Abstract
Introduction:
Ataxia telangiectasia (AT) is a rare, genetic, primary immune deficiency disease characterized by immunodeficiency and neurological manifestations, with an increased tendency to infection, malignancy, and autoimmune diseases. Both growth delay and endocrine abnormalities are occasionally reported in these patients.
Patients and Methods:
We studied growth parameters height (Ht), weight, body mass index (BMI) and calculated the Ht standard deviation scores (HtSDS) of 13 patients (age 7.7 ± 3.5 years-age range: 3–14.5 years) with AT in relation to their mid-parental Ht SDS (MPHtSDS). We measured their serum calcium (Ca), phosphorus (PO4), alkaline phosphatase, alanine transferase (ALT), serum ferritin, creatinine and albumin concentrations. Endocrine investigations included the assessment of serum free thyroxine (FT4), thyrotropin (TSH), insulin-like growth factor-I (IGF-I) and morning cortisol. Complete blood count and serum immunoglobulins (IgG, IgM and IgA antibodies) were also measured. Growth data were correlated to hormonal and immune data.
Results:
About 31% of patients with AT had short stature (HtSDS <−2). However, their MPHtSDS denoted that their short stature was familial because four out of 13 had MPHtSDS <−2. They had low BMI, and two of them had low serum albumin and IGF-I, denoting malnutrition or disturbed growth hormone secretion. Elevated serum ALT and ferritin in some patients suggest immune-related inflammation in the liver. 30% of patients had high TSH, two of them had low FT4 diagnosing overt (15%) and sub-clinical (15%) hypothyroidism. Anti-thyroid peroxidase antibodies were high in two out of 13 patients denoting immune-related thyroid aggression. Eight out of 13 patients had Vitamin D deficiency (<20 ng/ml) however, their serum Ca and PO4 levels were in the normal range. One adolescent girl (14.5 years) had hyper-gonadotropic hypogonadism (low estradiol and high follicle stimulating hormone). All patients had normal 8 AM cortisol and renal function. None of the growth parameters were correlated with the IgG, IgM or IgA levels.
In summary:
Patients with AT had a high prevalence of growth retardation and endocrine dysfunction in the form of low IGF-I, overt and subclinical hypothyroidism and hypogonadism. Physicians should be aware of these possible endocrinopathies for an early diagnosis and proper treatment.
Keywords: Ataxia telangiectasia, endocrine functions, growth
INTRODUCTION
Ataxia telangiectasia (AT) develops when there are alterations in a specific gene known as AT mutated (ATM), located on chromosome 11 at position q22.3. The ATM gene can produce an enzyme “serine/threonine kinase” that has several important functions. It acts as a tumor suppressor and coordinates DNA repair by activating other proteins that are essential for the repair process to occur. Primary immunodeficiency diseases encompass a rare, genetically diverse, group of disorders that affect distinct components of the innate and adaptive immune system resulting in defective differentiation, function, or both of the components.[1] They make the affected individuals quite susceptible to infections, malignancies and autoimmune disorders.[2,3,4]
Retardation of somatic growth with significant dwarfism is observed in a large proportion of the patients. The heights (Hts) and weights of children aged 4–7 years are typically at the 10th percentile. Only an exceptional patient with AT achieves somatic growth at the 50th percentile or beyond. Patients with AT who develop normal puberty are the ones most likely to achieve somatic growth within the normal range.[5,6,7]
The stunting of growth is not well-understood and appears to be multifactorial. Although chronic sino-pulmonary disease may be a contributing factor, stunting of growth also occurs in its absence. Other factors may include the possible affection of the hypothalamic-pituitary axis, e.g. growth hormone-insulin like growth factor-I (IGF-I) axis and hypothalamic pituitary gonadal axis secondary to the neurodegenerative process in the brain and/or due to immune aggression against the endocrine glands (thyroid, ovary). Endocrine studies performed on patients with AT provided different results. Although growth retardation and abnormal puberty are common manifestations of AT, endocrine data are conflicting.[6,8,9,10] While some Authors have not documented endocrine dysfunction, other investigators have reported hypothyroidism and hypogonadism in a considerable number of their patients.[10,11,12,13,14,15,16,17]
In this study, we report linear growth parameters in relation to endocrine and immune functions in a cohort of children with AT.
