Abstract
Chronic rhinosinusitis (CRS) is a common disease worldwide, with a prevalence rate of 5%-15% in the general population. CRS is currently classified into two types: CRS with and without nasal polyps. CRS may also be divided into eosinophilic CRS (ECRS) and non-ECRS subtypes based on the presence of tissue eosinophilic infiltration or not. There are significant geographic and ethnic differences in the tissue eosinophilic infiltration, which is predominant in Western white patients and less common in East Asians, despite an increasing tendency for its prevalence in East Asia countries. ECRS differs significantly from non-ECRS in clinical characteristics, treatment outcomes and strategies, and underlying pathogenic mechanisms. ECRS commonly demonstrates more severe symptoms, polyp diseases with a higher incidence of bilateral polyps and sinonasal diseases on computed tomography, and the increase in blood eosinophils. ECRS is considered a special and recalcitrant subtype of CRS, commonly with poor treatment outcomes compared to non-ECRS. The differentiation of specific subtypes and clinical features of CRS will be important for developing novel treatment strategies and improving treatment outcomes for individual phenotypes of CRS. This review discusses clinical features, diagnosis, treatment and prognosis of ECRS in East Asians.
Keywords: Chronic rhinosinusitis, Eosinophilic chronic rhinosinusitis, Eosinophils, Chronic rhinosinusitis with nasal polyps, Nasal polyps
Core tip: Chronic rhinosinusitis (CRS) is a common disease and currently classified into two types based on presence or absence of nasal polyps. CRS may also be subtyped into eosinophilic CRS (ECRS) and non-ECRS according to the presence of predominant tissue eosinophilic infiltration or not. ECRS differs significantly from non-ECRS in clinical characteristics, treatment outcomes and strategies, and underlying pathogenic mechanisms. ECRS is considered a special and recalcitrant subtype of CRS. The identification of ECRS is helpful to develop treatment strategies for this CRS subtype. Herein we review the clinical features, diagnosis, treatment and prognosis of ECRS in East Asians.
INTRODUCTION
Chronic rhinosinusitis (CRS) is one of the most common chronic diseases worldwide, with a prevalence rate of 5%-15% in the general population in Europe and the United States[1] and 7% in South Korea[2]. CRS remains a significant public health problem with a considerable socioeconomic burden[3]. In the current practice guidelines of Europe, the United States and China, CRS is classified into two types based on the presence or absence of nasal polyps: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP)[1,4,5]. Eosinophilic inflammation is considered a major pathologic hallmark of CRS. Histological studies demonstrate the predominant tissue eosinophilic infiltration with a high proportion of CRS cases, most prominently with CRSwNP cases[1]. Thus, CRS may be classified into two subtypes: eosinophilic CRS (ECRS) and non-eosinophilic CRS (NECRS). Similarly, CRSwNP may also be subclassified into ECRSwNP and NECRSwNP[6-19]. However, the tissue eosinophilic infiltration in CRS shows significant geographic and ethnic differences. Eosinophilic infiltration is predominantly observed in Western white patients with CRS, accounting for more than 80% of CRS cases[1,4,18], while the eosinophilic phenotype is less than 50% of CRS cases in East Asia countries including Japan[13-15,20], South Korea[17,21,22] and China[11,16,23,24]. However, recent studies indicate an increasing tendency for the prevalence of ECRS in these Asia countries[12,13,15,20,21,25]. Studies show that ECRS differs significantly from NECRS in clinical characteristics, underlying pathogenic mechanisms, treatment outcomes and strategies[1,6,10,11,13,15,16,20,26-28]. ECRS is considered a special subtype of CRS[10,13,15] and also a subtype of recalcitrant CRS, which commonly has worse disease severity[8,18,19,29] and poorer treatment outcomes[19,28,30] compared to NECRS. For example, ECRSwNP is refractory to the combined treatments of endoscopic sinus surgery (ESS) and macrolide therapy and shows a strong tendency for recurrence after surgery but responds to systemic steroid therapy[13-15]. Thus, identifying specific subtypes of CRS and underlying pathogenic mechanisms will be important for developing novel treatment strategies and improving treatment outcomes for individual phenotypes of CRS[10,29].
ROLES OF EOSINOPHILS IN ECRS
CRS is a heterogeneous disease to which numerous etiologies contributed. Although intensive investigations have been performed, the etiology, pathogenesis and underlying mechanisms of CRS are not fully understood[1,15,31,32]. The dominant eosinophilic inflammation for CRS indicates that eosinophils play a key role in the pathogenesis of CRS, especially in CRSwNP[1], although many kinds of other inflammatory cells including neutrophils, mass cells, lymphocytes and plasma cells also have important roles in the pathogenesis of CRS[27,33] (Figure 1).
Figure 1.
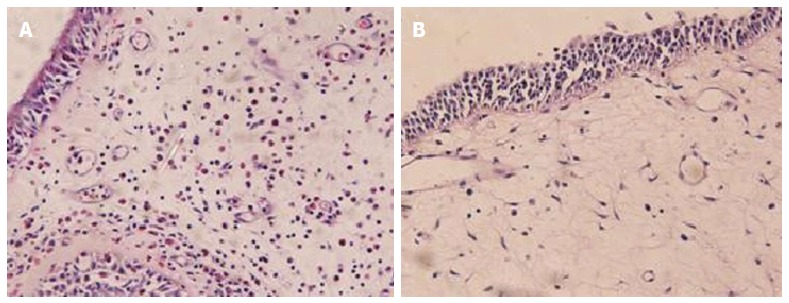
Hematoxylin and eosin staining for nasal polyp tissues. Predominant eosinophil infiltration is showed in the subtype of eosinophilic chronic rhinosinusitis with nasal polyps (A), but other forms of inflammatory cell infiltration in the subtype of non-eosinophilic chronic rhinosinusitis with nasal polyps (B) (× 400).
Eosinophils develop from CD34+ progenitors in the bone marrow and migrate into the bloodstream, and they are recruited to disease sites by chemokines or cytokines, where eosinophils can perform and participate in a variety of functions, including antigen presentation, cytokine or chemokine production, and secretion of granule mediators[34-36]. The ability of eosinophils to process and present antigens has been generally underestimated and this function can be added to the growing list of mechanisms by which eosinophils regulate the immune system[34]. Eosinophils store preformed cytokines in granules that can be released rapidly upon antigenic provocation[36]. These eosinophil-derived cytokines can be T helper 2 (Th2) cytokines such as interleukin (IL)-13 that act directly on T cells, as well as other inflammatory cytokines that can prime antigen presenting cells and the vascular endothelium to secrete chemokines and cytokines that recruit and activate T cells[34].
