Abstract
Patients with interstitial lung disease commonly exhibit abnormal sleep architecture and increased sleep fragmentation on polysomnography. Fatigue is a frequent complaint, and it is likely that poor sleep quality is a significant contributor. A number of studies have shown that sleep disordered breathing is prevalent in this population, particularly in the idiopathic pulmonary fibrosis subgroup. The factors that predispose these patients to obstructive sleep apnoea are not well understood, however it is believed that reduced caudal traction on the upper airway can enhance collapsibility. Ventilatory control system instability may also be an important factor, particularly in those with increased chemo-responsiveness, and in hypoxic conditions. Transient, repetitive nocturnal oxygen desaturation is frequently observed in interstitial lung disease, both with and without associated obstructive apnoeas. There is increasing evidence that sleep-desaturation is associated with increased mortality, and may be important in the pathogenesis of pulmonary hypertension in this population.
Keywords: Sleep disordered breathing, Interstitial lung disease, Pulmonary fibrosis, Nocturnal hypoxia, Obstructive sleep apnoea
Core tip: This article reviews the literature on sleep disordered breathing in interstitial lung disease, seeking to define the important contributing factors and sequelae. The key concepts that are explored include the contribution of nocturnal hypoxaemia to the development of pulmonary hypertension, and the mechanisms behind the observed high prevalence of obstructive sleep apnoea in interstitial lung disease patients.
INTRODUCTION
The interstitial lung diseases (ILD) are a heterogeneous group of disorders characterized by varying degrees of fibrosis and inflammation of lung parenchyma. Sufferers exhibit lung restriction and exercise intolerance, often developing progressive hypoxia over time. Independent of the presence of daytime hypoxia, many individuals with ILD are observed to desaturate during sleep, with or without associated apnoea.
There is mounting evidence that nocturnal hypoxia and sleep-disordered breathing (SDB) may contribute to adverse outcomes. Aside from resulting in poor sleep quality and daytime fatigue, transient repetitive desaturation and associated sympathetic nervous system activation may play a role in the development of pulmonary hypertension and contribute to increased mortality[1-3].
Existing evidence on aspects of sleep physiology and pathophysiology in ILD will be considered within this review.
POPULATION AT RISK
There are estimates of more than two hundred known causes of ILD, leading to restrictive physiology, dyspnoea and often a pervasive cough. These diseases can be divided into broad subcategories: (1) those with known aetiology such as the pneumoconioses, drug-related ILD, and connective tissue disease-associated ILD; (2) the granulomatous diseases such as sarcoidosis and chronic hypersensitivity pneumonitis; (3) the idiopathic interstitial pneumonias such as idiopathic pulmonary fibrosis (IPF), non-specific interstitial pneumonia, cryptogenic organizing pneumonia (COP); and (4) a miscellaneous group including pulmonary Langerhans cell histiocytosis[4,5]. A classification scheme is depicted in Figure 1.
Figure 1.
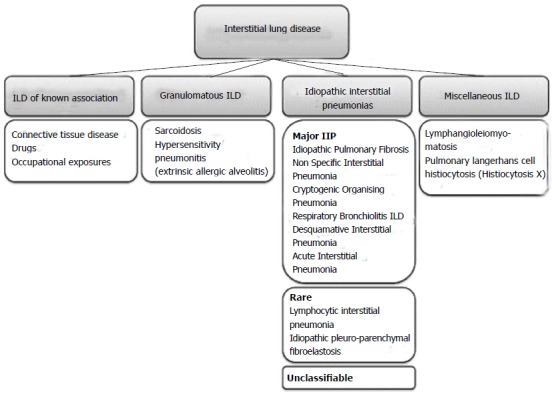
Classification scheme of interstitial lung disease (Adapted from[4,5]). ILD: Interstitial lung disease; IIP: Idiopathic interstitial pneumonia.
Many of these diseases evolve from an initial inflammatory process involving the lung interstitium with varying inclusion of the lung vasculature and airways. Over time, inflammation may give way to advancing fibrosis, especially in cases where diagnosis and treatment are delayed and inflammation persists unabated. In contrast, IPF is a distinct entity, in which inflammation does not appear to play an important role in the pathogenesis of fibrosis, which often progresses rapidly and relentlessly.