PATIENTS AND METHODS
We studied growth parameters: Ht, weight, body mass index (BMI) and calculated the Ht standard deviation scores (HtSDS) of 13 patients (age 7.7 ± 3.5 years-age range: 3–14.5 years) with AT in relation to their mid-parental Ht SDS (MPHtSDS). We measured their serum calcium (Ca), phosphorus (PO4), alkaline phosphatase (ALP), alanine transferase (ALT), serum ferritin, creatinine and albumin concentrations. Endocrine investigations included: Measurement of serum levels of free thyroxine (FT4), thyrotropin (TSH), anti-thyroid peroxidase antibodies (ATPO), IGF-I, and morning cortisol. Complete blood count and serum immunoglobulins (IgG, IgM and IgA antibodies) were also measured, simultaneously. Growth parameters were correlated to hormonal and immune data.
RESULTS
Patients with AT (n = 13) had a HtSDS −1.4 ± 1.2. 38% of them had a HtSDS <−2. However, their midparental HtSDS was −1.3 ± 1.1 and 31% had a HtSDS <−2. Patients HtSDS was correlated significantly with their MPHtSDS (r = 0.425, P < 0.001), [Figure 1]. Patients BMI was low in 31% of them (BMISDS <−2) [Table 1]. All AT patients had normal renal function, serum calcium, phosphate, ALP and fasting glucose concentrations. Two patients with low BMISDS (<-2) had low serum albumin and IGF-I concentrations denoting malnutrition.
Figure 1.
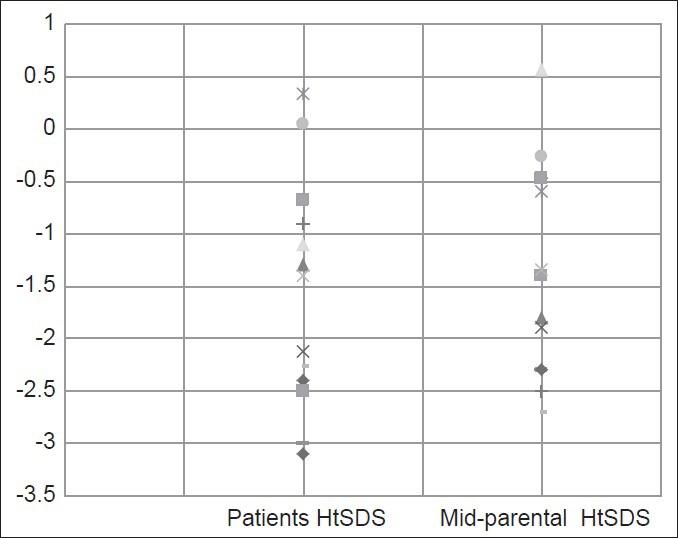
Height standard deviation scores of 13 patients with ataxia telangiectasia and their parents
Table 1.