Studies indicate significant roles of T cell regulation in CRS. CRS appears to be a disease mediated by CD4+ T cells that can be functionally divided into Th1 or Th2 phenotype based on their patterns of cytokine secretion. It is found that among West white patients with CRS, CRSsNP is characterized by Th1 polarization, whereas CRSwNP by predominant Th2, with high levels of Th2-type cytokines including IL-4, IL-5 and IL-13[37]. CRSwNP also is characterized by a Th2-driven eosinophilic inflammation in tissue[37,38]. However, studies suggest that East Asians with CRSwNP present different immunopathologic features compared with West white patients[17,24,39]. For example, CRSwNP in Chinese demonstrates a Th1/Th17 cell pattern with minor eosinophilic inflammation[24]. Th2-dominated reactions can only be found in ECRSwNP instead of all CRSwNP cases, suggesting that Th cell responses may exert different impacts on the pathogenesis of ECRSwNP and NECRSwNP[16,17,24]. There are interactions between T cells and eosinophils. It is conventionally viewed that the T cell–eosinophil interactions are primarily based on the activation of eosinophils by T cells via cytokines, but it is suggested that eosinophils also have the capacity to activate T cells to produce cytokines[40,41]. Eosinophils by secreting specific cytokines or chemokines have a more central role in Th2 responses in CRS[34].
CLINICAL FEATURES OF ECRS
Many studies have shown that ECRS differs from NECRS in clinical features[13,14,15]: (1) ECRS often shows the symptom of olfactory dysfunction in its early stage; (2) ECRS commonly demonstrates multiple and bilateral nasal polyps, with highly viscous mucus secretion, while NECRS mostly with mucopurulent discharge; (3) ECRS tends to have bilateral sinus diseases on sinonasal computed tomography (CT), with a predominant disease in the ethmoid sinus especially in early stage, while NECRS in the maxillary sinus; (4) Co-existence of asthma is more common in ECRS; (5) Most of ECRS cases show the increase of peripheral blood eosinophils; (6) ECRS demonstrates dominant tissue eosinophilic infiltration; (7) In medical treatments, local or systemic steroid therapy is more effective for ECRS compared to macrolide therapy, while macrolide is effective for NECRS; and (8) ECRS shows strong tendency for nasal polyp recurrence after surgery, but systemic steroid is effective for the recurrent nasal polyps.
Symptoms of ECRS
Many studies indicate that ECRS commonly has more severe disease and higher symptom score compared to NECRS[8,18,19,29]. A recent study shows the mean severity score of symptoms including olfactory dysfunction, nasal obstruction, and nasal discharge in ECRS is significantly higher than that in NECRS[42]. Previous studies have shown that there is a close correlation between symptoms and tissue eosinophil infiltration in CRS[18,43]. However, a recent study shows no significant differences in the symptom severities of nasal obstruction, nasal discharge, and facial pain aside from smell dysfunction between ECRS and NECRS cases[10]. Another study also shows no difference in visual analogue scale (VAS) score or duration of symptoms between ECRSwNP and NECRSwNP patients[11], suggesting that the two subtypes may have an equivalent severity of symptoms. Similarly, ECRSwNP and NECRSwNP patients may present with comparable symptom scores[44]. In our recent study, a significant difference in the mean VAS score of symptoms between the ECRSwNP and NECRSwNP patients was also not found (Table 1).
Table 1.
Demographic and clinical characteristics of eosinophilic chronic rhinosinusitis with nasal polyps and non-eosinophilic chronic rhinosinusitis with nasal polyps
| ECRSwNP | NECRSwNP | |
| n (%) | 27 (45%) | 33 (55%) |
| Age (yr), mean ± SD | 46.93 ± 12.35 | 40.27 ± 13.47 |
| M/F | 20/7 | 24/9 |
| With AR (%) | 74.10% | 48.5% |
| With asthma (%) | 18.50% | 12.1% |
| Duration of symptom (yr) | 5.50 ± 3.92 | 8.55 ± 6.93 |
| VAS | 4.04 ± 1.01 | 3.99 ± 1.09 |
| Score of olfactory dysfunction | 5.59 ± 2.54 | 5.21 ± 2.66 |
| Score of polyps | 3.59 ± 1.11b | 2.06 ± 0.82 |
| Incidence of bilateral polyps | 92.6%b | 39.9% |
| Score of disease on CT | 14.42 ± 3.84b | 9.64 ± 3.37 |
| Serum IgE (kU/L) | 236.72 ± 157.77 | 167.97 ± 176.77 |
| Blood eosinophil count (× 109/L) | 0.44 ± 0.24b | 0.21 ± 0.11 |
| Blood eosinophil percentage (%) | 6.49 ± 3.27b | 3.42 ± 1.87 |
| Tissue eosinophil count/HPF | 31.56 ± 21.37b | 0.91 ± 0.80 |
P < 0.01 vs NECRSwNP. ECRSwNP: Eosinophilic chronic rhinosinusitis with nasal polyps; NECRSwNP: Non-eosinophilic chronic rhinosinusitis with nasal polyps; M/F: Male/female; AR: Allergic rhinitis; VAS: Visual analogue scale; HPF: High power field; CT: Computed tomography.
CRS is one of the most frequent causes of olfactory dysfunction (reduction or loss of smell) and accounts for 21%-25% of cases with smell loss[45-48]. Meanwhile, olfactory dysfunction affects about 60% of CRS patients[4]. Olfactory dysfunction is related to the severity of CRS, especially when with nasal polyps[49]. A study shows that 38% of CRS patients present with olfactory dysfunction, which is affected by nasal polyps, and the prevalence of olfactory dysfunction is 57% in CRSwNP and 13.7% in CRSsNP, respectively[20]. A recent report indicates that smell dysfunction is a very common symptom in CRSwNP, even accounting for 96.5% of cases[41]. Olfactory dysfunction is a more predominant and characteristic symptom of ECRS and tends to occur in the early stage of ECRS[10,13-15,20,50]. This symptom is more severe and common in ECRS compared to NECRS[42]. A study shows that there is a high prevalence of olfactory dysfunction in ECRS (78.9%) compared to NECRS (25.9%)[20]. Olfactory dysfunction is reported to be associated with olfactory cleft opacification on CT images[51]. Nasal polyps occur more commonly in the olfactory cleft in ECRS compared to NECRS[42]. Edematous swelling or polyposis of the middle turbinate, which is often observed in ECRS patients, increases the opacification of the olfactory cleft and causes olfactory impairments[13]. Studies indicate that olfaction score is influenced by mucosal eosinophilic infiltration, with lower olfaction score in ECRSwNP as compared to NECRSwNP[29,52]. A study shows that there are no statistically significant differences in the VAS scores of nasal obstruction, nasal discharge, headache or overall symptoms, but a statistically significant difference is found in relation to problems of smell between the patients with high and low infiltration of eosinophils in the ethmoidal sinus mucosa[50]. But in our recent study, no statistically significant difference in olfactory dysfunction scores was found between ECRSwNP and NECRSwNP (Table 1). The patients with ECRSwNP seemed to have a shorter duration of symptoms than NECRSwNP patients although this difference was not significant statistically (Table 1).