Irrespective of underlying aetiology, advancing fibrosis often leads to worsening gas exchange across a thickened collagen-dense interstitium, and respiratory failure may eventually ensue. Hypoxic vasoconstriction, endothelial remodelling and vascular obliteration all contribute to the development of pulmonary hypertension, a poor prognostic feature when present in ILD patients[6,7].
SLEEP CHARACTERISTICS IN ILD
Breathing pattern during sleep
During wakefulness, ILD patients are known to have a rapid, shallow breathing pattern both at rest and with exercise[8,9]. This is thought to be due to increased intrinsic elastic loading of respiratory muscles and stimulation of peripheral mechanoreceptors[8,10]. Even in the face of severely impaired gas exchange, this respiratory pattern allows ILD patients to maintain ventilation and daytime eucapnia until very advanced stages of disease. During sleep, some investigators have found respiratory frequency, f to be no different to that when awake[11-14]. Others have shown that f falls, but with an attendant increase in tidal volume, so that overall, the increased minute ventilation is preserved during sleep[15].
Sleep architecture and sleep quality
Not surprisingly, sleep quality is comparatively poorer than that of the normal population. Nocturnal cough, medications, breathing difficulties, hypoxia and obstructive apneas have all been implicated in disrupting sleep in this population. Perez-Padilla et al[14] in 1985, compared 11 ILD patients with age- and sex-matched controls. They reported decreased amounts of rapid eye movement (REM) sleep as well as significant sleep fragmentation in the patient group[14]. Further prospective studies, with particular focus on IPF confirm these findings, and also report increased stage 1 and 2 sleep, reduced slow wave sleep and poorer overall sleep efficiency[13,15-20]. Sleep characteristics in ILD subjects are shown in Table 1.
Table 1.
Sleep characteristics in interstitial lung disease
| Sleep characteristic | Abnormality | Patient group | Ref. |
| Respiratory rate | Decreased | ILD | [15] |
| Unchanged | ILD, IPF | [11-14] | |
| Stage 1/2 sleep | Increased | ILD | [14] |
| IPF | [19,20] | ||
| REM sleep | Reduced | ILD | [11,14,15,25] |
| Scleroderma | [26] | ||
| IPF | [2,19,20] | ||
| Slow wave sleep | Reduced | IPF | [13] |
| ILD | [17,25] | ||
| Arousal index | Increased | ILD | [12,14,15] |
| IPF | [2,19,20] | ||
| Sleep efficiency | Reduced | ILD | [25] |
| IPF | [13,19,20] |
ILD: Interstitial lung disease; IPF: Idiopathic pulmonary fibrosis; REM: Rapid eye movement.
Daytime symptoms and quality of life
The symptoms of sleepiness and fatigue often co-exist in ILD patients. Common causative factors include systemic inflammation, treatment side effects, age and comorbidities. Depression and disease-related stressors also affect many. In addition, sleep fragmentation appears to be a substantial contributor, as demonstrated in studies using numerous sleep and health-related quality of life (QoL) questionnaires in ILD subjects. Fatigue is frequently reported, impacting on wellbeing and daytime function[13,21,22]. The Epworth Sleepiness Scale (ESS), and Pittsburgh Sleep Quality Index (PSQI) scores are higher in unselected ILD patients than in normal controls, indicating poorer quality sleep[22-24]. However, in ILD cohorts with polysomnography, the ESS and other tools do not reliably predict severity of sleep-disordered breathing[2,19,25]. The PSQI does appear to correlate with health-related QoL indices, particularly physical function and vitality, highlighting the pervasive influence of sleep fragmentation[22]. Nocturnal hypoxia in ILD patients is also independently associated with a reduction in energy levels, as well as physical and social functioning, as assessed by a variety of QoL instruments[13,21].
Non-respiratory disturbances to sleep
Increased periodic limb movements and restless legs syndrome (RLS) have been documented in IPF and scleroderma patients[17,26]. A self-reported study in IPF patients and normal controls, however, did not find any difference in the incidence of RLS[27]. Gastro-oesophageal reflux disease may also play a role in sleep disruption, particularly in high-risk groups including scleroderma patients[26].
