Abstract
Background: Advanced glycation end products (AGEs) are a heterogeneous group of compounds present in uncooked foods as well as in foods cooked at high temperatures. AGEs have been associated with insulin resistance, oxidative stress, and chronic inflammation in patients with diabetes. Dietary AGEs are an important contributor to the AGE pool in the body. Nϵ-(carboxymethyl)lysine (CML) AGE is one of the major biologically and chemically well-characterized AGE markers. The consumption of red meat, which is CML-AGE rich, has been positively associated with pancreatic cancer in men.
Objectives: With the use of a published food CML-AGE database, we estimated the consumption of CML AGE in the prospective NIH-AARP Diet and Health Study and evaluated the association between CML-AGE consumption and pancreatic cancer and the mediating effect of CML AGE on the association between red meat consumption and pancreatic cancer.
Design: Multivariate Cox proportional hazard regression models were used to estimate HRs and 95% CIs for pancreatic cancer.
Results: During an average of 10.5 y of follow-up, we identified 2193 pancreatic cancer cases (1407 men and 786 women) from 528,251 subjects. With the comparison of subjects in the fifth and the first quintiles of CML-AGE consumption, we observed increased pancreatic cancer risk in men (HR: 1.43; 95% CI: 1.06, 1.93, P-trend = 0.003) but not women (HR: 1.14; 95% CI: 0.76, 1.72, P-trend = 0.42). Men in the highest quintile of red meat consumption had higher risk of pancreatic cancer (HR: 1.35; 95% CI: 1.07, 1.70), which attenuated after adjustment for CML-AGE consumption (HR: 1.20; 95% CI: 0.95, 1.53).
Conclusion: Dietary CML-AGE consumption was associated with modestly increased risk of pancreatic cancer in men and may partially explain the positive association between red meat and pancreatic cancer. The NIH-AARP Diet and Health Study was registered at clinicaltrials.gov as NCT00340015.
Keywords: advanced glycation end products, diet, inflammation, pancreatic cancer, risk
INTRODUCTION
Cigarette smoking, type 2 diabetes, and possibly a high-fat and red meat diet are modifiable risk factors for pancreatic cancer (1). All of these factors are related to advanced glycation end products (AGEs)6. AGEs are a heterogeneous group of compounds formed irreversibly via nonenzymatic reaction of reducing sugars with free amino groups on proteins, lipids, and nucleic acids (2, 3). AGEs are naturally present in uncooked foods, and prolonged high-temperature cooking further prompts the formation of large amounts of AGEs within these foods (4, 5).
AGEs induce insulin resistance, oxidative stress, and chronic inflammation by interacting with the pro-oxidant receptor for advanced glycation end products [RAGEs (AGER in Human Genome Organization nomenclature)] (6, 7). Diet-derived AGEs from proteins and fats are major contributors to the AGE pool in both normal and diabetic individuals (8). Nϵ-(carboxymethyl)lysine (CML) arises from the glycation of lysine on proteins and aminolipids and lipid peroxidation reactions (9, 10). CML is one of the chemically and biologically well-characterized AGEs (11). The dietary CML AGE in commonly consumed foods has been assessed in 2 studies (4, 5). However, its implication in diseases remains a subject of open debate (12, 13) and has not been evaluated in a large epidemiologic study.
In a previous serologic study, we showed that soluble receptor for advanced glycation end product (sRAGE) concentrations were inversely associated with pancreatic cancer, whereas the ratio of CML AGE:sRAGE was positively associated with risk (14). In the current study, we linked the published information on the CML-AGE contents of 549 commonly consumed foods in North America (5) to the NIH-AARP food-frequency questionnaire (FFQ). We estimated CML-AGE consumption in participants of the NIH-AARP Diet and Health Study and subsequently examined the association between the daily consumption of CML-AGE and risk of pancreatic cancer. We hypothesized that CML-AGE consumption was positively associated with risk of pancreatic cancer. Because red meat consumption was one of the major contributors of dietary AGEs and has been positively associated with pancreatic cancer in the NIH-AARP Diet and Health Study (15), we further hypothesized that CML-AGE consumption explained the association between red meat and pancreatic cancer.
SUBJECTS AND METHODS
Study population and data collection
The NIH-AARP Diet and Health Study is a large prospective study of AARP members conducted between 1995 and 1996. At baseline, the study used a self-administered AARP FFQ (an early version of the National Cancer Institute Diet History Questionnaire) (16) to assess the usual frequencies of consumption and portion sizes of 124 line items over the previous 12 mo, which led to 170 nutritionally distinct food and beverage items queried. The FFQ also included 21 questions on low-fat, high-fiber foods and food preparation. Nutrient values for these items were derived from the USDA's Continuing Survey of Food Intakes by Individuals (CSFII) 1994–1996 (17) that includes more than 5000 individual food and beverage items reported on 24-h dietary recalls in a representative survey sample of the U.S. population. A sex-specific weighted mean approach was used to determine nutrient values for these items (18). The AARP FFQ also elicited information on demographic factors, medical history, body weight and height, physical activity, and other health-related behaviors. Participants reported whether they smoked ≥100 cigarettes during their entire life to distinguish ever smokers from never smokers. Ever smokers reported whether they currently smoked, when they had stopped smoking (<1 or 1–4, 5–9, or ≥10 y ago), and their daily smoking dose (1–10, 11–20, 21–30, 31–40, 41–60, and ≥61 cigarettes). The AARP FFQ was mailed to 3.5 million AARP members aged 50–71 y who resided in 6 U.S. states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and 2 metropolitan areas (Atlanta, Georgia, and Detroit, Michigan). A total of 617,119 members returned questionnaires, and 567,169 members completed the questionnaire satisfactorily.
Approximately 6 mo after the baseline questionnaire was sent, respondents were sent a second questionnaire that included a meat-cooking module (19). The meat-cooking module queried the consumption of hamburgers, steak, bacon, and chicken, as well as the usual cooking method of these meats (pan fried; grilled or barbecued; oven broiled; or other such as sautéed, baked, or microwaved), and the level of doneness on the outside (not browned, lightly browned, well browned, black, or charred). Meat mutagens were evaluated by using the meat-cooking module.
Linking published CML-AGE values to the AARP FFQ
A published database has reported protein- and lipid-associated CML-AGE values in 549 commonly consumed foods in North America (5, 20). The estimates were based on an ELISA by using a widely used, well-validated, non–cross-reactive monoclonal antibody (4G9) raised against HPLC-characterized synthetic CML bovine serum albumin (21). CML bovine serum albumin was also used for plate coating (22). A blocking buffer, containing nonmodified albumin, was used against a nonspecific or matrix effect (20). Values reflected stable protein- or lipid-associated CML, such as are present in tissues, blood, and foods. However, they account for less for free CML, which is a terminal nonactive product of extensive proteolysis. Values are expressed in KU (KU/100 g solid goods or 100 mL liquid) (5). We assigned CML-AGE estimates to the CSFII 1994–1996 database by using methods similar to those used for all other nutrients on the questionnaire (18, 23). Published CML-AGE values were reviewed to determine how closely they matched food codes in the CSFII representing the 170 food groups comprising the line items on the AARP FFQ. When cooking methods were reported in CSFII food codes, we used the available information in assigning CML-AGE values. When multiple CML-AGE values were given for one food because of different preparation methods, we generated a mean value of all listed values to be used for matching. For example, we used mean CML-AGE values listed for “Bacon, fried 5 min no added oil” and for “Bacon, microwaved, 2 slices, 3 min” to create a mean value for “bacon” (5). The value for cheese was the average of CML-AGE values for all types of cheese assuming that each type of cheese was consumed equally in quantity and frequency. When the CML-AGE value was not available for a food, we used values for similar foods. For example, we used mean available CML-AGE values for fruit and uncooked vegetables to assign values to fruit and vegetables for which the CML-AGE value had not been directly measured, thereby providing a value for all CSFII foods needed to calculate nutrient values for the FFQ. For food mixtures, we assigned values to individual food components in recipes to calculate a value.