Polyps in ECRS
ECRS commonly exhibits multiple and bilateral nasal polyps compared to NECRS[13-15], and the polyps commonly exist in the olfactory cleft[42]. Although a previous study shows that there is not a significant difference in endoscopic scores of nasal polyps between ECRSwNP and NECRSwNP subtypes[29], many studies demonstrate that ECRSwNP often present with a higher endoscopic score of nasal polyps compared with NECRSwNP[10,18]. Our recent study showed that ECRSwNP presented with a higher score of nasal polyps and a higher incidence of bilateral nasal polyps when compared with NECRSwNP (Table 1 and Figure 2).
Figure 2.
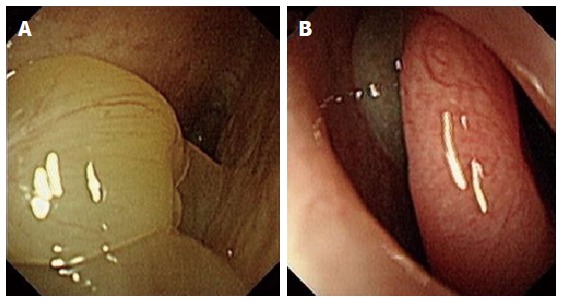
Nasal endoscopic findings. Polyps in eosinophilic chronic rhinosinusitis with nasal polyps (A) and in non-eosinophilic chronic rhinosinusitis with nasal polyps (B).
In addition, endoscopic examination indicates that most of patients with ECRS demonstrate sinonasal mucus secretion with high viscosity, while NECRS is common with mucopurulent discharge[13-15]. It was found in our recent study that more than half (55.6%) of 27 ECRSwNP patients showed highly viscous mucus secretion, but less than a third (30.3%) of 33 NECRSwNP patients presented with this condition.
CT findings in ECRS
The Lund-Mackay scoring system is widely used to evaluate the disease severity of CRS on sinonasal CT[1,4,53,54]. CRSwNP tends to have a higher score of disease on CT compared with CRSsNP[41]. CT imaging also is a powerful tool to differentiate ECRS from NECRS[13]. Studies show that there are significant differences in the disease scores of most sinuses aside from maxillary sinus between ECRS and NECRS[13,15]. ECRSwNP presents with higher disease scores on CT compared to NECRSwNP[18,27], although an obvious difference in CT scores between ECRSwNP and NECRSwNP subtypes is not found in some studies[11,29]. In addition, CT studies show that sinus diseases commonly occur bilaterally in ECRS compared to NECRS[13-15]. Our recent study showed significant differences in the mean score of total diseases in all sinuses, the mean number of involved sinuses, the percentage of cases with involvement of all sinuses, and the incidence of bilateral diseases in individual sinuses between ECRSwNP and NECRSwNP (Table 2 and Figure 3).
Table 2.
Computed tomography features of eosinophilic chronic rhinosinusitis with nasal polyps and non-eosinophilic chronic rhinosinusitis with nasal polyps
| ECRSwNP (n = 27) | NECRSwNP (n = 33) | |
| Total disease score of sinuses | 14.42 ± 3.84b | 9.64 ± 3.37 |
| Number of involved sinuses | 7.88 ± 1.22b | 5.64 ± 1.49 |
| Percentage of involvement in all sinuses | 23.1%a | 3.0% |
| Incidence of bilateral diseases in individual sinuses | ||
| Frontal | 53.8%b | 12.1% |
| Sphenoid | 38.5%a | 9.1% |
| Anterior ethmoid | 53.8%a | 12.1% |
| Posterior ethmoid | 100.0%b | 75.8% |
| Maxillary | 96.2%a | 69.7% |
| OMC | 69.2%a | 36.4% |
| Score of diseases in individual sinuses | ||
| Frontal | 1.81 ± 1.51a | 0.88 ± 0.91 |
| Sphenoid | 1.23 ± 1.28a | 0.55 ± 0.76 |
| Anterior ethmoid | 3.27 ± 0.90b | 2.27 ± 0.87 |
| Posterior ethmoid | 3.04 ± 1.04b | 1.52 ± 0.95 |
| Maxillary | 2.23 ± 0.43 | 2.15 ± 0.69 |
| OMC | 2.85 ± 1.60 | 2.27 ± 1.20 |
P < 0.05,
P < 0.01 vs NECRSwNP. Scoring for sinus diseases on computed tomography (CT): 0 = normal, 1 = partial opacification, and 2 = total opacification; these points are applied to individual sinuses on each side; OMC is graded as 0 = not occluded, or 2 = occluded; deriving a maximum score of 12 per side. ECRSwNP: Eosinophilic chronic rhinosinusitis with nasal polyps; NECRSwNP: Non-eosinophilic chronic rhinosinusitis with nasal polyps; OMC: Ostiomeatal complex.
Figure 3.
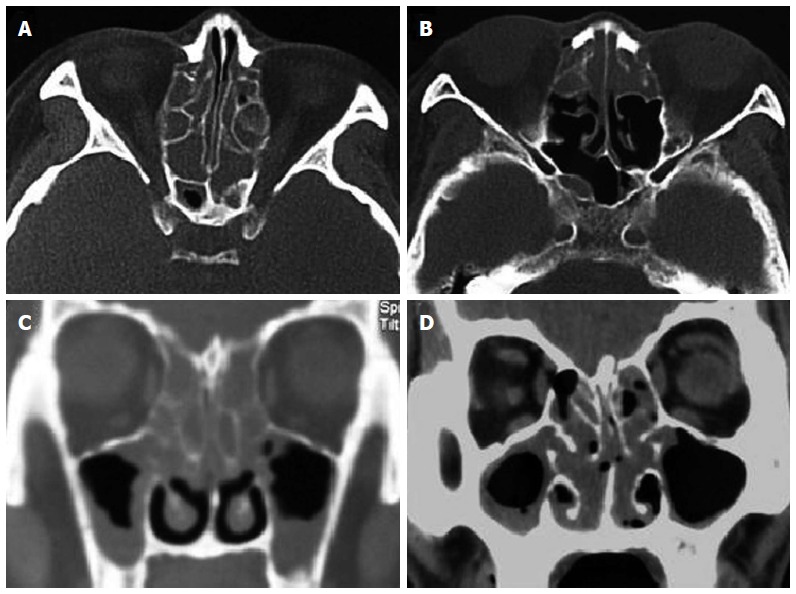
Computed tomography findings. Axial and frontal sections in the subtypes of eosinophilic chronic rhinosinusitis with nasal polyps (ECRSwNP) (A and C) and non-eosinophilic chronic rhinosinusitis with nasal polyps (NECRSwNP) (B and D). Predominant diseases in bilateral anterior and posterior ethmoid sinuses are showed in ECRSwNP, while predominant diseases in anterior ethmoid sinuses in NECRSwNP.
