6,41,48].
The underlying principles to describe the complex and coupled fluid-aerosol dynamics inside the human respiratory systems are the physical conservation laws, using the Eulerian or Lagrangian modeling approach. Specifically, the Eulerian approach can be employed to calculate continuity, momentum and energy equations for the continuous airflow phase, while both Eulerian and Lagrange approaches can be used for the discrete drug aerosol phases[6,48]. Supplementary equations may be necessary for the description of complex mechanisms, e.g., evaporation or condensation of aerosol droplets[50], magnetic-force driven drug-targeting delivery[51], rotational motion of non-spherical particles[52], etc. Several computational fluid-particle dynamics (CF-PD) models are available for the calculation of air-drug mixture dynamics[53], such as the discrete phase model (DPM), two-fluid model, mixture model, dense dispersed phase model (DDPM), and the discrete element method (DEM).
The deposition mechanisms of aerosol particles or droplets - impaction, sedimentation, diffusion and combinations in the respiratory tract - have been extensively discussed[6,48,54]. Specifically, for microparticles, the deposition may occur due to: (1) secondary airflow (laminar or turbulent) induced wall impaction; (2) inertial wall impaction; (3) gravity induced sedimentation; (4) particle-particle interaction induced wall impaction; and/or (5) diffusion. For nanoparticles, diffusion, caused by Brownian motion, may become most significant.
Other than treating local diseases in the respiratory system, pulmonary drug delivery is also promising for systemic drug delivery which is intended to utilize the alveolar region to swiftly absorb drug aerosols. However, the processes that take place after an aerosol particle has landed on the pulmonary epithelial surfaces, i.e., the dissolution, absorption, mucociliary clearance, and systemic translocation, still lack information[55]. Using complex pharmacokinetic modeling will be helpful in understanding the absorption process through the lung epithelium, including parcellular transport and transcellular transport. Considering numerical modeling of this process, mass transfer advection-diffusion equations can be employed with proper boundary conditions[56]. Furthermore, systemic drug transport can be modeled using multi-compartment mass transfer models for drug-aerosol migration into the mucus-tissue-blood system[57,58]. Factors affecting drug absorption are physiochemical properties of drug aerosol particles (e.g., hydrophobicity), co-administration conditions, lung pathophysiology, etc.[55].
With decreasing computational limitations and the advancements in commercial CFD software development, more realistic numerical models are becoming available for the simulation of dense particle suspensions as well as droplets with heat and mass transfer. Those models can be used for the design of drug delivery devices[59]. Although experiments can provide some information about the airflow field of the drug delivery device[60], using CFD-techniques provide more detailed information when compared to experiments. Specifically, computer simulation models require initial and boundary conditions as well as the geometry of the flow domain of the device. Once fully validated, they are cost-effective tools to analyze factors influencing the performance of drug delivery devices, e.g., turbulence in the inhaler, spray momentum and inlet jet effects, as well as best possible geometric design and operation.
Originally, CFD was only utilized for airflow field analysis of the drug inhalers[61,62]. With the development of numerical multiphase flow methods, simulation techniques were used for the design and analysis. For example, Kleinstreuer et al[63] numerically investigated the transport and deposition of drug droplet aerosols from a pMDI into a model of the human respiratory system. The parametric analyses with different propellants, nozzle diameters, and releasing positions were presented. Based on the numerical results, they found that using a smaller nozzle provides a better atomization effect and finer droplets with more uniform dispersion.
Recently, based on the numerical model established by Worth Longest et al[64,65], Longest et al[13] investigated different aerosol deposition in human lung airways between a specific MDI and DPI. Fluent 12.0 with user-defined functions (UDFs) was employed for the Euler-Lagrange numerical simulations. They claimed that for the specific inhaler models they investigated, MDI is able to deliver significantly more drugs to the tracheobronchial region when compare to DPI. It is worth mentioning that they did not consider any particle-particle interaction effects. Jiang et al[66] investigated the design impact on a commercial DPI using the LRN κ-ω model in ANSYS Fluent 6.2.16. However, they did not simulate powder transport and subsequent deposition by using any multiphase flow model. Based on CFD analysis, Longest et al[67] evaluated associations between aerodynamic parameters and DPI performance for a carrier-free formulation, forming micron/submicron-scale drug aerosols. Factors which may influence the dispersion of aerosol particles were discussed.
With the development of computational fluid dynamics-discrete element method (CFD-DEM), the discrete element method is a robust and computational economic model to simulate the highly dynamic process (i.e., particle-particle interaction) for dense powder dispersions in inhalers[68]. Inhaler developments based on CFD-DEM simulations have been focused on pharmaceutical agglomerate break-up in DPIs[59]. For example, Tong et al[68,69] recently employed ANSYS Fluent with in-house UDFs, describing powder dispersion in a commercial Aerolizer® Inhaler model. They also investigated the factors influencing the performance of the inhaler based on their numerical simulation results. They found that at low flow velocities, agglomerates consisting of particulate matters with smaller diameters were more difficult to disperse. They also claimed that the dispersion efficiency is proportional to the ratio of the particle impact energy and particle-particle cohesion energy.
In summary, it is promising to use CFD-DEM or DDPM-DEM for the simulation of dense drug-powder suspensions in pMDIs and DPIs, because of the DEM capability of taking into account the particle-particle contact interaction as well as the computational economy aspect. CF-PD models can also provide guidance for drug delivery and hence enhancing methodology developments which are discussed in Section 2.2. For a recent review see Ruzycki et al[70].
Design and strategies for direct drug-targeting
Existing drug aerosol delivery devices, including those that attempt to target specific areas in the lung, exhibit poor efficiencies (e.g., from 5% to 20%). Efforts are being made to improve direct drug delivery through the pulmonary route. The goal is to provide high doses of drugs to lung tumor tissue via inhalation, resulting in treatment efficiencies and low adverse side effects.
