Abstract
Skeletal age assessment (SAA) is a clinical procedure which is used in determining the SA of children and adolescents. Bone development is influenced by a number of factors, including nutrition, hormonal secretions, and genetics. There are several factors to be borne in mind when using methods of assessing skeletal maturity. These include: Variability among methods, degree of variability in the estimation of skeletal maturation, sources of low accuracy, and dispersion of the values of skeletal maturation. Currently, the main clinical methods for SAA are the Greulich and Pyle (GP) and Tanner and Whitehouse (TW) methods. The GP method has the advantage of being quick and easy to use. A well-trained radiologist takes few minutes to determine the bone age (BA) from a single hand radiograph. The method of TW, however, seems to be more reliable than the GP method. In recent years, the increasing speed in computer sciences and reduction of their cost has given the opportunity to create and use computerized BA estimation system. Despite the fact that the number of automated systems for BAA have increased, most are still within the experimental phase. The use of automated BA determination system, cleared for clinical use in Europe (BoneXpert), has been validated for various ethnicities and children with endocrine disorders. Ultrasound imaging has some limitations that include operator dependence, lower intra-rater and inter-rater reliability of assessment and difficulties with standardization of documentation and imaging transfer. Magnetic resonance imaging (MRI) is noninvasive alternative tool for SA assessment in children. However, few studies have been reported on this topic, and further research is needed to evaluate the reliability and validity of MRI BAAs. In conclusion, at present radiographic methods for the assessment of BA remain the gold standards. Whatever method one adopts, it is essential to minimize the causes of imprecision by taking care to consider the quality of the X-ray. Moreover, it is imperative to assume a correct hand positioning because poor positioning can change the appearance of some bones. It is also preferable to employ scoring methods to these techniques and percentiles rather than BA in years and months. In addition, the possible differences in maturation among different population should be kept in mind.
Keywords: Growth, pediatric endocrinology, puberty, skeletal age assessment
INTRODUCTION
The use of appropriate methods for determining the age is necessary for medical, legal, and sporting contexts. Skeletal age assessment (SAA) and understanding of growth events have an essential role in everyday practice of pediatrician as well as of endocrinologist. Though the developmental status of a child can be assessed from various parameters such as height, weight, secondary sexual characteristics, chronological age (CA), and dental age, the SAA has been considered the most reliable method.[1,2,3]
The level of skeletal maturity can essentially be determined based on two characteristics: The level of growth in areas undergoing the ossification and the level of calcium accumulation in those areas. From infancy to adulthood, these two characteristics follow a certain and specific pattern and timeline.[1,2,3] It is not clear which factors determine a normal maturational pattern, but it is certain that genetics, nutritional, metabolic, social, emotional, environmental factors and hormones, such as thyroxine, growth hormone, and sex steroids play important roles.[1,2,3] SAA is a common procedure in pediatric radiology and is frequently requested as part of the evaluation of children who are either too tall or too short for their CA. Such assessment can also be useful in the management of children with various endocrinopathies and growth disorders because a significant discrepancy between the bone age (BA) and the actual age of a child indicates abnormalities in skeletal development.[3,4,5]
This short review provides bone development information that is necessary to understand the process of skeletal maturation of the hand and wrist and the concept of BA.
BACKGROUND OF SKELETAL AGE ASSESSMENT
There are two ages of a child: The CA and the SA. The CA is the actual age in years, determined from the child's birth date. The SA describes the degree of maturation of a child's bones. The basis for SA is that a particular ossification center appears and matures at a particular time of age.[2,5,7] Therefore, SA is the age at which an average child reaches a particular stage of bone maturation. Changes in human skeletal development are basically similar, as the development process of each bone has continuity and runs through the same stages. At each stage, bones have specific characteristics. Therefore, comparing with CA, SAA is a more accurate way to reflect the level of individual growth development and the degree of maturation.[2,5,6,7]
Nevertheless, we cannot ignore that the rate, duration, and amount of growth and maturation varies widely. Actually, the skeleton of children of the same CA may show marked differences in maturity. Furthermore, the duration and amount of growth varies considerably during the pubertal growth spurt; some individuals mature early with a relatively short pubertal growth spurt, whereas others mature late with a larger pubertal growth spurt.[1,2,5,7]
Conditions that delay skeletal maturation are associated with a postponed onset of puberty, while conditions that accelerate skeletal maturation advance the onset of pubertal development.[8] This synchrony between different maturational processes has suggested the concept of “tempo” to refer to the whole process of maturation.[9]
Skeletal maturation and development
There are two types of hand and wrist bones: Long bones and short bones. Long bones include the radius, ulna, and phalanges. At birth, long bones have more than one ossification center. These grow during childhood until the ends of the bone (epiphyseal plates) become fused with the shaft of the bone (the diaphysis). Many factors influence the proliferation and transformation of cells within the growth plate and the interaction of the growth plate with the metaphysis. For example, growth hormone has an effect on cellular proliferation.[10] A deficiency of thyroid hormone or excess corticosteroids results in a reduction in cell division in the proliferation zone, causing growth retardation.[10]
The timing of epiphyseal ossification and fusion of bones does not happen uniformly across the body. In some bones ossification starts directly after birth, whereas in other bones between 14 and 17 years of age.[11] Females, at any age, have advanced BA when compared to boys. The difference is present at birth and persists throughout growth, although it is slightly more pronounced after the onset of puberty. Moreover, the skeletal maturation process lasts longer in boys than in girls.[12,13] The reasons for these gender discrepancies in skeletal maturity remain unknown. The time period for epiphyseal fusion and closure of the physis also varies; between 10 and 25 years of age, and in girls approximately 2 years earlier than boys.
