Abstract
Puberty is a period of development characterized by partially concurrent changes which includes growth acceleration, alteration in body composition and appearance of secondary sex characteristics. Puberty is characterized by an acceleration and then deceleration in skeletal growth. The initiation, duration and amount of growth vary considerably during the growth spurt. Pubertal growth and biological maturation are dynamic processes regulated by a variety of genetic and environmental factors. Changes in skeletal maturation and bone mineral accretion concomitant with the stage of pubertal development constitute essential components in the evaluation of growth during this pubertal period. Genetic, endocrine and nutritional factors and ethnicity contribute variably to the amount of growth gained during this important period of rapid changes. Many studies investigated the possibility of increasing pubertal growth to gain taller final adult height in adolescents with idiopathic short stature (ISS). The pattern of pubertal growth, its relation to sex maturity rating and factors affecting them has been addressed in this review. The results of different trials to increase final adult height of adolescents using different hormones have been summarized. These data enables Endocrinologists to give in-depth explanations to patients and families about the efficacy and clinical significance as well as the safety of using these therapies in the treatment of adolescents with ISS.
Keywords: Genetics, gonadotrophin-releasing hormone analog, growth hormone, hormones, idiopathic short stature, nutrition, pubertal growth
INTRODUCTION
Adolescent growth spurt is the fast and intense increase in the rate of growth in height and weight that occurs during the adolescent stage of the human life cycle. This growth practically occurs in all of the long bones and most other skeletal elements. No other primate species, including the chimpanzee, is known to have such a global post-pubertal increase in skeletal growth velocity.
The pubertal growth spurt begins on average at 9-10.0 years for girls and 11-12.0 for boys, however there is considerable variation between individuals and populations. The peak growth rate as well as the duration of this spurt is greater for boys than for girls, and this accounts for the average difference of 11-13 cm in height between adult males and females. Up to 10% of clinically normally girls, usually those who sexually mature at a late age, experience a reduced or absent growth spurt.[1,2]
Pattern of growth during normal puberty
Pubertal growth, because of its distinct features, must be analyzed differently from the relatively slow prepubertal growth. The age at take-off is highly variable and sex-dependent. The mean take-off age in adolescents growing and peak height velocity (PHV) differ between boys and girls as well as the contribution of pubertal growth to final height (FH) are summarized in Table 1. Age at take-off correlates highly with pubertal stage, but correlates negatively with duration and magnitude of puberty. The PHV is highest in early-maturing children and lowest in late-maturing children [Table 1].
Table 1.
Pubertal changes in different clinical conditions
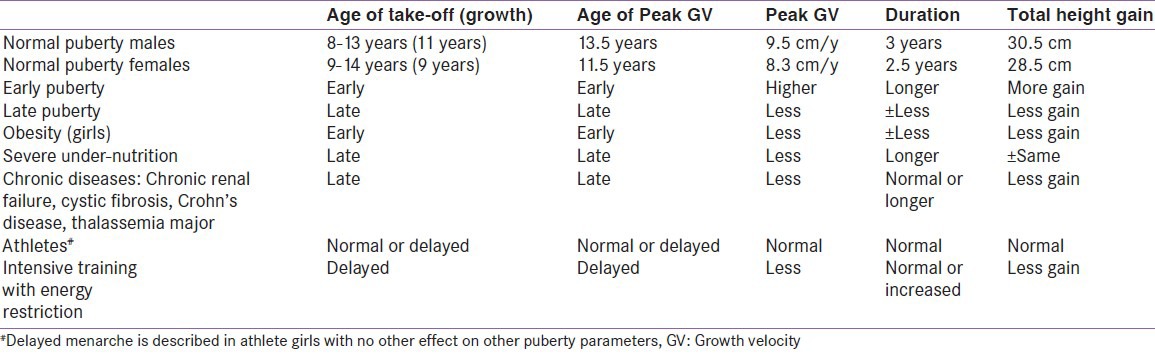
The duration of puberty is quite variable, due to considerable variation in the onset of puberty. In one study, linear-growth velocity begins to increase in males at genital stage III and pubic-hair stage II, but PHV is not attained until age 14 years in boys and 12 years in girls. Lean body mass, which primarily reflects muscle mass, begins to increase during early puberty in both boys and girls. Fat mass increases during the late stages of puberty in girls. Sex differences in the adolescent growth spurt produce the characteristics sexual dimorphism in shape and proportions seen in young adults.[3,4,5,6,7,8,9,10,11] Two large studies found that the earlier the start of pubertal growth spurt PGS onset, the higher were PHV and the total pubertal growth (TPG) gain. However, adult heights were similar among early maturers and late maturers when these values were compared with adult height values obtained.[12,13] In another longitudinal study a statistically significant correlation was found between height values at the start of pubertal growth and adult height.[14]
In summary, although the duration of postnatal growth is shorter in earlier than in later maturers, the former gain more centimeters during pubertal growth and reach similar adult height.[13,14] In both sexes, genetic factors, and not the time when pubertal growth begins, influenced final adult height (FAH).[13,14]
Genetic control of pubertal growth
Growth during puberty appears to be strictly genetically regulated. From 50% to 80% of the variation in the timing of puberty has been found to be because of genetic differences between individuals.[15] Twin studies showed that pubertal growth is under strong genetic control. A Swedish cohort of 99 monozygotic and 76 dizygotic twin pairs showed that the heritability estimate was 0.91 for age at onset of pubertal growth spurt, 0.93 for age at PHV, and 0.97 for adult height. Age at onset of pubertal growth spurt was negatively associated with BMI from 1 to 10 years of age and stature in early adulthood. The age at PHV was found to correlate with childhood BMI and stature in early adulthood. These associations were explained by common genetic factors. There is also evidence that a new set of genes affecting height may be turned on during the pubertal growth spurt and then turned off.[15,16,17]
The onset of puberty is almost certainly polygenic. Recently, the neuropeptides kisspeptin (encoded by KISS1 gene located on the long arm of chromosome 1 (1q32) and neurokinin B (NKB, encoded by TAC3 in humans) have been placed as essential gatekeepers of puberty. Studies in humans and rodents have revealed that loss-of-function mutations in the genes encoding either kisspeptin and NKB or their receptors, Kiss1r and neurokinin 3 receptor (NK3R), lead to impaired sexual maturation and infertility. Kisspeptin, NKB, and dynorphin A are co-expressed in neurons of the arcuate nucleus (ARC), the so-called “Kisspeptin/NKB/Dyn (KNDy)” neurons. Compelling evidence suggests a stimulatory role of NKB (or the NK3R agonist, senktide) on luteinizing hormone (LH) release through shaping the pulsatile release of kisspeptin and hence GnRH.[18] Data from mammals are consistent with the concept that KISS1 gene product activates the GPR54 on hypothalamic GnRH neurons.[19] Inactivating mutations of either the ligand or the receptor are associated with delayed pubertal development and activating mutations with central precocious puberty. An increase in KISS1 mRNA and/or GPR54 mRNA expression occurs during puberty.[18,19,20,21,22]
Pubertal growth assessment using velocity charts: Relationship with various common indexes of maturation
Longitudinal, rather than cross-sectional, growth data are necessary for constructing height-velocity charts. Clinical use of these growth–velocity charts requires calculating the child's growth velocity and knowing his or her pubertal status. In calculating growth velocity, the increment between two measurements should be not <0.85 yr and not >1.15 yrs. Velocities calculated over shorter periods can reflect only seasonal effects. By plotting the height values of a child in a chart containing pre-pubertal reference values, the onset of the pubertal growth spurt can be identified by a change in the pre-pubertal height standard deviation score values of 0.3 standard deviations or more over a period of 1 year.[23] Once the pubertal onset is established, an accurate FH prediction method for both sexes can be applied to the data.[3,23]
Another important fact to consider is the concordance between the growth spurt and different stages of pubertal development. When using auxological parameters to detect the spurt, it is possible to evaluate its concordance with clinical Tanner stages. In girls, peak growth velocity occurs at stage B2 in 40% of individuals, B3 in 30%, B4 in 20% and B1 (before breast development) in 10%.[1] Similarly, in boys, peak growth velocity occurs at stage G3 in 60% of individuals, G4 in 28%, G2 in 8% and G5 in 4%.[24,25,26]
A recent study showed that the onset of the pubertal growth spurts in height, facial size, and mandibular length occurred in girls at 9.3, 9.8, and 9.5 years, respectively, and in boys at 11.9, 12.0, and 11.9 years, respectively. The peak of the growth spurt in height, facial size, and mandibular length occurred at 10.9, 11.5, and 11.5 years in girls and 14.0, 14.4, and 14.3 years in boys.[27]
Prediction of adult height during pubertal growth
Adult height prediction is a common procedure in pediatric endocrinology. The prediction may reassure the family or indicate a need for laboratory tests to establish the cause of the unusual growth. Early maturing individuals are closer to their adult height than average and late maturing individuals of the same chronological age. In addition, at the PHV a child has reached 92% of their adult stature. Therefore, prediction of adult height is based on both the assessment of the child's current biological maturity and the amount of growth remaining until full maturity.[28]
The most popular predictive equations of adult height are the Bayley and Pinneau method, the Roche–Wainer–Thissen method, Khamis–Roche and the Tanner–Whitehouse methods. To predict adult height the following variables are required: gender, date of birth, date of measurement, height and skeletal maturity (bone age (BA)).[29,30,31,32,33,34,35,36] Although all these methods are clinically useful, wide variation exists among formulas used to predict adult heights. Because these algorithms may be used in decisions regarding whether to initiate GH treatment and assessment of the efficacy of GH in research trials, it is important for parents, pediatricians, and investigators to recognize the considerable variation involved in height predictions.
