Abstract
Twins are two independent babies delivered during the same pregnancy and are divided as monozygotic or dizygotic based on their origin. Dizygotic twins are similar to two siblings and have different genetic information. In contrary, monozygotic twins have a similar genetic identity and provide a unique opportunity to evaluate the contribution of genetic and environmental factors of the disease. The endocrine and metabolic disorders affect a large number of the population including the twins. Diabetes, obesity, and autoimmune thyroid disease are the most common endocrine disorders in general practice. It is essential to understand the genetic basis of endocrine disorders for therapy, prognostication and risk assessment for future generations. In this article, we review the endocrine disorders in relation to their occurrence in monozygotic twins to highlight the genetic and environmental contribution.
Keywords: Diabetes, endocrinology, monozygotic twins, thyroid disorders, twins
INTRODUCTION
Twins or multiple pregnancies were an enigma during the last century prior to the understanding of the genetics. Advances in the reproductive physiology lead us to fathom the basis behind the twin pregnancy.[1] Dizygotic twins are formed when two independent eggs are fertilized by different sperm cells and are implanted in the uterus simultaneously. They can be male-male, female-female or male-female twins and genetically they are two siblings born simultaneously. Monozygotic twins occur when the zygote splits and results in two embryos. They can be either two similar looking males or females and are genetically identical. The twin birth rates are increasing worldwide due to increased exposure to the ovulation inducing agents, phytoestrogens and endocrine disruptors.[2] India has a twin pregnancy rate of 10–15/1000 live births. High twin rates are observed in the African countries where the rates are reported in excess of 20/1000 births.[3,4]
Twin studies help to determine the contribution of a particular genetic or environmental trait on the phenotype of the disease. The developed nations have established twin registries enabling the researchers for a long follow-up studies.[5] These studies compare the concordance and discordance of a disease in the twins in an attempt to isolate the influence of the genetic and environmental factors. Monozygotic twins reared apart in different circumstances offer an excellent opportunity for exploring the disease pathogenesis.[6] Diabetes, obesity and autoimmune thyroid diseases are the most common endocrine disorders in the population. These disorders affect about 10–20% of the population, including the twins.[7] In this review, we present the physiology of twin pregnancy, followed by the relevant literature pertaining to the endocrine disorders.
BASIC PHYSIOLOGY OF TWINS
Zygosity determines the genetic makeup of the twins and is the cornerstone for understanding all the twin studies. Monozygotic twins have similar genetic makeup, whereas dizygotic twins have only 50% similarities. Twin analysis is based on the concordance of the disease among monozygotic versus dizygotic twins. The concordance rates help in deducing the penetrance and “heritability” of the disease, which explains the phenotypic variation in the population.[8] Any discordance is taken as the effect of reduced penetrance and could be due to multiple effects like altered immune repertoire, effects of epigenetic and environmental factors. The immune repertoire is different between the monozygotic twins due to the varying somatic recombination of T- and B-cells and also the individual immune exposures.[9] The concordance rates are expressed as pairwise and proband wise in general. Pair-wise concordance indicates the proportion of affected pairs concordant for the disease and proband wise estimates the risk of the disease in the other twin if one has developed the disease.
Familial clustering of the diseases is due to the genetic and non-genetic factors. The non-genetic factors that contribute to familial clustering are the sharing of environmental exposure through food, air and household items.[10] Sibling risk ratio helps in identifying the genetic susceptibility and non-genetic factors in familial clustering of the diseases. A ratio of more than 5 indicates significant genetic influence on the disease causation.[11] The interpretation of twin data is related to certain limitations as explained below. First, monozygotic twin pairs may inherit the same DNA at conception but may develop in genetically non-identical pairs due to somatic mutations and gene rearrangements. Second, the gene-environment interaction leads to modification in the phenotypic expression and plays a substantial role in twins brought up at different locations.[12] Twin samples recruited through advertising or hospital clinics have the potential for ascertainment bias due to disproportionate sampling of the diseased twins. Consequently, the reported concordance rates may have been overestimated owing to selection bias. Hence, the ideal method for sampling twins is by means of population-based twin registries.