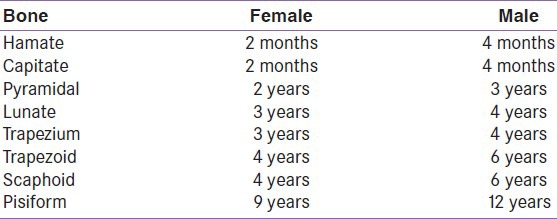
Short bones have no dominant long axis and develop differently from the long bones. Like long bones, significant variations in carpal development can occur due to varying order of appearance of bones, unexpected fusion or partitioning of bones, and the formation of accessory elements from nodules of cartilage. The carpal bones are not ossified at birth, and they ossify solely via their primary ossification centers.[10] Capitate and uncinate bones are the first to show ossification centers (2nd to 4th month) while the pisiform is the last (9–12th year)[11] [Table 1].
Table 1.
Mean age of ossification center appearance in carpal bones
Conventional methods used for skeletal age assessment
Pediatric endocrinologists are the physicians who most commonly order and interpret BA X-rays and evaluate children for advanced or delayed growth and physical development.[3,4,5]
Many different areas of the body (the hand, foot, knee, elbow, shoulder, and hip) have been studied in the hope of developing a method that can provide an accurate assessment of development.[1,2,4] The hand and wrist conveniently possess many bones and epiphyses that mature in a well-defined progression over time and which are also easily evaluated on a single radiograph. This informs the clinician of the relative maturity of a patient at a particular time in his or her life. When integrated with other clinical findings, clinicians can separate the normal from the relatively advanced or retarded.[1,14]
Todd created one of the first atlases describing the progressive maturation of the bones of the hand and wrist in male and female standards at 6-month intervals and also described specific maturity indicators characteristic of each age.[14] At present, the most commonly applied methods used worldwide are the Greulich and Pyle (GP) and Tanner and Whitehouse (TW2, TW3), both are based on radiographs of the left hand and the wrist.[5,7,15] The Fels method is less frequently used.[15]
Greulich and pyle method
The standards determined by GP were developed between 1931 and 1942 from the hand and wrist radiographies of white, upper-middle class male and female children included in the Brush Foundation Growth Study. For this reason, the GP method is commonly used in the United States of America. The standard templates developed for male children consist of 31 radiography images covering the growth stages between 0 and 19 years of age, while the standard templates for female children consists of 27 radiography images covering the growth stages between 0 and 18 years of age. The reason the template series were developed separately for female and male children is related to the fact that bone growth and development displays gender-related differences.
The radiography samples in this template series are then compared with the radiographies of the evaluated case. The comparisons are performed until the radiography that corresponds the most to the studied case's radiography is found. Finding a 100% match is generally difficult. In such cases, the closest matching template radiography is selected.
Tanner-whitehouse method
The TW method is based on obtaining a score for the relevant bones through a detailed structural analysis and the sum of points assigned to the bones based on this analysis.[6] The bones that are subject to ossification analysis are the radius, the ulna, the short bones (RUS), and the carpal bones. For each stage of every bone, separate scores are used for female and male children.
The original system (TW1) was refined and published as TW2 and recently, as TW3.[15]
The TW2 of BAA (BAA) is based on a series of eight maturity indicators for each bone of the hand and wrist and nine for the radius. These maturity indicators were then evaluated not in relation to CA, but in relation to their appearance within the full passage of each specific bone from immaturity to maturity. The reference standards that are used were established in the 1950s and 1960s, and there is evidence that bone maturity is reached sooner now than four or five decades ago. In order to improve accuracy and reproducibility of the TW method, the scoring system, maturity stages, SAs and even the equations for the prediction of adult height have been modified throughout the years. This process led to the publication of the new version of this method, called TW3. Furthermore, the 20-bone score was abolished, and the reference values and charts for RUS were changed based on data from North American children.[13] To date, several scales, converting the skeletal maturity score to BA, have been developed in Belgium,[16] Italy,[17,18] Argentina,[19] Sweden,[20] Japan,[21] and the United States,[22] as well as in other countries. For 10–12-year-olds, the new TW3 reference values are about a year ahead of those described in TW2 but are less at odds with earlier ages.
The TW3 SAA terminates at 16.5 for boys and 15 years for girls, while the GP SAA terminates at 19 and 18 years. In other words, the TW3 method stops 2.5–3 years earlier.
The TW method was developed in the United Kingdom, and its use is preferred in Europe. The GP method is used by over 76% of pediatricians. The average time spent on the assessments was 7.9 min for the TW2 method and 1.4 min for the GP method.