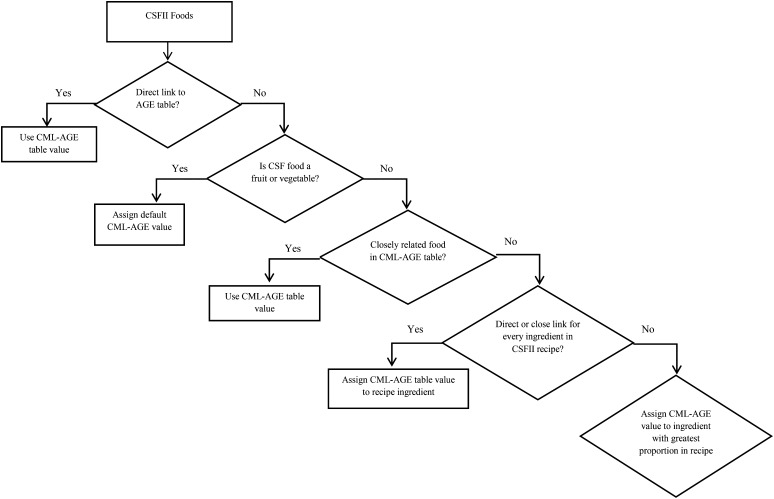
The multiple process of linking CML-AGE values to CSFII food codes is summarized in Figure 1. Once all CSFII food codes were linked to CML-AGE values, the weighted average CML-AGE value for the FFQ line item and sex-specific portion range was calculated as described previously (18, 23).
FIGURE 1.
Procedure for linking CML-AGE values to CSFII food code. AGE, advanced glycation end product; CML, Nϵ-(carboxymethyl)lysine; CSFII, Continuing Survey of Food Intakes by Individuals.
Cohort follow-up and case ascertainment
To alleviate concerns regarding reverse causality (i.e., a biased association attributed to diet or a lifestyle change as a result of latent pancreatic cancer), the follow-up time was calculated from 2 y after the baseline questionnaire to the date of pancreatic cancer diagnosis or death, move out of study areas, death from any cause, or 31 December 2006, whichever came first. The NIH-AARP Diet and Health Study followed participants yearly by using the National Change of Address database (U.S. Postal Service) and MaxCoA (Anchor Computer Inc.). Approximately 4% of participants were lost to follow-up. Vital status was ascertained by annual linkage to the Social Security Administration Death Master File. Incident pancreatic cancer cases were identified by linkage to 11 state cancer registries (the 8 original states where participants were recruited as well as Arizona, Nevada, and Texas), and mortality cases were identified by linkage to the National Death Index. We included adenocarcinoma of the exocrine pancreas (International Classification of Diseases for Oncology, Third Edition, codes C25.0–C25.3 and C25.7–C25.9) and excluded histology types 8150–8155, 8240, 8246, and 8502.
From 567,169 study subjects who completed the baseline AARP FFQ satisfactorily, we excluded people with duplicate representation of the questionnaire (n = 179), who moved out of the study areas before returning the questionnaire (n = 321), died before study entry (n = 261), or withdrew (n = 9). From the remaining 566,399 participants, we further excluded people whose questionnaire was completed by proxy respondents (n = 15,760), prevalent cancer cases as identified through cancer registries at baseline (n = 8587) except for nonmelanoma skin cancer, people with extreme daily energy intake (i.e., >2 IQRs below the sex-specific 25th percentile or above the 75th percentile of log-transformed energy intake; n = 4810), and those who had diagnosis of pancreatic cancer, died, or were lost to follow-up within 2 y after the return of the baseline questionnaire (n = 8991). Our final analytic cohort consisted of 528,251 individuals, including 310,458 men and 217,793 women. Of 528,251 individuals, 319,895 subjects also completed the meat-cooking module questionnaire 6 mo later and formed the subcohort in our analysis; after the exclusion of 3239 participants whose questionnaires were completed by proxy respondents, 316,656 individuals (183,005 men and 133,651 women) remained in the subcohort. The scheme of study subject selection is summarized in Supplemental Figure 1. During follow-up, 2193 pancreatic cancer cases (1407 men and 786 women), including 1933 incident cases and 260 mortality cases, developed from the baseline cohort in 528,251 study subjects, and 1337 pancreatic cancer cases (841 men and 496 women), including 1184 incident cases and 153 mortality cases, developed in the subcohort (i.e., those 316,656 study subjects who completed the meat-cooking module questionnaire). The study was approved by the National Cancer Institute Special Studies Institutional Review Board.
Statistical analysis
Dietary intakes of foods and nutrients, including CML AGE (KU), were energy adjusted by using the nutrient-density method. We described the distribution of CML-AGE consumption and calculated the contribution of each food group to CML-AGE consumption in our study population. Foods were grouped into the following categories: fats, nuts, oils and seeds, red meats, white meats, processed meats, grains, mixed foods, dairy products and eggs, vegetables, miscellaneous [5% of cookies and brownies (95% counted toward grain), candy with or without chocolate, sugar or honey added to coffee and tea, coffee no sugar, tea, coffee no sugar, tea, soft drinks, beer, wine, and liquor, and saccharine and aspartame), and fruit. Partial correlation coefficients (r) adjusted for age were calculated for CML AGE and nutrients thought to contribute to their formation or potential dietary risk factors for pancreatic cancer, which included protein, total fat, saturated fat, MUFAs, PUFAs, carbohydrate, fructose, calcium, and BMI.