In terms of individual sinuses, ECRS patients especially in their early stages often have predominant diseases in the ethmoid sinuses[13-15,20,42]. A previous study shows that there is a significant correlation between the severity of eosinophilic infiltration in the ethmoidal mucosa and the disease on CT[55]. Ethmoidal sinus lesions are readily detected by CT in patients with CRS accompanied by severe eosinophil infiltration[50]. Involvement of the posterior ethmoid sinus is one of the most apparent differences in CT images between ECRS and NECRS. In the early stage of ECRS, CT images can demonstrate the opacification of the posterior ethmoid sinus[15]. A study shows that the posterior ethmoid sinus is more commonly involved in ECRSwNP compared to NECRSwNP, whereas both the anterior and posterior ethmoid sinuses are similarly involved in NECRSwNP, and CT score of the posterior ethmoid has a good accuracy as a predictor of ECRSwNP in a Japanese population[13]. Our recent study showed that ECRSwNP had a higher incidence of bilateral diseases and a higher disease score in the anterior or posterior ethmoid sinus compared to NECRS, but ECRSwNP had similar disease scores in its anterior and posterior ethmoid sinuses, while NECRS showed a higher disease score in the anterior ethmoid sinus compared with the posterior ethmoid sinus (Table 2).
The maxillary sinus is most often involved in CRS. The middle meatus or ostiomeatal complex (OMC) has a fundamental role in the pathogenesis of CRS[1]. As the drainage from the sinus to the middle meatus or OMC is impaired, the sinus becomes secondarily involved. According to this pathogenesis, sinuses that are most likely to be affected are the maxillary sinus and anterior ethmoid sinus that connect to the middle meatus or OMC through the small ostia. However, ECRS has predominant disease in the ethmoid sinus, while NECRS in the maxillary sinus[13-15,20], and the posterior ethmoid sinus that does not directly connect with the middle meatus is involved in similar to the anterior ethmoid sinus even in the early stage for ECRS patients. This suggests that pathological changes in the middle meatus or OMC may be of less importance for the pathogenesis of ECRS, namely, the pathogenesis of ECRS is different from that of NECRS[15]. A recent study reveals that OMC obstruction is correlated with sinus disease only for patients with CRSsNP but not CRSwNP[56]. It is thought that ECRS may not be associated with OMC occlusion[8].
In contrast to NECRS patients who have often a predominant disease in the maxillary sinus, patients with ECRS have commonly a predominant disease in the ethmoid sinus especially in the early stage[9,13-15,20]. Our recent study showed that ECRSwNP had higher disease scores in frontal, sphenoid, anterior and posterior ethmoid sinuses than NECRSwNP, but there was not a significant difference in maxillary or OMC disease score between ECRSwNP and NECRSwNP (Table 2), which indicated that ECRSwNP had predominant disease in the ethmoid sinus including the anterior and posterior ethmoid sinuses, while NECRSwNP had similar involvement of the anterior ethmoid and maxillary sinuses but with less involvement in the posterior ethmoid sinus.
Co-morbid allergic rhinitis or asthma in ECRS
Inflammation in the upper respiratory tract affects the lower respiratory tract and vice versa. The concept of the unified airway is proposed based on evidence from epidemiological, pathophysiological, and treatment outcome studies, indicating the existence of similar inflammatory responses and the shared pathophysiological mechanisms between allergic rhinitis (AR), asthma and CRS[20,57,58].
Some studies demonstrate that 25%-58% of individuals with CRS have AR[59,60]. A recent study shows that 67.2% of 418 patients with CRS have AR, and 76.8% of 190 patients with ECRS and 59.2% of 228 patients with NECRS have AR[42]. However, some studies show that there is not a statistically significant difference in the coexistent rate of AR between ECRSwNP and NECRSwNP[11,22], and a similar finding was also found in our case cohort (Table 1).
The clinical relationship between CRS and asthma has been known for many years. CRS and asthma coexist often clinically and they share some histopathologic features such as chronic eosinophilic inflammation, epithelial damage, and basement membrane thickening of the airway mucosa[61]. It is reported that the prevalence of asthma in CRS patients is 20%-50%[13,18,20,41,42,62,63] and even more than 50%[61]. However, there is a lower prevalence of asthma (2%-3%) in CRS patients in China compared with the Western population[64]. This difference may result from distinct immunopathologic characteristics of CRS in Chinese patients, specifically from lower levels of eosinophilic inflammation[16,24,64-66]. CRS especially CRSwNP is commonly associated with asthma[31]. The association of CRSwNP and asthma is well established, and CRSwNP in white population of Europe and the United States represents often a form of severe and difficult-to-treat eosinophilic airway inflammation, which frequently is linked to co-morbid asthma[1]. Eosinophilic inflammation is considered a common mechanism in both CRSwNP and asthma[67]. It is reported that among 2176 cases with CRSwNP, 37.5% present with asthma[68]. A recent study shows that among 182 patients with CRSwNP, the percentage of patients with asthma is as high as 94%[69]. Asthma is known to be often concurrent with ECRS[14,42,70]. A study shows that 34.7% of 190 patients with ECRS, but only 9.6% of 228 patients with NECRS, present the coexistence with asthma[42]. Co-morbid asthma is one of typical features for ECRS[13]. Association of ECRSwNP with asthma is widely accepted[12]. Some authors believe that ECRS and asthma share similar histopathologic features and are the same inflammatory process demonstrating in different sites of the respiratory tract[61,67].
A study shows that in Chinese patients with CRSwNP, the incidence of asthma (15.9%) in ECRSwNP is higher than that (3.6%) in NECRSwNP[9]. Another study shows that the prevalence of asthma in ECRSwNP is higher than that in NECRSwNP, but the difference does not reach statistical significance[11]. And also, there are the studies showing no significant difference in the prevalence of asthma between ECRSwNP and NCRSwNP patients[11,22], which may be due to the low prevalence of asthma among CRS patients in China[64]. A statistically significant difference in the incidence of asthma between ECRSwNP and NECRSwNP patients was also not found in our recent study (Table 1). Although asthma is often seen in patients with ECRS, co-morbidity with asthma may not be a diagnostic criterion for ECRS because about half of ECRS cases are not associated with asthma[15].
IgE and ECRS
CRS is a form of eosinophil-dominated inflammation. Some factors result in local production of IgE, which may contribute to severe eosinophilic inflammation. There is a significant correlation between the concentration of IgE and the number of eosinophils in nasal polyp tissue[38]. Some ECRS patients show the elevation of total or specific IgE level[6,15,44]. ECRSwNP patients demonstrate increased blood IgE levels compared with NECRSwNP[11,27]. A study shows that the amount of tissue eosinophils in CRSwNP is related to eosinophilia of the peripheral blood, but no significant correlation exists between elevated serum IgE and the increase of tissue or blood eosinophils, indicating that atopic conditions may play a minor role in the pathogenesis of CRSwNP in Koreans[17]. It is showed that only less than half of CRS patients present with the increased blood IgE and thus eosinophilic inflammation is not likely driven by an IgE mechanism[61]. A study shows the absence of a significant difference in total serum IgE levels between ECRS and NECRS patients, suggesting that systemic IgE does not greatly contribute to the pathophysiology of ECRS[13]. Also, a recent study shows that although total serum IgE in ECRS is higher than that in NECRS (120.3 vs 48.0 kU/L), the difference is not statistically significant[10]. Similarly, a significant difference in serum IgE levels between ECRSwNP and NECRSwNP was not found in our recent study (Table 1).