Twins and diabetes
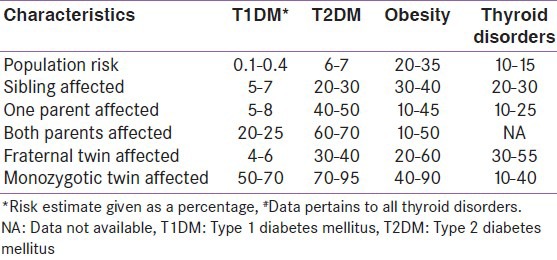
Diabetes is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. The disease spectrum is heterogeneous, and the patients are divided broadly into type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM). Previous reports suggest that the subdivision is rather simplistic one and many patients have overlapping features of both types.[13] Genetic and environmental factors play an important role in the etiopathogenesis of T1DM and T2DM, respectively. Our knowledge about the vice versa is limited due to a concept known as missing heritability. The missing heritability exceeds 80% for T2DM and 20% for T1DM.[14] The risk of T1DM is increased in relatives of patients as shown in Tables 1 and 2. The lifetime risk is 50% for T1DM, and the risk is more within 10 years of diagnosis of the first twin.[15] The lifetime risk of developing T2DM is 40% for individuals who have one parent with T2DM and almost 70% if both parents are affected. The concordance rate of T2DM in monozygotic twins is about 70%, while the concordance in dizygotic twins is only 20–30%. Proband wise concordance rates for T1DM among monozygotic twin were 61% and 12% among dizygotic twins.[16] T2DM had a monozygotic proband wise concordance of 58% and a dizygotic concordance of 11%.
Table 1.
Twin concordance rates for the endocrine disorders
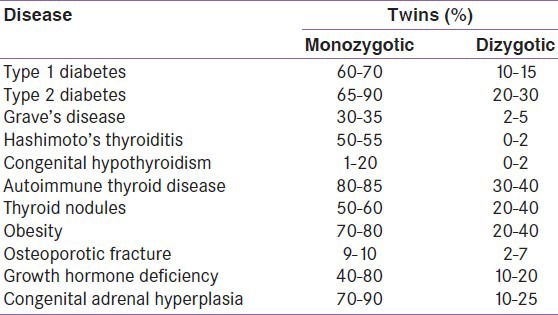
Table 2.
Risk estimates of the common endocrine disorders
Type 1 diabetes mellitus results from a T lymphocyte-dependent, selective destruction of the insulin-producing pancreatic beta-cells. The T-cell receptor (TCR), is mainly involved in the recognition of the peptide-major histocompatibility complex, and twin sets discordant for T1DM represent the ideal scenario to explore the factors involved in the TCR repertoire and the risk of T1DM.[17] The large-scale twin studies have studied the concordance rates of twins with T1DM and T2DM.[18,19] The wide variation in the concordance is explained by the variable heritability of the metabolic components and the influence of the non-genetic factors. The twin study data also showed that the largest discordance time between diagnosis of monozygotic pairs, was 6.9 years, compared with 23.6 years among dizygotic pairs.[20] The risk of developing T1DM is influenced by the age of the onset in the first twin, and the concordance was increased in twins who were heterozygous for human leukocyte antigen DR3-DQ2 and DR4-DQ8.[21] These data suggest that genetics plays an important role in the development, severity and progression of T1DM. Sex differences have also been observed in twin studies on diabetes mellitus.[22] Females showed higher proband and pairwise concordance rates when compared to males in the twin registry studies. The influence of the sex steroids on the DNA methylation and the epigenetic alterations explain the sex differences.[23]
Twins and obesity
Obesity is a multifactorial disorder leading to significant morbidity and mortality. The studies establishing the etiopathogenesis of obesity are complicated by the influence of the genetic factors of the disease.[24] Body mass index (BMI) is a simple measure of obesity derived from the height and weight of an individual. The BMI is the basis of classifying individuals into different weight categories such as normal, overweight and obesity. Previous studies have shown that BMI has a heritability estimate between 50% and 90%. The heritability factor of other body composition variables like total body fat mass and lean mass are less studied.[25] Monozygotic twin studies, especially from the Finnish twin cohorts have enabled the fine characterization of the genetic basis and acquired factors in twins discordant for the obesity. The factors leading to the development of obesity are higher birth weight, presence of variability genes involving Ghrelin, overexpression of the 11-beta-hydroxysteroid dehydrogenase gene, physical inactivity in adolescence and higher insulin resistance.[26]
Mitochondrial dysfunction also plays a significant role towards the discordance of obesity in monozygotic twins.[27] The adipose tissue of obese co-twin shows reduction of mitochondrial DNA and down-regulation of the genes involved in the oxidative phosphorylation pathway. There is also a reduction in the mitochondrial metabolism of branched chain amino acids leading to increasing in the visceral and hepatic fat content. The enlarged adipocyte releases inflammatory mediators and cytokines, leading to low-grade inflammation, and this propagates further insulin resistance. Previous studies also suggest that the monozygotic twins with restrained eating have higher ghrelin levels than the twins with unrestrained eating.[28] These findings support the concept of cognitive control in relation to the physiological stimulation.