The fels method
Fels method was developed by Roche et al. in order to provide an objective assessment of SA together with a confidence limit. It is based on a sample of North American children followed in the Fels longitudinal study. The data for developing this method were based on 13,823 serial radiographs of the left hand/wrist of 355 boys and 322 girls born between 1928 and 1974. The radiographs were taken from 1 month to 22 years of age.[23] The subject child's radiograph is then scored using both relative maturation levels and measured ratios of epiphyseal and diaphyseal diameters of the appropriate bones of the hand and the wrist. The resultant scores are then entered into a computer program, which calculates the SA.[24,25]
Intra-observer and inter-observer variability
The use of an atlas is inherently subjective; interpretation can vary among observers, and it can prove difficult to find an exact match with an item in the standard series.[26] Intra-observer variability or reliability, refers to the stability of an individual's assessment of BA between two points in time. Inter-observer variability is the agreement between two or more individuals performing an assessment. Both are important parameters for any BAA method because they lead to a reduction in accuracy and reproducibility of BA results. Because the two most commonly used methods of SAA do not give equivalent estimates, Bull et al. suggested that one method only (preferably the TW2) should be used.[27]
Both GP and TW methods suffer from inconsistencies due to the subjective nature of the analysis as performed by various observers with different levels of training. In a study conducted by King et al., BAAs were performed by three evaluators using both the GP and TW2 techniques.[28] The average spread of intraobserver variation was 0.74 years for TW2 method and 0.96 years for the GP method. The difference was statistically significant at the 5% level. The average intraobserver variation to TW2 was 0.33 years but with 95% confidence limits of −0.87 to +1.53 years.[28] In another study, the 95% confidence interval for the difference between the two methods was 2.28−1.52 years. Intra-observer variation was greater for the GP method than for the TW2 method (95% confidence limit: −2.46–2.18 vs. −1.41–1.43).[27]
In addition, GP method stayed unchanged since its initial publication. Some authors have shown that the GP standards must be used with reservations to determine BA in children of today and those with diverse ethnicity, particularly when making clinical decisions requiring accurate SAA in black and Hispanic girls and in Asian and Hispanic boys in late childhood and adolescence.[19,29,30] Another recent study found the GP standards are also imprecise for American children of European and African descent born after 1980, concluding that new standards are needed to make clinical decisions that require reliable BAs and accurately represent a multi-ethnic pediatric population.[31]
Larsen et al. performed a comparative study on GP, TW2-RUS, TW3-RUS and Fels methods. A total of 174 hand-wrist radiographs from young Danes (12–20 years) and young asylum seekers of mixed ethnic origin were blindly assessed by all methods by two of the authors. Reproducibility was least with the GP method. TW2-RUS had the highest accuracy for boys. Fels had the highest accuracy for girls. The authors concluded that more than one method should be considered for SAA.[32]
The Growth Hormone Research Society, in its consensus guidelines in 2000, recommended the routine estimation of BA for children over the age of 1 year for those children with growth failure.[33] They also recommended that the SAA should be performed by an “experienced person,” although they did not specify acceptable accuracy and reproducibility requirements for BAAs in order to gauge what is meant by an “experienced person.”[28,34,35,36]
Knowledge of chronologic age does not affect the reliability of BAAs. However, observers are more likely to interpret the radiograph as normal when chronologic age is known than when it is not. Therefore, it is important that each radiologist, group, or institution adopt a policy indicating whether each will consistently interpret BA studies with or without knowledge of the patient's chronologic age.[37] In general, when a single measurement of BA is required for diagnosis, it has been suggested that a tolerance of ±0.5 years would be acceptable.[36]
Computerized systems for assessing skeletal maturity
The findings of large intra- and inter-observer variability and the unresolved questions of what variability is considered acceptable stimulated the development of an automated system for BAA. The objective of the automated system is to eliminate observer variability and to leave human maturational variance as the main contributor to uncertainty in BAA.
In 1991, Pietka et al.[38] carried out a computer assisted BAA method using phalanx lengths, atlas lengths, and atlas matching under some restrictions of the quality of hand radiographs. Computerized BA estimation has the obvious advantage of saving the radiologist's time because the computer, not the operator, usually rates the bone. The image is digitalized and then represented by a large number of mathematical coefficients. Most of the automated systems for estimation of BA derived the state of skeletal maturity from X-ray images of the left-hand wrist.
In the following years, more than 15 attempts have been developed to automate the SAA procedure. The analysis starts with a series of preprocessing steps, which is used to correct the image orientation and remove unwanted background, especially soft tissues. The next procedure of the analysis is localization of the regions of interest (ROI), which can extract all the bones of interest in a digitized hand/wrist radiograph. Then, the indicators of each bone are analyzed by a sequence of image processing algorithms.[39] However, it should be taken into account that the ROI extraction stage presents the main challenge for the current automated system. This is the reason why the number of algorithms based on the hand wrist presented in the literature suffered the problem of segmentation of special regions in the X-ray image. In addition, the lack of sufficient image processing techniques leads to low accuracy of assessment of BA. Therefore, validation remains the main challenge for the current automated systems.[40,41]
A fully automated BA method (BoneXpert; Visiana, Denmark), which appears to be clinically acceptable, was presented in 2008. The architecture of BoneXpert divides the processing into three layers. Layer A reconstructs the borders of 15 bones. Layer B determines bone maturity values, called intrinsic BAs, for 13 of these 15 bones based on the appearance of the bone. If a BA value deviates more than 2.4 years from an average of all the bones, it is deemed unacceptable. Layer C transforms the computed intrinsic BAs to agree on average with GP BA based on the training set of images with manual ratings.[40,41] The software does not take into account the development of the carpal bones and is restricted to a BA range of 2.5–17 years for boys and 2.0–15.0 years for girls.
The accuracy of the automated BA determination of BoneXpert, at present the only automated system cleared for clinical use in Europe, is 0.71–0.72 year, and the precision is 0.17–0.18 year.[40,41,42] In addition, the use of this software has been validated for various ethnicities and children with endocrine disorders.[41,42] The time required to automatically analyze each image varies from 1.5 min to 4 min. It highly depends on the orientation correction step. If the original image has a standard orientation of the hand/wrist, it is not necessary to rotate the image; therefore, sometimes is saved. On the other hand, if a high degree of hand/wrist rotation presents in the original image, or, even worse, if also an angle exists between hand and forearm, more time is needed to perform the rotation.