Recent longitudinal study assessing the value of prediction of adult height by Tanner–Whitehouse method in young Caucasian male athletes (n = 477) proved accurate relationship between estimated and FAH was high (r = 0.96). Sherar et al.[28] developed maturity and sex-specific cumulative height velocity curves for early, average, and late maturers. The technique is a valid, nonintrusive, inexpensive, and simple method of predicting adult height in.[26,27,28,29,30,31,32,33,34] If automated BA measurements are found to be more precise than measurements by human readers, then introduction of this technology might help to reduce errors in height predictions and might allow more accurate comparisons of the different algorithms.[29]
Endocrine control of pubertal growth
Pubertal growth is a complex process regulated and modified by hormonal and genetic factors, environment and nutrition [Figures 1 and 2].
Figure 1.
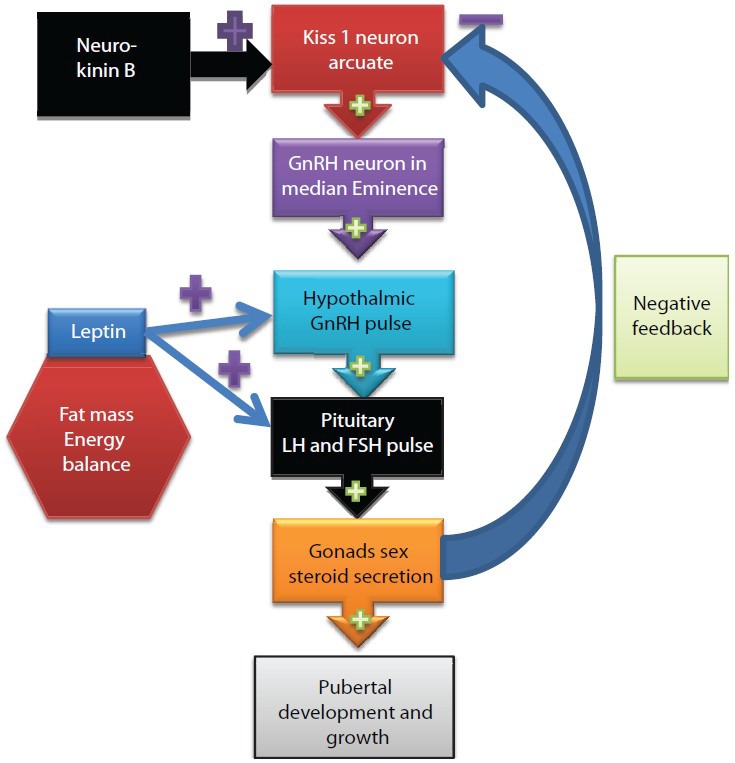
A schematic representation of hormonal control of pubertal development. Kisspeptin from Kiss neurons stimulates GnRH secretion by a direct effect on GnRH neurons. KiSS-1 neurons in the ARC appear to be involved in the negative feedback regulation of GnRH/LH by sex steroids. The expression of KiSS-1 mRNA in the arcuate is inhibited by estradiol (E), and testosterone (T). + = stimulation, – = inhibition
Figure 2.
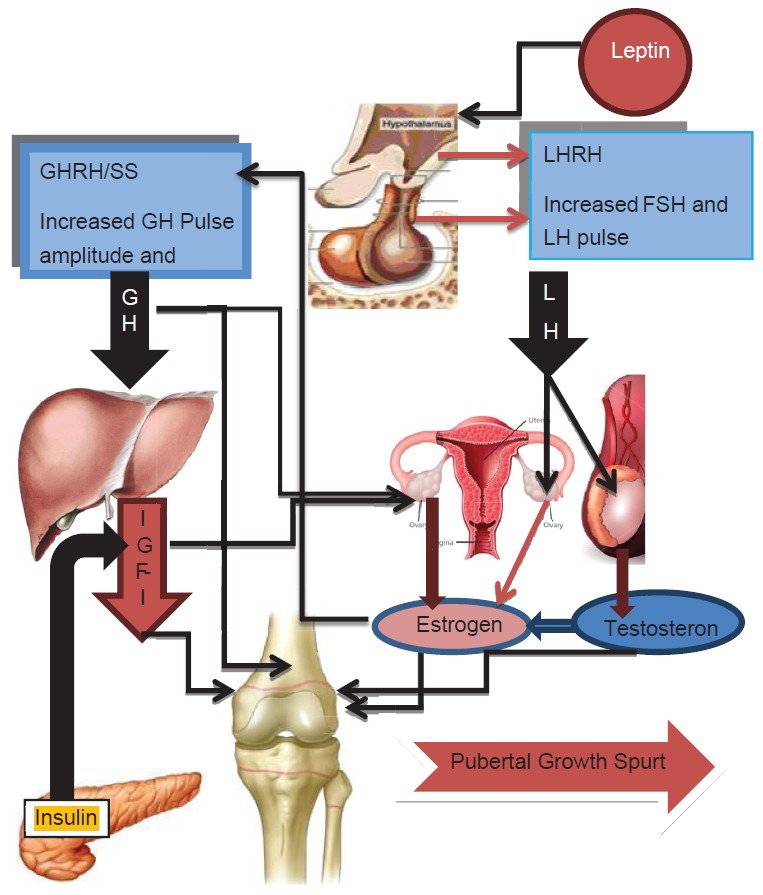
Activation of hypothalamic pituitary gonadal axis and GHIGF-1 axis leading to pubertal growth spurt and sexual development. → = stimulation
Compelling evidence suggests a stimulatory role of NKB on LH release through shaping the pulsatile release of kisspeptin and hence GnRH. Studies reported that kisspeptins stimulate the secretion of gonadotropins from the pituitary by stimulating the release of GnRH from the forebrain after the activation of GPR54, which is expressed by GnRH neurons. Kisspeptin neurons express the estrogen receptor (ER) and the androgen receptor, and these cells are direct targets for the action of gonadal steroids in both male and female animals, suggesting that kisspeptin signaling could mediate the neuroendocrine events that trigger the onset of puberty. Animal observations suggest that kisspeptin neurons in the ARC and anteroventral periventricular nucleus (AVPV) of both the male and female provide tonic drive to GnRH-neuronal activity, which is modulated by the negative/positive feedback effects of gonadal steroids.[30,31,32,33,34] It is suggested that Kisspeptin released by neurons in the AVPV and Arc stimulates GnRH release, which induces the release of LH and FSH. The gonads respond to gonadotropins by secreting sex steroids, which then feedback to regulate the activity of kisspeptin neurons, inhibiting KISS1 expression in the Arc and inducing its expression in the AVPV. Kisspeptin signaling in the brain has been implicated in triggering and guiding the tempo of sexual maturation at puberty and generating the preovulatory GnRH/LH surge.[35,36,37] Exogenous kisspeptin administered to prepubertal rodents and monkeys initiates various aspects of precocious puberty such as LH secretion or vaginal opening. Manipulation of kisspeptin signaling has the potential for novel therapies in patients with pathologically low or high LH pulsatility[37] [Figure 1].
