Abstract
Background: Experimental evidence suggests that hepatic de novo lipogenesis (DNL) affects insulin homeostasis via synthesis of saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs). Few prospective studies have used fatty acid biomarkers to assess associations with type 2 diabetes.
Objectives: We investigated associations of major circulating SFAs [palmitic acid (16:0) and stearic acid (18:0)] and MUFA [oleic acid (18:1n–9)] in the DNL pathway with metabolic risk factors and incident diabetes in community-based older U.S. adults in the Cardiovascular Health Study. We secondarily assessed other DNL fatty acid biomarkers [myristic acid (14:0), palmitoleic acid (16:1n–7), 7-hexadecenoic acid (16:1n–9), and vaccenic acid (18:1n–7)] and estimated dietary SFAs and MUFAs.
Design: In 3004 participants free of diabetes, plasma phospholipid fatty acids were measured in 1992, and incident diabetes was identified by medication use and blood glucose. Usual diets were assessed by using repeated food-frequency questionnaires. Multivariable linear and Cox regression were used to assess associations with metabolic risk factors and incident diabetes, respectively.
Results: At baseline, circulating palmitic acid and stearic acid were positively associated with adiposity, triglycerides, inflammation biomarkers, and insulin resistance (P-trend < 0.01 each), whereas oleic acid showed generally beneficial associations (P-trend < 0.001 each). During 30,763 person-years, 297 incident diabetes cases occurred. With adjustment for demographics and lifestyle, palmitic acid (extreme-quintile HR: 1.89; 95% CI: 1.27, 2.83; P-trend = 0.001) and stearic acid (HR: 1.62; 95% CI: 1.09, 2.41; P-trend = 0.006) were associated with higher diabetes risk, whereas oleic acid was not significantly associated. In secondary analyses, vaccenic acid was inversely associated with diabetes (HR: 0.56; 95% CI: 0.38, 0.83; P-trend = 0.005). Other fatty acid biomarkers and estimated dietary SFAs or MUFAs were not significantly associated with incident diabetes.
Conclusions: In this large prospective cohort, circulating palmitic acid and stearic acid were associated with higher diabetes risk, and vaccenic acid was associated with lower diabetes risk. These results indicate a need for additional investigation of biological mechanisms linking specific fatty acids in the DNL pathway to the pathogenesis of diabetes. This trial was registered at clinicaltrials.gov as NCT00005133.
Keywords: diabetes mellitus, fatty acids, metabolism, biomarker, diet
INTRODUCTION
De novo lipogenesis (DNL)6 is the process whereby excess carbohydrate and protein are converted into SFAs and MUFAs (1). Emerging evidence suggests that DNL might play an important role in metabolic regulation and influence the pathogenesis of type 2 diabetes. In short-term metabolic studies, low-fat, high-carbohydrate diets induce hepatic DNL, which may be a primary mechanism that drives increased fasting triglycerides (2, 3). Elevated DNL also contributes to hepatic fat accumulation in subjects with nonalcoholic fatty acid liver disease (4, 5), which is a risk factor for insulin resistance and diabetes, whereas short-term weight loss induced by low-carbohydrate diets reduces hepatic fat substantially more than does similar weight loss induced by low-fat diets (6). However, whether differences in DNL in adults consuming habitual diets are associated with diabetes risk remains unknown.