The time for a BoneXpert analysis is 5 s, and it automatically accepts any hand orientation as well as both left and right hands. BoneXpert rejected 14 of 1097 radiographs due to abnormal morphology (n = 3), bad image quality (n = 3), BA being below the software's limit of 2/2.5 years (n = 3), or inefficiency of the method due to poor contrast (n = 1) or too small hands, which were analyzed correctly when magnified by 20% (n = 3).[42]
Automated SAA does not completely eliminate the radiologist and/or pediatric evaluation. Several disorders can be read from the image, such as: Defects of chondrogenesis and/or osteogenesis (hypochondroplasia), irregular metaphyses (frayed radial and ulnar metaphyses with some expansion in rickets; metaphyseal condrodysplasia), shortness of 4th metacarpal, triangularization of the distal radial epiphysis, pyramidalization of the distal carpal row or lucency of the distal ulnar border of the radius (Leri-Weil and Turner Syndrome), shortness of 4th and 5th metacarpal (pseudohypoparathyroidism), Harris lines (expression of temporary arrest of long bone growth) and disorders of bone mineralization (osteochondrodysplasias).[43,44]
The main obstacles for BoneXpert introduction seem to be: Opposition from the local administrators toward installing the software, the omission of the carpals, the incomplete BA range or the reluctance to use money on an automated system for a procedure, which can be done manually.[44]
Other methods and future directions and research
There also are other available, proven methods for assessing BA, in particular:
Ultrasound imaging
Sonographic evaluation is based on the maturation of an epiphysis, by virtue of enchondral ossification, which is strongly related to the systemic bone development. Quantitative ultrasound technology is a radiation-free method currently used for bone composition assessment. It measures the speed of sound of US waves propagating along a specific bone distance.
The method uses two transducers, one that produces ultrasonic waves with a frequency of 750 kHz directed at the epiphysis of the distal end of ulna and radius whereas the other acts a receiver. The technique utilizes the width of the growth plate as a hypoechoic area to determine the width of epiphysis of distal of radius, in three views: Anterior, posterior and lateral.[45,46,47,48] The entire process takes about 5 min in which eleven cycles of measurement are completed to provide accurate results.
Zadik et al.[49] assessed the ability of the Sunlight Medical ultrasonic system to accurately predict BA obtained by the GP method and showed that the BA device measurements were highly reproducible and highly correlated with conventional BA readings. Shimura et al. measured BA ultrasonically with sunlight BA (SBA) and compared the results with radiographs. They showed that a good correlation existed with SBA results and TW2 method.[50] In contrast, Khan et al. suggested that ultrasonic assessment should not yet be considered a valid replacement for radiographic BA determination.[47]
In summary, BA calculated using ultrasound is still in initial stages and needs further refinement. The limitations of ultrasound assessment include its operator dependence, the likely lower intrarater and interrater reliability of assessment, and the difficulties with standardization of documentation and imaging transfer. Although, initial studies on a comparison with the GP atlas standards show promising results,[41,51] this method needs to be evaluated in a multiethnic population with a large sample size before its wide-scale use.[52,53,54,55]
Hand magnetic resonance imaging
Magnetic resonance imaging (MRI) is s noninvasive and alternative tool for SA assessment in children, although few studies have reported on this topic. MRI has the advantage of having excellent soft tissue contrast with a multiplanar cross-sectional imaging capability. Cartilage is well-visualized in MRI, and its growth assessment might provide additional important information on skeletal maturity during infancy.[56,57] The SA assessed by MR rating demonstrated a strong positive correlation with CA. The intrarater and inter-rater reproducibilities were significantly high. These findings indicate that MRI could be a potential powerful, non-invasive, and non-irradiative method for assessment of SAA in children.[56,57]
Clinical application of bone age readings