Cox proportional hazards regression models, with person-years as the underlying time metric, were used to estimate HRs and 95% CIs for pancreatic cancer according to quintiles of CML-AGE consumption or meat variables. Because the association between CML AGE and risk of pancreatic cancer differed by sex (P-interaction = 0.05), sex-specific quintiles of consumption were used for risk estimation that was reported for men and women separately. We evaluated the proportional hazards assumption (hazards were proportional over time) graphically by using a log-log plot for CML AGE and red meat (quintiles) for all models. No significant violation of the assumption was noted because the plots essentially showed the parallel pattern. The linear trend was tested by using Wald's test and treating quintiles as a continuous variable. Age, energy intake, race (non-Hispanic white, non-Hispanic black, Hispanic, Asian Pacific islanders or American Indian/Alaskan Native, and missing), educational levels (<11 y, high school graduate, post–high school, some college, college and postgraduate, and unknown), diabetes (yes compared with no), BMI (in kg/m2; categorized as <25, 25–30, ≥30, and missing), daily alcohol consumption (never consume alcohol and <3 or ≥3 drinks/d), first-degree family history of any cancer except for basal cell skin cancer (yes compared with no), and intakes of carbohydrate, calcium, and red meat (continuous) were included in the multivariable models. These covariates were associated with both CML AGE and pancreatic cancer risk, or their addition in the stepwise selection model changed the risk estimate by >10%. Intakes of total fat, saturated fat, and protein and physical activity were not confounders and were not included in the final multivariable models. A smoking variable with finer categories was included in all multivariable models to account for its confounding effect as follows: never smokers, quit ≥10 y and smoked <20 cigarettes/d, quit ≥10 y and smoked ≥20 cigarettes/d, quit 5–9 y and smoked <20 cigarettes/d, quit 5–9 y and smoked ≥20 cigarettes/d, quit 1–4 y and smoked <20 cigarettes/d, quit 1–4 y and smoked ≥20 cigarettes/d, and current smokers. The use of a more-detailed smoking variable with 31 categories did not affect risk estimates.
Because red and processed meat consumption has been positively associated with pancreatic cancer in the NIH-AARP Diet and Health study as well as other studies (15, 24), and they are AGE rich, we examined the association between meat groups and pancreatic cancer while adjusting models for saturated fat and, in addition, for CML AGE. Because high-temperature cooking–generated meat mutagens, such as heterocyclic amines, have been associated with increased risk of pancreatic cancer, we further adjusted models for 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (DiMeIQx), 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), and CML AGE separately and jointly in the subcohort of participants who completed the meat-cooking module questionnaire.
Stratified analyses were performed according to smoking status (never compared with ever smokers), diabetes status (no compared with yes), and BMI (<25 compared with ≥25) in sex-specific models. The interaction effect was tested by using Wald's test. An additional lag analysis was performed by excluding study subjects with follow-up <5 y. Because the catabolism of AGEs is partially dependent on renal elimination (25), we conducted a sensitivity analysis by excluding individuals who self-reported renal diseases at baseline (n = 967). All analyses were performed with SAS 9.2 software (SAS institute) with P < 0.05 used to indicate statistical significance for 2-sided tests.
RESULTS
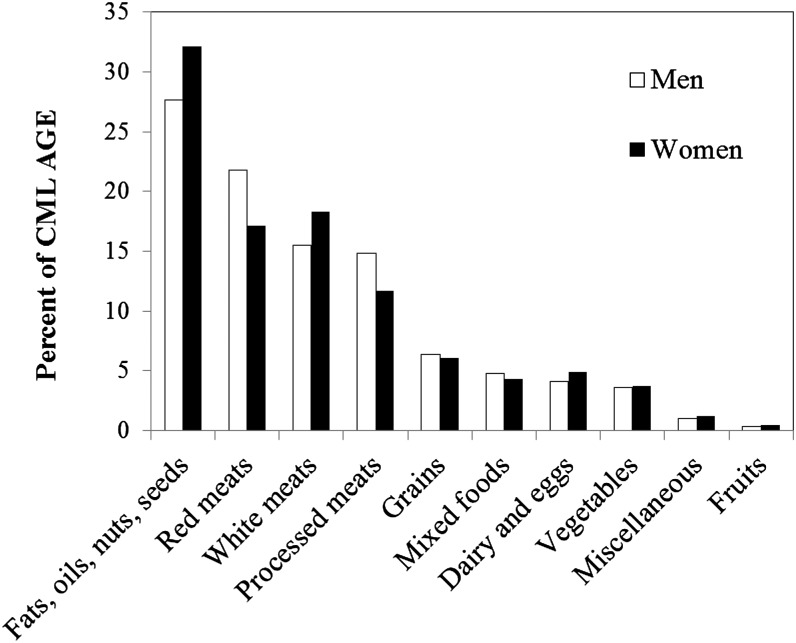
The average (±SD) age at baseline for study subjects was 62.1 ± 5.4 y, and the average number of years of follow up was 10.5 ± 2.4 y. The consumption of dietary CML AGE followed a normal distribution in our study population. The range of daily consumption was from 118 to 83,308 KU/1000 kcal for men (mean ± SD: 6191 ± 2129 KU/1000 kcal; median: 6056 KU/1000 kcal) and 211 to 45,124 KU/1000 kcal for women (mean ± SD: 6073 ± 2107 KU/1000 kcal; median: 5936 KU/1000 kcal). Food groups that contributed mostly to CML AGE consumption were fats, oils, nuts, and seeds and meats, whereas fruit contribute minimally (Figure 2).
FIGURE 2.
Dietary source of CML AGE (percentages) from different food groups in men and women in the NIH-AARP Diet and Health Study. For the miscellaneous group, we included 5% of cookies and brownies (95% of cookies and brownies counted toward the grain group), candy with or without chocolate, sugar or honey added to coffee and tea, coffee with no sugar, tea, soft drinks, beer, wine, liquor, and saccharine and aspartame. AGE, advanced glycation end product; CML, Nϵ-(carboxymethyl)lysine.
Both men and women in the higher quintiles of CML-AGE consumption were less educated, less physically active, and consumed less alcohol, total carbohydrate, fructose, and calcium but more energy, fat, protein, saturated fat, and red meat; in addition, they were more likely to have renal diseases and diabetes and be obese or current smokers (Table 1).
TABLE 1.