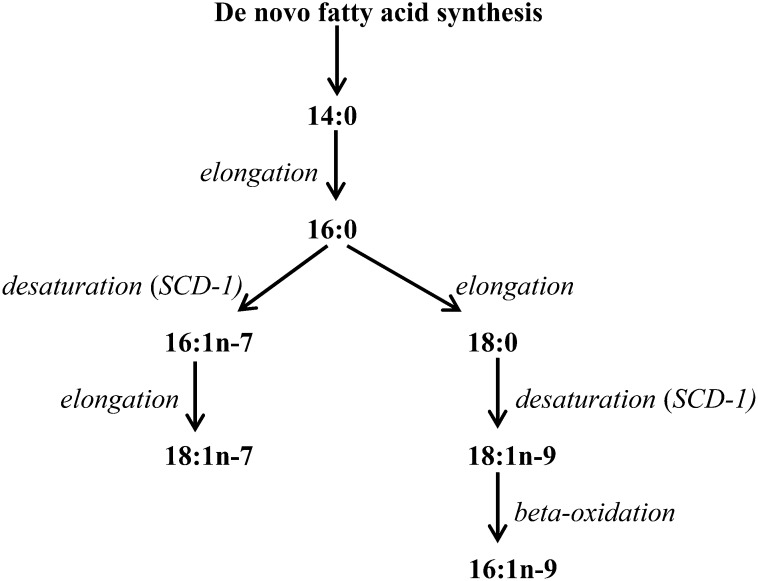
In addition to increasing triglycerides and hepatic steatosis, DNL may influence diabetes through effects of its SFA and MUFA products, including myristic acid (14:0), palmitic acid (16:0), palmitoleic acid (16:1n–7), vaccenic acid (18:1n–7), stearic acid (18:0), oleic acid (18:1n–9), and 7-hexadecenoic acid (16:1n–9) (Figure 1). Experimentally, several of these SFAs and MUFAs affect metabolic pathways related to diabetes. For example, in animal and in vitro models, the major SFA palmitic acid induces inflammation, endoplasmic reticulum stress, and insulin resistance (7–11). Similarly, stearic acid may promote adiposity and cause insulin resistance (12–14). Conversely, oleic acid may prevent palmitate-induced metabolic harms (10, 15, 16). In previous work by our group and others (17, 18), factors known to stimulate DNL including carbohydrate consumption and alcohol use were independently associated with higher concentrations of several of these fatty acids in free-living adults. These findings suggest that, even at usual ranges of dietary exposures, circulating concentrations of specific SFAs and MUFAs at least partly reflect DNL activity (19). These results are consistent with controlled dietary interventions in which increased carbohydrate and decreased total fat consumption increased circulating concentrations of these fatty acids (20–22).
FIGURE 1.
SFAs and MUFAs produced during de novo lipogenesis, whereby carbohydrates, protein, and alcohol are converted to fatty acids. SCD-1 is a key rate-limiting enzyme for the synthesis of 16:1n–7 and 18:1n–9. SCD-1, stearoyl-CoA desaturase-1.
Despite experimental evidence that linked SFAs and MUFAs to metabolic regulation and insulin homeostasis, only limited previous studies have used circulating SFA and MUFA biomarkers to assess prospective associations with diabetes risk (18, 23–30). Findings have been inconsistent perhaps because of insufficient sample size and cases, different tissues in which fatty acids were measured (serum, plasma, or erythrocyte membranes), or a lack of adjustment for risk factors that stimulate hepatic DNL. Furthermore, previous studies have not investigated independent associations of SFA and MUFA biomarkers with metabolic risk factors. To elucidate potential determinants and metabolic effects of circulating SFAs and MUFAs, we investigated demographic and lifestyle correlates of circulating SFAs and MUFAs, and whether these fatty acids were related to metabolic risk factors and incident diabetes in the CHS (Cardiovascular Health Study; registered at clinicaltrials.gov as NCT00005133), which is a community-based cohort of older U.S. adults. To evaluate the concordance of our findings for circulating SFAs and MUFAs, which are influenced by both endogenous synthesis and diet, with their dietary intake, we also examined associations of the dietary consumption of these SFAs and MUFAs with diabetes risk.
SUBJECTS AND METHODS
Study design and population
The CHS is a community-based, prospective cohort study funded by the National Heart, Lung, and Blood Institute to identify risk factors for cardiovascular diseases in older adults (31). Participants were randomly selected from Medicare eligibility lists from 4 U.S. communities (Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; Allegheny County, Pennsylvania). Initially, 5201 noninstitutionalized men and women aged ≥ 65 y were recruited in 1989–1990; an additional subcohort of 687 mostly black participants was recruited in 1992–1993. In total, 57% of randomly sampled adults agreed to participate in the study. Each center's institutional review committee approved the study, and all participants gave informed written consent.