Twins and thyroid disorders
Autoimmune thyroid disorders (AITD) are seen in approximately 2–4% of the population.[29] The spectrum of AITD extends from clinically silent presence of autoantibodies to overt thyroid dysfunction. Thyroid dysfunction ranges from the hypothyroidism (Hashimoto's thyroiditis) to hyperthyroidism (Graves’ disease). AITD is developed due to a combination of genetic susceptibility and environmental encounters leading to the breakdown of immunological self-tolerance.[30] The studies comparing the concordance rates between monozygotic and dizygotic twins shows that approximately 75% of the total phenotypic variance in AITD is because of the genetic effects. In contrary, the role of environmental and epigenetic factors is evident by the lack of complete concordance in monozygotic twin pairs.[31] The environmental factors implicated as the triggers for AITD are cigarette smoking, Yersinia infection, microchimerism and degree of X chromosome inactivation. The studies of AITD in discordant twins suggest that few environmental factors like smoking and infection may be causally associated with overt AITD.[32] The presence of thyroid autoantibodies in euthyroid subjects did not show any heritability in the twin pairs.
The concordance rate for Graves’ disease in monozygotic twins was found to be approximately 30–35% and about 2–5% of dizygotic twins.[33] The concordance rate for the Hashimoto's disease is 55% and 0% respectively for monozygotic and dizygotic twins. The same rates for the thyroid antibodies include 80% and 40% respectively. The sexual differences in the concordance manifest more in the thyroid disorders and studies suggest females have a higher concordance than males.[34] Few studies suggest a link between the X-chromosome inactivation and the presence of the AITD in twin studies.[35] These studies demonstrate that a mixture of genetic, epigenetic, and environmental factors are involved in the pathogenesis of the autoimmune thyroid disease.
Twins and other endocrine disorders
Twin studies pertaining to other endocrine disorders are limited by the lack of numbers affected by these diseases. Cortisol is an essential hormone for maintenance of the normal physical and mental health. The cortisol secretion has a characteristic diurnal rhythm, and the secretory rates have shown a heritability varying between 50% and 60% in twin studies.[36] The discordant presentation of adrenogonadal disorders like cryptorchidism and congenital adrenal hyperplasia have been described in the twins earlier.[37,38] The literature is conflicting regarding the prevalence of testicular germ cell tumors (TGCT) in twin pregnancies. Few reports suggest that dizygotic twins have a higher risk of TGCT when compared with monozygotic twins and recent reports suggest otherwise.[39,40] The increased prevalence in dizygotic twins is explained by the higher concentration of estrogen levels in the dizygotic pregnancies. The discordance of the hypospadias observed in monozygotic twins is explained by the low birth weight of the affected twins.[39] Placental inadequacy leads to the scanty provision of the nutrients and gonadotrophic hormones, and the effect is more apparent in the small for gestational age baby with hypersensitive gonads.