Smart inhaler system methodology: Kleinstreuer et al[63] analyzed computationally the performance of pMDIs with and without spacers and compared their deposition efficiencies with that of a smart inhaler system (US Patent 7900625 issued 03/08/2011) based on a new optimal targeting methodology[71]. A novel smart inhaler system (SIS), which achieves up to 85% drug-aerosol deposition efficiency, is being prototyped and experimentally tested. The SIS is a device for directed aerosol delivery to predetermined lung sites, facilitated by an adaptive nozzle and a mechanism for inhalation waveform modulation. The aerosol particles are released through a nozzle which incorporates lightweight, multifunctional shape memory materials that allows to move the nozzle’s optimal radial position based on subject-specific numerical data. The SIS is promising to notably improve the aerosol delivery efficiency to specific locations through pulmonary routes, thereby reducing unwanted deposition in healthy lung airways.
Enhanced deeper lung delivery of nano- and micro-pharmaceutical aerosols via condensational growth: Drug aerosol losses occur because of high deposition in the nasal passages or in the oral cavity due to impaction. To enhance drug-aerosol delivery into deeper lung airways, enhanced condensational growth (ECG) and excipient enhanced growth (EEG) methods have been proposed and validated by experiments in vitro[13,72-74]. Based on the fact that the larger mass mean aerodynamic diameter of drug aerosols indicates strong impacting, deposition of particles before entering the trachea as well as most submicron particles inhaled will be exhaled without depositing in the lung airways. Thus one can utilize the high relative humidity of the ambient air or inside the human respiratory system to control the trajectories and depositing sites of the particles in human pulmonary routes, relying on different condensational growth rates of such sub-micron aerosols of different formulations. Specifically, submicron particles are emitted from the inhalers which are able to initially penetrate through the oral or nasal cavity, thereby reducing the deposition before entering the trachea. With the condensation effect, those particles will grow in size and most of them will deposit in deeper lung airways. For ECG, the sub-micron aerosols are inhaled with highly humidified air at a temperature higher than that of the human body. The droplets will grow due to the condensation of surrounding water vapors when they enter the human respiratory system[72]. For EEG, hygroscopic excipients are formulated with the drug and the formulated drug aerosol will absorb water inside the human respiratory system. Although these methods result in higher pulmonary deposition, they are not able to provide location-specific delivery (see Level 2).
Magnetic nanoparticles for site-specific pulmonary drug delivery: Another active targeting strategy is magnetic targeting, which can be realized by combining magnetic nanoparticles (i.e., γ-Fe2O3 and Fe3O4) with micron particles or droplets[51,75-79]. These types of particles are also called magnetic nano-in-microparticles (NIMs). An external magnetic field will be enforced to guide drug aerosols to specific regions of the lung (Figure 4), thereby reducing undesired side effects, e.g., mitigating deposition on healthy lung tissues. To succeed in direct drug delivery, those magnetic nanoparticles should have characteristics such as mono-dispersity, superparamagnetism, stability and biocompatibility[80].
Figure 4.
Targeted deliveries of magnetic aerosol particles. (Reprinted from Ref.[12], with permission from Elsevier).
However, further translation of magnetic nanoparticles may cause potential health problems and need further clinical investigations. The long-term effects of magnetic nanoparticles need to be studied as well[77]. For example, concerns associated with long-term tissue damage, toxicity, carcinogenesis, immunogenicity, and inflammation need to be investigated to improve the production of magnetic nanoparticles[81].
Shape engineering for novel drug carriers: For pulmonary drug delivery, the deposition pattern and clearance from deposition sites are two key parameters for a proper design of drug-delivery carriers[82]. For example the particle shape of drug carriers has a profound impact on optimizing performance of drug delivery. Compared to spherical particles, numerical studies have shown that fiber-like particles are more likely to reach the deeper lung airways[52,83]. Also, fiber-like carriers have better internalization abilities than spherical particles for drug delivery[84]. This finding demonstrates that when targeting drugs into deeper lung airways, fiber-like drug carriers may perform more efficiently than spherical ones. A recent study demonstrated that using elongated fine mannitol particles enhance the aerosolization performance of inhalable drugs which may improve the efficiency of drug delivery from the devices[37,85]. It is promising to explore the shape as an important parameter for improved drug delivery performance.
Multifunctional Nanoparticles: Today, the size of drug aerosol particles can be reduced from tens of micrometers to tens of nanometers (i.e., less than 100 nm in size), which is a significant technological and medical breakthrough. Drug-delivery systems for nanoparticles have been developed which can potentially enhance the efficacy and reduce side effects for a wide range of drugs. Due to the small inertia of nanoparticles, they can avoid impacting the oral cavity when being inhaled, and hence they are transported deeper into human lung airways. Those nanoparticles with diameters around 50 nm have been reported to be most efficiently internalized by cells[86]. The design of multifunctional nanoparticles for treatment of pulmonary diseases (i.e., lung cancer) is also a promising methodology. Additional functionality such as image contrast enhancement can be realized by adding other constituents into multifunctional nanoparticles, thereby assisting better drug-targeting control. Multifunctional nanoparticles can be designed for detecting infected cells, and delivering drugs specifically to those cells, and leaving healthy organs and lung cells unaffected[87]. Multifunctional nanoparticles can also deliver multiple therapeutic agents in a single formulation[49]. A multifunctional nanoparticle consists of a surface coating, imaging agent for detection, and therapeutic drugs. The aims of multifunctional nanoparticles are[49]: (1) enable specific targeting and aid in uptake realized by surface modification; (2) avoid fast endothelial system clearance via surface coating technology[88], and thereby extending circulation time and enhance uptake; and (3) load higher concentrations of multiple remedial agents that can override multidrug resistance and result in therapeutic effects.
However, more work is needed to understand the fate of nanoparticles after inhalation, including interactions with biological cells and nano-toxicity. Also, in a recent review article, Cheng et al[89] discussed the costs and regulatory hurdles for using multifunctional nanoparticles vs their potential benefits.
INTRAVASCULAR DRUG-TARGETING METHODOLOGIES FOR SOLID TUMORS
In addition to drug-targeting in the pulmonary system, much focus has been placed on treating unresectable tumors via intravascular therapies. These therapies involve either systemic or local, intra-arterial delivery of therapeutic agents such as chemotherapeutic drugs, multifunctional nanoparticles (NPs), radioactive microspheres, or embolic agents. While micron-sized agents must be delivered locally due to their embolic potential, this local drug administration is also beneficial for delivering higher therapeutic concentrations to cancer cells.