Plasma phospholipid fatty acids were measured in 3941 participants by using specimens drawn in 1992–1993, which was the baseline for all current biomarker analyses. After the exclusion of participants with prevalent diabetes in 1992–1993 (n = 902) and those with missing follow-up data on incident diabetes (n = 35), 3004 participants were evaluated in the analysis of biomarker SFAs and MUFAs and incident diabetes.
In dietary analyses, we assessed dietary SFAs and MUFAs on the basis on validated questionnaires administered in 1989–1990 for the original cohort and again in 1995–1996 for both cohorts. From the entire cohort of 5888 adults, we excluded participants with prevalent diabetes (n = 1227) at the time of their first diet assessment and those with missing data on diet (n = 112) or incident diabetes (n = 328), which resulted in 4221 participants in the analysis of dietary SFAs and MUFAs and incident diabetes.
Plasma phospholipid fatty acids
At the 1992–1993 study visit, blood was drawn after a 12-h fast and stored at −80°C until 2010. Plasma phospholipid fatty acids are stable under prolonged storage at −80°C for 10 y (32). Fatty acids were measured as a weight percentage of total fatty acids by the Fred Hutchinson Cancer Research Center Biomarker Laboratory by using previously described methods (31). Total lipids were extracted from plasma (33), and phospholipids were separated from neutral lipids by using one-dimensional thin-layer chromatography. Samples of fatty acid methyl esters were prepared by direct transesterification (34) and separated by using gas chromatography [initially 160°C for 16 min, ramped to 3°C/min to 240°C, and held for 15 min; Agilent 5890 Gas Chromatograph Flame Ionization Detector and Supelco fused silica capillary column SP-2560 (100 m × 0.25 mm, 0.2 μm; Sigma-Aldrich)]. Identification, precision, and accuracy were evaluated by using model mixtures of known fatty acid methyl esters and an established in-house control pool with identification confirmed by using gas chromatography–mass spectrometry at the USDA lipid laboratory.
On the basis of experimental evidence for potential effects on the pathogenesis of diabetes (7–16), our prespecified primary exposures of interest were palmitic acid, stearic acid, and oleic acid. We had previously evaluated the association of palmitoleic acid with diabetes in the CHS (19), and evaluated palmitoleic acid again because of a larger number of incident diabetes cases in this more-updated follow-up. We also evaluated myristic acid, 7-hexadecenoic acid, and vaccenic acid in secondary analyses because of insufficient experimental evidence to allow the formulation of a priori hypothesis with regard to their effects on diabetes.
Estimation of stearoyl-CoA desaturase-1 activity
The altered activity of stearoyl-CoA desaturase-1 (SCD-1), which is the rate-limiting enzyme for the endogenous production of palmitoleic acid and oleic acid, could also influence the pathogenesis of diabetes. Mice deficient in SCD-1 showed increased fatty acid oxidation and insulin sensitivity (35, 36). We used plasma phospholipid oleic acid:stearic acid ratio to estimate hepatic SCD-1 activity because SCD-1 preferentially uses stearic acid-CoA as the substrate for desaturation (37), and the fasting VLDL oleic acid:stearic acid ratio was closely correlated with hepatic SCD-1 messenger RNA expression (38). We confirmed that the ratio of the 2 fatty acids in plasma phospholipids was correlated with the equivalent ratio in fasting triglycerides in a subset of 104 CHS participants as shown in Supplemental Table 1 (Spearman's r = 0.46). We also evaluated other possible alternative estimators of SCD-1 activity such as the residual of oleic acid after a linear regression adjusted for stearic acid, the log2 transformation of the residual, and the log2 transformation of oleic acid:stearic acid. The potential performance of each of these estimators was compared by assessing their association with carbohydrate and alcohol intakes [(inducers of SCD-1 expression and activity in experimental studies (3, 39)]. On the basis of these comparisons (not shown), the oleic acid:stearic acid ratio was selected as the primary estimator for SCD-1 activity.