OBSTRUCTIVE SLEEP APNOEA IN ILD
Increasing attention has been focused on the prevalence of obstructive sleep apnoea (OSA) in ILD, with much of the cross-sectional data coming from studies in IPF patients. Three prospective studies showed the incidence of OSA in IPF subjects to markedly exceed that reported in healthy age-matched populations, with estimates between 59% and 90%[2,19,20]. This increased risk however, does not appear to be unique to IPF, with studies of mixed ILD populations (and in particular sarcoidosis and scleroderma subgroups) demonstrating similar findings[18,25,28]. These results are summarized in Table 2.
Table 2.
Prevalence of obstructive sleep apnoea in interstitial lung disease populations n (%)
| Ref. | Population | Prevalence of OSA | M/F | Age (mean) | BMI (mean) | Comment |
| Aydoğdu et al[18], 2006 | ILD | 24 (65) | Abstract only | |||
| Mermigkis et al[17], 2007 | IPF | 11 (61) | 12/6 | 68.1 | 33.2 | Retrospective study; subjects with symptoms of SDB |
| Lancaster et al[19], 2009 | IPF | 44 (88) | 34/16 | 65.7 | 32.2 | Prospective study of unselected patients; 16 subjects used oxygen during PSG |
| Mermigkis et al[20], 2010 | IPF | 20 (59) | 21/13 | 65.0 | 27.3 | Prospective study of subjects with newly diagnosed IPF |
| Kolilekas et al[2], 2013 | IPF | 28 (90) | 24/7 | 68.0 | 28.7 | Increased AHI associated with decreased survival, after exclusion of those prescribed CPAP |
| Pihtili et al[25], 2013 | ILD | 34 (68) | 14/36 | 53.9 | 25.9 | Prospective study; excluded obese subjects (BMI ≥ 30) |
| IPF | 14 (82) | |||||
| Sarcoidosis | 10 (67) | |||||
| Scleroderma | 10 (56) |
OSA: Obstructive sleep apnoea; BMI: Body mass index; ILD: Interstitial lung disease; IPF: Idiopathic pulmonary fibrosis; SDB: Sleep disordered breathing; PSG: Polysomnography; AHI: Apnoea hypopnoea index; CPAP: Continuous positive airway pressure.
Association between degree of OSA and severity of lung disease
Although retrospective analyses of ILD subjects suggest an association between the degree of lung restriction and the risk and severity of sleep disordered breathing, this has not been demonstrated in larger, prospective studies[2,17,19,20,25,29]. The only correlation between measured lung volumes and PSG parameters, reported by Mermigkis et al[20], was in total lung capacity and REM sleep apnoea hypopnoea index (AHI). Kolilekas et al[2] found an association between overall AHI and peak VO2 on exercise testing, but this may simply reflect poorer daytime function and more deconditioning in those with more fragmented sleep.
Hypotheses for why SDB is increased in patients with ILD
In the general OSA literature, recent attention has been turned towards the inherent characteristics that predispose individuals to sleep disordered breathing. The two key components believed to underscore the pathogenesis of OSA are increased upper airway collapsibility and enhanced ventilatory control system instability[30]. It is useful to consider these processes in the ILD population.
Upper airway collapsibility: It is generally believed that restrictive lung disease leads to increased upper airway collapsibility through reduced caudal traction on these structures[31,32]. The inability to demonstrate a relationship between lung function parameters and AHI seems to refute this theory. One limitation with all reported data, however, is the assessment of lung function in the upright position only. There may be more robust associations between supine measurements and SDB, but this is yet to be investigated in ILD subjects. Increased body mass index (BMI) is associated with deposition of adipose tissue around upper airway structures, enhancing collapsibility. BMI correlates with AHI in some, but not all studies of ILD subjects, suggesting that other mechanisms are important[2,18-20,25].
Ventilatory control system instability: In sleep, any rise in arterial CO2 (such as occurs with an apnoea) will stimulate carotid and medullary chemoreceptors, resulting in a central respiratory motor output response[33]. The direct activation of respiratory pump muscles and upper airway dilator muscles (e.g., genioglossus) restores upper airway patency, and is often accompanied by an arousal. Some individuals with heightened chemo-responsiveness may overshoot the eucapnic range, by overventilating in response to apnoea-induced hypercapnia. The ensuing hypocapnia will then cause a further apnoea, sometimes leading to a cyclical pattern of repetitive apnoeas as the ventilatory system continues to attempt to achieve homeostasis. This ventilatory system instability is believed to be an important contributing factor in many with OSA[34].