Intra-arterial delivery is often achieved using a locally placed drug-infusion catheter. However, due to the often tortuous and small size of the complex arterial systems leading to tumors, it is difficult or impossible to manually position this catheter directly in tumor-supplying arteries. As a result, therapeutic agents can still deposit in healthy tissue and/or travel to other organs. Thus, techniques are needed to better target predetermined cancer sites from upstream. Some existing methods include passive and active targeting for multifunctional nanoparticles as well as magnetic drug targeting. As will be discussed in the next sections, the shortcomings of these methods are that the nanoparticles must be very close to the tumor for passive and active targeting to be effective, and the magnetic particles must be near the body’s surface and in slow blood-flow systems for magnetic drug targeting to be successful. As a result, a direct drug-targeting strategy has been proposed which uses knowledge of the patient-specific, local blood flow field to precisely position a smart micro-catheter radially and deliver the therapeutic agents to the tumor directly. Current research is focused on developing and testing such a device.
Passive and active targeting
Being less than 1 μm in at least one dimension, multifunctional nanoparticles can more readily extravasate through tumor vessels and attach to cancer cells with the help of passive and active targeting. Specifically, passive targeting takes advantage of the leaky walls and poor lymphatic drainage of many tumor vessels. This allows NPs to more readily enter the tumor interstitium and linger for extended periods (i.e., the enhanced permeability and retention effect)[90-100]. Active targeting can then enhance tumor accumulation through ligand-receptor binding which is achieved by incorporating ligands on the drug’s surface to selectively attach to over-expressed antigens or receptors on tumor cells[101,102]. This targeting can also enhance therapeutic efficacy through receptor-mediated endocytosis.
The limitation of these passive/active-targeting events is that the NPs must come in close proximity to the tumors for both strategies to be effective. Thus, the NPs’ size, shape, surface properties, and targeting ligands have been modified in attempts to lengthen their circulation time and increase site specific accumulation[91,103-105]. For example, drug-loaded nanoparticles are often coated with polyethylene glycol to minimize the attraction of proteins which trigger immunogenic responses leading to system clearance. However, while such characteristics are beneficial for prolonging the NPs circulation time, they can also be detrimental once the NPs are near the tumor cells due to their resistance to endocytosis. Thus, the possibility of dynamically altering these characteristics in vivo is currently being investigated[106]. Such functionality has been achieved by taking advantage of the different pH, temperature, and enzyme levels in and around tumors. Despite these efforts, NPs still face filtration by non-target organs or clearance by the immune system before reaching the tumor[91,103-105,107].
Magnetic drug targeting
Magnetic drug targeting is one technique for increasing NP accumulation at solid tumors. In this method, drugs are bound to magnetic nanoparticles, and an external magnetic field is applied to attract the particles to a target site. Several studies have demonstrated the feasibility of this approach through computational and animal studies, and a few have demonstrated this targeting in human trials. Recently, studies have focused on obtaining a better understanding of the parameters which affect magnetic targeting success. For example, Kayal et al[108] experimentally and computationally analyzed the effects of the flow, magnet, nanoparticle properties, and injection site on deposition efficiency. As expected, they found that the efficiency was lower for increased flow rates, lower magnetic field strength, and smaller NPs. These, and other studies, demonstrate that the current applications are limited due to restrictions on the particle size and magnetic field strength. Specifically, this technique is only applicable when the tumor is located near the body surface and the flow rate is small. To overcome this, implant-assisted magnetic drug targeting has been proposed in which a magnetic implant is inserted near the target site to increase the magnetic field gradient in deep tissue[109]. However, this technique is still not likely to resolve the downstream tumor-targeting problem because it may not be feasible to place these implants as near to the tumor as necessary.
Direct drug-targeting
As an alternative to the current strategies, a novel direct tumor-targeting methodology has been proposed. In this technique, the catheter position (i.e., radial position in the arterial particle-injection plane), infusion speed, and injection timing are precisely controlled so that the injected drug-loaded particles are carried by the blood flow directly to the target site[110]. While previous work has demonstrated that the particle infusion speed should be relatively equal to the surrounding blood flow[111,112], the remaining conditions are determined by computational simulations which mimic the targeted arterial system. Specifically, a vast amount of particles are infused over the selected injection position of the truncated arterial system, and their transport is modeled through the system. By backtracking along the particle trajectories (as indicated in Figure 5), a patient-specific particle release map (PRM) can be generated, which visually links particle injection regions with associated exit branches, some potentially connected to tumors. Such PRMs can then be used to determine radial micro-catheter positions to achieve optimal targeting. For example, in the scenario given in Figure 5, the catheter should be placed in the red zone (zone 1) of the PRM while avoiding the remaining zones. By generating multiple PRMs at subsequent intervals throughout the cardiac cycle, the best injection interval can also be determined[113].
Figure 5.
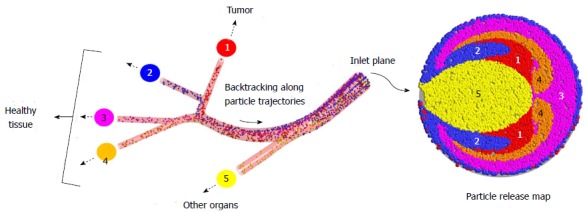
Illustration of the direct tumor-targeting methodology. (Reprinted from Ref.[115], with permission from Springer).
The computational medical management program: The Computational Medical Management Program has been proposed to implement this targeting methodology into clinical practice. As illustrated in Figure 6, there are three basic stages in this program: (1) the patient evaluation stage; (2) the computer modeling stage; and (3) the clinical implementation stage.
Figure 6.
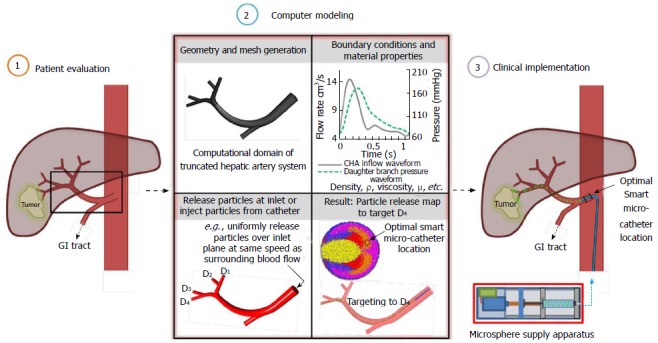
Computational medical management program. SMC: Smart micro-catheter.