The discordant presentation of pituitary disorders such as Cushing's disease and growth hormone deficiency have also been reported as isolated case reports.[41,42] These reports do not give sufficient information to derive conclusions regarding the genetic basis of the disease. Bone mineral density (BMD) is influenced by the interactions of the genetic, metabolic, environmental and nutritional factors.[43] Osteoprotegerin and receptor activator of nuclear factor kappa-B ligand (RANKL) are important players in the determination of bone density. Previous reports suggest that the heritability was 82% for BMD and close to 50% for RANKL.[44] The levels of bone turnover markers also differ markedly between singleton and twin pregnancies. The high demand of calcium during twin pregnancy is met by the early, and high bone turnover observed in mothers with twin pregnancies.[45] We reported a case of discordant twins with severe achondroplasia, developmental delay and acanthosis nigricans syndrome.[46] The expression of multiple endocrine neoplasia (MEN) is also different between the monozygotic twins, lending support to the two mutation model of tumor formation.[47] MEN syndrome discordance suggests that the first mutation is inherited, but the tumor formation occurs only after a second mutation resulting in loss of the normal allele. The factors other than the heredity are responsible for the expression of the MEN-I syndromes, and similar phenomenon are described in pituitary adenomas also.[48]
CONCLUSION
Twin studies offer a unique opportunity to study the genetics and environmental interactions in disease etiopathogenesis. It is essential to have a national twin registry to capture the data and further study the discordant twin pairs in a large sample. Future twin studies should record the information on genetic, epigenetic and environmental variation including the birth details. This information enhances our ability further to quantify the precise contribution of genetic risk factors in endocrine and metabolic disorders.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
REFERENCES
- 1.Goslin D. The wonderful world of twin birth. Midwifery Today Int Midwife. 2014:18–22. [PubMed] [Google Scholar]
- 2.Lambalk CB, De Koning CH, Braat DD. The endocrinology of dizygotic twinning in the human. Mol Cell Endocrinol. 1998;145:97–102. doi: 10.1016/s0303-7207(98)00175-0. [DOI] [PubMed] [Google Scholar]
- 3.Satija M, Sharma S, Soni RK, Sachar RK, Singh GP. Twinning and its correlates: Community-based study in a rural area of India. Hum Biol. 2008;80:611–21. doi: 10.3378/1534-6617-80.6.611. [DOI] [PubMed] [Google Scholar]
- 4.Onah HE, Ugwu GO. Trends in the twinning rate in Enugu, Nigeria. J Obstet Gynaecol. 2008;28:590–2. doi: 10.1080/01443610802091404. [DOI] [PubMed] [Google Scholar]
- 5.Madsen M, Andersen PK, Gerster M, Andersen AM, Osler M, Christensen K. Are familial factors underlying the association between socioeconomic position and prescription medicine? A register-based study on Danish twins. BMJ Open. 2013;3:e003292. doi: 10.1136/bmjopen-2013-003292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackhurst G, McElroy KP, Kenyon CJ, Fraser R, Swan L, Anderson N, et al. Glucocorticoid receptor binding in twin pairs is affected by shared environment but not by shared genes. J Steroid Biochem Mol Biol. 2002;80:395–400. doi: 10.1016/s0960-0760(02)00034-1. [DOI] [PubMed] [Google Scholar]