Selected baseline characteristics of participants according to quintiles of dietary CML AGE in 310,458 men and 217,793 women in the NIH-AARP Diet and Health Study (1995–2006)1
| Men, quintiles |
Women, quintiles |
|||||
| Characteristics | 1 | 3 | 5 | 1 | 3 | 5 |
| CML-AGE consumption,2 KU/1000 kcal | 50–6866 | 9647–12,677 | 17,261–101,909 | 258–5200 | 7341–9697 | 13,255–76,156 |
| Age at entry to the cohort, y | 62.6 ± 5.33 | 62.3 ± 5.3 | 61.8 ± 5.4 | 62.0 ± 5.4 | 62.0 ± 5.4 | 62.6 ± 5.4 |
| Race (non-Hispanic white), % | 89.4 | 93.4 | 93.0 | 88.2 | 91.3 | 90.1 |
| Education (college or postgraduate), % | 48.5 | 45.3 | 40.2 | 36.0 | 30.4 | 25.6 |
| Diabetes (yes), % | 6.27 | 8.91 | 16.5 | 4.1 | 6.6 | 12.2 |
| Renal disease (yes), % | 0.18 | 0.19 | 0.28 | 0.14 | 0.14 | 0.24 |
| BMI, kg/m2 | 26.2 ± 3.9 | 27.2 ± 4.1 | 28.4 ± 4.8 | 25.3 ± 5.2 | 26.8 ± 5.8 | 28.3 ± 6.7 |
| BMI >30 kg/m2, % | 13.3 | 20.1 | 29.8 | 13.9 | 22.0 | 31.3 |
| Physical activity >3 times/wk, % | 58.9 | 49.4 | 41.6 | 51.5 | 40.9 | 42.3 |
| Smoking status, % | ||||||
| Never smokers | 31.7 | 29.5 | 25.9 | 46.2 | 44.6 | 39.7 |
| Former smokers | 56.8 | 57.3 | 57.0 | 40.1 | 38.9 | 38.4 |
| Current smokers | 7.4 | 9.5 | 14.1 | 9.9 | 13.3 | 18.7 |
| Missing | 4.1 | 3.7 | 4.0 | 3.5 | 3.2 | 3.1 |
| First degree family history of any cancer (yes),4 % | 46.6 | 48.2 | 48.2 | 51.6 | 52.9 | 51.9 |
| Alcohol (>3 drinks/d), % | 18.4 | 10.4 | 6.11 | 4.35 | 2.50 | 1.58 |
| Total energy intake, kcal | 1967 ± 927 | 1996 ± 797 | 2106 ± 864 | 1463 ± 645 | 1544 ± 635 | 1581 ± 702 |
| Protein, g · 1000 kcal−1· d−1 | 33.5 ± 8.2 | 38.2 ± 6.5 | 43.5 ± 7.9 | 35.3 ± 8.1 | 38.5 ± 7.1 | 42.7 ± 8.6 |
| Total fat, g · 1000 kcal−1· d−1 | 24.1 ± 6.7 | 34.4 ± 5.6 | 42.0 ± 6.3 | 24.0 ± 6.3 | 33.7 ± 5.9 | 41.8 ± 6.9 |
| Saturated fat, g · 1000 kcal−1· d−1 | 7.42 ± 2.8 | 10.7 ± 2.4 | 13.4 ± 2.8 | 7.40 ± 2.7 | 10.3 ± 2.5 | 13.2 ± 3.1 |
| Red meat, g · 1000 kcal−1· d−1 | 18.1 ± 11.4 | 37.2 ± 15.6 | 59.4 ± 24.9 | 13.7 ± 9.4 | 28.9 ± 14.1 | 46.7 ± 23.4 |
| Red meat cooked at high temperature, g · 1000 kcal−1· d−1 | 6.3 ± 5.4 | 14.7 ± 8.8 | 27.9 ± 16.8 | 4.5 ± 4.4 | 10.8 ± 7.6 | 20.7 ± 14.8 |
| Total carbohydrate, g · 1000 kcal−1· d−1 | 148 ± 29 | 128 ± 17 | 108 ± 16 | 160 ± 23 | 135 ± 16 | 113 ± 16 |
| Fructose, g · 1000 kcal−1· d−1 | 17.6 ± 10.5 | 12.5 ± 6.5 | 9.2 ± 5.0 | 19.7 ± 11.2 | 13.9 ± 7.0 | 10.2 ± 5.4 |
| Calcium, mg · 1000 kcal−1· d−1 | 475 ± 224 | 414 ± 153 | 347 ± 116 | 556 ± 240 | 457 ± 166 | 372 ± 126 |
AGE, advanced glycation end product; CML, Nϵ-(carboxymethyl)lysine.
All values are ranges.
Mean ± SD (all such values).
Not including basal cell skin cancer.
Table 2 shows the significant correlation between CML AGE and nutrients that were related to the formation of CML AGE or potentially influenced risk of pancreatic cancer. In addition, CML AGE was significantly positively correlated with BMI in both men and women (r = 0.17, P < 0.05). Of individuals who completed the meat-cooking module, we also showed that CML-AGE consumption had a significant positive correlation with meat mutagens including MeIQx (r = 0.30 for men and r = 0.29 for women), PhIP (r = 0.27 for men and r = 0.24 for women), and DiMeIQx (r = 0.16 for men and r = 0.17 for women).
TABLE 2.
Partial correlation coefficients between daily intake of CML AGE and selected nutrients in men and women in the NIH-AARP Diet and Health Study (1995–2006)1
| Protein | Total fat | Saturated fat | MUFA | PUFA | Carbohydrate | Fructose | Calcium | |
| Men | 0.44 | 0.73 | 0.64 | 0.73 | 0.48 | −0.58 | −0.37 | −0.25 |
| Women | 0.32 | 0.72 | 0.62 | 0.71 | 0.48 | −0.70 | −0.39 | −0.33 |
For all of the correlations, P < 0.05. AGE, advanced glycation end product; CML, Nϵ-(carboxymethyl)lysine.
Table 3 shows that CML-AGE consumption was positively associated with pancreatic cancer in men (HR for comparison of fifth with first quintiles: 1.43; 95% CI: 1.06, 1.93; P-trend = 0.003). Risk was unchanged when saturated fat or protein consumption was added to the model (data not shown). In the subcohort, additional adjustment for meat mutagens (PhIP, MeIQx, and DiMeIQx) augmented the risk estimate slightly for men. In women, we did not observe an association between CML AGE and pancreatic cancer. Because the findings from the entire cohort and subcohort were essentially the same, we present data for the entire cohort in the stratified analysis by sex.
TABLE 3.
HRs (95% CIs) for pancreatic cancer according to quintiles of baseline CML-AGE consumption in the NIH-AARP Diet and Health Study (1995–2006)1
| CML-AGE quintile |
||||||
| 1 | 2 | 3 | 4 | 5 | P-trend (Wald's test) | |
| Men (n = 310,458) | ||||||
| Intake, KU/1000 kcal | 50–68662 | 6867–9046 | 9047–12,677 | 12,678–17,260 | 17,261–101,909 | — |
| Cases, n | 233 | 258 | 276 | 328 | 312 | — |
| Age-adjusted incidence rate/100,000 person-years | 38.7 | 42.4 | 46.5 | 55.4 | 56.3 | — |
| HR3 (95% CI) | 1.00 | 1.12 (0.94, 1.34) | 1.21 (1.02, 1.45) | 1.48 (1.25, 1.75) | 1.46 (1.23, 1.73) | <0.0001 |
| HR4 (95% CI) | 1.00 | 1.18 (0.93, 1.49) | 1.10 (0.86, 1.42) | 1.56 (1.21, 2.02) | 1.43 (1.06, 1.93) | 0.003 |
| HR5 (95% CI) | 1.00 | 1.17 (0.93, 1.48) | 1.09 (0.84, 1.41) | 1.54 (1.18, 2.00) | 1.40 (1.02, 1.92) | 0.007 |
| Subcohort6 (n = 183,005) | ||||||