Skeletal maturity is one of the most commonly used index used by pediatrician and endocrinologists in routine clinical work because it is closely related to somatic and sexual and maturity. Substantially, the main applications of SAA in growth disorders (e.g. growth hormone deficiency, hypothyroidism, hyperthyroidism, precocious or delayed puberty, and adreno-genital syndrome) include measuring the effect of the disease on bone maturation, prediction of adult height and monitoring of growth hormone placement therapy [Figure 1].[1,3,54,58] An early detection of such conditions is very important for the treatment and thus SAA has come to the center of public attention. Apart from the effect of endocrine diseases, SAA is delayed in the constitutional delay of growth and puberty and nutritional disorders. A greater delay in BA has been found in undernourished children who were small for gestational age.[59]
Figure 1.
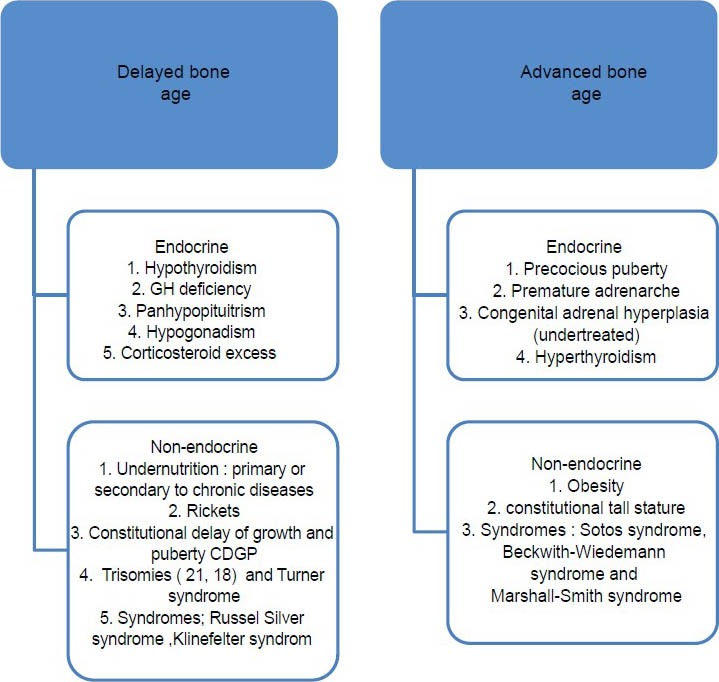
Important causes for delayed and advanced bone age
Greater discordance between SA and CA occurs in children who start puberty early, as their SA is accelerated.[58] BA may be significantly advanced in genetic overgrowth syndromes, such as Sotos syndrome, Beckwith-Wiedemann syndrome and Marshall-Smith syndrome. Those with an advanced BA typically hit a growth spurt early on but stop growing at an earlier age. The BA is often marginally advanced in premature adrenarche, when a child is overweight from a young age or when a child has lipodystrophy.[60]
Focal increases in maturation may occur following infection, burns, frostbites, radiation therapy or trauma, particularly epiphyseal separations. Evaluation of width of the carpus can be useful in the evaluation of juvenile rheumatoid arthritis as well as many bone dysplasias.[59] The premature closure of the epiphysis may occur as the result of bone infarcts, particularly in sickle cell disease. A disharmonic maturation or a bilateral asymmetry has been reported in congenital malformation syndromes.[61,62] Many genetic disorders can often be diagnosed by clinical and imaging examination of the patient's hand and feet. An interesting description of the these abnormalities has been reported by Mankin et al.[63]
Skeletal age assessment is also used for consultation in planning orthopedic procedures (e.g. surgical management of scoliosis or leg-length discrepancy), determination of CA for adopted children, youth sports participation, forensic scientists when the date of birth of the child/adolescent is unknown or cannot be confirmed and for the identification of human remains especially in mass fatalities.[64,65]
CONCLUSIONS
Skeletal age or BAA using a radiograph of the left hand is frequently requested as part of the evaluation of children with various endocrinopathies or in children with malformation syndromes, and in planning orthopedic procedures in which the outcome may be influenced by subsequent growth of the child. It is also used when CA is not available for minors without known birth dates. The literature review shows that there are three main methods of BAA: The GP method, TW method and the Fels method from the Fels Research Institute. A discrepancy between CA and SAA indicates abnormalities in skeletal development and has some important implications in the diagnosis and management of endocrine disorders. An accurate and reproducible SAA is a difficult and time-consuming. Part of the problem with the accuracy arises from the subjectivity in interpreting the skeletal maturity indicators of the radiograph. All manual methods suffer from this, but the TW and Fels methods are thought to be more objective than the GP method. In recent years, a newer method for automatic BAA called BoneXpert is very promising, and the software has been validated for various ethnicities. Therefore, it is hoped that the development of a computerized method of assessment will lead to greater accuracy in the determination of skeletal maturity.
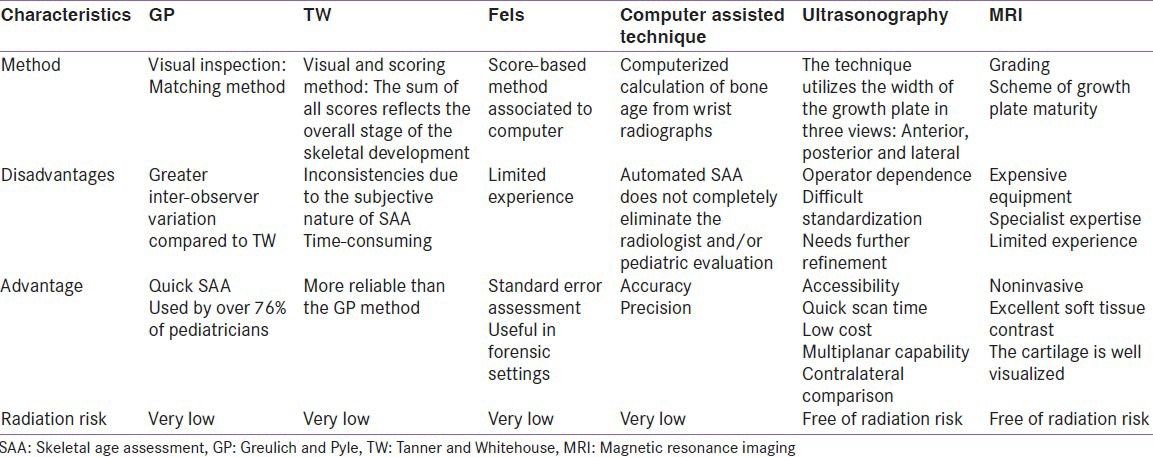
Ultrasound has some limitations that include operator dependence, lower intra-rater and inter-rater reliability of assessment and difficulties with standardization of documentation and imaging transfer. MRI is s noninvasive and alternative tool for SAA in children, although further research is needed to evaluate the reliability and validity of BAAs with this procedure. The pros and cons of different methods for the hand SAA are summarized in Table 2.
Table 2.
Pros and cons of different methods for the hand skeletal maturity assessment
In conclusion, at present radiographic methods for the assessment of BA remains the gold standard. Whatever method one adopts, it is important to minimize the causes of imprecision by taking very good quality X-ray image and assure a correct hand positioning [Table 3]. It is also preferable to employ scoring methods for increasing accuracy and to bear in mind the possible differences in maturation among different population.
Table 3.
Correct positioning for hand skeletal maturity assessment

Footnotes
Source of Support: Nil
Conflict of Interest: No.