DEFINITION OR DIAGNOSIS OF ECRS
Currently ECRS is determined primarily based on tissue eosinophilic infiltration, but there is not a well-defined criterion of the tissue eosinophilic infiltration for diagnosis of ECRS. In some studies, ECRS including ECRSwNP is defined as tissue eosinophil count per high power field (HPF) more than 5 eosinophils[18,21,29,64], 10 eosinophils[8,30], or 20 eosinophils[9], even more than 100 eosinophils[27,42], as well as the percentage of eosinophils in tissue-infiltrated inflammatory cells exceeding 5%[17], 10%[16,66,71] or 15%[20]. In our recent study tissue eosinophil count more than 5 eosinophils/HPF was used as a criterion for ECRSwNP based on the frequency of cases with individual eosinophil counts in nasal polyp tissues (Figure 4).
Figure 4.
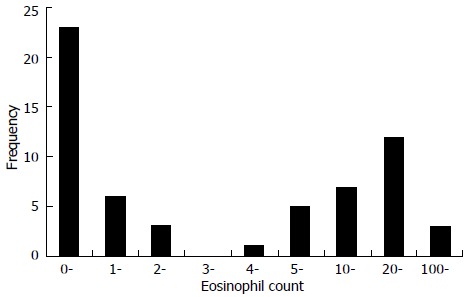
Frequency distribution and range of tissue eosinophil count per high power field for 60 patients with chronic rhinosinusitis with nasal polyps.
Tissue eosinophilic infiltration, based on which ECRS is determined, is commonly identified after surgery by histopathological examination. Therefore, this approach may be quite unpractical because it is difficult to obtain the diagnostic information before surgery or from the patients treated only with medicines. While peripheral blood eosinophilia has a certain diagnostic value for ECRS[15], because the close correlation between the number of peripheral blood and tissue-infiltrated eosinophils has been shown in several studies[9-11,15,17,18,27,42]. It is easy to understand the close association of blood eosinophils with ECRS because the tissue-infiltrated eosinophils are recruited via bloodstream to disease sites of ECRS. Many studies have shown that ECRSwNP presents with a significant increase in the peripheral blood eosinophil count or percentage compared to NECRSwNP[9,11,13,52]. Our recent study also showed the existence of a close correlation between tissue eosinophil count and blood eosinophil count or percentage in ECRSwNP patients, but not in NECRSwNP patients. Thus, the increased peripheral blood eosinophil count or percentage is considered a good marker or predictor of ECRSwNP[9,11,13,52]. Some studies show that blood eosinophil count or percentage in ECRS subtype is significantly higher than that in NECRS subtype[9,11,13,52]. It is found by receiver operating characteristic curve analysis that blood eosinophil count or percentage has high sensitivity and specificity for the diagnosis of ECRS[9,11,13,52] (Table 3).
Table 3.
Diagnostic sensitivity and specificity of blood eosinophil count or percentage for eosinophilic chronic rhinosinusitis
| Ref. |
Blood eosinophil count |
Blood eosinophil percentage |
||||||
| AUC | Cutoff value | Sensitivity | Specificity | AUC | Cutoff value | Sensitivity | Specificity | |
| Zuo et al[52] | 0.873 | 0.16 × 109/L | 84.9% | 84.4% | 0.863 | 2.05% | 89.0% | 84.4% |
| Wang et al[9] | - | - | - | - | 0.818 | 5.65% | 79.0% | 78.2% |
| Hu et al[11] | 0.871 | 0.22 × 109/L | 74.2% | 86.5% | 0.864 | 3.05% | 80.3% | 75.3% |
| Sakuma et al[13] | - | - | - | - | 0.880 | 6.00% | 97.4% | 70.7% |
ECRS: Eosinophilic chronic rhinosinusitis; AUC: Area under receiver operating characteristic curve.
Our recent study also showed that there was a statistically significant difference in mean blood eosinophil count or percentage between ECRSwNP and NECRSwNP patients (Table 1). However, it was notable that neither all patients with ECRSwNP had the increased circulating eosinophils nor all patients with NECRSwNP showed a normal level of blood eosinophil count. For example, only 10 of 27 patients with ECRSwNP showed blood eosinophil counts more than normal range and 2 of 33 patients with NECRSwNP had the increase of eosinophil count. Therefore, ECRS or NRECR can not be determined only based on if blood eosinophils increase.
The definition or diagnostic criterion for ECRS is very important since ECRS differs from NECRS in treatment strategy. However, there is not yet a clear definition or diagnostic criterion to differentiate ECRS and NECRS subtypes. Recently, new diagnostic criteria for ECRS have been proposed[13], in which the diagnosis of ECRS is finally determined by the clinical symptoms, nasal endoscopy, sinonasal CT imaging, peripheral blood test, and histological examination[13,15].
TREATMENT AND PROGNOSIS FOR ECRS
ESS has been used widely for the treatment of CRS. Outstanding short- and long-term results of ESS in CRS have previously been reported in the literature[41,68,72-75]. The impact of ESS on the improvement in CRS-related symptoms postoperatively is remarkable. However, some of CRS patients are inadequately controlled despite receiving combination of maximal medical therapy and ESS[1]. A wide variety of factors contribute to poor disease control, including patient-related factors such as ECRS[76]. It is believed that NECRS can be relatively well controlled with a combination of ESS and macrolide therapy, whereas ECRS is unresponsive to macrolide therapy[13]. Many studies indicate that ECRS commonly has poorer treatment outcomes compared to NECR[14,19,28,30,76,77]. For example, ECRSwNP is refractory to the combined treatment of ESS and macrolide therapy and shows a strong tendency for recurrence after surgery[13-15,27].
However, a recent study suggests that eosinophilic inflammation in CRS may not be related to the surgical outcome in South Koreans[22]. Another study also shows that the presence or absence of tissue eosinophilic infiltration does not impact significantly on the time interval to revision surgery[78]. Our recent study showed that in terms of the short-term efficacy of ESS in CRSwNP, both ECRSwNP and NECRSwNP patients had significant improvement in symptoms aside from smell dysfunction at one-week follow-up after ESS, but there was no significant difference in symptom improvement between the two subgroups.
CONCLUSION
In conclusion, CRS can be subclassified into two subtypes: ECRS and NECRS. The prevalence of ECRS is increasing in East Asians in the recent years. ECRS differs from NECRS in clinical features and treatment outcomes; however, there is not yet a universally accepted definition or diagnostic criterion for ECRS, and also the underlying pathogenic mechanisms of ECRS are not well-understood. Identification of ECRS subtypes and underlying pathogenic mechanisms is key to developing treatment strategies for the phenotypes of CRS.