Intermittent hypoxia enhances chemo-responsiveness, and is likely to exacerbate ventilatory instability in susceptible individuals[35]. This may at least partly explain the frequent observation in some ILD patients, where repetitive apnoeas are unmasked during REM sleep when hypoxia is most pronounced[20].
NOCTURNAL OXYGEN DESATURATION IN ILD
Nocturnal hypoxia is common in ILD, both with and without concomitant OSA. The relative importance of this has been debated, with some early studies concluding that desaturation was minimal, having little clinical impact in ILD[15,16,36]. Other observational studies found that ILD patients experience transient or sustained hypoxia repetitively throughout sleep, leading to a substantial cumulative period of time with SpO2 below 90%[11-14,18,21]. More recently, sleep-desaturation has been found to be an independent predictor of poorer prognosis[1,2].
Whilst obstructive events will undoubtedly be the cause for a proportion of the transient desaturations, there are many other pathophysiologic contributions. Perez-Padilla et al[14], did not observe OSA in any of their subjects, but transient desaturation was a frequent occurrence amounting to an average of nearly 50% of total sleep time with SpO2 below 90%. Further studies found the degree of desaturation that subjects experienced during sleep was independent from oxygen desaturation during moderate and maximal exercise[2,11,16].
There are a number of reasons why ILD patients might be more vulnerable to desaturation during sleep than normal subjects, and indeed than sufferers of OSA with normal lungs. Firstly, many patients will be on the steep portion of the oxygen-haemoglobin dissociation curve, whereby small changes in arterial oxygen tension lead to large decrements in saturation. In support of this is the observation of greater degrees of sleep desaturation in those with lower awake resting PaO2 and SaO2[16,21,36,37]. Hypoxia may also occur due to worsening ventilation/perfusion inhomogeneity, and also alveolar hypoventilation, particularly during REM sleep[3]. Findley et al[38] studied the effect of lung volume on apnoea-related desaturation in normal subjects lying supine. Apnoeas were initiated at a range of lung volumes between total lung capacity and residual volume. The most severe desaturations occurred with apneas at the lower volumes, presumably because of the greater relative impact of dependent airway closure. This is a further mechanism of sleep-related hypoxia that may be extrapolated to individuals with lung restriction from ILD and concomitant sleep apnoea.
Predicting nocturnal desaturation in ILD patients
As might be expected, daytime hypoxia has been identified as a predictor of night-time desaturation in a number of studies[16,21,36,37]. Severity of lung restriction and degree of desaturation with exercise, on the other hand, do not correlate well with nocturnal hypoxia[1,16,21,37,39]. Respiratory drive during wakefulness, (measured as the change in ventilation in response to changes in PaCO2), shows a tight negative correlation with the degree of desaturation in both REM and NREM sleep in ILD patients[37], suggesting the innate chemo-responsiveness of the individual will also influence susceptibility to hypoxia. It is possible also that repeated severe episodes of nocturnal desaturation will eventually blunt this responsiveness, further perpetuating the problem as the disease advances.
Oxygen supplementation during sleep
Although there is widespread practice to provide supplemental oxygen for chronic lung disease patients with significant sleep desaturation, there is very little supportive evidence. In the COPD literature, the survival benefit derived from continuous oxygen therapy has not been demonstrated with overnight supplementation in those with nocturnal hypoxia only[40-42]. There is some suggestion that pulmonary haemodynamics may be improved in COPD patients with long-term nocturnal oxygen[43].
Only two studies have looked at the acute use of nocturnal oxygen in ILD. By eliminating sleep-hypoxia with supplemental oxygen, Shea and co-workers were able to demonstrate a fall in both f and minute ventilation compared with awake values, approximating those of normal controls[44]. Vazquez studied ILD subjects at 2240 m above sea-level, breathing air or oxygen on two separate nights[45]. Not surprisingly, all were hypoxic at rest (mean PaO2 51 mmHg). With the addition of low-flow oxygen during sleep, heart rate and f were reduced and oxygenation improved. Sleep architecture, efficiency and arousal index were not significantly altered. To date, there have been no studies to address whether sleep quality or haemodynamics may improve with long-term use in ILD.