During puberty GnRH is secreted in pulses and in greater amounts at first only at night. Pituitary gonadotropes respond to GnRH, by LH is secretion which stimulates the testis or ovary to produce testosterone or estradiol.[38] Gonadal steroid hormone levels promote the development and growth of the secondary sexual characteristics and the changes in body composition associated with pubertal development. Sex steroids play a crucial role in pubertal growth both at the systemic level via the growth hormone/insulin like growth factor-1 (GH/IGF-1) axis and at the local level of the epiphyseal growth plate. In both sexes estrogen is the critical hormone in controlling growth plate acceleration and fusion.[39] Estrogen stimulation of growth is largely dependent on pituitary GH and is mediated via the ERs, estrogen receptor-alpha (ER-α) and estrogen receptor-beta (ER-ß), which are expressed in the anterior pituitary as well as the hypothalamus. GH and estrogen levels show positive correlations in prepubertal girls and boys. Estrogen increases the basal GH secretion rate and the irregularity of GH release patterns, whereas testosterone stimulates greater GH secretory burst mass and greater IGF-1 concentrations [Figure 2].[40,41,42]
During puberty the pulsatile secretion of GH increases (1.5 to 3-fold) along with a greater than three-fold increase in serum IGF-1 levels. Twenty-four hour GH secretion peaks during adolescence, contributing to the high serum levels of IGF-1 that are characteristic of puberty. The increase in GH production during middle to late puberty is caused by enhanced pulse amplitude and increased mass of GH per secretory burst, rather than by a change in pulse frequency.[38,40] In girls, an increase in circulating GH is seen relatively early in puberty at Tanner breast stage 2 (B2) with peak levels coinciding with B3-4. In boys, this increase in GH is seen later with the peak occurring at Tanner genital stage 4 (G4). Peak IGF-1 levels occur at 14.5 years in girls and 1 year later in boys. During puberty, IGF-1 levels rise two to three times the adult range. IGF-1 levels during adolescence correlate better with Tanner stage or BA than with chronologic age. After secondary sexual development is complete, GH and IGF-1 levels fall to prepubertal levels in both sexes. Both systemic and local IGF-1 contribute to longitudinal growth.[37,38,39]
In experimental animals, GH seems to stimulate FSH-induced differentiation of granulosa cells directly, increase ovarian levels of IGF-1, and amplify the ovarian response to gonadotropins. IGF-1, in turn, enhances the gonadotropin effect on the granulosa cell, and GH seems to act synergistically to facilitate ovarian maturation postmenarche.[41] The local production or accumulation of GH and IGF-1 contributes an intra-ovarian paracrine control on steroidogenesis.[42] Patients with isolated GH deficiency frequently have delayed puberty and their Leydig cell function is diminished. GH administration can restore testicular responsiveness to LH and Leydig cell steroidogenesis.[43,44]
Insulin is also important for normal growth. Plasma insulin levels increase throughout childhood, but the rise is particularly pronounced during puberty with a strong positive correlation with IGF-1. The pubertal transition from Tanner stage I to Tanner stage III or IV is associated with a 32% reduction in insulin sensitivity (SI) and subsequently recovered by the end of puberty. SI was not associated with changes in body fat, testosterone, or estradiol.[45,46,47,48]
Thyroid hormones are an integral and essential prerequisite for normal pubertal development. Thyroid hormone regulates chondrocyte proliferation and stimulates terminal differentiation, mineralization, and angiogenesis. In particular, thyroid hormone is essential for hypertrophic chondrocyte differentiation. Thyroid hormones potentiate the actions of IGF-1 on cartilage and stimulate GH synthesis by the anterior pituitary gland. Hypothyroidism can cause growth and pubertal delay and delayed skeletal maturation, whereas hyperthyroidism can accelerate linear growth and skeletal maturation.[48,49] Longitudinal assessment of free T4 levels revealed a marked decrease from 10 years of age (15.7 pmol/L) to 12.5 years of age (13 pmol/L) before rising to prepubertal (15.9 pmol/L) levels at 15 years of age. The nadir occurred during pubertal developmental Tanner stages III to IV.[50]
Leptin is a protein product of the obesity (ob) gene. It is secreted as a hormone mainly from white adipose tissue and serves as a signal for the brain of the body's energy stores.[53] Leptin receptors have been identified in the hypothalamus, gonadotrope cells of the anterior pituitary, and ovarian follicular cells, as well as Leydig cells. It accelerates gonadotropin-releasing hormone (GnRH) pulsatility in hypothalamic neurons, and it has a direct effect on the anterior pituitary. Leptin administration at low doses may have a permissive, threshold effect on the central networks that regulate gonadotropin secretion. Recent data indicate that leptin has a specific role in stimulating the activity of enzymes essential for the synthesis of adrenal androgens.[51,53] Leptin has been shown to act as a skeletal growth factor, with a direct effect on skeletal growth.[49,51,52]
It appears in obese girls with early puberty the elevated leptin levels might have a permissive effect on the pubertal process and links energy regulation with pubertal development and growth.[54,55]
Effect of nutrition on pubertal growth spurt
In rural Hyderabad (India) longitudinal data on height measurements were studied in pre-school children available during an 18 year period. Boys with severe height deficit at age 5+ (severe under-nutrition) entered late into puberty significantly later compared to British and normal Indian boys. They grew with significantly depressed intensity, but gained a similar amount of height, as a result of prolonged adolescent growth spurt period which continued till 19.2 years. Thus, a childhood background of undernutrition did not lead to any additional deficit in height during puberty. However, pre-pubertal height deficits were carried into adult height.[56]
Interestingly, in childhood and early adolescence, early maturers were taller, had higher body mass index (BMI) and thicker skinfolds than later maturers.[57] This points to the tight interactions between fat mass and pubertal development. This means that a compensatory mechanism occurs where individuals with earlier puberty grow less before puberty and more during puberty while those with late pubertal development start their puberty taller but grow less during puberty. Whether this compensation is complete and whether those who enter puberty at the earlier end of the normal spectrum end up shorter than those who mature later is still a matter of debate.[58,59]
A longitudinal study of normal children showed that overweight and obesity have significant effect on pubertal growth.[57] When the evolution of the adolescent growth spurt was related to the evolution of BMI between the ages of 2 and 8, the findings indicate that an additional gain of 1 BMI point (1 kg/m2) decreases the adolescent growth spurt by a mean of 0.5 cm in girls and 0.9 cm in boys.[60]
Puberty, bone growth and bone mineral accretion
Puberty is a crucial time in bone mineral mass development. The lumbar spine bone mineral mass doubles from 9 to 15 years and from 11 to 17 years in females and males, respectively. Approximately 40% of peak bone mass is acquired between Tanner stages II and V and the rate of acquisition is particularly high between stage III and stage IV.[61]
Bone mass increases throughout puberty in both sexes. Bone formation markers, dual energy X-ray absorptiometry (DEXA) and quantitative computerized tomography (CT) imaging indicate that there is an increase in bone mass throughout childhood with a marked acceleration in accumulation at puberty.[62,63,64] Estrogen level makes the greatest contribution to bone mineral acquisition in boys and girls.[66,67,68] The achievement of peak bone mass is sustained by estrogen in both sexes. Strong correlation coefficients are found between BMD and serum estradiol levels and height. Estradiol has imperative effects, through increasing bone density and by suppression of bone resorption at the endocortical surface leading to an increase in cortical thickness.[57,58,59]
In healthy adolescent males and females, bone mass and density at skeletal maturity are inversely related to the timing of puberty. The BMD at all skeletal sites in Childhood Study indicated that the age of onset of puberty is a strong negative predictor of DXA bone measurements at skeletal maturity, independent of bone values at the beginning of puberty, and the length of puberty.[58,60] In contrast, length of puberty has no relation to any measures of bone accretion. BMD at the radius and lumbar spine levels has been found to be significantly higher in girls with precocious puberty compared with prepubertal girls and delayed puberty is associated with reduced bone mass. In delayed or late puberty, reduced bone mass gain can be observed before the onset of sexual maturation.[60,65,66,67,68,69]
In addition to estrogen, activation of the GH and IGF-1 axis positively affects bone turnover by stimulating osteoblast proliferation and differentiation. The prepubertal increase in adrenal androgens, specifically dehydroepiandrosterone metabolites, also has beneficial effects on the accretion of bone strength. Moreover, pubertal increase in muscularity (anabolic effects of the sex hormones) is likely to stimulates bone formation.[70,71,72]
Can we increase adolescent growth?