Footnotes
P- Reviewer: Di Lorenzo G, (William) Wan YL, Xavier-Elsas P S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Wu HL
References
- 1.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;(23):3 p preceding table of contents, 1–298. [PubMed] [Google Scholar]
- 2.Kim YS, Kim NH, Seong SY, Kim KR, Lee GB, Kim KS. Prevalence and risk factors of chronic rhinosinusitis in Korea. Am J Rhinol Allergy. 2011;25:117–121. doi: 10.2500/ajra.2011.25.3630. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharyya N, Orlandi RR, Grebner J, Martinson M. Cost burden of chronic rhinosinusitis: a claims-based study. Otolaryngol Head Neck Surg. 2011;144:440–445. doi: 10.1177/0194599810391852. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld RM, Andes D, Bhattacharyya N, Cheung D, Eisenberg S, Ganiats TG, Gelzer A, Hamilos D, Haydon RC, Hudgins PA, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137:S1–S31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 5.Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery, Subspecialty Group of Rhinology, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Guidelines for diagnosis and treatment of chronic rhinosinusitis (2012, Kunming) Zhonghua Erbiyan Houtoujing Waike Zazhi. 2013;48:92–94. [PubMed] [Google Scholar]
- 6.Cao PP, Zhang YN, Liao B, Ma J, Wang BF, Wang H, Zeng M, Liu WH, Schleimer RP, Liu Z. Increased local IgE production induced by common aeroallergens and phenotypic alteration of mast cells in Chinese eosinophilic, but not non-eosinophilic, chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2014;44:690–700. doi: 10.1111/cea.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czerny MS, Namin A, Gratton MA, Antisdel JL. Histopathological and clinical analysis of chronic rhinosinusitis by subtype. Int Forum Allergy Rhinol. 2014;4:463–469. doi: 10.1002/alr.21304. [DOI] [PubMed] [Google Scholar]
- 8.Snidvongs K, Chin D, Sacks R, Earls P, Harvey RJ. Eosinophilic rhinosinusitis is not a disease of ostiomeatal occlusion. Laryngoscope. 2013;123:1070–1074. doi: 10.1002/lary.23721. [DOI] [PubMed] [Google Scholar]
- 9.Wang MJ, Zhou B, Li YC, Huang Q. The role of peripheral blood eosinophil percentage in classification of chronic rhinosinusitis with nasal polyps. Zhonghua Erbiyan Houtoujing Waike Zazhi. 2013;48:650–653. [PubMed] [Google Scholar]
- 10.Ouyang Y, Fan E, Li Y, Wang X, Zhang L. Clinical characteristics and expression of thymic stromal lymphopoetin in eosinophilic and non-eosinophilic chronic rhinosinusitis. ORL J Otorhinolaryngol Relat Spec. 2013;75:37–45. doi: 10.1159/000346929. [DOI] [PubMed] [Google Scholar]
- 11.Hu Y, Cao PP, Liang GT, Cui YH, Liu Z. Diagnostic significance of blood eosinophil count in eosinophilic chronic rhinosinusitis with nasal polyps in Chinese adults. Laryngoscope. 2012;122:498–503. doi: 10.1002/lary.22507. [DOI] [PubMed] [Google Scholar]
- 12.Jeong WJ, Lee CH, Cho SH, Rhee CS. Eosinophilic allergic polyp: a clinically oriented concept of nasal polyp. Otolaryngol Head Neck Surg. 2011;144:241–246. doi: 10.1177/0194599810391738. [DOI] [PubMed] [Google Scholar]
- 13.Sakuma Y, Ishitoya J, Komatsu M, Shiono O, Hirama M, Yamashita Y, Kaneko T, Morita S, Tsukuda M. New clinical diagnostic criteria for eosinophilic chronic rhinosinusitis. Auris Nasus Larynx. 2011;38:583–588. doi: 10.1016/j.anl.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Takeno S, Hirakawa K, Ishino T. Pathological mechanisms and clinical features of eosinophilic chronic rhinosinusitis in the Japanese population. Allergol Int. 2010;59:247–256. doi: 10.2332/allergolint.10-RAI-0202. [DOI] [PubMed] [Google Scholar]
- 15.Ishitoya J, Sakuma Y, Tsukuda M. Eosinophilic chronic rhinosinusitis in Japan. Allergol Int. 2010;59:239–245. doi: 10.2332/allergolint.10-RAI-0231. [DOI] [PubMed] [Google Scholar]
- 16.Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH, Wang DY, Desrosiers M, Liu Z. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009;124:478–484, 484.e1-e2. doi: 10.1016/j.jaci.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Kim JW, Hong SL, Kim YK, Lee CH, Min YG, Rhee CS. Histological and immunological features of non-eosinophilic nasal polyps. Otolaryngol Head Neck Surg. 2007;137:925–930. doi: 10.1016/j.otohns.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 18.Kountakis SE, Arango P, Bradley D, Wade ZK, Borish L. Molecular and cellular staging for the severity of chronic rhinosinusitis. Laryngoscope. 2004;114:1895–1905. doi: 10.1097/01.mlg.0000147917.43615.c0. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson BJ. Categorization of eosinophilic chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2004;12:237–242. doi: 10.1097/01.moo.0000124938.46948.c7. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimura K, Kawata R, Haruna S, Moriyama H, Hirakawa K, Fujieda S, Masuyama K, Takenaka H. Clinical epidemiological study of 553 patients with chronic rhinosinusitis in Japan. Allergol Int. 2011;60:491–496. doi: 10.2332/allergolint.10-OA-0234. [DOI] [PubMed] [Google Scholar]
- 21.Kim SJ, Lee KH, Kim SW, Cho JS, Park YK, Shin SY. Changes in histological features of nasal polyps in a Korean population over a 17-year period. Otolaryngol Head Neck Surg. 2013;149:431–437. doi: 10.1177/0194599813495363. [DOI] [PubMed] [Google Scholar]
- 22.Kim SY, Park JH, Rhee CS, Chung JH, Kim JW. Does eosinophilic inflammation affect the outcome of endoscopic sinus surgery in chronic rhinosinusitis in Koreans? Am J Rhinol Allergy. 2013;27:e166–e169. doi: 10.2500/ajra.2013.27.3959. [DOI] [PubMed] [Google Scholar]
- 23.Zhang N, Holtappels G, Claeys C, Huang G, van Cauwenberge P, Bachert C. Pattern of inflammation and impact of Staphylococcus aureus enterotoxins in nasal polyps from southern China. Am J Rhinol. 2013;20:445–450. doi: 10.2500/ajr.2006.20.2887. [DOI] [PubMed] [Google Scholar]
- 24.Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, Van Cauwenberge P, Bachert C. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961–968. doi: 10.1016/j.jaci.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Shin SH, Ye MK, Kim JK, Cho CH. Histological characteristics of chronic rhinosinusitis with nasal polyps: Recent 10-year experience of a single center in Daegu, Korea. Am J Rhinol Allergy. 2014;28:95–98. doi: 10.2500/ajra.2014.28.4003. [DOI] [PubMed] [Google Scholar]