NOCTURNAL DESATURATION AND PULMONARY HYPERTENSION
The link between intermittent desaturation and increased pulmonary arterial pressure was hypothesised nearly forty years ago[46,47]. Cross-sectional data in ILD subjects confirms the association between severity of nocturnal desaturation and the presence of pulmonary hypertension on echocardiography and right heart catherisation[1,39,48]. Furthermore, prolonged exposure to transient, repetitive hypoxia in both animals and humans leads to measurable changes in pulmonary haemodynamics[49]. Tatsumi and colleagues studied subjects with both obstructive and restrictive lung diseases, comparing those with significant nocturnal oxygen desaturation (NOD) to those without NOD, but matched for other disease variables[3]. Daytime, supine pulmonary arterial pressures (PAP) and pulmonary vascular resistances (PVR) were significantly higher in the NOD group. Under hypoxic conditions, these differences became more marked. Hyperoxia, on the other hand, improved PVR and PAP, but not to the normal range seen in the non-NOD patients. This experimental data suggests that the effects may last well beyond the acute period, due to permanent structural changes in the vasculature.
Biomarkers in pulmonary hypertension
Serum Endothelin-1 (ET-1), a vasoactive peptide believed to be important in the pathogenesis of pulmonary hypertension, has been measured in ILD patients during sleep, in a novel study by Trakada and colleagues[50]. During wakefulness, ET-1 was significantly higher in those with elevated pulmonary pressures. During sleep, ET-1 rose acutely in all patients during episodes of desaturation below 90%, and correlated with simultaneously measured PaO2 concentrations and pulmonary arterial pressures. This very interesting research highlights a putative mechanistic pathway in the evolution of pulmonary vascular disease in ILD patients.
In summary, there is increasing evidence that nocturnal desaturation is not a benign phenomenon in ILD patients. In a large proportion, NOD occurs transiently and repeatedly throughout sleep, both with and without associated apnoeas. This may promote development of pulmonary hypertension, and is independently associated with higher mortality. Mechanisms of SDB and NOD are illustrated in Figure 2.
Figure 2.
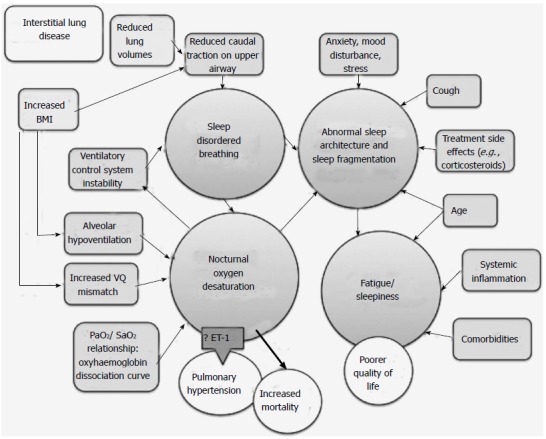
Mechanisms of sleep-related pathology in interstitial lung disease. BMI: Body mass index; VQ: Ventilation-perfusion; ET-1: Endothelin-1.
CONCLUSION
A small but growing body of literature suggests that SDB and NOD are common in patients with IPF and other ILD. There is still much to learn regarding the true impact that these have on the natural history of disease. A large area of uncertainty remains in whether targeted treatment (e.g., positive pressure ventilation, oxygen or other novel therapies) will offer any quality of life or mortality benefits.