Hormonal treatment using GH with or without GnRH and/or aromatase inhibitors (AIs) are the main hormonal therapies tried by endocrinologists to enhance FAH in short adolescents with idiopathic short stature (ISS).
Gonadotropin-releasing hormone agonists in short children at onset of puberty
The good results of using GnRH in precocious puberty[73] and the hope that interrupting puberty might increase adult height has led to several attempts to use GnRH agonists in patients other than those with strict criteria for precocious puberty, in particular children with normal puberty and poor growth prognosis due to ISS.[74]
In 31 girls with ISS and pubertal onset around the age of 12 years, the use of GnRH agonist for 2 years increased of adult over pretreatment-predicted height only 1:2.3 cm. Growth velocity markedly declined during treatment and the height deficit increased by 0.4 SDS on average in these already short girls.[84] Another placebo-controlled randomized study used GnRH agonist for treatment of adolescents with ISS for an average of 3.5 years (one third of them were also treated with growth hormone (GH)). Covariance analysis of adult height SDS, adjusted on sex, GH treatment showed an increase of 0.6 SDS (4.2 cm) with the use of GnRH agonist. However, the treatment was associated with a decrease in BMD, measured 1 year after the discontinuation of the treatment.[75]
It appears that when these treatments are used for short periods of time the effect on adult height is close to zero. However, when duration of treatment increases, the slow growth rate observed in the absence of BA progression eventually converts into increased adult height, roughly 1 cm per treatment year.[76,77] This is similar to the effect of untreated hypogonadism that leads to increased adult height only if untreated to the age of 20 years. Similarly, in males with ER or aromatase deficiency, height is normal or low around the age of puberty in the absence of a growth spurt. However, persistent growth in the absence of growth plate fusion leads to tall stature when patients are older than 20 years.[77,78]
In summary, the data accumulated so far allow the clinician to give in-depth explanations to patients and families that short treatments are completely ineffective and long treatments have some efficacy with questionable clinical significance (4 cm) and possible safety concerns about decreased BMD. In addition, the psychological sequelae of drug-induced severe pubertal delay have to be evaluated. Therefore, GnRH agonist treatments to increase height outside of precocious puberty are not currently advised outside research protocols.[79,80]
GH in short children at onset of puberty
A large multicenter observational study monitored the effect of GH treatment of short children with ISS at onset of puberty until the near-end of growth. Results showed that all groups gained about 1.1 SDS. Patients who had used GH for the shortest period of time (less than 18 months) did similarly to those who used treatment for the longest periods. These results raised the question whether this was due to spontaneous catch-up in individuals with delayed puberty or to the effect of GH. The mean effect was close to 1 cm of adult height gain by year of treatment. The limits of this observational study included the relative heterogeneity of the patients and the relatively low dose of GH used (0.4 U/kg per week or 0.02 mg/kg per week).[81,82] Using high doses of GH (1.4 U/kg per week or 0.067 mg/kg per day) in short adolescents born small for gestational age resulted in increased height SDS by 0.6 SDS in 2.7 years (0.2 SDS/year).[83] Other short-term studies have addressed similar questions and have obtained quite similar results.[84,85]
The FAH of children with ISS (n = 194) treated with GH for 5.9 ± 1.1 years showed that GH treatment significantly increased FAH in a dose-dependent manner, with a mean gain of 1.3 SDS (8 cm) and a broad range of response from no gain to 3 SDS compared to a mean gain of 0.2 SDS in the untreated controls.[86] In addition GH treatment showed positive effect on lean body mass, bone turnover, and bone mass accrual over 2 years.[87]
Altogether, these results demonstrate that GH given alone can increase the pubertal growth spurt in short adolescents. Similar to the conclusions with GnRH agonists, the physician now has precise information to give to patients and families on what to expect from a GH treatment in such a situation. The constraints and risks of such treatments and the uncertainties concerning long-term consequences should be discussed with the adolescent and parents.[88,89,90]
Combination of GH and GnRH agonists in short adolescents
The possibility to block pubertal development and skeletal maturation as a tool to increase the effect of GH treatment has long been considered in the KIGS database, children on combined GH and GnRH agonists compared to those treated with GH only, followed up to near-adult showed a lower total height gain (from start of GH treatment to adult) in the combination patients than with GH alone. Another study showed similar observation and, in multivariate analysis, use of GnRH agonist was associated with a 0.3 SDS decrease in adult height gain.[91,92] Thirty-two adolescents with Tanner stage II–III, age and BA less than 12 yrs for girls or less than 13 yrs for boys, height SD score (SDS) less than −2.0 plus a predicted adult height (PAH) less than −2.0 SDS were randomly allocated to receive GH plus GnRHa (n = 17) or no treatment (n = 15) for 3 yrs. FH was assessed at the age of 18 yrs or older in girls or 19 yrs or older in boys. FH was not different between treatment and control groups.[93]
In contrast to these findings, Pasquino et al.[94] have compared 12 short normal girls treated with the combination versus 12 treated with GH alone. At adult height, the gain over predicted height was 10 ± 2.9 cm with the combination versus 6.1 ± 4.4 cm with GH alone (a mean difference of 4 cm). In the Dutch study, 36 patients were randomized to combination treatment or observation. The predicted height at the end of the 3-year treatment period was higher by a mean of 1.2 SDS in the treated versus control group.[104] Other small-scale trials with a the mean durations of the combination was quite variable ranging from 1.4 to 3 years concluded a benefit from the combination ranging from 0.8 to 1.4 SDS.[95,96,97]
More recently, the use of AIs has been proposed to specifically decrease the growth maturating effects of estrogens on the growth plate and therefore increase adult height.[98,99,100] The primary data suggest that the use of AIs in ISS hold promise for increasing adult height in short children, apparently by maintaining growth velocity while decreasing BA progression. The available data on near adult height indicate that gains noted during initial therapy may be (although not always fully) sustained into adulthood. A randomized study on adolescent males with GH deficiency compared growth of those on treatment with GH and either daily co-treatment with anastrozole or placebo for up to 36 months. A significant slower advancement in the BA in anastrozole versus the placebo group resulted in a net increase in mean PAH of 4.5 cm in the anastrozole group at 24 months and 6.8 cm at 36 months compared with a 1-cm gain at both time points in placebo group. All boys on anastrozole had a normal tempo of pubertal virilization and, as expected.[101]