| Intake, KU/1000 kcal | 118–4414 | 4415–5515 | 5516–6494 | 6495–7720 | 7721–83,308 | — |
| Cases, n | 135 | 156 | 150 | 204 | 196 | — |
| HR3 (95% CI) | 1.00 | 1.17 (0.93, 1.47) | 1.13 (0.90, 1.43) | 1.58 (1.27, 1.96) | 1.57 (1.26, 1.95) | <0.0001 |
| HR4 (95% CI) | 1.00 | 1.16 (0.92, 1.48) | 1.12 (0.87, 1.45) | 1.53 (1.18, 1.98) | 1.46 (1.08, 1.97) | 0.003 |
| HR5 (95% CI) | 1.00 | 1.15 (0.91, 1.47) | 1.11 (0.86, 1.43) | 1.50 (1.15, 1.96) | 1.42 (1.04, 1.94) | 0.007 |
| HR7 (95% CI) | 1.00 | 1.17 (0.92, 1.48) | 1.12 (0.87, 1.45) | 1.53 (1.18, 1.99) | 1.48 (1.10, 2.00) | 0.002 |
| Women (n = 217,793) | ||||||
| Intake, KU/1000 kcal | 258–5200 | 5201–7340 | 7341–9647 | 9648–13254 | 13,255–76,156 | — |
| Cases, n | 157 | 170 | 140 | 165 | 154 | — |
| Age adjusted incidence rate (per 100,000 person-years) | 35.4 | 39.0 | 33.1 | 39.3 | 38.3 | — |
| HR3 (95% CI) | 1.00 | 1.09 (0.88, 1.35) | 0.90 (0.72, 1.14) | 1.08 (0.87, 1.35) | 1.04 (0.84, 1.31) | 0.77 |
| HR4 (95% CI) | 1.00 | 1.11 (0.83, 1.50) | 0.94 (0.67, 1.30) | 1.24 (0.87, 1.75) | 1.14 (0.76, 1.72) | 0.42 |
| HR5 (95% CI) | 1.00 | 1.11 (0.82, 1.49) | 0.93 (0.67, 1.29) | 1.22 (0.86, 1.73) | 1.11 (0.73, 1.69) | 0.52 |
| Subcohort6 (n = 133,651) | ||||||
| Intake, KU/1000 kcal | 373–4309 | 4310–5403 | 5404–6386 | 6387–7599 | 7600–33,168 | — |
| Cases, n | 94 | 102 | 84 | 109 | 107 | — |
| HR3 (95% CI) | 1.00 | 1.09 (0.82, 1.44) | 0.91 (0.68, 1.22) | 1.20 (0.91, 1.58) | 1.22 (0.93, 1.61) | 0.12 |
| HR4 (95% CI) | 1.00 | 1.09 (0.81, 1.46) | 0.90 (0.64, 1.25) | 1.17 (0.82, 1.65) | 1.16 (0.77, 1.73) | 0.43 |
| HR5 (95% CI) | 1.00 | 1.08 (0.80, 1.46) | 0.90 (0.64, 1.24) | 1.15 (0.81, 1.63) | 1.13 (0.74, 1.71) | 0.52 |
| HR7 (95% CI) | 1.00 | 1.09 (0.81, 1.46) | 0.90 (0.64, 1.25) | 1.17 (0.82, 1.65) | 1.16 (0.77, 1.73) | 0.43 |
P-interaction by sex = 0.05. AGE, advanced glycation end product; CML, Nϵ-(carboxymethyl)lysine.
Range (all such values).
Adjusted for age (continuous).
Adjusted for race, education, diabetes, smoking status, first degree family history of cancer, BMI, and alcohol (all on a categorical scale), and for age, calories, carbohydrate intake, and calcium (all on a continuous scale).
Adjusted further for red meat intake on a continuous scale.
Study subjects who completed the meat-cooking module questionnaire 6 mo after baseline.
Adjusted further for 3 types of heterocyclic amine simultaneously on a continuous scale.
Table 4 shows that, in men, the positive association between red meat consumption and pancreatic cancer was attenuated slightly when saturated fat was adjusted in the model. The addition of CML AGE in the model attenuated the association markedly. Combined adjustment for saturated fat and CML AGE attenuated associations for red meat cooked at a higher temperature and processed meat. In an analysis of subjects who completed the meat-cooking module, the addition of heterocyclic amines to models without CML AGE did not materially change risk estimates for red meats and pancreatic cancer (data not shown). The consumption of white meats and red meat cooked at a low temperature was not associated with risk of pancreatic cancer in men. Meat consumption was not associated with risk of pancreatic cancer in women (data not shown).
TABLE 4.
HRs (95% CIs) for pancreatic cancer according to quintiles of meat consumption in men in the NIH-AARP Diet and Health Study (1995–2006)
| Quintile |
||||||
| 1 | 2 | 3 | 4 | 5 | P-trend (Wald's test) | |
| Red meat | ||||||
| Intake, g/1000 kcal | 0–30.21 | 30.3–51.8 | 51.9–76.6 | 76.7–115.5 | 115.6–972.8 | — |
| Cases, n | 242 | 268 | 282 | 302 | 313 | — |
| HR2 (95% CI) | 1.00 | 1.25 (1.05, 1.48) | 1.18 (0.99, 1.41) | 1.29 (1.09, 1.54) | 1.54 (1.31, 1.83) | <0.0001 |
| HR3 (95% CI) | 1.00 | 1.20 (1.01, 1.44) | 1.12 (0.92, 1.35) | 1.20 (0.99, 1.46) | 1.40 (1.12, 1.76) | 0.02 |
| HR4 (95% CI) | 1.00 | 1.19 (0.99, 1.42) | 1.09 (0.90, 1.32) | 1.17 (0.95, 1.43) | 1.35 (1.07, 1.70) | 0.05 |
| HR5 (95% CI) | 1.00 | 1.16 (0.97, 1.38) | 1.04 (0.86, 1.26) | 1.09 (0.89, 1.34) | 1.20 (0.95, 1.53) | 0.36 |
| Red meat cooked at high temperature | ||||||
| Intake (range, g/1000 kcal) | 0–9.2 | 9.3–18.0 | 18.1–29.7 | 29.8–49.2 | 49.3–693.7 | — |
| Cases, n | 245 | 255 | 294 | 300 | 313 | — |
| HR2 (95% CI) | 1.00 | 1.03 (0.86, 1.23) | 1.38 (1.17, 1.63) | 1.23 (1.04, 1.46) | 1.50 (1.27, 1.78) | <0.0001 |
| HR3 (95% CI) | 1.00 | 0.98 (0.81, 1.17) | 1.27 (1.06, 1.52) | 1.10 (0.91, 1.34) | 1.29 (1.04, 1.59) | 0.005 |
| HR4 (95% CI) | 1.00 | 0.87 (0.69, 1.10) | 1.23 (0.98, 1.54) | 1.01 (0.78, 1.30) | 1.18 (0.89, 1.56) | 0.01 |
| HR5 (95% CI) | 1.00 | 0.96 (0.80, 1.15) | 1.23 (1.02, 1.47) | 1.03 (0.85, 1.26) | 1.15 (0.93, 1.44) | 0.17 |
| Processed meat | ||||||
| Intake (range, g/1000 kcal) | 0–6.4 | 6.5–12.7 | 12.8–21.9 | 22.0–37.5 | 37.6–602.2 | — |
| Cases, n | 232 | 289 | 284 | 316 | 286 | — |
| HR2 (95% CI) | 1.00 | 1.29 (1.09, 1.54) | 1.35 (1.14, 1.60) | 1.42 (1.20, 1.69) | 1.33 (1.11, 1.58) | 0.0009 |
| HR3 (95% CI) | 1.00 | 1.22 (1.02, 1.45) | 1.23 (1.03, 1.47) | 1.27 (1.06, 1.53) | 1.15 (0.95, 1.40) | 0.19 |
| HR4 (95% CI) | 1.00 | 1.21 (1.01, 1.44) | 1.21 (1.01, 1.45) | 1.25 (1.04, 1.50) | 1.03 (0.92, 1.37) | 0.28 |
| HR5 (95% CI) | 1.00 | 1.18 (0.99, 1.41) | 1.16 (0.97, 1.39) | 1.18 (0.98, 1.42) | 1.04 (0.85, 1.27) | 0.86 |
Range (all such values).