As in current intra-arterial procedures, the patient evaluation stage includes classification of the tumor and determination of the best treatment route. In the proposed procedure, the patient’s geometry and flow conditions are also collected in this stage. In the next stage, computational case studies are run in the truncated geometry to determine the best injection region and interval for targeting as well as the appropriate time to terminate injection. In the final stage, optimal catheter positioning and injection is achieved using the proposed Smart Micro-Catheter (SMC) and Medicine Supply Apparatus (MSA). As in current procedures, success of the treatment is then evaluated.
SMC system for optimal drug-delivery: As introduced in the previous section, a SMC and MSA have been proposed to achieve direct tumor-targeting. The main objective of the SMC is to provide precise intra-arterial positioning of the particle injection stream, while the main objective of the MSA is to supply the particle stream to the SMC at the appropriate interval and speed for targeting. Figure 7 illustrates sample concepts for each device.
Figure 7.
Proposed components of the medicine supply apparatus. (Reprinted from Ref.[118] with permission from Elsevier).
Experimental and computational studies: As an initial validation of this direct tumor-targeting strategy, Richards et al[114] performed experimental studies in a scaled-up, rigid hepatic artery system with steady Newtonian-fluid flow. Using the generated particle release map from the corresponding computational simulation, it was demonstrated that specific downstream arteries could be targeted by precisely positioning the particle injection region upstream. While additional computational studies have demonstrated the feasibility of this technique under more realistic conditions such as transient pulsatile flow, a patient-specific geometry, and flexible arterial walls[111-113,115], future experimental studies will need to verify these findings.
CONCLUSION
In this review article, we compared and discussed different drug-delivery devices and drug-targeting methodologies using pulmonary or intravascular routes, as well as related computational fluid dynamics techniques and applications. Drug-targeting has the potential to greatly enhance drug-delivery efficacy, reduce side effects, and lower treatment cost. However, the vast majority of drug-targeting studies assume that the drug-particles are already at the target site or at least in its direct vicinity.
In this review, drug-delivery methodologies, drug types and drug-delivery devices are discussed with examples in two major application areas: (1) inhaled drug-aerosol delivery into human lung-airways; and (2) intravascular drug-delivery for solid tumor targeting. The major problem addressed is how to deliver efficiently the drug-particles from the entry/infusion point to the target site. Experimental results so far are based on simple laboratory studies and restrictive animal tests. Concerning computational fluid-particle dynamics, further advancements in software and hardware are needed to develop faster, more realistic and accurate computer simulation models.
Pulmonary drug targeting
As mentioned, the selection of drug delivery device and drug aerosol formulation has a critical influence on pulmonary drug-targeting efficiency. To further optimize the pulmonary drug-delivery process and provide more effective therapy, the focus should be on the following aspects: (1) control the aerosol generation process[116]; (2) control the aerosol deposition patterns in lung airways; and (3) control the aerosol transport after penetrating the air-blood barriers. Specifically, due to the scarcity of air-blood barrier transport of drug aerosols via the lung route, it is of interest that to know to what extent drug-aerosols can be absorbed or cleared. That will affect the systemic drug delivery effectiveness, including modulation of solubility in the airway-surface layers and the permeability across the epithelial barrier to improve pulmonary bio-availability of the active pharmaceutical ingredients. It should also control the clearance process to prolong the action of the active pharmaceutical ingredients.
Solid-tumor targeting
It is evident that the micron- or nano-drugs have to be delivered directly from the infusion point to the pre-determined tumor site to guarantee high treatment efficiency, minimal side-effects, and low cost. Such a direct drug delivery equates to optimal tumor targeting. Concerning the promising smart micro-catheter system, i.e., a combined and synchronized SMC and MSA assembly, SMC-device miniaturization and system testing in the lab and clinical environment are ongoing and planned projects.
Footnotes
Supported by National Science Foundation, No. NSF-CBET 1232988 and ANSYS Inc. (Canonsburg, PA)
P- Reviewer: Aggarwal D S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Ruge CA, Kirch J, Lehr CM. Pulmonary drug delivery: from generating aerosols to overcoming biological barriers-therapeutic possibilities and technological challenges. Lancet Respir Med. 2013;1:402–413. doi: 10.1016/S2213-2600(13)70072-9. [DOI] [PubMed] [Google Scholar]
- 2.Kunda NK, Somavarapu S, Gordon SB, Hutcheon GA, Saleem IY. Nanocarriers targeting dendritic cells for pulmonary vaccine delivery. Pharm Res. 2013;30:325–341. doi: 10.1007/s11095-012-0891-5. [DOI] [PubMed] [Google Scholar]
- 3.Sahane S, Nikhar A, Bhaskaran S, Mundhada D. Dry powder inhaler: An advance technique for pulmonary drug delivery system. IJPCS. 2012;1:1027–1034. Available from: http://www.ijpcsonline.com/files/63-209A.pdf. [Google Scholar]
- 4.Sandhya P, Fatima A, Afreen G, Afreen A. A review on drug targeting to respiratory system. IJPCS. 2013;1:277–291. Available from: http://www.ijprronline.com/15.pdf. [Google Scholar]