REFERENCES
- 1.Tanner JM, Whitehouse RH. 2nd ed. Springfield, Illinois, USA: Blackwell Scientific Publications; 1962. Growth at Adolescence. [Google Scholar]
- 2.Gilsanz, Ratib O. Berlin, Heidelberg: Springer-Verlag; 2005. Hand Bone Age. [Google Scholar]
- 3.Gaskin CM, Kalm SL. USA: Oxford University Press; 2011. Skeletal Development of the Hand and Wrist: A Radiographis Atlas and Digital Bone Age Companion; pp. 30–42. [Google Scholar]
- 4.Oestreich AE. Berlin-Heidelberg: Springer; 2010. Growth of the Pediatric Skeleton: A Primer for Radiologists. Kindle edition; pp. 20–42. [Google Scholar]
- 5.Greulich WW, Pyle SI. 2nd ed. Stanford, California, USA: Stanford University Press; 1959. Radiograph Atlas of Skeletal Development of the Hand and Wrist. [Google Scholar]
- 6.Fishman LS. Radiographic evaluation of skeletal maturation. A clinically oriented method based on hand-wrist films. Angel Orthod. 1982;52:88–112. doi: 10.1043/0003-3219(1982)052<0088:REOSM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Tanner JM, Whitehouse RH, Cameron N, Marshall WA, Healy MJ, Goldstein H. London: Academic Press; 1983. Assessment of skeletal maturity and prediction of adult height. (TW 2 method) [Google Scholar]
- 8.Flor-Cisneros A, Leschek EW, Merke DP, Barnes KM, Coco M, Cutler GB, Jr, et al. In boys with abnormal developmental tempo, maturation of the skeleton and the hypothalamicpituitary-gonadal axis remains synchronous. J Clin Endocrinol Metab. 2004;89:236–41. doi: 10.1210/jc.2002-021954. [DOI] [PubMed] [Google Scholar]
- 9.Tanner JM. Issues and advances in adolescent growth and development. J Adolesc Health Care. 1987;8:470–8. doi: 10.1016/0197-0070(87)90048-9. [DOI] [PubMed] [Google Scholar]
- 10.Buckwalter JA, Einhorn TA, Simon SR, editors. 2nd ed. Rosemont, Illinois: American Academy of Orthopaedic Surgery; 2000. Orthopaedic Basic Science. [Google Scholar]
- 11.Garn SM, Rohmann CG. Variability in the order of ossification of the bony centers of the hand and wrist. Am J Phys Anthropol. 1960;18:219–30. doi: 10.1002/ajpa.1330180309. [DOI] [PubMed] [Google Scholar]
- 12.Mora S, Boechat MI, Pietka E, Huang HK, Gilsanz V. Skeletal age determinations in children of European and African descent: Applicability of the Greulich and Pyle standards. Pediatr Res. 2001;50:624–8. doi: 10.1203/00006450-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Gilsanz V, Kovanlikaya A, Costin G, Roe TF, Sayre J, Kaufman F. Differential effect of gender on the sizes of the bones in the axial and appendicular skeletons. J Clin Endocrinol Metab. 1997;82:1603–7. doi: 10.1210/jcem.82.5.3942. [DOI] [PubMed] [Google Scholar]
- 14.Todd Y. St. Louis: C. V Mosby Co; 1937. Atlas of Skeletal Maturation (Hand) [Google Scholar]
- 15.Tanner JM, Whitehouse RH, Cameron N, Marshall WA, Healy MJ, Goldstein NH. 3rd ed. London: WB Saunders; 2001. Assessment of Skeletal Maturity and Prediction of Adult Height (TW3 Method) [Google Scholar]
- 16.Beunen G, Lefevre J, Ostyn M, Renson R, Simons J, Van Gerven D. Skeletal maturity in Belgian youths assessed by the Tanner-Whitehouse method (TW2) Ann Hum Biol. 1990;17:355–76. doi: 10.1080/03014469000001142. [DOI] [PubMed] [Google Scholar]
- 17.Vignolo M, Naselli A, Magliano P, Di Battista E, Aicardi M, Aicardi G. Use of the new US90 standards for TW-RUS skeletal maturity scores in youths from the Italian population. Horm Res. 1999;51:168–72. doi: 10.1159/000023352. [DOI] [PubMed] [Google Scholar]
- 18.Vignolo M, Milani S, DiBattista E, Naselli A, Mostert M, Aicardi G. Modified Greulich-Pyle, Tanner-Whitehouse, and Roche-Wainer-Thissen (knee) methods for skeletal age assessment in a group of Italian children and adolescents. Eur J Pediatr. 1990;149:314–7. doi: 10.1007/BF02171555. [DOI] [PubMed] [Google Scholar]
- 19.Lejarraga H, Guimarey L, Orazi V. Skeletal maturity of the hand and wrist of healthy Argentinian children aged 4-12 years, assessed by the TWII method. Ann Hum Biol. 1997;24:257–61. doi: 10.1080/03014469700004982. [DOI] [PubMed] [Google Scholar]
- 20.Taranger J, Karlberg J, Bruning B, Engström I. Standard deviation score charts of skeletal maturity and its velocity in Swedish children assessed by the Tanner-Whitehouse method (TW2-20) Ann Hum Biol. 1987;14:357–65. doi: 10.1080/03014468700009141. [DOI] [PubMed] [Google Scholar]
- 21.Ashizawa K, Kumakura C, Zhou X, Jin F, Cao J. RUS skeletal maturity of children in Beijing. Ann Hum Biol. 2005;32:316–25. doi: 10.1080/03014460500087725. [DOI] [PubMed] [Google Scholar]
- 22.Tanner J, Oshman D, Bahhage F, Healy M. Tanner-Whitehouse bone age reference values for North American children. J Pediatr. 1997;131:34–40. doi: 10.1016/s0022-3476(97)90000-7. [DOI] [PubMed] [Google Scholar]
- 23.Roche AF, Chumlea WC, Thissen D. 1st ed. Illinois, USA: Charles C Thomas, Springfield; 1988. Assessing the Skeletal Maturity of the Hand-Wrist: FELS Method. [DOI] [PubMed] [Google Scholar]