AIs are well tolerated and are available as a convenient once-daily oral dose; however, safety information regarding long-term effects on indices such as bone mineral acquisition, spermatogenesis, and exposure to supra-physiologic testosterone levels are still needed. Data on adult height are still lacking, and there is no information identifying which children are likely to benefit most.[102,103,104,105,106]
In summary
Individual adolescents vary in the timing of pubertal onset, its duration or “tempo,” and its termination. Little is known about the “offset” mechanisms of puberty. There are a number of variables that may influence directly or indirectly pubertal growth spurt including, gender, genetics, nutrition, endocrine regulation, physical activity and ethnicity [Table 1]. It is the interaction among several of these variables that may affect pubertal growth and maturation in complex ways. Children with short stature, who enter puberty at normal time, comprise a large portion of the typical pediatric endocrinologist's practice. Possible strategies for increasing adult height include growth hormone treatment alone or together with GnRH analog or with AIs therapy to suppress pubertal development. These approaches are expensive and invasive (requiring repeated injections), and GnRH analog and AIs therapy may result in potentially adverse effects in children whose puberty is occurring at a physiologically normal time. Additional controlled studies for their efficacy and safety are required before this therapy can be routinely recommended to augment adult height.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bogin B. Evolutionary perspective on human growth. Annu Rev Anthropol. 1999;28:109–53. doi: 10.1146/annurev.anthro.28.1.109. [DOI] [PubMed] [Google Scholar]
- 2.Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. Williams Textbook of Endocrinology. 12th ed. chapter 23. Philadelphia, PA: Saunders; 2012. Endocrine Regulation of Growth; pp. 1009–37. [Google Scholar]
- 3.Val Abbassi V. Growth and Normal Puberty. Pediatrics. 1998;102(Suppl 3):507–11. [PubMed] [Google Scholar]
- 4.Wheeler MD. Physical changes of puberty. Endocrinol Metab Clin North Am. 1991;20:1–14. [PubMed] [Google Scholar]
- 5.Marshall WA, Tanner JM. Puberty. In: Falkner F, Tanner JM, editors. Human Growth. II. Postnatal Growth. New York, NY: Plenum Press; 1986. pp. 171–209. [Google Scholar]
- 6.Thissen DR, Bock D, Wainer H, Roche AF. Individual growth in stature: A comparison of four growth studies in the USA. Ann Hum Biol. 1976;3:529–42. doi: 10.1080/03014467600001791. [DOI] [PubMed] [Google Scholar]
- 7.Berkey CS, Dockery DW, Wang X, Wypij D, Ferris B., Jr Longitudinal height velocity standards for US adolescents. Stat Med. 1992;12:403–14. doi: 10.1002/sim.4780120321. [DOI] [PubMed] [Google Scholar]
- 8.Lee PA. Normal ages of pubertal events among American males and females. J Adolesc Health Care. 1980;1:26–9. doi: 10.1016/s0197-0070(80)80005-2. [DOI] [PubMed] [Google Scholar]
- 9.Biro FM, Lucky AW, Huster GA, Morrison JA. Pubertal staging in boys. J Pediatr. 1995;127:100–2. doi: 10.1016/s0022-3476(95)70265-2. [DOI] [PubMed] [Google Scholar]
- 10.Hamill PV, Terence AD, Johnson CL. NCHS growth curves for children birth–18 years. Vital Health Stat. 1977;165:1–74. [PubMed] [Google Scholar]
- 11.Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107:317–29. doi: 10.1016/s0022-3476(85)80501-1. [DOI] [PubMed] [Google Scholar]
- 12.Tanner JM, Whitehouse RH. The adolescent growth spurt of boys and girls of the Harpenden Growth Study. Ann Hum Biol. 1976;3:109–26. doi: 10.1080/03014467600001231. [DOI] [PubMed] [Google Scholar]
- 13.Ferrández A, Carrascosa A, Audí L, Baguer L, Rueda C, Bosch-Castañé J, et al. Longitudinal pubertal growth according to age at pubertal growth spurt onset: Data from a Spanish study including 458 children (223 boys and 235 girls) J Pediatr Endocrinol Metab. 2009;22:715–26. doi: 10.1515/jpem.2009.22.8.715. [DOI] [PubMed] [Google Scholar]
- 14.Carrascosa A, Audí L, Bosch-Castañé J, Gussinyé M, Yeste D, Albisu MA, et al. Influence of the age at the start of pubertal growth on adult height. Med Clin (Barc) 2008;130:645–9. doi: 10.1157/13120692. [DOI] [PubMed] [Google Scholar]
- 15.Silventoinen K, Haukka J, Dunkel L, Tynelius P, Rasmussen F. Genetics of pubertal timing and its associations with relative weight in childhood and adult height: The Swedish Young Male Twins Study. Pediatrics. 2008;12:e885–91. doi: 10.1542/peds.2007-1615. [DOI] [PubMed] [Google Scholar]
- 16.Fischbein S, Pedersen NL. Multivariate analysis of genetic and environmental influences for longitudinal height and weight data. Acta Genet Med Gemellol. 1987;36:171–80. doi: 10.1017/s0001566000004402. [DOI] [PubMed] [Google Scholar]
- 17.Phillips K, Matheny AP., Jr Quantitative genetic analysis of longitudinal trends in height: Preliminary results from the Louisville Twin Study. Acta Genet Med Gemellol. 1990;39:143–63. doi: 10.1017/s0001566000005389. [DOI] [PubMed] [Google Scholar]
- 18.Navarro VM. Interactions between kisspeptins and neurokinin B. Adv Exp Med Biol. 2013;784:325–47. doi: 10.1007/978-1-4614-6199-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–8. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teles MG, Silveira LF, Tusset C, Latronico AC. New genetic factors implicated in human GnRH-dependent precocious puberty: The role of kisspeptin system. Mol Cell Endocrinol. 2011;346:84–90. doi: 10.1016/j.mce.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Silveira LG, Noel SD, Silveira-Neto AP, Abreu AP, Brito VN, Santos MG, et al. Mutations of the KISS1 gene in disorders of puberty. J Clin Endocrinol Metab. 2010;95:2276–80. doi: 10.1210/jc.2009-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plant TM, Ramaswamy S, Pietro MJ. Repetitive activation of hypothalamic G Protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (macaca mulatta) elicits a sustained train of gonadotropins-releasing hormone discharges. Endocrinology. 2006;147:1007–13. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- 23.Karlberg J, Kwan CW, Gelander L, Albertsson-Wikland K. Pubertal growth assessment. Horm Res. 2003;60(Suppl 1):S27–35. doi: 10.1159/000071223. [DOI] [PubMed] [Google Scholar]
- 24.Coste J, Ecosse E, Lesage C, Chaussain JL, Carel JC. Evaluation of adolescent statural growth in health and disease: Reliability of assessment from height measurement series and development of an automated algorithm. Horm Res. 2002;58:105–14. doi: 10.1159/000063577. [DOI] [PubMed] [Google Scholar]