Adjusted for age (on a continuous scale).
Adjusted for race, education, diabetes, smoking status, first degree family history of cancer, BMI, alcohol consumption (all on a categorical scale), age, calories, and carbohydrate intake (on a continuous scale).
Adjusted further for saturated fat (on a continuous scale).
Adjusted further for Nϵ-(carboxymethyl)lysine advanced glycation end product (on a continuous scale) on the basis of the multivariate model with saturated fat.
There was no significant interaction between CML AGE and smoking status, BMI, or diabetes in men or women. In women, significant increased risk was seen in never-smoking women and women with self-reported diabetes as well as ever-smoking men and men without diabetes or with normal weight (Supplemental Table 1).
The positive association between CML AGE and pancreatic cancer was also observed in the 5-y lag analysis that was based on 1652 cases ascertained from 510,138 study participants (multivariate HR for fifth compared with first quintiles:1.33; 95% CI: 1.01, 1.76, P-trend = 0.02). Finally, the sensitivity analysis that excluded renal diseases did not change the association between CML AGE and pancreatic cancer.
DISCUSSION
In the NIH-AARP Diet and Health Study, we linked published CML-AGE values to the AARP FFQ. We observed a diverse range of dietary CML-AGE consumption in our study population. Foods high in fat and protein, such as meats, were rich in CML AGE probably because of protein glycation, lipid glycoxidation, and oxidation of PUFAs, especially if consumed after heat treatment, as is most often the case, whereas fruit were low in CML AGE. The consumption of CML AGE was positively correlated with protein and fat but inversely correlated with carbohydrates, fructose, and calcium. The correlation differed for men and women for protein and carbohydrates. This result indicated the dietary source of CML AGE might not be completely the same in men compared with women. We showed that higher consumption of CML AGE was positively associated with pancreatic cancer in men, independent of other risk factors for pancreatic cancer. We further showed that dietary CML AGE might be one of the contributing factors that underlies the association between red meat consumption and pancreatic cancer risk in men.
The evidence on the detrimental role of dietary CML AGE in a typical Western diet in chronic diseases has been supported by a body of research (26–31). CML AGE exerts its biological function via receptor-dependent or –independent mechanisms. By binding to pro-oxidant RAGE, CML AGE may contribute to insulin resistance, oxidative stress, and chronic inflammation (8, 32, 33). Advanced glycation end product receptor 1 (AGER1) is the other major receptor involved in the cellular uptake and degradation of AGEs and is responsible for countering RAGE upregulation and inflammation. Concentrations of AGER1 were shown to be suppressed under chronic inflammatory conditions (27, 34). One study that using pancreatic cancer cell lines has shown that AGE ligand-receptor interactions could play an active role in the progression of pancreatic cancer through the induction of autocrine platelet-derived growth factor-B (35). We previously showed a significant positive association between the ratio of circulating concentrations of CML AGE to sRAGE and risk of pancreatic cancer in Finnish men smokers (14). sRAGE may bind to CML AGE or other ligands of RAGE and neutralize the effect caused by RAGE and ligand binding (14). Our research collectively indicated that internal regulatory mechanisms of CML AGE, such as sRAGE and anti-AGE receptors (such as AGER1) (30, 34), are also important in determining the physiologic role of CML AGE, the undue accumulation of which might play a role in pancreatic carcinogenesis. Uncleared CML AGE and other AGEs can accumulate in the tissues and form stable cross-links with long-lived proteins such as collagen to promote vascular stiffness and, thus, alter the vascular structure and function (36, 37). It is unknown whether the pancreas extracellular matrix is modified by CML AGE. However, pancreatic cancer cells are surrounded by extracellular matrix and type IV collagen, which are modified by glycation during the aging process (38, 39). Therefore, we speculated that CML AGE may be involved in pancreatic carcinogenesis by changing the tissue stroma environment.
Our analysis confirmed the positive association between red meat intake and pancreatic cancer in men as shown in a previous study that had shorter follow-up (15). Our current analysis further showed that such a positive association may partially be explained by the consumption of CML AGE because the adjustment of CML AGE attenuated the association between meat intake and risk of pancreatic cancer. Because CML AGE has been implicated in insulin resistance, oxidative stress, and chronic inflammation, our study raises the possibility that meat consumption contributes to pancreatic cancer through mechanisms other than meat mutagens.
If exposure to CML AGE is confirmed to be a risk factor for pancreatic cancer, a novel yet simple preventive approach can be proposed. It has been shown that boiling and stewing, short-time and high-moisture cooking reduces the formation of new AGEs in foods, especially in meats (5). This reduction is an important benefit of low-temperature cooking in addition to the benefit of the reduced formation of meat mutagens (40). Moreover, both synthetic compounds and natural products have been evaluated as inhibitors against the formation of AGEs (41). For instance, metformin has been shown to reduce concentrations of glyoxal and methylglyoxal, which are 2 well-known reactive precursors of AGEs (42, 43).
To our knowledge, our study is among the first few studies that attempted to evaluate the health impact of dietary exposure to CML AGE in a large population-based study. Several limitations of our research should be discussed. First, the estimate of CML AGE by using the FFQ may not have been entirely accurate because cooking practices by this population were not specifically evaluated for all foods consumed. The potential nondifferential misclassification because of our method of assigning a CML-AGE value to individual foods would likely have driven risk estimates toward the null. Future studies weighting food-processing methods for all foods consumed in the study population would provide more-accurate estimates of the intake of AGEs. Second, although we observed significantly increased risk of pancreatic cancer in never-smoking women or women who had self-reported diabetes, the sample sizes on which these observations were based were extremely small. Third, we could not exclude the possibility that the moderate association observed in men was the result of residual confounding or unrecognized confounding factors such as unhealthy lifestyles that track with CML-AGE consumption.
Last but not least, there is currently no consensus regarding quantification methods for CML AGE in foods. Iimmunochemical methods (such as ELISA) provide estimates on protein and lipid CML as present in vivo. This approach has enabled numerous cellular, animal, and human studies that show the involvement of dietary AGEs in chronic diseases (44–46) as well as the fact that a reduced consumption of AGEs is associated with improved chronic inflammation, oxidant stress, insulin sensitivity, the prevention of diabetes, and an extended life span (30, 47). Alternatively, the instrumental methods use HPLC, gas chromatography–mass spectrometry, and LC–mass spectrometry to report on CML free of its carrier, such as protein, lipoprotein, or fat. By excluding large sources of AGEs during sample hydrolysis and preparation, these methods likely underestimate AGE concentrations in complex clinical or food samples (48). Thus far, neither ELISA nor instrumental methods have been standardized against universal standards or units (49, 50). Nevertheless, although a comprehensive and valid reference database for CML AGE or other AGE compounds in different foods would be a prerequisite to accurately evaluate dietary exposure to AGEs, the ELISA assay seems to have opened useful inroads with respect to the pathophysiologic properties of AGEs.
The limitations mentioned on measuring AGEs in foods did not compromise the internal validity of our study because values were generated in one laboratory by using the same anti-CML monoclonal antibody (21). This measure was shown to track important pathologic events in cells, animals, and humans, and thus the relevant measure of CML AGE is a qualified marker for chronic diseases (44, 47). However, the external validity of the study findings should be tested in other study populations.