- 26.Wei Y, Xia W, Ye X, Fan Y, Shi J, Wen W, Yang P, Li H. The antimicrobial protein short palate, lung, and nasal epithelium clone 1 (SPLUNC1) is differentially modulated in eosinophilic and noneosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2014;133:420–428. doi: 10.1016/j.jaci.2013.09.052. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda K, Shiozawa A, Ono N, Kusunoki T, Hirotsu M, Homma H, Saitoh T, Murata J. Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope. 2013;123:E1–E9. doi: 10.1002/lary.24154. [DOI] [PubMed] [Google Scholar]
- 28.Haruna S, Shimada C, Ozawa M, Fukami S, Moriyama H. A study of poor responders for long-term, low-dose macrolide administration for chronic sinusitis. Rhinology. 2009;47:66–71. [PubMed] [Google Scholar]
- 29.Soler ZM, Sauer DA, Mace J, Smith TL. Relationship between clinical measures and histopathologic findings in chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2009;141:454–461. doi: 10.1016/j.otohns.2009.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soler ZM, Sauer D, Mace J, Smith TL. Impact of mucosal eosinophilia and nasal polyposis on quality-of-life outcomes after sinus surgery. Otolaryngol Head Neck Surg. 2010;142:64–71. doi: 10.1016/j.otohns.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan BK, Chandra RK, Pollak J, Kato A, Conley DB, Peters AT, Grammer LC, Avila PC, Kern RC, Stewart WF, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;131:1350–1360. doi: 10.1016/j.jaci.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Crombruggen K, Zhang N, Gevaert P, Tomassen P, Bachert C. Pathogenesis of chronic rhinosinusitis: inflammation. J Allergy Clin Immunol. 2011;128:728–732. doi: 10.1016/j.jaci.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen LH, Fakhri S, Frenkiel S, Hamid QA. Molecular immunology and immunotherapy for chronic sinusitis. Curr Allergy Asthma Rep. 2003;3:505–512. doi: 10.1007/s11882-003-0062-1. [DOI] [PubMed] [Google Scholar]
- 34.Walsh ER, August A. Eosinophils and allergic airway disease: there is more to the story. Trends Immunol. 2010;31:39–44. doi: 10.1016/j.it.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akuthota P, Wang HB, Spencer LA, Weller PF. Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin Exp Allergy. 2008;38:1254–1263. doi: 10.1111/j.1365-2222.2008.03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 37.Van Bruaene N, Pérez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, Schmidt-Weber C, Akdis C, Van Cauwenberge P, Bachert C, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008;121:1435–1441, 1441e1-3. doi: 10.1016/j.jaci.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Bachert C, Gevaert P, Holtappels G, Johansson SG, van Cauwenberge P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol. 2001;107:607–614. doi: 10.1067/mai.2001.112374. [DOI] [PubMed] [Google Scholar]
- 39.Sejima T, Holtappels G, Kikuchi H, Imayoshi S, Ichimura K, Bachert C. Cytokine profiles in Japanese patients with chronic rhinosinusitis. Allergol Int. 2012;61:115–122. doi: 10.2332/allergolint.10-OA-0290. [DOI] [PubMed] [Google Scholar]
- 40.Zimmermann N, Hershey GK, Foster PS, Rothenberg ME. Chemokines in asthma: cooperative interaction between chemokines and IL-13. J Allergy Clin Immunol. 2003;111:227–242; quiz 243. doi: 10.1067/mai.2003.139. [DOI] [PubMed] [Google Scholar]
- 41.Abdalla S, Alreefy H, Hopkins C. Prevalence of sinonasal outcome test (SNOT-22) symptoms in patients undergoing surgery for chronic rhinosinusitis in the England and Wales National prospective audit. Clin Otolaryngol. 2012;37:276–282. doi: 10.1111/j.1749-4486.2012.02527.x. [DOI] [PubMed] [Google Scholar]
- 42.Mori E, Matsuwaki Y, Mitsuyama C, Okushi T, Nakajima T, Moriyama H. Risk factors for olfactory dysfunction in chronic rhinosinusitis. Auris Nasus Larynx. 2013;40:465–469. doi: 10.1016/j.anl.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Baudoin T, Cupić H, Geber G, Vagić D, Grgić M, Kalogjera L. Histopathologic parameters as predictors of response to endoscopic sinus surgery in nonallergic patients with chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2006;134:761–766. doi: 10.1016/j.otohns.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 44.Zhang XH, Lu X, Long XB, You XJ, Gao QX, Cui YH, Liu Z. Chronic rhinosinusitis with and without nasal polyps is associated with decreased expression of glucocorticoid-induced leucine zipper. Clin Exp Allergy. 2009;39:647–654. doi: 10.1111/j.1365-2222.2008.03198.x. [DOI] [PubMed] [Google Scholar]
- 45.Konstantinidis I, Triaridis S, Printza A, Vital V, Ferekidis E, Constantinidis J. Olfactory dysfunction in nasal polyposis: correlation with computed tomography findings. ORL J Otorhinolaryngol Relat Spec. 2007;69:226–232. doi: 10.1159/000101543. [DOI] [PubMed] [Google Scholar]
- 46.Raviv JR, Kern RC. Chronic rhinosinusitis and olfactory dysfunction. Adv Otorhinolaryngol. 2006;63:108–124. doi: 10.1159/000093757. [DOI] [PubMed] [Google Scholar]
- 47.Landis BN, Konnerth CG, Hummel T. A study on the frequency of olfactory dysfunction. Laryngoscope. 2004;114:1764–1769. doi: 10.1097/00005537-200410000-00017. [DOI] [PubMed] [Google Scholar]
- 48.Temmel AF, Quint C, Schickinger-Fischer B, Klimek L, Stoller E, Hummel T. Characteristics of olfactory disorders in relation to major causes of olfactory loss. Arch Otolaryngol Head Neck Surg. 2002;128:635–641. doi: 10.1001/archotol.128.6.635. [DOI] [PubMed] [Google Scholar]
- 49.Litvack JR, Fong K, Mace J, James KE, Smith TL. Predictors of olfactory dysfunction in patients with chronic rhinosinusitis. Laryngoscope. 2008;118:2225–2230. doi: 10.1097/MLG.0b013e318184e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haruna S, Otori N, Moriyama H, Nakanishi M. Olfactory dysfunction in sinusitis with infiltration of numerous activated eosinophils. Auris Nasus Larynx. 2006;33:23–30. doi: 10.1016/j.anl.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Chang H, Lee HJ, Mo JH, Lee CH, Kim JW. Clinical implication of the olfactory cleft in patients with chronic rhinosinusitis and olfactory loss. Arch Otolaryngol Head Neck Surg. 2009;135:988–992. doi: 10.1001/archoto.2009.140. [DOI] [PubMed] [Google Scholar]