- 25.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–24. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mellion ZJ, Behrents RG, Johnston LE., Jr The pattern of facial skeletal growth and its relationship to various common indexes of maturation. Am J Orthod Dentofacial Orthop. 2013;143:845–54. doi: 10.1016/j.ajodo.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Sherar LB, Mirwald RL, Baxter-Jones AD, Thomas M. Prediction of adult height using maturity based cumulative height velocity curves. J Pediatr. 2005;147:508–14. doi: 10.1016/j.jpeds.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 29.Thodberg HH, Jenni OG, Caflisch J, Ranke MB, Martin DD. Prediction of adult height based on automated determination of bone age. J Clin Endocrinol Metab. 2009;94:4868–74. doi: 10.1210/jc.2009-1429. [DOI] [PubMed] [Google Scholar]
- 30.Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: Revised for use with greulich-Pyle hand standards. J Pediatr. 1952;40:423–41. doi: 10.1016/s0022-3476(52)80205-7. [DOI] [PubMed] [Google Scholar]
- 31.Ostojic SM. Prediction of adult height by Tanner–Whitehouse method in young Caucasian male athletes. QJM. 2013;106:341–5. doi: 10.1093/qjmed/hcs230. [DOI] [PubMed] [Google Scholar]
- 32.Roche AF, Wainer H, Thissen D. The RWT method for the prediction of adult stature. Pediatrics. 1975;56:1026–33. [PubMed] [Google Scholar]
- 33.Tanner JM, Whitehouse RH, Cameron N, Marshall WA, Healy MJ, Goldstein H. 2nd ed. New York: Academic Press; 1983. Assessment of skeletal maturity and prediction of adult height. [Google Scholar]
- 34.Tanner JM, Healy MJ, Goldstein H, Cameron N. 3rd ed. London: Saunders; 2001. Assessment of skeletal maturity and prediction of adult height. (TW2 Method) [Google Scholar]
- 35.Khamis HJ, Roche AF. Predicting adult stature without using skeletal age: The Khamis-Roche method. Pediatrics. 1994;94:504–7. [PubMed] [Google Scholar]
- 36.Topor LS, Feldman HA, Bauchner H, Cohen LE. Variation in methods of predicting adult height for children with idiopathic short stature. Pediatrics. 2010;126:938–44. doi: 10.1542/peds.2009-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skorupskaite K, George JT, Anderson RA. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update. 2014;20:485–500. doi: 10.1093/humupd/dmu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perry RJ, Farquharson C, Ahmed SF. the role of sex steroids in controlling pubertal growth. Clin Endocrinol. 2008;68:4–15. doi: 10.1111/j.1365-2265.2007.02960.x. [DOI] [PubMed] [Google Scholar]
- 39.Kerrigan JR, Rogol AD. The impact of gonadal steroid hormone action on growth hormone secretion during childhood and adolescence. Endocr Rev. 1992;13:281–98. doi: 10.1210/edrv-13-2-281. [DOI] [PubMed] [Google Scholar]
- 40.Veldhuis JD, Roemmich JN, Rogol AD. Gender and sexual maturation-dependent contrasts in the neuroregulation of growth hormone secretion in prepubertal and late adolescent males and females-A general clinical research center-based study. J Clin Endocrinol Metab. 2000;85:2385–94. doi: 10.1210/jcem.85.7.6697. [DOI] [PubMed] [Google Scholar]
- 41.Hugues JN, Miro F, Smyth CD, Hillier SG. Effects of growth hormone releasing hormone on rat ovarian steroidogenesis. Hum Reprod. 1996;11:50–4. doi: 10.1093/oxfordjournals.humrep.a019033. [DOI] [PubMed] [Google Scholar]
- 42.Thomas FH, Campbell BK, Armstrong DG, Telfer EE. Effects of IGF-I bioavailability on bovine preantral follicular development in vitro. Reproduction. 2007;133:1121–8. doi: 10.1530/REP-06-0382. [DOI] [PubMed] [Google Scholar]
- 43.Frohman LA. Disorders of the anterior pituitary. In: Felig P, Baxter JD, Frohman LA, editors. Endocrinology and Metabolism. Ohio: McGraw-Hill; 1995. [Google Scholar]
- 44.Hull KL, Harvey S. Growth hormone: A reproductive endocrine–paracrine regulator? Rev Reprod. 2000;5:175–82. doi: 10.1530/ror.0.0050175. [DOI] [PubMed] [Google Scholar]
- 45.Caprio S. Insulin: The other anabolic hormone of puberty. Acta Paediatr Suppl. 1999;88:84–7. doi: 10.1111/j.1651-2227.1999.tb14410.x. [DOI] [PubMed] [Google Scholar]
- 46.Hannon TS, Janosky J, Arslanian SA. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res. 2006;60:759–63. doi: 10.1203/01.pdr.0000246097.73031.27. [DOI] [PubMed] [Google Scholar]
- 47.Guercio G, Rivarola MA, Chaler E, Maceiras M. Belgorosky A Relationship between the GH/IGF-I axis, insulin sensitivity, and adrenal androgens in normal prepubertal and pubertal boys. J Clin Endocrinol Metab. 2002;87:1162–9. doi: 10.1210/jcem.87.3.8330. [DOI] [PubMed] [Google Scholar]
- 48.Dubuis JM. Hormonal changes of puberty. 10th Postgraduate Course for Training in Reproductive Medicine and Reproductive Biology. [last accessed August 17, 2012]. Available from: http://www.gfmer.ch/Endo/Lectures_10/Puberty_%20Physiology.htm .
- 49.Soliman AT, De Sanctis V, Bedair ES. Eliška Potluková., editor. Congenital Hypothyroidism: Effects on Linear Growth, Catch- Up Growth, GH-IGF-I Axis and Bones. Current Topics in Hypothyroidism with Focus on Development. [last accessed February 13, 2013]. ISBN 978-953-51-0970-9, Published. Available from: http://www.intechopen.com/books/current-topics-in-hypothyroidism-with-focus-on-development/congenital-hypothyroidism-effects-on-linear- growth-catch-up-growth-gh-igf-i-axis-and-bones .
- 50.Dunger DB, Perkins JA, Jowett TP, Edwards PR, Cox LA, Preece MA, et al. A longitudinal study of total and free thyroid hormones and thyroxine binding globulin during normal puberty. Acta Endocrinol. 1990;123:305–10. doi: 10.1530/acta.0.1230305. [DOI] [PubMed] [Google Scholar]
- 51.Roemmich JN, Clark PA, Berr SS, Mai V, Mantzoros CS, Flier JS, et al. Gender differences in leptin levels during puberty are related to subcutaneous fat distribution and sex steroids. Am J Physiol. 1998;275:E543–51. doi: 10.1152/ajpendo.1998.275.3.E543. [DOI] [PubMed] [Google Scholar]
- 52.Ahmed ML, Ong KK, Morrell DJ, Cox L, Drayer N, Perry L, et al. Longitudinal study of leptin concentrations during puberty: Sex differences and relationship to changes in body composition. J Clin Endocrinol Metab. 1999;84:899–905. doi: 10.1210/jcem.84.3.5559. [DOI] [PubMed] [Google Scholar]
- 53.Matkovic V, Ilich JZ, Skugor M, Badenhop NE, Goel P, Clairmont A, et al. Leptin is inversely related to age at menarche in human females. J Clin Endocrinol Metab. 1997;82:3239–45. doi: 10.1210/jcem.82.10.4280. [DOI] [PubMed] [Google Scholar]
- 54.Veldhuis JD, Roemmich JN, Richmond EJ, Rogol AD, Lovejoy JC, Scheffield-Moore M, et al. Endocrine control of body composition in infants, children and puberty. Endocr Rev. 2005;26:114–46. doi: 10.1210/er.2003-0038. [DOI] [PubMed] [Google Scholar]
- 55.Shalitin S, Phillip M. Role of obesity and leptin in the pubertal process and pubertal growth: A review. Int J Obes Relat Metab Disord. 2003;27:869–74. doi: 10.1038/sj.ijo.0802328. [DOI] [PubMed] [Google Scholar]