In conclusion, our study raised the valid question on whether chronic dietary CML-AGE consumption as a long-term lifestyle exposure is an unrecognized causing factor for pancreatic cancer related to lifestyle. Our study proposes a novel mechanism by which thermally processed red meat consumption contributes to chronic inflammation and, subsequently, pancreatic cancer. Additional studies that use precise analytic tools and biomarkers in evaluating exposure to and health effects of specific dietary AGEs on the human body are needed (48). Should our study findings be confirmed, a cost-effective strategy to improve healthy aging, i.e., a low-AGE diet, may also have implications in the prevention of pancreatic cancer.
Supplementary Material
Acknowledgments
Cancer-incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia. Cancer-incidence data from California were collected by the California Cancer Registry, California Department of Public Health's Cancer Surveillance and Research Branch, Sacramento, California. Cancer-incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, Lansing, Michigan. Florida cancer-incidence data used in this report were collected by the Florida Cancer Data System (Miami, Florida) under a contract with the Florida Department of Health, Tallahassee, Florida. Cancer-incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Health Sciences Center School of Public Health, New Orleans, Louisiana. Cancer-incidence data from New Jersey were collected by the New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey State Department of Health, Trenton, New Jersey. Cancer-incidence data from North Carolina were collected by the North Carolina Central Cancer Registry, Raleigh, North Carolina. Cancer-incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. Cancer-incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services, Phoenix, Arizona. Cancer-incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, Austin, Texas. Cancer-incidence data from Nevada were collected by the Nevada Central Cancer Registry, State Health Division, State of Nevada Department of Health and Human Services, Las Vegas, Nevada.
We thank Arthur Schatzkin for his support of our research at the National Cancer Institute. We thank Yikyung Park for study coordination. We thank Robert F Todd and Hashem B El-Serag for critical support at Baylor College of Medicine.
The authors’ responsibilities were as follows—LJ and RS: designed the research; ZD and LC: analyzed data; LJ, RS-S, TPZ, ZD, LC, LK, AFS, AJC, AH, HV, GS, and RS: conducted research; LJ, RS-S, TPZ, AFS, AJC, RS, HV, and GS: wrote the manuscript; AFS, AH, and RS: acquired data; LJ: had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. HV and GS are authors of a book titled The AGE-less way: escape America's over-eating epidemic and were not involved in data analysis. LJ, RS-S, TPZ, ZD, LC, LK, AR, AFS, AJC, AH, and RS had no conflicts of interest.
Footnotes
Abbreviations used: AGE, advanced glycation end product; AGER1, advanced glycation end products receptor 1; CML, Nϵ-(carboxymethyl)lysine; CSFII, Continuing Survey of Food Intakes by Individuals; DiMeIQx, 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline; FFQ, food-frequency questionnaire; MeIQx, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline; PhIP, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine; RAGE, receptor for advanced glycation end product; sRAGE, soluble receptor for advanced glycation end product.
REFERENCES
- 1.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity and the prevention of cancer: a global perspective. Washington, DC: AICR;2007.
- 2.Ahmed MU, Thorpe SR, Baynes JW. Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J Biol Chem 1986;261:4889–94. [PubMed] [Google Scholar]
- 3.Vistoli G, De MD, Cipak A, Zarkovic N, Carini M, Aldini G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic Res 2013;47(Suppl 1):3–27. [DOI] [PubMed] [Google Scholar]
- 4.Hull GL, Woodside JV, Ames JM, Cuskelly GLN. N-(carboxymethyl)lysine content of foods commonly consumed in a Western style diet. Food Chem 2012;131:170–4. [Google Scholar]
- 5.Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, Yong A, Striker GE, Vlassara H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc 2010;110:911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song F, Schmidt AM. Glycation and insulin resistance: novel mechanisms and unique targets? Arterioscler Thromb Vasc Biol 2012;32:1760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unoki H, Yamagishi S. Advanced glycation end products and insulin resistance. Curr Pharm Des 2008;14:987–9. [DOI] [PubMed] [Google Scholar]
- 8.Uribarri J, Cai W, Sandu O, Peppa M, Goldberg T, Vlassara H. Diet-derived advanced glycation end products are major contributors to the body's AGE pool and induce inflammation in healthy subjects. Ann N Y Acad Sci 2005;1043:461–6. [DOI] [PubMed] [Google Scholar]
- 9.Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem 1996;271:9982–6. [DOI] [PubMed] [Google Scholar]
- 10.Glomb MA, Monnier VM. Mechanism of protein modification by glyoxal and glycolaldehyde, reactive intermediates of the Maillard reaction. J Biol Chem 1995;270:10017–26. [DOI] [PubMed] [Google Scholar]
- 11.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia 2001;44:129–46. [DOI] [PubMed] [Google Scholar]
- 12.Henle T. Dietary advanced glycation end products–a risk to human health? A call for an interdisciplinary debate. Mol Nutr Food Res 2007;51:1075–8. [DOI] [PubMed] [Google Scholar]
- 13.Sebeková K, Somoza V. Dietary advanced glycation endproducts (AGEs) and their health effects–PRO. Mol Nutr Food Res 2007;51:1079–84. [DOI] [PubMed] [Google Scholar]
- 14.Jiao L, Weinstein SJ, Albanes D, Taylor PR, Graubard BI, Virtamo J, Stolzenberg-Solomon RZ. Evidence that serum levels of the soluble receptor for advanced glycation end products are inversely associated with pancreatic cancer risk: a prospective study. Cancer Res 2011;71:3582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stolzenberg-Solomon RZ, Cross AJ, Silverman DT, Schairer C, Thompson FE, Kipnis V, Subar AF, Hollenbeck A, Schatzkin A, Sinha R. Meat and meat-mutagen intake and pancreatic cancer risk in the NIH-AARP cohort. Cancer Epidemiol Biomarkers Prev 2007;16:2664–75. [DOI] [PubMed] [Google Scholar]
- 16.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol 2001;154:1089–99. [DOI] [PubMed] [Google Scholar]
- 17.USDA Food and nutrient database for dietary studies, 3.0. Beltsville (MD): Agricultural Research Service, Food Surveys Research Group; 2008.
- 18.Subar AF, Midthune D, Kulldorff M, Brown CC, Thompson FE, Kipnis V, Schatzkin A. Evaluation of alternative approaches to assign nutrient values to food groups in food frequency questionnaires. Am J Epidemiol 2000;152:279–86. [DOI] [PubMed] [Google Scholar]
- 19.Sinha R, Cross A, Curtin J, Zimmerman T, McNutt S, Risch A, Holden J. Development of a food frequency questionnaire module and databases for compounds in cooked and processed meats. Mol Nutr Food Res 2005;49:648–55. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg T, Cai W, Peppa M, Dardaine V, Baliga BS, Uribarri J, Vlassara H. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc 2004;104:1287–91. [DOI] [PubMed] [Google Scholar]
- 21.Founds HW, Sadeghi H, inventors. Alteon Inc., assignee. Monoclonal antibodies specific for advanced glycoxylation endproducts in biological samples. United States patent US5892000. 1995 Dec 29.