Dietary fatty acids
Usual dietary habits were assessed in 1989–1990 by using a picture-sort food-frequency questionnaire (FFQ) and again in 1995–1996 by using the Willett FFQ, both of which have shown reproducibility and validity compared with repeated 24-h dietary recalls or 1-wk dietary records (40, 41). Participants were asked to report usual dietary intakes in standardized portion sizes over the previous year. The 2 measurements of dietary fatty acids correlated reasonably well, with correlations over time that ranged from 0.36 (palmitoleic acid) to 0.47 (stearic acid). Estimated dietary SFAs and MUFAs were evaluated as a percentage of total energy intake. Dietary habits were cumulatively updated as time-varying exposures in participants who completed both questionnaires. Participants in the second cohort enrolled in 1992–1993 entered the dietary analysis in 1995–1996, which was the visit at which their dietary habits were first assessed.
Metabolic outcomes and covariates
Information on demographics, medical history, lifestyle, and other risk factors was collected during annual clinical visits on the basis of standardized interviews, physical examinations, and laboratory testing (31). Physical activity was evaluated by using the modified Minnesota Leisure Time Activities (42). Weight, height, and waist circumference were measured by using standardized methods with BMI (in kg/m2) calculated as weight divided by height squared. Blood lipids were measured by using standardized methods with LDL cholesterol calculated according to Friedewald's equation in participants without hypertriglyceridemia. Fibrinogen was measured by using the modified Clauss method, and C-reactive protein (CRP) was measured by using a high-sensitivity enzyme-linked immunosorbent assay (43). Fasting serum glucose was measured by using a Kodak Ektachem 700 Analyzer (Eastman Kodak Corp.), and insulin was measured by using serum-based standards (Diagnostics Products Corp.). For indicators of glucose-insulin homeostasis, we calculated the HOMA-IR and homeostasis model assessment of β cell function (HOMA-β) according to a homeostasis model assessment 2 model (44). For fatty acid biomarker analyses, we used covariates measured at the same visit in 1992–1993; for dietary fatty acid analyses, we used covariates measured at the same visits as diet assessments.
Ascertainment of incident diabetes
Fasting glucose was measured during annual clinic visits in 1989–1990, 1992–1993, 1996–1997, 1998–1999, and 2005–2006, nonfasting glucose was measured in 1994–1995, and 2-h postchallenge glucose was measured in 1989–1990 and 1996–1997. Information on prescription medications taken in the previous 2 wk was collected annually by using standardized methods at clinic visits for the first 10 y and by telephone contact thereafter. Diabetes was defined as a single measure of fasting glucose concentration ≥126 mg/dL (7.0 mmol/L), nonfasting or 2-h postchallenge glucose concentration ≥200 mg/dL (11.1 mmol/L), or new use of an insulin or oral hypoglycemic medication. In sensitivity analyses, we also evaluated diabetes defined by fasting glucose, nonfasting glucose, or medications only (i.e., excluding postchallenge glucose data from the definition).
Statistical analysis
Unadjusted interrelations between phospholipid fatty acids, estimated SCD-1 activity, and estimated dietary fatty acids were evaluated by using Spearman's correlation. Independent demographic, lifestyle, and dietary correlates of each fatty acid biomarker were assessed using multivariable-adjusted linear regression with fatty acid concentrations as dependent variables. The multivariable-adjusted relation of fatty acid biomarkers with metabolic risk factors was similarly evaluated with metabolic risk factors as the dependent variable. For dietary fatty acids, we used the multivariate nutrient density method to estimate isocaloric replacement effects between various nutrients (45).
A Cox proportional hazards model was used to estimate the association of each exposure with incident diabetes with time at risk until first diagnosis, last follow-up visit with information on medications, or administrative censoring in 2010 (the latest date of currently available adjudicated medication information). Exposures were evaluated in quintiles with significance of trends across categories assessed by assigning participants the median value in their quintile and evaluating this as a continuous variable. The Cox proportional hazards assumption was tested by using Schoenfeld's residuals.