- 7.Garg A, Anand T, Sharma U, Kishore J, Chakraborty M, Ray PC, et al. Prevalence of Risk Factors for Chronic Non-communicable Diseases Using WHO Steps Approach in an Adult Population in Delhi. J Family Med Prim Care. 2014;3:112–8. doi: 10.4103/2249-4863.137617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minihane AM. The genetic contribution to disease risk and variability in response to diet: Where is the hidden heritability? Proc Nutr Soc. 2013;72:40–7. doi: 10.1017/S0029665112002856. [DOI] [PubMed] [Google Scholar]
- 9.Bresson D, Fradkin M, Manenkova Y, Rottembourg D, von Herrath M. Genetic-induced variations in the GAD65 T-cell repertoire governs efficacy of anti-CD3/GAD65 combination therapy in new-onset type 1 diabetes. Mol Ther. 2010;18:307–16. doi: 10.1038/mt.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seabra AF, Mendonça DM, Göring HH, Thomis MA, Maia JA. Genetic and environmental factors in familial clustering in physical activity. Eur J Epidemiol. 2008;23:205–11. doi: 10.1007/s10654-008-9222-x. [DOI] [PubMed] [Google Scholar]
- 11.Queitsch C, Carlson KD, Girirajan S. Lessons from model organisms: Phenotypic robustness and missing heritability in complex disease. PLoS Genet. 2012;8:e1003041. doi: 10.1371/journal.pgen.1003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuk O, Hechter E, Sunyaev SR, Lander ES. The mystery of missing heritability: Genetic interactions create phantom heritability. Proc Natl Acad Sci U S A. 2012;109:1193–8. doi: 10.1073/pnas.1119675109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leiter EH, Lee CH. Mouse models and the genetics of diabetes: Is there evidence for genetic overlap between type 1 and type 2 diabetes? Diabetes. 2005;54(Suppl 2):S151–8. doi: 10.2337/diabetes.54.suppl_2.s151. [DOI] [PubMed] [Google Scholar]
- 14.Poulsen P, Andersen G, Fenger M, Hansen T, Echwald SM, Vølund A, et al. Impact of two common polymorphisms in the PPARgamma gene on glucose tolerance and plasma insulin profiles in monozygotic and dizygotic twins: Thrifty genotype, thrifty phenotype, or both? Diabetes. 2003;52:194–8. doi: 10.2337/diabetes.52.1.194. [DOI] [PubMed] [Google Scholar]
- 15.Vaag A, Brøns C, Gillberg L, Hansen NS, Hjort L, Arora GP, et al. Genetic, nongenetic and epigenetic risk determinants in developmental programming of type 2 diabetes. Acta Obstet Gynecol Scand. 2014;93:1099–108. doi: 10.1111/aogs.12494. [DOI] [PubMed] [Google Scholar]
- 16.Kaprio J, Tuomilehto J, Koskenvuo M, Romanov K, Reunanen A, Eriksson J, et al. Concordance for type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in a population-based cohort of twins in Finland. Diabetologia. 1992;35:1060–7. doi: 10.1007/BF02221682. [DOI] [PubMed] [Google Scholar]
- 17.Fozza C, Contini S, Corda G, Virdis P, Galleu A, Bonfigli S, et al. T-cell receptor repertoire analysis in monozygotic twins concordant and discordant for type 1 diabetes. Immunobiology. 2012;217:920–5. doi: 10.1016/j.imbio.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Hyttinen V, Kaprio J, Kinnunen L, Koskenvuo M, Tuomilehto J. Genetic liability of type 1 diabetes and the onset age among 22,650 young Finnish twin pairs: A nationwide follow-up study. Diabetes. 2003;52:1052–5. doi: 10.2337/diabetes.52.4.1052. [DOI] [PubMed] [Google Scholar]
- 19.Redondo MJ, Yu L, Hawa M, Mackenzie T, Pyke DA, Eisenbarth GS, et al. Heterogeneity of type I diabetes: Analysis of monozygotic twins in Great Britain and the United States. Diabetologia. 2001;44:354–62. doi: 10.1007/s001250051626. [DOI] [PubMed] [Google Scholar]