Anthropometric and hormonal data in children with AT
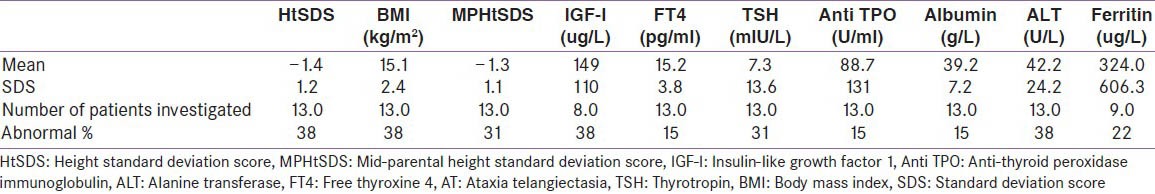
Patients with AT had low IGF-I (IGF-I SDS <−1.5). Elevated serum ALT (38%) and serum ferritin (22%) were found in some patients suggesting an immune-related inflammation in the liver. About 31% of patients had high TSH, two of them (15%) had low FT4 diagnosing clinical hypothyroidism, and another 2 (15%) had normal FT4 (sub-clinical hypothyroidism). Thyroid ATPO was high in two out of 13 patients denoting an immune-related thyroid aggression. Eight out of 13 patients had Vitamin D deficiency (<20 ng/ml) however, their serum Ca and PO4 levels were in the normal laboratory range. One adolescent girl (14.5 year) had hyper-gonadotropic hypogonadism. She presented with delayed puberty, no breast development (Tanner-1) and low serum estradiol and high follicle stimulating hormone concentrations. All patients had normal AM cortisol [Table 1]. None of the growth parameters, FT4, TSH or IGF-I concentrations were correlated significantly with the IgG, IgM or IgA levels.
DISCUSSION
In this study, we report a high prevalence of short stature and low IGF-I secretion in children with AT. Many factors can explain this growth delay in our patients. It is possible that their short parents (MPHtSDS: −1.3 ± 1.1), carrying the gene (carrier state-recessive transmission), constituted a genetic background explanation for their short stature. Recent data suggest that ATM gene is a modulator of IGF-1 signaling downstream of insulin receptor substrate one in skeletal muscle.[11,12,18] In addition, our patients had low IGF-I concentrations that may be explained by their undernourished state (recurrent infections and poor appetite). In the agreement to our observations, Schubert et al. reported IGF-1 and IGF-binding protein 3 deficiency in 56% and 83% respectively of their patients with AT.[10] Kieslich et al. reported pronounced deficiency of the growth hormone (GH)/IGF-1 axis accompanied by markedly reduced body weight and high ataxia scores especially in patients with central cerebral white matter affection, spinal atrophy, and extrapyramidal symptoms.[11]
Low IGF-I secretion may also be secondary to defective secretion of GH secondary to the neuro-degenerative process affecting the hypothalamic-pituitary region.[19] Three autopsy pituitaries from patients with AT showed adenohypophyseal cells with cyto-and nucleomegaly, as well as pleomorphism.[13]
Impaired hepatic function in the form of low albumin and high ALT may also lead to defective hepatic synthesis of IGF-I in these patients. Moreover, overt and subclinical hypothyroidism found in our patients and reported by other investigators, may negatively affect GH-IGF-I axis and contribute to the slow growth and short stature in these patients.[20,21,22] The presence of high thyroid antibodies in our patients and reported by other authors pointed out to an immune aggression against the thyroid gland.[21]
Delayed or failure of puberty is another cause of short stature and may explain slow growth of adolescents with AT because of the absence of sex steroid effect on linear growth. Many girls with AT had delayed onset or incomplete pubertal development, and very early menopause is common. The absence of ATM gene causes defects in folliculogenesis in defective mouse mutant and can explain the premature ovarian failure/or atrophy (hypergonadotrophic hypogonadism) in girls with AT. On the other hand, neurodegeneration affecting the brain, may theoretically affect the hypothalamic-pituitary axis to cause hypogonadotropic hypogonadism.[14,15,16,17,23]
Other associated factors that may contribute to the growth and pubertal delay in AT patients include chronic catabolic state due to recurrent infections and pulmonary complications, as well as the severity of neurological affection.[24,25]
Besides the immune therapy and proper treatment of recurrent infections, improvement of nutrition and early correction of hypothyroidism, Vitamin D deficiency, and delayed puberty can improve linear growth and bone mineral accretion in these patients. Appropriate use of IGF-I therapy also is suggested by some authors as a treatment for cerebellar ataxia, may have a positive effect on linear growth.[24,25,26]
IN SUMMARY
Our patients with AT had a high prevalence of growth delay and endocrine dysfunction in the form of low IGF-I, overt and subclinical hypothyroidism and hypogonadism.