- 5.Dalby R, Spallek M, Voshaar T. A review of the development of Respimat Soft Mist Inhaler. Int J Pharm. 2004;283:1–9. doi: 10.1016/j.ijpharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Kleinstreuer C. Biofluid dynamics: Principles and selected applications. Florida: CRC Press; 2006. [Google Scholar]
- 7.Sumby B, Slater A, Atkins PJ, Prime D. Review of dry powder inhalers. Adv Drug Deliv Rev. 1997;26:51–58. doi: 10.1016/s0169-409x(97)00510-3. [DOI] [PubMed] [Google Scholar]
- 8.Sanders M. Inhalation therapy: an historical review. Prim Care Respir J. 2007;16:71–81. doi: 10.3132/pcrj.2007.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delgado A, Chou KJ, Silver EJ, Crain EF. Nebulizers vs metered-dose inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med. 2003;157:76–80. doi: 10.1001/archpedi.157.1.76. [DOI] [PubMed] [Google Scholar]
- 10.Gangurde H, Chordiya M, Baste N, Tamizharasi S, Upasani C. Approaches and devices used in pulmonary drug delivery system: a review. AJPRHC. 2012;4:11–27. Available from: http://www.jprhc.in/index.php/ajprhc/article/download/18/17. [Google Scholar]
- 11.Newman SP. Principles of metered-dose inhaler design. Respir Care. 2005;50:1177–1190. [PubMed] [Google Scholar]
- 12.Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet. 2011;377:1032–1045. doi: 10.1016/S0140-6736(10)60926-9. [DOI] [PubMed] [Google Scholar]
- 13.Longest PW, Tian G, Walenga RL, Hindle M. Comparing MDI and DPI aerosol deposition using in vitro experiments and a new stochastic individual path (SIP) model of the conducting airways. Pharm Res. 2012;29:1670–1688. doi: 10.1007/s11095-012-0691-y. [DOI] [PubMed] [Google Scholar]
- 14.Newman SP, Pavia D, Morén F, Sheahan NF, Clarke SW. Deposition of pressurised aerosols in the human respiratory tract. Thorax. 1981;36:52–55. doi: 10.1136/thx.36.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverman R, Sellers J, Greene S, Flaster E, Colice G. Comparison of the Maxair Autohaler to wet nebulizer in patients with acute asthma. Chest. 1998;114:766–770. doi: 10.1378/chest.114.3.766. [DOI] [PubMed] [Google Scholar]
- 16.Lenney J, Innes JA, Crompton GK. Inappropriate inhaler use: assessment of use and patient preference of seven inhalation devices. EDICI. Respir Med. 2000;94:496–500. doi: 10.1053/rmed.1999.0767. [DOI] [PubMed] [Google Scholar]
- 17.Price D, Thomas M, Mitchell G, Niziol C, Featherstone R. Improvement of asthma control with a breath-actuated pressurised metred dose inhaler (BAI): a prescribing claims study of 5556 patients using a traditional pressurised metred dose inhaler (MDI) or a breath-actuated device. Respir Med. 2003;97:12–19. doi: 10.1053/rmed.2002.1426. [DOI] [PubMed] [Google Scholar]
- 18.Barry PW, O’Callaghan C. Inhalational drug delivery from seven different spacer devices. Thorax. 1996;51:835–840. doi: 10.1136/thx.51.8.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kesser KC, Geller DE. New aerosol delivery devices for cystic fibrosis. Respir Care. 2009;54:754–767; discussion 767-768. doi: 10.4187/002013209790983250. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Ma Y, Zhang L, Zhu J, Jin F. The development of a novel dry powder inhaler. Int J Pharm. 2012;431:45–52. doi: 10.1016/j.ijpharm.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Islam N, Gladki E. Dry powder inhalers (DPIs)--a review of device reliability and innovation. Int J Pharm. 2008;360:1–11. doi: 10.1016/j.ijpharm.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 22.Newman SP, Busse WW. Evolution of dry powder inhaler design, formulation, and performance. Respir Med. 2002;96:293–304. doi: 10.1053/rmed.2001.1276. [DOI] [PubMed] [Google Scholar]
- 23.Finlay WH. The mechanics of inhaled pharmaceutical aerosols: an introduction. Pittsburgh: Academic Press; 2001. [Google Scholar]
- 24.Waldrep JC, Berlinski A, Dhand R. Comparative analysis of methods to measure aerosols generated by a vibrating mesh nebulizer. J Aerosol Med. 2007;20:310–319. doi: 10.1089/jam.2007.0538. [DOI] [PubMed] [Google Scholar]
- 25.Rau JL. The inhalation of drugs: advantages and problems. Respir Care. 2005;50:367–382. [PubMed] [Google Scholar]
- 26.Hochrainer D, Hölz H, Kreher C, Scaffidi L, Spallek M, Wachtel H. Comparison of the aerosol velocity and spray duration of Respimat Soft Mist inhaler and pressurized metered dose inhalers. J Aerosol Med. 2005;18:273–282. doi: 10.1089/jam.2005.18.273. [DOI] [PubMed] [Google Scholar]
- 27.Hess DR. Aerosol delivery devices in the treatment of asthma. Respir Care. 2008;53:699–723; discussion 723-725. [PubMed] [Google Scholar]
- 28.Anderson P. Use of Respimat Soft Mist inhaler in COPD patients. Int J Chron Obstruct Pulmon Dis. 2006;1:251–259. doi: 10.2147/copd.2006.1.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geller DE. New liquid aerosol generation devices: systems that force pressurized liquids through nozzles. Respir Care. 2002;47:1392–1404; discussion 1404-1405. [PubMed] [Google Scholar]
- 30.Vecellio L. The mesh nebuliser: A recent technical innovation for aerosol delivery. Breathe. 2006;2:253–260. [Google Scholar]
- 31.Amani A, Amini MA, Ali HS, York P. Alternatives to conventional suspensions for pulmonary drug delivery by nebulisers: a review. J Pharm Sci. 2011;100:4563–4570. doi: 10.1002/jps.22665. [DOI] [PubMed] [Google Scholar]
- 32.Chou KJ, Cunningham SJ, Crain EF. Metered-dose inhalers with spacers vs nebulizers for pediatric asthma. Arch Pediatr Adolesc Med. 1995;149:201–205. doi: 10.1001/archpedi.1995.02170140083015. [DOI] [PubMed] [Google Scholar]
- 33.Batra V, Sethi GR, Sachdev HP. Comparative efficacy of jet nebulizer and metered dose inhaler with spacer device in the treatment of acute asthma. Indian Pediatr. 1997;34:497–503. [PubMed] [Google Scholar]