- 56.Satyanarayana K, Radhaiah G, Mohan KR, Thimmayamma BV, Rao NP, Rao BS, et al. The adolescent growth spurt of height among rural Indian boys in relation to childhood nutritional background: An 18 year longitudinal study. Ann Hum Biol. 1989;16:289–300. doi: 10.1080/03014468900000422. [DOI] [PubMed] [Google Scholar]
- 57.Biro FM, McMahon RP, Striegel-Moore R, Crawford PB, Obarzanek E, Morrison JA, et al. Impact of timing of pubertal maturation on growth in black and white female adolescents: The National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 2001;138:636–43. doi: 10.1067/mpd.2001.114476. [DOI] [PubMed] [Google Scholar]
- 58.Rogol AD, Clark PA, Roemmich JN. Growth and pubertal development in children and adolescents: effects of diet and physical activity. Am J Clin Nutr. 2000;72:521s–8. doi: 10.1093/ajcn/72.2.521S. [DOI] [PubMed] [Google Scholar]
- 59.Vizmanos B, Marti-Henneberg C, Cliville R, Moreno A, Fernandez-Ballart J. Age of pubertal onset affects the intensity and duration of pubertal growth peak but not final height. Am J Hum Biol. 2001;13:409–16. doi: 10.1002/ajhb.1065. [DOI] [PubMed] [Google Scholar]
- 60.Bogin B. Secular Changes in Childhood, Adolescent and Adult Stature. In: Gillman MW, Gluckman PD, Rosenfeld RG, Karger AG, editors. Recent Advances in Growth Research: Nutritional, Molecular and Endocrine Perspectives. Vol. 71. Basel: Nestlé Nutr Inst Workshop Ser. Nestec Ltd., Vevey/S; 2013. pp. 115–26. [Google Scholar]
- 61.He Q, Karlberg J. BMI in childhood and its association with height gain, timing of puberty, and final height. Pediatr Res. 2001;49:244–51. doi: 10.1203/00006450-200102000-00019. [DOI] [PubMed] [Google Scholar]
- 62.Grimston SK, Morrison K, Harder JA, Hanley DA. Bone mineral density during puberty in western Canadian children. Bone Miner. 1992;19:85–96. doi: 10.1016/0169-6009(92)90846-6. [DOI] [PubMed] [Google Scholar]
- 63.Gilsanz V, Chalfant J, Kalkwarf H, Zemel B, Lappe J, Oberfield S, et al. Age at onset of puberty predicts bone mass in young adulthood. J Pediatr. 2011;158:100–5. doi: 10.1016/j.jpeds.2010.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yilmaz D, Ersoy B, Bilgin E, Gümüşer G, Onur E, Pinar ED. Bone mineral density in girls and boys at different pubertal stages: Relation with gonadal steroids, bone formation markers, and growth parameters. J Bone Miner Metab. 2005;23:476–82. doi: 10.1007/s00774-005-0631-6. [DOI] [PubMed] [Google Scholar]
- 65.Doneray H, Orbak Z. Association between anthropometric hormonal measurements and bone mineral density in puberty and constitutional delay of growth and puberty. West Indian Med J. 2010;59:125–30. [PubMed] [Google Scholar]
- 66.Davies JH, Evans BA, Gregory JW. Bone mass acquisition in healthy children. Arch Dis Child. 2005;90:373–8. doi: 10.1136/adc.2004.053553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takahashi Y, Minamitani K, Kobayashi Y, Minagawa M, Yasuda T, Niimi H. Spinal and femoral bone mass accumulation during normal adolescence: Comparison with female patients with sexual precocity and with hypogonadism. J Clin Endocrinol Metab. 1996;81:1248–53. doi: 10.1210/jcem.81.3.8772607. [DOI] [PubMed] [Google Scholar]
- 68.Chevalley T, Bonjour JP, Ferrari S, Rizzoli R. Influence of age at menarche on forearm bone microstructure in healthy young women. J Clin Endocrinol Metab. 2008;93:2594–601. doi: 10.1210/jc.2007-2644. [DOI] [PubMed] [Google Scholar]
- 69.Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun. 2005;328:688–96. doi: 10.1016/j.bbrc.2004.11.097. [DOI] [PubMed] [Google Scholar]
- 70.Frenkel B, Hong A, Baniwal SK, Coetzee GA, Ohlsson C, Khalid O, et al. Regulation of adult bone turnover by sex steroids. J Cell Physiol. 2010;224:305–10. doi: 10.1002/jcp.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shalet SM, Shavrikova E, Cromer M. Effect of growth hormone (GH) treatment on bone in postpubertal GH-deficient patients: A 2-year randomized, controlled, dose-ranging study. J Clin Endocrinol Metab. 2003;88:4124–9. doi: 10.1210/jc.2003-030126. [DOI] [PubMed] [Google Scholar]
- 72.Wang Q, Nicholson PH, Suuriniemi M. Relationship of sex hormones to bone geometric properties and mineral density in early pubertal girls. J Clin Endocrinol Metab. 2004;89:1698–703. doi: 10.1210/jc.2003-031113. [DOI] [PubMed] [Google Scholar]
- 73.Carel JC, Lahlou N, Roger M, Chaussain JL. Precocious puberty and statural growth. Hum Reprod Update. 2004;10:135–47. doi: 10.1093/humupd/dmh012. [DOI] [PubMed] [Google Scholar]
- 74.Carel JC, Hay F, Coutant R, Rodrigue D, Chaussain JL. Gonadotropin releasing hormone agonist treatment of girls with constitutional short stature and normal pubertal development. J Clin Endocrinol Metab. 1996;81:3318–22. doi: 10.1210/jcem.81.9.8784090. [DOI] [PubMed] [Google Scholar]
- 75.Lazar L, Kauli R, Pertzelan A, Phillip M. Gonadotropin-suppressive therapy in girls with early and fast puberty affects the pace of puberty but not total pubertal growth or final height. J Clin Endocrinol Metab. 2002;87:2090–4. doi: 10.1210/jcem.87.5.8481. [DOI] [PubMed] [Google Scholar]
- 76.Bouvattier C, Coste J, Rodrigue D, Teinturier C, Carel JC, Chaussain JL, et al. Lack of effect of GnRH agonists on final height in girls with advanced puberty: A randomized long-term pilot study. J Clin Endocrinol Metab. 1999;84:3575–8. doi: 10.1210/jcem.84.10.6032. [DOI] [PubMed] [Google Scholar]
- 77.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–61. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 78.Bilezikian JP, Morishima A, Bell J, Grumbach MM. Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. N Engl J Med. 1998;339:599–603. doi: 10.1056/NEJM199808273390905. [DOI] [PubMed] [Google Scholar]
- 79.Yanovski JA, Rose SR, Municchi G, Pescovitz OH, Hill SC, Cassorla FG, et al. Treatment with a luteinizing hormone-releasing hormone agonist in adolescents with short stature. N Engl J Med. 2003;348:908–17. doi: 10.1056/NEJMoa013555. [DOI] [PubMed] [Google Scholar]
- 80.Lee MM. Is treatment with a luteinizing hormone-releasing hormone agonist justified in short adolescents? N Engl J Med. 2003;348:942–5. doi: 10.1056/NEJMe030003. [DOI] [PubMed] [Google Scholar]
- 81.Carel JC, Ecosse E, Nicolino M, Tauber M, Leger J, Cabrol S, et al. Adult height after long-term recombinant growth hormone treatment for idiopathic isolated growth hormone deficiency: Observational follow-up study of the French population-based registry. Br Med J. 2002;325:70–3. doi: 10.1136/bmj.325.7355.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carel JC, Tresca JP, Letrait M, Le Bouc Y, Job JC, Chaussain JL, et al. Growth hormone testing for the diagnosis of growth hormone deficiency in childhood: A population register-based study. J Clin Endocrinol Metab. 1997;82:2117–21. doi: 10.1210/jcem.82.7.4106. [DOI] [PubMed] [Google Scholar]
- 83.Carel JC, Chatelain P, Rochiccioli P, Chaussain JL. Improvement in adult height after growth hormone treatment in adolescents with short stature born small for gestational age: Results of a randomized controlled study. J Clin Endocrinol Metab. 2003;88:1587–93. doi: 10.1210/jc.2002-021123. [DOI] [PubMed] [Google Scholar]