Physicians should be aware of these possible endocrinopathies for an early diagnosis and proper treatment.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
REFERENCES
- 1.Rezaei N, Bonilla FA, Sullivan KE. An Introduction to primary immunodeficiency diseases. In: Rezaei N, Aghamohammadi A, Notarangelo LD, editors. Primary Immunodeficiency Diseases, Definition Diagnosis and Management. Ch. 1. Germany: Springer-Verlag Berlin Heidelberg; 2008. pp. 1–38. [Google Scholar]
- 2.Rezaei N, Hedayat M, Aghamohammadi A, Nichols KE. Primary immunodeficiency diseases associated with increased susceptibility to viral infections and malignancies. J Allergy Clin Immunol. 2011;127:1329–41.e2. doi: 10.1016/j.jaci.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 3.Leechawengwongs E, Shearer WT. Lymphoma complicating primary immunodeficiency syndromes. Curr Opin Hematol. 2012;19:305–12. doi: 10.1097/MOH.0b013e328353fa13. [DOI] [PubMed] [Google Scholar]
- 4.Goyal R, Bulua AC, Nikolov NP, Schwartzberg PL, Siegel RM. Rheumatologic and autoimmune manifestations of primary immunodeficiency disorders. Curr Opin Rheumatol. 2009;21:78–84. doi: 10.1097/BOR.0b013e32831cb939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janniger KC, Jozwiak S, Kmiec T, Bernatowska E. Ataxia-Telangiectasia Clinical Presentation. Medscape. [Last accessed 2014 Oct 08]. Available from: http://www.emedicine.medscape.com/article/1113394-clinical#a0217 .
- 6.Xu Y, Ashley T, Brainerd EE, Bronson RT, Meyn MS, Baltimore D. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 1996;10:2411–22. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- 7.Baple EL, Chambers H, Cross HE, Fawcett H, Nakazawa Y, Chioza BA, et al. Hypomorphic PCNA mutation underlies a human DNA repair disorder. J Clin Invest. 2014;124:3137–46. doi: 10.1172/JCI74593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ammann AJ, Duquesnoy RJ, Good RA. Endocrinological studies in ataxiatelangiectasia and other immunological deficiency diseases. Clin Exp Immunol. 1970;6:587–95. [PMC free article] [PubMed] [Google Scholar]
- 9.Busiguina S, Fernandez AM, Barrios V, Clark R, Tolbert DL, Berciano J, et al. Neurodegeneration is associated to changes in serum insulin-like growth factors. Neurobiol Dis. 2000;7:657–65. doi: 10.1006/nbdi.2000.0311. [DOI] [PubMed] [Google Scholar]
- 10.Schubert R, Reichenbach J, Zielen S. Growth factor deficiency in patients with ataxia telangiectasia. Clin Exp Immunol. 2005;140:517–9. doi: 10.1111/j.1365-2249.2005.02782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kieslich M, Hoche F, Reichenbach J, Weidauer S, Porto L, Vlaho S, et al. Extracerebellar MRI-lesions in ataxia telangiectasia go along with deficiency of the GH/IGF-1 axis, markedly reduced body weight, high ataxia scores and advanced age. Cerebellum. 2010;9:190–7. doi: 10.1007/s12311-009-0138-0. [DOI] [PubMed] [Google Scholar]
- 12.Ching JK, Luebbert SH, Collins RL, 4th, Zhang Z, Marupudi N, Banerjee S, et al. Ataxia telangiectasia mutated impacts insulin-like growth factor 1 signalling in skeletal muscle. Exp Physiol. 2013;98:526–35. doi: 10.1113/expphysiol.2012.066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovacs K, Giannini C, Scheithauer BW, Stefaneanu L, Lloyd RV, Horvath E. Pituitary Changes in Ataxia-Telangiectasia Syndrome: An Immunocytochemical, in situ Hybridization, and DNA Cytometric Study of Three Cases. Endocr Pathol. 1997;8:195–203. doi: 10.1007/BF02738786. [DOI] [PubMed] [Google Scholar]