- 20.Vaag A, Henriksen JE, Madsbad S, Holm N, Beck-Nielsen H. Insulin secretion, insulin action, and hepatic glucose production in identical twins discordant for non-insulin-dependent diabetes mellitus. J Clin Invest. 1995;95:690–8. doi: 10.1172/JCI117715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blasetti A, Chiuri RM, Tocco AM, Di Sabatino G, Chiarelli F, Verrotti A. Simultaneous onset and similar course of type 1 diabetes mellitus in monozygotic twins (a 4-year follow-up) J Pediatr Endocrinol Metab. 2012;25:557–9. doi: 10.1515/jpem.2011.376. [DOI] [PubMed] [Google Scholar]
- 22.Poulsen P, Kyvik KO, Vaag A, Beck-Nielsen H. Heritability of type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerance - A population-based twin study. Diabetologia. 1999;42:139–45. doi: 10.1007/s001250051131. [DOI] [PubMed] [Google Scholar]
- 23.Korsoff P, Bogl LH, Korhonen P, Kangas AJ, Soininen P, Ala-Korpela M, et al. A comparison of anthropometric, metabolic, and reproductive characteristics of young adult women from opposite-sex and same-sex twin pairs. Front Endocrinol (Lausanne) 2014;5:28. doi: 10.3389/fendo.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrera BM, Lindgren CM. The genetics of obesity. Curr Diab Rep. 2010;10:498–505. doi: 10.1007/s11892-010-0153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poulsen P, Vaag A, Kyvik K, Beck-Nielsen H. Genetic versus environmental aetiology of the metabolic syndrome among male and female twins. Diabetologia. 2001;44:537–43. doi: 10.1007/s001250051659. [DOI] [PubMed] [Google Scholar]
- 26.Schousboe K, Visscher PM, Erbas B, Kyvik KO, Hopper JL, Henriksen JE, et al. Twin study of genetic and environmental influences on adult body size, shape, and composition. Int J Obes Relat Metab Disord. 2004;28:39–48. doi: 10.1038/sj.ijo.0802524. [DOI] [PubMed] [Google Scholar]
- 27.Bondia-Pons I, Maukonen J, Mattila I, Rissanen A, Saarela M, Kaprio J, et al. Metabolome and fecal microbiota in monozygotic twin pairs discordant for weight: A Big Mac challenge. FASEB J. 2014 doi: 10.1096/fj.14-250167. pii:fj14-250167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myhre R, Kratz M, Goldberg J, Polivy J, Melhorn S, Buchwald D, et al. A twin study of differences in the response of plasma ghrelin to a milkshake preload in restrained eaters. Physiol Behav. 2014;129:50–6. doi: 10.1016/j.physbeh.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar KV, Priya S, Sharma R, Kapoor U, Saini M, Bisht YS. Autoimmune thyroid disease in patients with vitiligo: Prevalence study in India. Endocr Pract. 2012;18:194–9. doi: 10.4158/EP11205.OR. [DOI] [PubMed] [Google Scholar]
- 30.Hansen PS, Brix TH, Sørensen TI, Kyvik KO, Hegedüs L. Major genetic influence on the regulation of the pituitary-thyroid axis: A study of healthy Danish twins. J Clin Endocrinol Metab. 2004;89:1181–7. doi: 10.1210/jc.2003-031641. [DOI] [PubMed] [Google Scholar]
- 31.Brix TH, Hansen PS, Kyvik KO, Hegedüs L. Cigarette smoking and risk of clinically overt thyroid disease: A population-based twin case-control study. Arch Intern Med. 2000;160:661–6. doi: 10.1001/archinte.160.5.661. [DOI] [PubMed] [Google Scholar]
- 32.Brix TH, Kyvik KO, Hegedüs L. A population-based study of chronic autoimmune hypothyroidism in Danish twins. J Clin Endocrinol Metab. 2000;85:536–9. doi: 10.1210/jcem.85.2.6385. [DOI] [PubMed] [Google Scholar]
- 33.Brix TH, Kyvik KO, Christensen K, Hegedüs L. Evidence for a major role of heredity in Graves’ disease: A population-based study of two Danish twin cohorts. J Clin Endocrinol Metab. 2001;86:930–4. doi: 10.1210/jcem.86.2.7242. [DOI] [PubMed] [Google Scholar]