- 84.Hintz RL, Attie KM, Baptista J, Roche A. Effect of growth hormone treatment on adult height of children with idiopathic short stature. N Engl J Med. 1999;340:502–7. doi: 10.1056/NEJM199902183400702. [DOI] [PubMed] [Google Scholar]
- 85.Leschek EW, Rose SR, Yanovski JA, Troendle JF, Quigley CA, Chipman JJ, et al. Effect of growth hormone treatment on adult height in peripubertal children with idiopathic short stature: A randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:3140–8. doi: 10.1210/jc.2003-031457. [DOI] [PubMed] [Google Scholar]
- 86.Albertsson-Wikland K, Aronson AS, Gustafsson J, Hagenäs L, Ivarsson SA, Jonsson B, et al. Dose-dependent effect of growth hormone on final height in children with short stature without growth hormone deficiency. J Clin Endocrinol Metab. 2008;93:4342–50. doi: 10.1210/jc.2008-0707. [DOI] [PubMed] [Google Scholar]
- 87.Högler W, Briody J, Moore B, Lu PW, Cowell CT. Effect of growth hormone therapy and puberty on bone and body composition in children with idiopathic short stature and growth hormone deficiency. Bone. 2005;37:642–50. doi: 10.1016/j.bone.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 88.Wilson TA, Rose SR, Cohen P, Rogol AD, Backeljauw P, Brown R, et al. Update of guidelines for the use of growth hormone in children: The Lawson Wilkins Pediatric Endocrinology Society Drug and Therapeutics Committee. J Pediatr. 2003;143:415–21. doi: 10.1067/s0022-3476(03)00246-4. [DOI] [PubMed] [Google Scholar]
- 89.Swerdlow AJ, Higgins CD, Adlard P, Preece MA. Risk of cancer in patients treated with human pituitary growth hormone in the UK, 1959-1985: A cohort study. Lancet. 2002;360:273–7. doi: 10.1016/s0140-6736(02)09519-3. [DOI] [PubMed] [Google Scholar]
- 90.Sperling MA, Saenger PH, Ray H, Tom W, Rose SR. Growth hormone treatment and neoplasia - coincidence or consequence? J Clin Endocrinol Metab. 2002;87:5351–2. doi: 10.1210/jc.2002-021467. [DOI] [PubMed] [Google Scholar]
- 91.Reiter EO, Lindberg A, Ranke MB, Price DA, Albertsson-Wikland K, Cowell CT, et al. The KIGS experience with the addition of gonadotropin-releasing hormone agonists to growth hormone (GH) treatment of children with idiopathic GH deficiency. Horm Res. 2003;60(Suppl 1):S68–73. doi: 10.1159/000071229. [DOI] [PubMed] [Google Scholar]
- 92.Carel JC, Ecosse E, Nicolino M, Tauber M, Leger J, Cabrol S, et al. Adult height after long-term recombinant growth hormone treatment for idiopathic isolated growth hormone deficiency: Observational follow-up study of the French population-based registry. Br Med J. 2002;325:70–3. doi: 10.1136/bmj.325.7355.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Van Gool SA, Kamp GA, Visser-van Balen H, Mul D, Waelkens JJ, Jansen M, et al. Final height outcome after three years of growth hormone and gonadotropin-releasing hormone agonist treatment in short adolescents with relatively early puberty. J Clin Endocrinol Metab. 2007;92:1402–8. doi: 10.1210/jc.2006-2272. [DOI] [PubMed] [Google Scholar]
- 94.Pasquino AM, Pucarelli I, Roggini M, Segni M. Adult height in short normal girls treated with gonadotropin-releasing hormone analogs and growth hormone. J Clin Endocrinol Metab. 2000;85:619–22. doi: 10.1210/jcem.85.2.6387. [DOI] [PubMed] [Google Scholar]
- 95.Kamp GA, Mul D, Waelkens JJ, Jansen M, Delemarre-van de Waal HA, Verhoeven-Wind L, et al. A randomized controlled trial of three years growth hormone and gonadotropin- releasing hormone agonist treatment in children with idiopathic short stature and intrauterine growth retardation. J Clin Endocrinol Metab. 2001;86:2969–75. doi: 10.1210/jcem.86.7.7650. [DOI] [PubMed] [Google Scholar]
- 96.Mericq MV, Eggers M, Avila A, Cutler GB, Jr, Cassorla F. Near final height in pubertal growth hormone (GH)-deficient patients treated with GH alone or in combination with luteinizing hormone-releasing hormone analog: Results of a prospective, randomized trial. J Clin Endocrinol Metab. 2000;85:569–73. doi: 10.1210/jcem.85.2.6343. [DOI] [PubMed] [Google Scholar]
- 97.Saggese G, Federico G, Barsanti S, Fiore L. The effect of administering gonadotropin-releasing hormone agonist with recombinant-human growth hormone (GH) on the final height of girls with isolated GH deficiency: Results from a controlled study. J Clin Endocrinol Metab. 2001;86:1900–4. doi: 10.1210/jcem.86.5.7439. [DOI] [PubMed] [Google Scholar]
- 98.Feuillan P, Merke D, Leschek EW, Cutler GB. Use of aromatase inhibitors in precocious puberty. Endocr Relat Cancer. 1999;6:303–6. doi: 10.1677/erc.0.0060303. [DOI] [PubMed] [Google Scholar]
- 99.Leschek EW, Jones J, Barnes KM, Hill SC, Cutler GB. Six-year results of spironolactone and testolactone treatment of familial male-limited precocious puberty with addition of deslorelin after central puberty onset. J Clin Endocrinol Metab. 1999;84:175–8. doi: 10.1210/jcem.84.1.5413. [DOI] [PubMed] [Google Scholar]
- 100.Dunkel L, Wickman S. Novel treatment of short stature with aromatase inhibitors. J Steroid Biochem Mol Biol. 2003;86:345–56. doi: 10.1016/s0960-0760(03)00344-3. [DOI] [PubMed] [Google Scholar]
- 101.Mauras N, Gonzalez de Pijem L, Hsiang HY, Desrosiers P, Rapaport R, Schwartz ID, et al. Anastrozole increases predicted adult height of short adolescent males treated with growth hormone: A randomized, placebo-controlled, multicenter trial for one to three years. J Clin Endocrinol Metab. 2008;93:823–31. doi: 10.1210/jc.2007-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Faglia G, Arosio M, Porretti S. Delayed closure of epiphyseal cartilages induced by the aromatase inhibitor anastrozole: Would it help short children grow up? J Endocrinol Invest. 2000;23:721–3. doi: 10.1007/BF03345059. [DOI] [PubMed] [Google Scholar]
- 103.Wickman S, Sipila I, Ankarberg-Lindgren C, Norjavaara E, Dunkel L. A specific aromatase inhibitor and potential increase in adult height in boys with delayed puberty: A randomised controlled trial. Lancet. 2001;357:1743–8. doi: 10.1016/S0140-6736(00)04895-9. [DOI] [PubMed] [Google Scholar]
- 104.Hero M, Norjavaara E, Dunkel L. Inhibition of estrogen biosynthesis with a potent aromatase inhibitor increases predicted adult height in boys with idiopathic short stature: A randomized controlled trial. J Clin Endocrinol Metab. 2005;90:6396–402. doi: 10.1210/jc.2005-1392. [DOI] [PubMed] [Google Scholar]
- 105.Hero M, Wickman S, Dunkel L. Treatment with the aromatase inhibitor letrozole during adolescence increases near-final height in boys with constitutional delay of puberty. Clin Endocrinol (Oxf) 2006;64:510–3. doi: 10.1111/j.1365-2265.2006.02499.x. [DOI] [PubMed] [Google Scholar]
- 106.Shulman DI, Francis GL, Palmert MP, Eugster EA Lawson Wilkins Pediatric Endocrine Society Drug and Therapeutics Committee. Use of aromatase inhibitors in children and adolescents with disorders of growth and adolescent development. Pediatrics. 2008;121:e 975–83. doi: 10.1542/peds.2007-2081. [DOI] [PubMed] [Google Scholar]