- 24.Roche AF. Cambridge: Cambridge University Press; 1992. Growth, Maturation and Body Composition: The Fels Longitudinal Study 1929-1991. [Google Scholar]
- 25.Chumlea WC, Roche AF, Thissen D. The FELS Method of assessing the skeletal maturity of the hand-wrist. Am J Hum Biol. 1989;1:175–83. doi: 10.1002/ajhb.1310010206. [DOI] [PubMed] [Google Scholar]
- 26.Cox LA. Tanner-Whitehouse method of assessing skeletal maturity: Problems and common errors. Horm Res. 1996;45:53–5. doi: 10.1159/000184848. [DOI] [PubMed] [Google Scholar]
- 27.Bull RK, Edwards PD, Kemp PM, Fry S, Hughes IA. Bone age assessment: A large scale comparison of the Greulich and Pyle, and Tanner and Whitehouse (TW2) methods. Arch Dis Child. 1999;81:172–3. doi: 10.1136/adc.81.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King DG, Steventon DM, O’Sullivan MP, Cook AM, Hornsby VP, Jefferson IG, et al. Reproducibility of bone ages when performed by radiology registrars: An audit of Tanner and Whitehouse II versus Greulich and Pyle methods. Br J Radiol. 1994;67:848–51. doi: 10.1259/0007-1285-67-801-848. [DOI] [PubMed] [Google Scholar]
- 29.Loder RT, Estle DT, Morrison K, Eggleston D, Fish DN, Greenfield ML, et al. Applicability of the Greulich and Pyle skeletal age standards to black and white children of today. Am J Dis Child. 1993;147:1329–33. doi: 10.1001/archpedi.1993.02160360071022. [DOI] [PubMed] [Google Scholar]
- 30.Ontell FK, Ivanovic M, Ablin DS, Barlow TW. Bone age in children of diverse ethnicity. AJR Am J Roentgenol. 1996;167:1395–8. doi: 10.2214/ajr.167.6.8956565. [DOI] [PubMed] [Google Scholar]
- 31.Mora S, Boechat MI, Pietka E, Huang HK, Gilsanz V. Skeletal age determinations in children of European and African descent: Applicability of the Greulich and Pyle standards. Pediatr Res. 2001;50:624–8. doi: 10.1203/00006450-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Larsen ST, Demant S, Lynnerup N. The 81st Annual Meeting of the American Association of Physical Anthropologists; 2012. GPA, TW2RUS, TW3RUS and FELS - A Comparative Study on Bone-Age of the Left Hand and Wrist. abstract. [Google Scholar]
- 33.Growth Hormone Research Society. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: Summary statement of the GH Research Society. GH Research Society. J Clin Endocrinol Metab. 2000;85:3990–3. doi: 10.1210/jcem.85.11.6984. [DOI] [PubMed] [Google Scholar]
- 34.Roche AF, Davila GH, Pasternack BA, Walton MJ. Some factors influencing the replicability of assessments of skeletal maturity (Greulich-Pyle) Am J Roentgenol Radium Ther Nucl Med. 1970;109:299–306. doi: 10.2214/ajr.109.2.299. [DOI] [PubMed] [Google Scholar]
- 35.Roche AF, Rohmann CG, French NY, Dávila GH. Effect of training on replicability of assessments of skeletal maturity (Greulich-Pyle) Am J Roentgenol Radium Ther Nucl Med. 1970;108:511–5. doi: 10.2214/ajr.108.3.511. [DOI] [PubMed] [Google Scholar]
- 36.Buckler JM. How to make the most of bone ages. Arch Dis Child. 1983;58:761–3. doi: 10.1136/adc.58.10.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berst MJ, Dolan L, Bogdanowicz MM, Stevens MA, Chow S, Brandser EA. Effect of knowledge of chronologic age on the variability of pediatric bone age determined using the Greulich and Pyle standards. AJR Am J Roentgenol. 2001;176:507–10. doi: 10.2214/ajr.176.2.1760507. [DOI] [PubMed] [Google Scholar]
- 38.Pietka E, McNitt-Gray MF, Kuo ML, Huang HK. Computer-assisted phalangeal analysis in skeletal age assessment. IEEE Trans Med Imaging. 1991;10:616–20. doi: 10.1109/42.108597. [DOI] [PubMed] [Google Scholar]
- 39.Adeshina SA. A Thesis Submitted to the University of Manchester for the Degree of Doctor of Philosophy in the Faculty of Medical and Human Sciences; 2010. Automatic Determination of Skeletal Maturity Using Statistical Models of Appearance; pp. 1–168. [Google Scholar]
- 40.van Rijn RR, Lequin MH, Thodberg HH. Automatic determination of Greulich and Pyle bone age in healthy Dutch children. Pediatr Radiol. 2009;39:591–7. doi: 10.1007/s00247-008-1090-8. [DOI] [PubMed] [Google Scholar]
- 41.Thodberg HH, Kreiborg S, Juul A, Pedersen KD. The BoneXpert method for automated determination of skeletal maturity. IEEE Trans Med Imaging. 2009;28:52–66. doi: 10.1109/TMI.2008.926067. [DOI] [PubMed] [Google Scholar]
- 42.Thodberg HH. Clinical review: An automated method for determination of bone age. J Clin Endocrinol Metab. 2009;94:2239–44. doi: 10.1210/jc.2008-2474. [DOI] [PubMed] [Google Scholar]
- 43.Binder G, Ranke MB, Martin DD. Auxology is a valuable instrument for the clinical diagnosis of SHOX haploinsufficiency in school-age children with unexplained short stature. J Clin Endocrinol Metab. 2003;88:4891–6. doi: 10.1210/jc.2003-030136. [DOI] [PubMed] [Google Scholar]
- 44.De Sanctis V, Soliman AT, Di Maio S, Bedair S. Are the new automated methods for bone age estimation advantageous over the manual approaches? Pediatr Endocrinol Rev. 2014 In press. [PubMed] [Google Scholar]