- 52.Zuo K, Guo J, Chen F, Xu R, Xu G, Shi J, Li H. Clinical characteristics and surrogate markers of eosinophilic chronic rhinosinusitis in Southern China. Eur Arch Otorhinolaryngol. 2014;271:2461–2468. doi: 10.1007/s00405-014-2910-0. [DOI] [PubMed] [Google Scholar]
- 53.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31:183–184. [PubMed] [Google Scholar]
- 54.Okushi T, Nakayama T, Morimoto S, Arai C, Omura K, Asaka D, Matsuwaki Y, Yoshikawa M, Moriyama H, Otori N. A modified Lund-Mackay system for radiological evaluation of chronic rhinosinusitis. Auris Nasus Larynx. 2013;40:548–553. doi: 10.1016/j.anl.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 55.Szucs E, Ravandi S, Goossens A, Beel M, Clement PA. Eosinophilia in the ethmoid mucosa and its relationship to the severity of inflammation in chronic rhinosinusitis. Am J Rhinol. 2002;16:131–134. [PubMed] [Google Scholar]
- 56.Leung RM, Kern RC, Conley DB, Tan BK, Chandra RK. Osteomeatal complex obstruction is not associated with adjacent sinus disease in chronic rhinosinusitis with polyps. Am J Rhinol Allergy. 2011;25:401–403. doi: 10.2500/ajra.2011.25.3672. [DOI] [PubMed] [Google Scholar]
- 57.Krouse JH. The unified airway--conceptual framework. Otolaryngol Clin North Am. 2008;41:257–266, v. doi: 10.1016/j.otc.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Bachert C, Vignola AM, Gevaert P, Leynaert B, Van Cauwenberge P, Bousquet J. Allergic rhinitis, rhinosinusitis, and asthma: one airway disease. Immunol Allergy Clin North Am. 2004;24:19–43. doi: 10.1016/S0889-8561(03)00104-8. [DOI] [PubMed] [Google Scholar]
- 59.Gutman M, Torres A, Keen KJ, Houser SM. Prevalence of allergy in patients with chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2004;130:545–552. doi: 10.1016/j.otohns.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 60.Emanuel IA, Shah SB. Chronic rhinosinusitis: allergy and sinus computed tomography relationships. Otolaryngol Head Neck Surg. 2000;123:687–691. doi: 10.1067/mhn.2000.110961. [DOI] [PubMed] [Google Scholar]
- 61.Ponikau JU, Sherris DA, Kephart GM, Kern EB, Gaffey TA, Tarara JE, Kita H. Features of airway remodeling and eosinophilic inflammation in chronic rhinosinusitis: is the histopathology similar to asthma? J Allergy Clin Immunol. 2003;112:877–882. doi: 10.1016/j.jaci.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 62.Seybt MW, McMains KC, Kountakis SE. The prevalence and effect of asthma on adults with chronic rhinosinusitis. Ear Nose Throat J. 2007;86:409–411. [PubMed] [Google Scholar]
- 63.Bousquet J, Vignola AM, Demoly P. Links between rhinitis and asthma. Allergy. 2003;58:691–706. doi: 10.1034/j.1398-9995.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 64.Fan Y, Chen S, Qu X, Zuo K, Li X, Huang J, Xu G, Mi J, Li H. A lower prevalence of asthma among patients with chronic rhinosinusitis in southern China. J Allergy Clin Immunol. 2011;127:520–522.e1-5. doi: 10.1016/j.jaci.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 65.Shi J, Fan Y, Xu R, Zuo K, Cheng L, Xu G, Li H. Characterizing T-cell phenotypes in nasal polyposis in Chinese patients. J Investig Allergol Clin Immunol. 2009;19:276–282. [PubMed] [Google Scholar]
- 66.Hao J, Pang YT, Wang DY. Diffuse mucosal inflammation in nasal polyps and adjacent middle turbinate. Otolaryngol Head Neck Surg. 2006;134:267–275. doi: 10.1016/j.otohns.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 67.Rinia AB, Kostamo K, Ebbens FA, van Drunen CM, Fokkens WJ. Nasal polyposis: a cellular-based approach to answering questions. Allergy. 2007;62:348–358. doi: 10.1111/j.1398-9995.2007.01323.x. [DOI] [PubMed] [Google Scholar]
- 68.Hopkins C, Browne JP, Slack R, Lund V, Topham J, Reeves B, Copley L, Brown P, van der Meulen J. The national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Clin Otolaryngol. 2006;31:390–398. doi: 10.1111/j.1749-4486.2006.01275.x. [DOI] [PubMed] [Google Scholar]
- 69.Fountain CR, Mudd PA, Ramakrishnan VR, Sillau SH, Kingdom TT, Katial RK. Characterization and treatment of patients with chronic rhinosinusitis and nasal polyps. Ann Allergy Asthma Immunol. 2013;111:337–341. doi: 10.1016/j.anai.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 70.Bachert C, Patou J, Van Cauwenberge P. The role of sinus disease in asthma. Curr Opin Allergy Clin Immunol. 2006;6:29–36. doi: 10.1097/01.all.0000200504.54425.0e. [DOI] [PubMed] [Google Scholar]
- 71.Zhang XH, Zhang YN, Li HB, Hu CY, Wang N, Cao PP, Liao B, Lu X, Cui YH, Liu Z. Overexpression of miR-125b, a novel regulator of innate immunity, in eosinophilic chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2012;185:140–151. doi: 10.1164/rccm.201103-0456OC. [DOI] [PubMed] [Google Scholar]
- 72.Bhattacharyya N. Symptom outcomes after endoscopic sinus surgery for chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg. 2004;130:329–333. doi: 10.1001/archotol.130.3.329. [DOI] [PubMed] [Google Scholar]
- 73.Damm M, Quante G, Jungehuelsing M, Stennert E. Impact of functional endoscopic sinus surgery on symptoms and quality of life in chronic rhinosinusitis. Laryngoscope. 2002;112:310–315. doi: 10.1097/00005537-200202000-00020. [DOI] [PubMed] [Google Scholar]
- 74.Deal RT, Kountakis SE. Significance of nasal polyps in chronic rhinosinusitis: symptoms and surgical outcomes. Laryngoscope. 2004;114:1932–1935. doi: 10.1097/01.mlg.0000147922.12228.1f. [DOI] [PubMed] [Google Scholar]
- 75.Rudmik L, Mace J, Soler ZM, Smith TL. Long-term utility outcomes in patients undergoing endoscopic sinus surgery. Laryngoscope. 2014;124:19–23. doi: 10.1002/lary.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Snidvongs K, Lam M, Sacks R, Earls P, Kalish L, Phillips PS, Pratt E, Harvey RJ. Structured histopathology profiling of chronic rhinosinusitis in routine practice. Int Forum Allergy Rhinol. 2012;2:376–385. doi: 10.1002/alr.21032. [DOI] [PubMed] [Google Scholar]
- 77.Scadding GK. Medical management of chronic rhinosinusitis. Immunol Allergy Clin North Am. 2004;24:103–118. doi: 10.1016/S0889-8561(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 78.Wu AW, Ting JY, Platt MP, Tierney HT, Metson R. Factors affecting time to revision sinus surgery for nasal polyps: a 25-year experience. Laryngoscope. 2014;124:29–33. doi: 10.1002/lary.24213. [DOI] [PubMed] [Google Scholar]