Footnotes
Supported by An Australian Postgraduate Award through the University of Sydney (Troy LK is supported)
P- Reviewer: Resch B, Turner AM S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Corte TJ, Wort SJ, Talbot S, Macdonald PM, Hansel DM, Polkey M, Renzoni E, Maher TM, Nicholson AG, Wells AU. Elevated nocturnal desaturation index predicts mortality in interstitial lung disease. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29:41–50. [PubMed] [Google Scholar]
- 2.Kolilekas L, Manali E, Vlami KA, Lyberopoulos P, Triantafillidou C, Kagouridis K, Baou K, Gyftopoulos S, Vougas KN, Karakatsani A, et al. Sleep oxygen desaturation predicts survival in idiopathic pulmonary fibrosis. J Clin Sleep Med. 2013;9:593–601. doi: 10.5664/jcsm.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fletcher EC, Luckett RA, Miller T, Costarangos C, Kutka N, Fletcher JG. Pulmonary vascular hemodynamics in chronic lung disease patients with and without oxyhemoglobin desaturation during sleep. Chest. 1989;95:757–764. doi: 10.1378/chest.95.4.757. [DOI] [PubMed] [Google Scholar]
- 4.American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, Costabel U, Hansell DM, King TE, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shlobin OA, Nathan SD. Pulmonary hypertension secondary to interstitial lung disease. Expert Rev Respir Med. 2011;5:179–189. doi: 10.1586/ers.11.11. [DOI] [PubMed] [Google Scholar]
- 7.Nadrous HF, Pellikka PA, Krowka MJ, Swanson KL, Chaowalit N, Decker PA, Ryu JH. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest. 2005;128:2393–2399. doi: 10.1378/chest.128.4.2393. [DOI] [PubMed] [Google Scholar]
- 8.Javaheri S, Sicilian L. Lung function, breathing pattern, and gas exchange in interstitial lung disease. Thorax. 1992;47:93–97. doi: 10.1136/thx.47.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Donnell DE, Chau LK, Webb KA. Qualitative aspects of exertional dyspnea in patients with interstitial lung disease. J Appl Physiol (1985) 1998;84:2000–2009. doi: 10.1152/jappl.1998.84.6.2000. [DOI] [PubMed] [Google Scholar]
- 10.Kornbluth RS, Turino GM. Respiratory control in diffuse interstitial lung disease and diseases of the pulmonary vasculature. Clin Chest Med. 1980;1:91–102. [PubMed] [Google Scholar]
- 11.Bye PT, Issa F, Berthon-Jones M, Sullivan CE. Studies of oxygenation during sleep in patients with interstitial lung disease. Am Rev Respir Dis. 1984;129:27–32. doi: 10.1164/arrd.1984.129.1.27. [DOI] [PubMed] [Google Scholar]
- 12.Hira HS, Sharma RK. Study of oxygen saturation, breathing pattern and arrhythmias in patients of interstitial lung disease during sleep. Indian J Chest Dis Allied Sci. 1997;39:157–162. [PubMed] [Google Scholar]
- 13.Mermigkis C, Stagaki E, Amfilochiou A, Polychronopoulos V, Korkonikitas P, Mermigkis D, Bregou M, Kouris N, Bouros D. Sleep quality and associated daytime consequences in patients with idiopathic pulmonary fibrosis. Med Princ Pract. 2009;18:10–15. doi: 10.1159/000163039. [DOI] [PubMed] [Google Scholar]
- 14.Perez-Padilla R, West P, Lertzman M, Kryger MH. Breathing during sleep in patients with interstitial lung disease. Am Rev Respir Dis. 1985;132:224–229. doi: 10.1164/arrd.1985.132.2.224. [DOI] [PubMed] [Google Scholar]
- 15.McNicholas WT, Coffey M, Fitzgerald MX. Ventilation and gas exchange during sleep in patients with interstitial lung disease. Thorax. 1986;41:777–782. doi: 10.1136/thx.41.10.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Midgren B, Hansson L, Eriksson L, Airikkala P, Elmqvist D. Oxygen desaturation during sleep and exercise in patients with interstitial lung disease. Thorax. 1987;42:353–356. doi: 10.1136/thx.42.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mermigkis C, Chapman J, Golish J, Mermigkis D, Budur K, Kopanakis A, Polychronopoulos V, Burgess R, Foldvary-Schaefer N. Sleep-related breathing disorders in patients with idiopathic pulmonary fibrosis. Lung. 2007;185:173–178. doi: 10.1007/s00408-007-9004-3. [DOI] [PubMed] [Google Scholar]