- 14.Miller ME, Chatten J. Ovarian changes in ataxia telangiectasia. Acta Paediatr Scand. 1967;56:559–61. doi: 10.1111/j.1651-2227.1967.tb15424.x. [DOI] [PubMed] [Google Scholar]
- 15.Christin-Maitre S, Vasseur C, Portnoï MF, Bouchard P. Genes and premature ovarian failure. Mol Cell Endocrinol. 1998;145:75–80. doi: 10.1016/s0303-7207(98)00172-5. [DOI] [PubMed] [Google Scholar]
- 16.Di Giacomo M, Barchi M, Baudat F, Edelmann W, Keeney S, Jasin M. Distinct DNA-damage-dependent and -independent responses drive the loss of oocytes in recombination-defective mouse mutants. Proc Natl Acad Sci U S A. 2005;102:737–42. doi: 10.1073/pnas.0406212102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, et al. Atm-deficient mice: A paradigm of ataxia telangiectasia. Cell. 1996;86:159–71. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 18.Lai A, Sarcevic B, Prall OW, Sutherland RL. Insulin/Insulin-like growth factor-1 and estrogen cooperate to stimulate cyclin E-Cdk2 activation and cell cycle progression in MCF-7 breast cancer cells through differential regulation of cyclin E and p21WAF1/Cip1. J Biol Chem. 2001;276:25823–33. doi: 10.1074/jbc.M100925200. [DOI] [PubMed] [Google Scholar]
- 19.Ehlayel M, Soliman A, Bedair S, De Sanctis V. Ataxia telangiectasia with multiple endocrine failures. Riv Ital Med Adolesc. 2014 In press. [Google Scholar]
- 20.Soliman AT. Catch-up growthCatch-Up growth: Role of GH-IGF-I axis and thyroxine. In: Preedy VR, editor. Handbook of Growth and Growth Monitoring in Health and Disease. New York: Springer; 2012. pp. 935–62. [Google Scholar]
- 21.Patiroglu T, Gungor HE, Unal E, Kurtoglu S, Yikilmaz A, Patiroglu T. Hashimoto thyroiditis associated with ataxia telangiectasia. J Pediatr Endocrinol Metab. 2012;25:349–52. [PubMed] [Google Scholar]
- 22.Soliman AT, De Sanctis V, Bedair S. Congenital hypothyroidism: Effects on linear growth, Catch-up growth, GH-IGF-I axis and bones. In: Potluková E, editor. Current Topics in Hypothyroidism with Focus on Development. Ch 4. Rijeka, Croatia: INTECH; 2013. [Google Scholar]
- 23.Zadik Z, Levin S, Prager-Lewin R, Laron Z. Gonadal dysfunction in patients with ataxia telangiectasia. Acta Paediatr Scand. 1978;67:477–9. doi: 10.1111/j.1651-2227.1978.tb16357.x. [DOI] [PubMed] [Google Scholar]
- 24.Nowak-Wegrzyn A, Crawford TO, Winkelstein JA, Carson KA, Lederman HM. Immunodeficiency and infections in ataxia-telangiectasia. J Pediatr. 2004;144:505–11. doi: 10.1016/j.jpeds.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 25.López-Calderón A, Soto L, Martín AI. Chronic inflammation inhibits GH secretion and alters the serum insulin-like growth factor system in rats. Life Sci. 1999;65:2049–60. doi: 10.1016/s0024-3205(99)00472-5. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez AM, Carro EM, Lopez-Lopez C, Torres-Aleman I. Insulin-like growth factor I treatment for cerebellar ataxia: Addressing a common pathway in the pathological cascade? Brain Res Brain Res Rev. 2005;50:134–41. doi: 10.1016/j.brainresrev.2005.05.003. [DOI] [PubMed] [Google Scholar]