- 34.Dhuper S, Chandra A, Ahmed A, Bista S, Moghekar A, Verma R, Chong C, Shim C, Cohen H, Choksi S. Efficacy and cost comparisons of bronchodilatator administration between metered dose inhalers with disposable spacers and nebulizers for acute asthma treatment. J Emerg Med. 2011;40:247–255. doi: 10.1016/j.jemermed.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 35.Tarsin WY, Pearson SB, Assi KH, Chrystyn H. Emitted dose estimates from Seretide Diskus and Symbicort Turbuhaler following inhalation by severe asthmatics. Int J Pharm. 2006;316:131–137. doi: 10.1016/j.ijpharm.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 36.Smith IJ, Bell J, Bowman N, Everard M, Stein S, Weers JG. Inhaler devices: what remains to be done? J Aerosol Med Pulm Drug Deliv. 2010;23 Suppl 2:S25–S37. doi: 10.1089/jamp.2010.0853. [DOI] [PubMed] [Google Scholar]
- 37.Nokhodchi A, Larhrib H. Overcoming the undesirable properties of dry-powder inhalers with novel engineered mannitol particles. Ther Deliv. 2013;4:879–882. doi: 10.4155/tde.13.64. [DOI] [PubMed] [Google Scholar]
- 38.Berlinski A, Waldrep JC. Four hours of continuous albuterol nebulization. Chest. 1998;114:847–853. doi: 10.1378/chest.114.3.847. [DOI] [PubMed] [Google Scholar]
- 39.D’Addio SM, Prud’homme RK. Controlling drug nanoparticle formation by rapid precipitation. Adv Drug Deliv Rev. 2011;63:417–426. doi: 10.1016/j.addr.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Heng D, Lee SH, Ng WK, Chan HK, Kwek JW, Tan RB. Novel alternatives to reduce powder retention in the dry powder inhaler during aerosolization. Int J Pharm. 2013;452:194–200. doi: 10.1016/j.ijpharm.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Smutney CC, Grant M, Kinsey PS. Device factors affecting pulmonary delivery of dry powders. Ther Deliv. 2013;4:939–949. doi: 10.4155/tde.13.77. [DOI] [PubMed] [Google Scholar]
- 42.Friebel C, Steckel H, Müller BW. Rational design of a dry powder inhaler: device design and optimisation. J Pharm Pharmacol. 2012;64:1303–1315. doi: 10.1111/j.2042-7158.2012.01525.x. [DOI] [PubMed] [Google Scholar]
- 43.Kakade PP, Versteeg HK, Hargrave GK, Genova P, Williams Iii RC, Deaton D. Design optimization of a novel pMDI actuator for systemic drug delivery. J Aerosol Med. 2007;20:460–474. doi: 10.1089/jam.2007.0595. [DOI] [PubMed] [Google Scholar]
- 44.Watts AB, McConville JT, Williams RO. Current therapies and technological advances in aqueous aerosol drug delivery. Drug Dev Ind Pharm. 2008;34:913–922. doi: 10.1080/03639040802144211. [DOI] [PubMed] [Google Scholar]
- 45.Chrystyn H, Niederlaender C. The Genuair® inhaler: a novel, multidose dry powder inhaler. Int J Clin Pract. 2012;66:309–317. doi: 10.1111/j.1742-1241.2011.02832.x. [DOI] [PubMed] [Google Scholar]
- 46.Addington WR, Miller SP, Phelipa MM, Stephens RE. Nebulizer having flow meter function. United States patent US 8333190 B2. Pittsburgh: Academic Press; 2012. p. Dec 18. [Google Scholar]
- 47.Behara SR, Farkas DR, Hindle M, Longest PW. Development of a high efficiency dry powder inhaler: effects of capsule chamber design and inhaler surface modifications. Pharm Res. 2014;31:360–372. doi: 10.1007/s11095-013-1165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kleinstreuer C, Zhang Z, Li Z, Roberts WL, Rojas C. A new methodology for targeting drug-aerosols in the human respiratory system. Int J Heat Mass Transfer. 2008;51:5578–5589. [Google Scholar]
- 49.Dawar S, Singh N, Kanwar RK, Kennedy RL, Veedu RN, Zhou SF, Krishnakumar S, Hazra S, Sasidharan S, Duan W, et al. Multifunctional and multitargeted nanoparticles for drug delivery to overcome barriers of drug resistance in human cancers. Drug Discov Today. 2013;18:1292–1300. doi: 10.1016/j.drudis.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z, Kleinstreuer C, Hyun S. Size-change and deposition of conventional and composite cigarette smoke particles during inhalation in a subject-specific airway model. J Aerosol Sci. 2012;46:34–52. [Google Scholar]
- 51.Babincova M, Babinec P. Magnetic drug delivery and targeting: principles and applications. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009;153:243–250. doi: 10.5507/bp.2009.042. [DOI] [PubMed] [Google Scholar]
- 52.Feng Y, Kleinstreuer C. Analysis of non-spherical particle transport in complex internal shear flows. Physics of Fluids. 2013;25:091904. [Google Scholar]
- 53.Feng Y, Kleinstreuer C. Micron-particle transport, interactions and deposition in triple lung-airway bifurcations using a novel modeling approach. J Aerosol Sci. 2014;71:1–15. [Google Scholar]
- 54.Tu J, Inthavong K, Ahmadi G. Computational Fluid and Particle Dynamics (CFPD): An Introduction. In: Computational Fluid and Particle Dynamics in the Human Respiratory System., editor. Germany: Springer; 2013. [Google Scholar]
- 55.Ibrahim M, Garcia-Contreras L. Mechanisms of absorption and elimination of drugs administered by inhalation. Ther Deliv. 2013;4:1027–1045. doi: 10.4155/tde.13.67. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Z, Kleinstreuer C, Feng Y. Vapor deposition during cigarette smoke inhalation in a subject-specific human airway model. J Aerosol Sci. 2012;53:40–60. [Google Scholar]
- 57.Kolanjiyil AV, Kleinstreuer C. Nanoparticle mass transfer from lung airways to systemic regions--Part I: Whole-lung aerosol dynamics. J Biomech Eng. 2013;135:121003. doi: 10.1115/1.4025332. [DOI] [PubMed] [Google Scholar]
- 58.Kolanjiyil AV, Kleinstreuer C. Nanoparticle mass transfer from lung airways to systemic regions--Part II: Multi-compartmental modeling. J Biomech Eng. 2013;135:121004. doi: 10.1115/1.4025333. [DOI] [PubMed] [Google Scholar]