- 18.Aydoğdu M, Ciftçi B, Firat Güven S, Ulukavak Ciftçi T, Erdoğan Y. [Assessment of sleep with polysomnography in patients with interstitial lung disease] Tuberk Toraks. 2006;54:213–221. [PubMed] [Google Scholar]
- 19.Lancaster LH, Mason WR, Parnell JA, Rice TW, Loyd JE, Milstone AP, Collard HR, Malow BA. Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest. 2009;136:772–778. doi: 10.1378/chest.08-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mermigkis C, Stagaki E, Tryfon S, Schiza S, Amfilochiou A, Polychronopoulos V, Panagou P, Galanis N, Kallianos A, Mermigkis D, et al. How common is sleep-disordered breathing in patients with idiopathic pulmonary fibrosis? Sleep Breath. 2010;14:387–390. doi: 10.1007/s11325-010-0336-5. [DOI] [PubMed] [Google Scholar]
- 21.Clark M, Cooper B, Singh S, Cooper M, Carr A, Hubbard R. A survey of nocturnal hypoxaemia and health related quality of life in patients with cryptogenic fibrosing alveolitis. Thorax. 2001;56:482–486. doi: 10.1136/thorax.56.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnan V, McCormack MC, Mathai SC, Agarwal S, Richardson B, Horton MR, Polito AJ, Collop NA, Danoff SK. Sleep quality and health-related quality of life in idiopathic pulmonary fibrosis. Chest. 2008;134:693–698. doi: 10.1378/chest.08-0173. [DOI] [PubMed] [Google Scholar]
- 23.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 24.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 25.Pihtili A, Bingol Z, Kiyan E, Cuhadaroglu C, Issever H, Gulbaran Z. Obstructive sleep apnea is common in patients with interstitial lung disease. Sleep Breath. 2013;17:1281–1288. doi: 10.1007/s11325-013-0834-3. [DOI] [PubMed] [Google Scholar]
- 26.Prado GF, Allen RP, Trevisani VM, Toscano VG, Earley CJ. Sleep disruption in systemic sclerosis (scleroderma) patients: clinical and polysomnographic findings. Sleep Med. 2002;3:341–345. doi: 10.1016/s1389-9457(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 27.Puthalapattu S, Roman-Rodriguez J, Perez RL, Bhadriraju S, Ioachimescu OC. Sleep apnea and other sleep disorders: survey in individuals with idiopathic pulmonary fibrosis (IPF) J Sleep Disorders Ther. 2013;2:1–4. [Google Scholar]
- 28.Turner GA, Lower EE, Corser BC, Gunther KL, Baughman RP. Sleep apnea in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:61–64. [PubMed] [Google Scholar]
- 29.Patterson KC, Huang F, Oldham JM, Bhardwaj N, Hogarth DK, Mokhlesi B. Excessive daytime sleepiness and obstructive sleep apnea in patients with sarcoidosis. Chest. 2013;143:1562–1568. doi: 10.1378/chest.12-1524. [DOI] [PubMed] [Google Scholar]
- 30.Dempsey JA, Xie A, Patz DS, Wang D. Physiology in medicine: obstructive sleep apnea pathogenesis and treatment--considerations beyond airway anatomy. J Appl Physiol (1985) 2014;116:3–12. doi: 10.1152/japplphysiol.01054.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradley TD, Brown IG, Grossman RF, Zamel N, Martinez D, Phillipson EA, Hoffstein V. Pharyngeal size in snorers, nonsnorers, and patients with obstructive sleep apnea. N Engl J Med. 1986;315:1327–1331. doi: 10.1056/NEJM198611203152105. [DOI] [PubMed] [Google Scholar]
- 32.Kairaitis K, Byth K, Parikh R, Stavrinou R, Wheatley JR, Amis TC. Tracheal traction effects on upper airway patency in rabbits: the role of tissue pressure. Sleep. 2007;30:179–186. doi: 10.1093/sleep/30.2.179. [DOI] [PubMed] [Google Scholar]
- 33.Nakayama H, Smith CA, Rodman JR, Skatrud JB, Dempsey JA. Carotid body denervation eliminates apnea in response to transient hypocapnia. J Appl Physiol (1985) 2003;94:155–164. doi: 10.1152/japplphysiol.00722.2002. [DOI] [PubMed] [Google Scholar]
- 34.Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol (1985) 2008;105:1389–1405. doi: 10.1152/japplphysiol.90408.2008. [DOI] [PubMed] [Google Scholar]