- 34.Brix TH, Kyvik KO, Hegedüs L. Major role of genes in the etiology of simple goiter in females: A population-based twin study. J Clin Endocrinol Metab. 1999;84:3071–5. doi: 10.1210/jcem.84.9.5958. [DOI] [PubMed] [Google Scholar]
- 35.Brix TH, Hansen PS, Kyvik KO, Hegedüs L. The pituitary-thyroid axis set point in women is uninfluenced by X chromosome inactivation pattern? A twin study. Clin Endocrinol (Oxf) 2010;73:666–70. doi: 10.1111/j.1365-2265.2010.03848.x. [DOI] [PubMed] [Google Scholar]
- 36.Hng TM, Cheung NW, McLean M. Growth hormone and cortisol dynamic function in relation to birth weight: A study in adult twins. J Clin Endocrinol Metab. 2005;90:2781–6. doi: 10.1210/jc.2004-2497. [DOI] [PubMed] [Google Scholar]
- 37.Jensen MS, Toft G, Thulstrup AM, Henriksen TB, Olsen J, Christensen K, et al. Cryptorchidism concordance in monozygotic and dizygotic twin brothers, full brothers, and half-brothers. Fertil Steril. 2010;93:124–9. doi: 10.1016/j.fertnstert.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 38.AvRuskin TW, Witchel SF, Taha DR, Juan CS. Monozygotic twins with congenital adrenal hyperplasia: Long-term endocrine evaluation and gene analysis. J Pediatr Endocrinol Metab. 2003;16:565–70. doi: 10.1515/jpem.2003.16.4.565. [DOI] [PubMed] [Google Scholar]
- 39.Lambalk CB, Boomsma DI. Genetic risk factors in tumours of the testis: Lessons from twin studies. Twin Res. 1998;1:154–5. doi: 10.1375/136905298320566302. [DOI] [PubMed] [Google Scholar]
- 40.Martin WM, Dane TE. Testicular germ cell tumors in monozygotic twins: Case report and review of the literature. J Urol. 1985;134:765–7. doi: 10.1016/s0022-5347(17)47431-3. [DOI] [PubMed] [Google Scholar]
- 41.Kouri JG, Oldfield EH. A twin with Cushing's disease. Lancet. 2005;365:1332. doi: 10.1016/S0140-6736(05)61031-8. [DOI] [PubMed] [Google Scholar]
- 42.Kumar KV, Shaikh A, Sharma R, Bisht YS. Palmoplantar keratoderma with growth hormone deficiency. J Pediatr Endocrinol Metab. 2012;25:327–9. doi: 10.1515/jpem-2011-0484. [DOI] [PubMed] [Google Scholar]
- 43.Albagha OM, Ralston SH. Genetic determinants of susceptibility to osteoporosis. Endocrinol Metab Clin North Am. 2003;32:65–81. doi: 10.1016/s0889-8529(02)00059-2. vi. [DOI] [PubMed] [Google Scholar]
- 44.Abrahamsen B, Hjelmborg JV, Kostenuik P, Stilgren LS, Kyvik K, Adamu S, et al. Circulating amounts of osteoprotegerin and RANK ligand: Genetic influence and relationship with BMD assessed in female twins. Bone. 2005;36:727–35. doi: 10.1016/j.bone.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 45.Nakayama S, Yasui T, Suto M, Sato M, Kaji T, Uemura H, et al. Differences in bone metabolism between singleton pregnancy and twin pregnancy. Bone. 2011;49:513–9. doi: 10.1016/j.bone.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 46.Kumar KV, Shaikh A, Sharma R, Prusty P. SADDAN syndrome. J Pediatr Endocrinol Metab. 2011;24:851–2. doi: 10.1515/jpem.2011.290. [DOI] [PubMed] [Google Scholar]
- 47.Bahn RS, Scheithauer BW, van Heerden JA, Laws ER, Jr, Horvath E, Gharib H. Nonidentical expressions of multiple endocrine neoplasia, type I, in identical twins. Mayo Clin Proc. 1986;61:689–96. doi: 10.1016/s0025-6196(12)62767-0. [DOI] [PubMed] [Google Scholar]
- 48.Namihira H, Sato M, Miyauchi A, Ohye H, Matsubara S, Bhuiyan MM, et al. Different phenotypes of multiple endocrine neoplasia type 1 (MEN1) in monozygotic twins found in a Japanese MEN1 family with MEN1 gene mutation. Endocr J. 2000;47:37–43. doi: 10.1507/endocrj.47.37. [DOI] [PubMed] [Google Scholar]