- 45.Manzoor Mughal A, Hassan N, Ahmed A. Bone age assessment methods: A critical review. Pak J Med Sci. 2014;30:211–5. doi: 10.12669/pjms.301.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mentzel HJ, Vilser C, Eulenstein M, Schwartz T, Vogt S, Böttcher J, et al. Assessment of skeletal age at the wrist in children with a new ultrasound device. Pediatr Radiol. 2005;35:429–33. doi: 10.1007/s00247-004-1385-3. [DOI] [PubMed] [Google Scholar]
- 47.Khan KM, Miller BS, Hoggard E, Somani A, Sarafoglou K. Application of ultrasound for bone age estimation in clinical practice. J Pediatr. 2009;154:243–7. doi: 10.1016/j.jpeds.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Karami M, Moshirfatemi A, Daneshvar P. Age determination using ultrasonography in young football players. Adv Biomed Res. 2014;3:174. doi: 10.4103/2277-9175.139192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zadik Z, Bistrizer T, Tsoref L, Schwartz T, Yaniv I. Prague: Europediatrics; 2003. [Last cited on 2012 Oct 19]. A Novel Method for Assessing Bone Age Using Ultrasound. Available from: http://www.sunlightnet.com/international/html/BAZadik2003.pdf . [Google Scholar]
- 50.Shimura N, Satomi K, Osamu A, Imataka M, Sato K, Matsuura M. Assessment of measurement of children's bone age ultrasonically with Sunlight BonAge. Clin Pediatr Endocrinol. 2005;14:17–20. [Google Scholar]
- 51.Shimura N, Koyama S, Arisaka O, Imataka M, Sato K, Matsuura M. Assessment of measurement of children's bone age ultrasonically with Sunlight BonAge. Clin Pediatr Endocrinol. 2005;14(Suppl 24):17–20. [Google Scholar]
- 52.Sato K, Mitani H. Evaluation of bone maturation with a computer. Clin Pediatr Endocrinol. 1999;8(Suppl 12):13–6. [Google Scholar]
- 53.Peloschek P, Nemec S, Widhalm P, Donner R, Birngruber E, Thodberg HH, et al. Computational radiology in skeletal radiography. Eur J Radiol. 2009;72:252–7. doi: 10.1016/j.ejrad.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 54.Martin DD, Wit JM, Hochberg Z, Sävendahl L, van Rijn RR, Fricke O, et al. The use of bone age in clinical practice - part 1. Horm Res Paediatr. 2011;76:1–9. doi: 10.1159/000329372. [DOI] [PubMed] [Google Scholar]
- 55.Rachmiel M, Naugolani L, Mazor-Aronovitch K, Levin A, Koren-Morag N, Bistritzer T. Bone age assessment by a novel quantitative ultrasound based device (SonicBone), is comparable to the conventional Greulich and Pyle method. Horm Res Pediatr. 2013;80(Suppl 1):35. [Google Scholar]
- 56.Terada Y, Kono S, Tamada D, Uchiumi T, Kose K, Miyagi R, et al. Skeletal age assessment in children using an open compact MRI system. Magn Reson Med. 2013;69:1697–702. doi: 10.1002/mrm.24439. [DOI] [PubMed] [Google Scholar]
- 57.Terada Y, Kono S, Uchiumi T, Kose K, Miyagi R, Yamabe E, et al. Improved reliability in skeletal age assessment using a pediatric hand MR Scanner with a 0.3T permanent magnet. Magn Reson Med Sci. 2014;13:215–9. doi: 10.2463/mrms.2013-0098. [DOI] [PubMed] [Google Scholar]
- 58.Martin DD, Wit JM, Hochberg Z, van Rijn RR, Fricke O, Werther G, et al. The use of bone age in clinical practice – Part 2. Horm Res Paediatr. 2011;76:10–6. doi: 10.1159/000329374. [DOI] [PubMed] [Google Scholar]
- 59.Poznanski AK. Useful measurements in the evaluation of hand radiographs. Hand Clin. 1991;7:21–36. [PubMed] [Google Scholar]
- 60.Pinhas-Hamiel O, Benary D, Mazor-Aronovich K, Ben-Ami M, Levy-Shraga Y, Boyko V, et al. Advanced bone age and hyperinsulinemia in overweight and obese children. Endocr Pract. 2014;20:62–7. doi: 10.4158/EP13193.OR. [DOI] [PubMed] [Google Scholar]
- 61.Poznanski AK, Garn SM, Kuhns LR, Shaw HA. Disharmonic skeletal maturation in the congenital malformation syndromes. Birth Defects Orig Artic Ser. 1977;13:45–65. [PubMed] [Google Scholar]
- 62.Poznanski AK, Garn SM, Kuhns LR, Sandusky ST. Dysharmonic maturation of the hand in the congenital malformation syndromes. Am J Phys Anthropol. 1971;35:417–32. doi: 10.1002/ajpa.1330350322. [DOI] [PubMed] [Google Scholar]
- 63.Mankin HJ, Jupiter J, Trahan CA. Hand and foot abnormalities associated with genetic diseases. Hand (N Y) 2011;6:18–26. doi: 10.1007/s11552-010-9302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bergeron MF, Bahr R, Bärtsch P, Bourdon L, Calbet JA, Carlsen KH, et al. International Olympic Committee consensus statement on thermoregulatory and altitude challenges for high-level athletes. Br J Sports Med. 2012;46:770–9. doi: 10.1136/bjsports-2012-091296. [DOI] [PubMed] [Google Scholar]
- 65.Vogelsang F, Kohnen M, Schneider H, Weiler F, Wein BB, Kilbinger M, et al. Skeletal maturity determination from hand radiograph by model based analysis. In: Hanson KM, editor. Processing Medical Imaging 2000; Image Processing. Vol. 3979. San Diego, USA: 2000. pp. 294–305. [Google Scholar]