- 59.Wong W, Fletcher DF, Traini D, Chan HK, Young PM. The use of computational approaches in inhaler development. Adv Drug Deliv Rev. 2012;64:312–322. doi: 10.1016/j.addr.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 60.Crosland BM, Johnson MR, Matida EA. Characterization of the spray velocities from a pressurized metered-dose inhaler. J Aerosol Med Pulm Drug Deliv. 2009;22:85–97. doi: 10.1089/jamp.2008.0687. [DOI] [PubMed] [Google Scholar]
- 61.Versteeg H, Hargrave G, Harrington L, Shrubb I, Hodson D. The use of computational fluid dynamics (CFD) to predict pMDI air flows and aerosol plume formation. In: Respiratory Drug Delivery; 2000. p. 7(1). [Google Scholar]
- 62.Shakked T, Katoshevski D, Broday DM, Amirav I. Numerical simulation of air flow and medical-aerosol distribution in an innovative nebulizer hood. J Aerosol Med. 2005;18:207–217. doi: 10.1089/jam.2005.18.207. [DOI] [PubMed] [Google Scholar]
- 63.Kleinstreuer C, Shi H, Zhang Z. Computational analyses of a pressurized metered dose inhaler and a new drug-aerosol targeting methodology. J Aerosol Med. 2007;20:294–309. doi: 10.1089/jam.2006.0617. [DOI] [PubMed] [Google Scholar]
- 64.Worth Longest P, Hindle M, Das Choudhuri S. Effects of generation time on spray aerosol transport and deposition in models of the mouth-throat geometry. J Aerosol Med Pulm Drug Deliv. 2009;22:67–83. doi: 10.1089/jamp.2008.0692. [DOI] [PubMed] [Google Scholar]
- 65.Worth Longest P, Hindle M. Evaluation of the Respimat Soft Mist Inhaler using a concurrent CFD and in vitro approach. J Aerosol Med Pulm Drug Deliv. 2009;22:99–112. doi: 10.1089/jamp.2008.0708. [DOI] [PubMed] [Google Scholar]
- 66.Jiang L, Tang Y, Zhang H, Lu X, Chen X, Zhu J. Importance of powder residence time for the aerosol delivery performance of a commercial dry powder inhaler Aerolizer(®) J Aerosol Med Pulm Drug Deliv. 2012;25:265–279. doi: 10.1089/jamp.2011.0908. [DOI] [PubMed] [Google Scholar]
- 67.Longest PW, Son YJ, Holbrook L, Hindle M. Aerodynamic factors responsible for the deaggregation of carrier-free drug powders to form micrometer and submicrometer aerosols. Pharm Res. 2013;30:1608–1627. doi: 10.1007/s11095-013-1001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tong Z, Zheng B, Yang R, Yu A, Chan H. CFD-DEM investigation of the dispersion mechanisms in commercial dry powder inhalers. Powder Technol. 2013;240:19–24. [Google Scholar]
- 69.Tong Z, Yang R, Chu K, Yu A, Adi S, Chan H. Numerical study of the effects of particle size and polydispersity on the agglomerate dispersion in a cyclonic flow. Chem Eng J. 2010;164:432–441. [Google Scholar]
- 70.Ruzycki CA, Javaheri E, Finlay WH. The use of computational fluid dynamics in inhaler design. Expert Opin Drug Deliv. 2013;10:307–323. doi: 10.1517/17425247.2013.753053. [DOI] [PubMed] [Google Scholar]
- 71.Kleinstreuer C, Seelecke S. Inhaler system for targeted maximum drug-aerosol delivery. United States patent US 7900625 B2. In: Respiratory Drug Delivery; 2011. p. May 8. [Google Scholar]
- 72.Hindle M, Longest PW. Condensational growth of combination drug-excipient submicrometer particles for targeted high-efficiency pulmonary delivery: evaluation of formulation and delivery device. J Pharm Pharmacol. 2012;64:1254–1263. doi: 10.1111/j.2042-7158.2012.01476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hindle M, Longest PW. Evaluation of enhanced condensational growth (ECG) for controlled respiratory drug delivery in a mouth-throat and upper tracheobronchial model. Pharm Res. 2010;27:1800–1811. doi: 10.1007/s11095-010-0165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Longest PW, Hindle M. Numerical Model to Characterize the Size Increase of Combination Drug and Hygroscopic Excipient Nanoparticle Aerosols. Aerosol Sci Technol. 2011;45:884–899. doi: 10.1080/02786826.2011.566592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dames P, Gleich B, Flemmer A, Hajek K, Seidl N, Wiekhorst F, Eberbeck D, Bittmann I, Bergemann C, Weyh T, et al. Targeted delivery of magnetic aerosol droplets to the lung. Nat Nanotechnol. 2007;2:495–499. doi: 10.1038/nnano.2007.217. [DOI] [PubMed] [Google Scholar]
- 76.Plank C. Nanomagnetosols: magnetism opens up new perspectives for targeted aerosol delivery to the lung. Trends Biotechnol. 2008;26:59–63. doi: 10.1016/j.tibtech.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 77.Yang HW, Hua MY, Liu HL, Huang CY, Wei KC. Potential of magnetic nanoparticles for targeted drug delivery. Nanotechnol Sci Appl. 2012;5:73–86. doi: 10.2147/NSA.S35506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McBride AA, Price DN, Lamoureux LR, Elmaoued AA, Vargas JM, Adolphi NL, Muttil P. Preparation and characterization of novel magnetic nano-in-microparticles for site-specific pulmonary drug delivery. Mol Pharm. 2013;10:3574–3581. doi: 10.1021/mp3007264. [DOI] [PubMed] [Google Scholar]
- 79.Tewes F, Ehrhardt C, Healy AM. Superparamagnetic iron oxide nanoparticles (SPIONs)-loaded Trojan microparticles for targeted aerosol delivery to the lung. Eur J Pharm Biopharm. 2014;86:98–104. doi: 10.1016/j.ejpb.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 80.Arruebo M. Drug delivery from structured porous inorganic materials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:16–30. doi: 10.1002/wnan.132. [DOI] [PubMed] [Google Scholar]
- 81.Kim JW, Xi J, Si XA. Dynamic growth and deposition of hygroscopic aerosols in the nasal airway of a 5-year-old child. Int J Numer Method Biomed Eng. 2013;29:17–39. doi: 10.1002/cnm.2490. [DOI] [PubMed] [Google Scholar]
- 82.Mitragotri S. In drug delivery, shape does matter. Pharm Res. 2009;26:232–234. doi: 10.1007/s11095-008-9740-y. [DOI] [PubMed] [Google Scholar]
- 83.Sturm R, Hofmann W. A theoretical approach to the deposition and clearance of fibers with variable size in the human respiratory tract. J Hazard Mater. 2009;170:210–218. doi: 10.1016/j.jhazmat.2009.04.107. [DOI] [PubMed] [Google Scholar]