- 35.Marcus NJ, Li YL, Bird CE, Schultz HD, Morgan BJ. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: role of the angiotensin II type 1 receptor. Respir Physiol Neurobiol. 2010;171:36–45. doi: 10.1016/j.resp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Midgren B. Oxygen desaturation during sleep as a function of the underlying respiratory disease. Am Rev Respir Dis. 1990;141:43–46. doi: 10.1164/ajrccm/141.1.43. [DOI] [PubMed] [Google Scholar]
- 37.Tatsumi K, Kimura H, Kunitomo F, Kuriyama T, Honda Y. Arterial oxygen desaturation during sleep in interstitial pulmonary disease. Correlation with chemical control of breathing during wakefulness. Chest. 1989;95:962–967. doi: 10.1378/chest.95.5.962. [DOI] [PubMed] [Google Scholar]
- 38.Findley LJ, Ries AL, Tisi GM, Wagner PD. Hypoxemia during apnea in normal subjects: mechanisms and impact of lung volume. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:1777–1783. doi: 10.1152/jappl.1983.55.6.1777. [DOI] [PubMed] [Google Scholar]
- 39.Miyahara Y, Miyahara Y, Naito T, Ikeda S. Monitoring of nocturnal oxygen desaturation using pulse oximeter and apnomonitor in patients with chronic pulmonary disease. Respiration. 1995;62:348–352. doi: 10.1159/000196478. [DOI] [PubMed] [Google Scholar]
- 40.Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;1:681–686. [PubMed] [Google Scholar]
- 41.Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93:391–398. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 42.Chaouat A, Weitzenblum E, Kessler R, Charpentier C, Enrhart M, Schott R, Levi-Valensi P, Zielinski J, Delaunois L, Cornudella R, et al. A randomized trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease patients. Eur Respir J. 1999;14:1002–1008. doi: 10.1183/09031936.99.14510029. [DOI] [PubMed] [Google Scholar]
- 43.Fletcher EC, Luckett RA, Goodnight-White S, Miller CC, Qian W, Costarangos-Galarza C. A double-blind trial of nocturnal supplemental oxygen for sleep desaturation in patients with chronic obstructive pulmonary disease and a daytime PaO2 above 60 mm Hg. Am Rev Respir Dis. 1992;145:1070–1076. doi: 10.1164/ajrccm/145.5.1070. [DOI] [PubMed] [Google Scholar]
- 44.Shea SA, Winning AJ, McKenzie E, Guz A. Does the abnormal pattern of breathing in patients with interstitial lung disease persist in deep, non-rapid eye movement sleep? Am Rev Respir Dis. 1989;139:653–658. doi: 10.1164/ajrccm/139.3.653. [DOI] [PubMed] [Google Scholar]
- 45.Vázquez JC, Pérez-Padilla R. Effect of oxygen on sleep and breathing in patients with interstitial lung disease at moderate altitude. Respiration. 2001;68:584–589. doi: 10.1159/000050576. [DOI] [PubMed] [Google Scholar]
- 46.Unger M, Atkins M, Briscoe WA, King TK. Potentiation of pulmonary vasoconstrictor response with repeated intermittent hypoxia. J Appl Physiol Respir Environ Exerc Physiol. 1977;43:662–667. doi: 10.1152/jappl.1977.43.4.662. [DOI] [PubMed] [Google Scholar]
- 47.Tilkian AG, Guilleminault C, Schroeder JS, Lehrman KL, Simmons FB, Dement WC. Hemodynamics in sleep-induced apnea. Studies during wakefulness and sleep. Ann Intern Med. 1976;85:714–719. doi: 10.7326/0003-4819-85-6-714. [DOI] [PubMed] [Google Scholar]
- 48.Pitsiou G, Bagalas V, Boutou A, Stanopoulos I, Argyropoulou-Pataka P. Should we routinely screen patients with idiopathic pulmonary fibrosis for nocturnal hypoxemia? Sleep Breath. 2013;17:447–448. doi: 10.1007/s11325-012-0716-0. [DOI] [PubMed] [Google Scholar]
- 49.Nattie EE, Bartlett D, Johnson K. Pulmonary hypertension and right ventricular hypertrophy caused by intermittent hypoxia and hypercapnia in the rat. Am Rev Respir Dis. 1978;118:653–658. doi: 10.1164/arrd.1978.118.4.653. [DOI] [PubMed] [Google Scholar]
- 50.Trakada G, Nikolaou E, Pouli A, Tsiamita M, Spiropoulos K. Endothelin-1 levels in interstitial lung disease patients during sleep. Sleep Breath. 2003;7:111–118. doi: 10.1007/s11325-003-0111-y. [DOI] [PubMed] [Google Scholar]