- 22.Boehm BO, Schilling S, Rosinger S, Lang GE, Lang GK, Kientsch-Engel R, Stahl P. Elevated serum levels of N(epsilon)-carboxymethyl-lysine, an advanced glycation end product, are associated with proliferative diabetic retinopathy and macular oedema. Diabetologia 2004;47:1376–9. [DOI] [PubMed] [Google Scholar]
- 23.Flood A, Subar AF, Hull SG, Zimmerman TP, Jenkins DJ, Schatzkin A. Methodology for adding glycemic load values to the National Cancer Institute Diet History Questionnaire database. J Am Diet Assoc 2006;106:393–402. [DOI] [PubMed] [Google Scholar]
- 24.Larsson SC, Wolk A. Red and processed meat consumption and risk of pancreatic cancer: meta-analysis of prospective studies. Br J Cancer 2012;106:603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornalley PJ. Advanced glycation end products in renal failure. J Ren Nutr 2006;16:178–84. [DOI] [PubMed] [Google Scholar]
- 26.Cai W, Gao QD, Zhu L, Peppa M, He C, Vlassara H. Oxidative stress-inducing carbonyl compounds from common foods: novel mediators of cellular dysfunction. Mol Med 2002;8:337–46. [PMC free article] [PubMed] [Google Scholar]
- 27.Cai W, He JC, Zhu L, Chen X, Wallenstein S, Striker GE, Vlassara H. Reduced oxidant stress and extended lifespan in mice exposed to a low glycotoxin diet: association with increased AGER1 expression. Am J Pathol 2007;170:1893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai W, He JC, Zhu L, Chen X, Zheng F, Striker GE, Vlassara H. Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan. Am J Pathol 2008;173:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung C, Herath CB, Jia Z, Goodwin M, Mak KY, Watt MJ, Forbes JM, Angus PW. Dietary glycotoxins exacerbate progression of experimental fatty liver disease. J Hepatol 2014;60:832–8. [DOI] [PubMed] [Google Scholar]
- 30.Vlassara H, Striker GE. AGE restriction in diabetes mellitus: a paradigm shift. Nat Rev Endocrinol 2011;7:526–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.West RK, Moshier E, Lubitz I, Schmeidler J, Godbold J, Cai W, Uribarri J, Vlassara H. Silverman JM, Beeri MS. Dietary advanced glycation end products are associated with decline in memory in young elderly. Mech Ageing Dev 2014;140:10–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 2005;15:16R–28R. [DOI] [PubMed] [Google Scholar]
- 33.Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, Vlassara H. Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci 2007;62:427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai W, Ramdas M, Zhu L, Chen X, Striker GE, Vlassara H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc Natl Acad Sci USA 2012;109:15888–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto Y, Yamagishi S, Hsu CC, Yamamoto H. Advanced glycation endproducts-receptor interactions stimulate the growth of human pancreatic cancer cells through the induction of platelet-derived growth factor-B. Biochem Biophys Res Commun 1996;222:700–5. [DOI] [PubMed] [Google Scholar]
- 36.Monnier VM, Sell DR. Prevention and repair of protein damage by the Maillard reaction in vivo. Rejuvenation Res 2006;9:264–73. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Jiang Y, Liu N, Ren L, Zhu Y, An Y, Chen D. Advanced glycation end-product Nepsilon-carboxymethyl-Lysine accelerates progression of atherosclerotic calcification in diabetes. Atherosclerosis 2012;221:387–96. [DOI] [PubMed] [Google Scholar]
- 38.Francis-Sedlak ME, Moya ML, Huang JJ, Lucas SA, Chandrasekharan N, Larson JC, Cheng MH, Brey EM. Collagen glycation alters neovascularization in vitro and in vivo. Microvasc Res 2010;80:3–9. [DOI] [PubMed] [Google Scholar]
- 39.Abe H, Matsubara T, Iehara N, Nagai K, Takahashi T, Arai H, Kita T, Doi T. Type IV collagen is transcriptionally regulated by Smad1 under advanced glycation end product (AGE) stimulation. J Biol Chem 2004;279:14201–6. [DOI] [PubMed] [Google Scholar]
- 40.Jägerstad M, Skog K. Genotoxicity of heat-processed foods. Mutat Res 2005;574:156–72. [DOI] [PubMed] [Google Scholar]
- 41.Peng X, Ma J, Chen F, Wang M. Naturally occurring inhibitors against the formation of advanced glycation end-products. Food Funct 2011;2:289–301. [DOI] [PubMed] [Google Scholar]
- 42.Desai K, Wu L. Methylglyoxal and advanced glycation endproducts: new therapeutic horizons? Recent Pat Cardiovasc Drug Discov 2007;2:89–99. [DOI] [PubMed] [Google Scholar]
- 43.Ruggiero-Lopez D, Lecomte M, Moinet G, Patereau G, Lagarde M, Wiernsperger N. Reaction of metformin with dicarbonyl compounds. Possible implication in the inhibition of advanced glycation end product formation. Biochem Pharmacol 1999;58:1765–73. [DOI] [PubMed] [Google Scholar]
- 44.Cai W, Uribarri J, Zhu L, Chen X, Swamy S, Zhao Z, Grosjean F, Simonaro C, Kuchel GA, Schnaider-Beeri M, et al. Oral glycotoxins are a modifiable cause of dementia and the metabolic syndrome in mice and humans. Proc Natl Acad Sci USA 2014;111:4940–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaens KH, Goossens GH, Niessen PM, van Greevenbroek MM, van der Kallen CJ, Niessen HW, Rensen SS, Buurman WA, Greve JW, Blaak EE, et al. Nepsilon-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler Thromb Vasc Biol 2014;34:1199–208. [DOI] [PubMed] [Google Scholar]
- 46.Uribarri J, Stirban A, Sander D, Cai W, Negrean M, Buenting CE, Koschinsky T, Vlassara H. Single oral challenge by advanced glycation end products acutely impairs endothelial function in diabetic and nondiabetic subjects. Diabetes Care 2007;30:2579–82. [DOI] [PubMed] [Google Scholar]
- 47.Mark AB, Poulsen MW, Andersen S, Andersen JM, Bak MJ, Ritz C, Holst JJ, Nielsen J, de Courten B, Dragsted LO, et al. Consumption of a diet low in advanced glycation end products for 4 weeks improves insulin sensitivity in overweight women. Diabetes Care 2014;37:88–95. [DOI] [PubMed] [Google Scholar]
- 48.Poulsen MW, Hedegaard RV, Andersen JM, de Courten B, Bügel S, Nielsen J, Skibsted LH, Dragsted LO. Advanced glycation endproducts in food and their effects on health. Food Chem Toxicol 2013;60:10–37. [DOI] [PubMed] [Google Scholar]
- 49.Ames JM. Determination of N epsilon-(carboxymethyl)lysine in foods and related systems. Ann N Y Acad Sci 2008;1126:20–4. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen HT, Van der Fels-Klerx HJ, Van Boekel MAJS. N-(carboxymethyl)lysine: a review on analytical methods, formation, and occurrence in processed food, and health impact. Food Rev Int 2013;30:36–52. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.