- 84.Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaialy W, Nokhodchi A. Engineered mannitol ternary additives improve dispersion of lactose-salbutamol sulphate dry powder inhalations. AAPS J. 2013;15:728–743. doi: 10.1208/s12248-013-9476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mansour HM. Advances in targeted lung nanomedicine as inhalable multifunctional microparticles & nanoparticles for pulmonary disease treatment and prevention. International Conference and Exhibition on Nanotechnology & nanomedicine. USA: Omaha Marriott; 2012. pp. 12–14. [Google Scholar]
- 87.Taratula O, Kuzmov A, Shah M, Garbuzenko OB, Minko T. Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J Control Release. 2013;171:349–357. doi: 10.1016/j.jconrel.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wei Y, Zhao L. Passive lung-targeted drug delivery systems via intravenous administration. Pharm Dev Technol. 2014;19:129–136. doi: 10.3109/10837450.2012.757782. [DOI] [PubMed] [Google Scholar]
- 89.Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338:903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jang SH, Wientjes MG, Lu D, Au JL. Drug delivery and transport to solid tumors. Pharm Res. 2003;20:1337–1350. doi: 10.1023/a:1025785505977. [DOI] [PubMed] [Google Scholar]
- 91.Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14:1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 92.Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994;54:3352–3356. [PubMed] [Google Scholar]
- 93.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuan F, Salehi HA, Boucher Y, Vasthare US, Tuma RF, Jain RK. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 1994;54:4564–4568. [PubMed] [Google Scholar]
- 95.Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55:3752–3756. [PubMed] [Google Scholar]
- 96.Bundgaard M. Transport pathways in capillaries--in search of pores. Annu Rev Physiol. 1980;42:325–336. doi: 10.1146/annurev.ph.42.030180.001545. [DOI] [PubMed] [Google Scholar]
- 97.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 98.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 99.Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjug Chem. 2010;21:797–802. doi: 10.1021/bc100070g. [DOI] [PubMed] [Google Scholar]
- 100.Muggia FM. Doxorubicin-polymer conjugates: further demonstration of the concept of enhanced permeability and retention. Clin Cancer Res. 1999;5:7–8. [PubMed] [Google Scholar]
- 101.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 103.De Jong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine. 2008;3:133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nie S. Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine (Lond) 2010;5:523–528. doi: 10.2217/nnm.10.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Blanco E, Kessinger CW, Sumer BD, Gao J. Multifunctional micellar nanomedicine for cancer therapy. Exp Biol Med (Maywood) 2009;234:123–131. doi: 10.3181/0808-MR-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yoo JW, Doshi N, Mitragotri S. Adaptive micro and nanoparticles: temporal control over carrier properties to facilitate drug delivery. Adv Drug Deliv Rev. 2011;63:1247–1256. doi: 10.1016/j.addr.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 107.Mornet S, Vasseur S, Grasset F, Duguet E. Magnetic nanoparticle design for medical diagnosis and therapy. J Mater Chem. 2004;14:2161–2175. [Google Scholar]
- 108.Kayal S, Bandyopadhyay D, Mandal TK, Ramanujan RV. The flow of magnetic nanoparticles in magnetic drug targeting. RSC Advances. 2011;1:238–246. [Google Scholar]
- 109.Cregg P, Murphy K, Mardinoglu A. Inclusion of interactions in mathematical modelling of implant assisted magnetic drug targeting. Appl Math Model. 2012;36:1–34. [Google Scholar]
- 110.Kleinstreuer C. Methods and devices for targeted injection of microspheres. United States patent US 20120190976 A1. USA: Omaha Marriott; 2010. p. Jul 26. [Google Scholar]
- 111.Kleinstreuer C, Basciano CA, Childress EM, Kennedy AS. A new catheter for tumor targeting with radioactive microspheres in representative hepatic artery systems. Part I: impact of catheter presence on local blood flow and microsphere delivery. J Biomech Eng. 2012;134:051004. doi: 10.1115/1.4006684. [DOI] [PubMed] [Google Scholar]
- 112.Basciano CA. Computational particle-hemodynamics analysis applied to an abdominal aortic aneurysm with thrombus and microsphere-targeting of liver tumors (2010) Available from: http://www.lib.ncsu.edu/resolver/1840.16/7113.
- 113.Childress EM, Kleinstreuer C, Kennedy AS. A new catheter for tumor-targeting with radioactive microspheres in representative hepatic artery systems--part II: solid tumor-targeting in a patient-inspired hepatic artery system. J Biomech Eng. 2012;134:051005. doi: 10.1115/1.4006685. [DOI] [PubMed] [Google Scholar]
- 114.Richards AL, Kleinstreuer C, Kennedy AS, Childress E, Buckner GD. Experimental microsphere targeting in a representative hepatic artery system. IEEE Trans Biomed Eng. 2012;59:198–204. doi: 10.1109/TBME.2011.2170195. [DOI] [PubMed] [Google Scholar]
- 115.Childress EM, Kleinstreuer C. Impact of fluid-structure interaction on direct tumor-targeting in a representative hepatic artery system. Ann Biomed Eng. 2014;42:461–474. doi: 10.1007/s10439-013-0910-7. [DOI] [PubMed] [Google Scholar]
- 116.Niven R. Prospects and challenges: inhalation delivery systems. Ther Deliv. 2013;4:519–522. doi: 10.4155/tde.13.18. [DOI] [PubMed] [Google Scholar]
- 117.Muchão FP, Filho LV. Advances in inhalation therapy in pediatrics. J Pediatr (Rio J) 2010;86:367–376. doi: 10.2223/JPED.2024. [DOI] [PubMed] [Google Scholar]
- 118.Kleinstreuer C, Childress EM, Kennedy AS. Targeted Drug Delivery: Multifunctional Nanoparticles and Direct Micro-drug Delivery to Tumors. In: Becker S, Kuznetsov , A , editors. Transport in Biological Media. San Diego: Academic Press; 2012. pp. 392–414. [Google Scholar]