To minimize potential confounding, covariates were selected on the basis of biological interest, which are well-established risk factors for diabetes in older adults, associations with exposures or outcomes in the cohort, and changes in the risk estimate of interest when covariates were included in the model. We separately evaluated factors that could be potential confounders or mediators of effects of SFAs and MUFAs, including the total:HDL cholesterol ratio, plasma triglycerides, CRP, fibrinogen, and HOMA-IR. In models that estimated SCD-1 activity by ratios of fatty acids, we also adjusted for component fatty acids to assess the independent additional information imparted by the ratio. Potential effect modification by age and sex was assessed by using multiplicative interaction terms, with statistical significance determined by using Wald's test. Missing covariates (<1% for most; <10–14% for dietary covariates) were imputed by a single imputation on the basis of age, sex, race, education, smoking status, alcohol consumption, leisure-time physical activity, BMI, prevalent ischemic heart disease, and stroke.
To minimize exposure misclassification with an increasing duration of follow-up, we performed sensitivity analyses limited to the midpoint of follow-up (9 y). To assess potential bias because of reverse causation, we performed analyses excluding participants with incident diabetes in the first 2 y of follow-up. In addition, we also restricted analyses to nondrinkers to reduce confounding by alcohol intake. All P values were 2-tailed (α = 0.05), and analyses were performed with Stata 11.2 software.
RESULTS
Distributions of lifestyle, dietary, and metabolic risk factors at baseline in 1992–1993 are shown in Table 1. The average age was 74 y (range: 65–97 y), and 60% of subjects were women. Mean concentrations of circulating fatty acids in the DNL pathway ranged from 0.09% of total phospholipid fatty acids (7-hexadecenoic acid) to 25.3% (palmitic acid). These fatty acids had generally positive intercorrelations (Supplemental Table 1) except for stearic acid, which was inversely correlated with the other fatty acids (r = −0.15 to −0.40). Consistent with endogenous production and metabolism being primary determinants, these circulating SFAs and MUFAs were generally weakly correlated with their estimated direct dietary intake with correlations that ranged from 0.01 (palmitic acid) to 0.21 (myristic acid) (Supplemental Table 2).
TABLE 1.
Characteristics of 3004 U.S. men and women with phospholipid fatty acid measurements and free of prevalent diabetes in the CHS at baseline in 1992–19931
| Characteristics | Values |
| Age, y | 74 ± 52 |
| Male sex, % | 40 |
| White race, % | 89 |
| Education more than high school, % | 47 |
| Current smoking, % | 9 |
| Alcohol, drinks/wk | 2.2 ± 6.5 |
| Leisure-time physical activity, kcal/wk | 1093 ± 1493 |
| BMI, kg/m2 | 26 ± 5 |
| Waist circumference, cm | 96 ± 13 |
| LDL cholesterol, mg/dL | 127 ± 33 |
| HDL cholesterol, mg/dL | 54 ± 15 |
| Triglycerides, mg/dL | 137 ± 77 |
| Total cholesterol:HDL cholesterol ratio | 4.1 ± 1.2 |
| C-reactive protein, mg/L | 4.8 ± 9.1 |
| Fibrinogen, mg/dL | 325 ± 64 |
| HOMA-IR, U | 1.4 ± 0.9 |
| HOMA-β, U | 102 ± 33 |
| Ischemic heart disease, % | 21 |
| Drug-treated hypertension, % | 45 |
| Dietary habits3 | |
| Total fat, % of energy | 30 ± 6 |
| Saturated fat, % of energy | 10 ± 2 |
| Monounsaturated fat, % of energy | 11 ± 2 |
| Polyunsaturated fat, % of energy | 6.5 ± 1.7 |
| Carbohydrate, % of energy | 54 ± 7 |
| Protein, % of energy | 18 ± 3 |
| Total energy intake, kcal/d | 2007 ± 582 |
Baseline characteristics of 4221 participants included in the dietary analyses were similar. Percentages are listed for categorical variables. CHS, Cardiovascular Health Study; FFQ, food-frequency questionnaire; HOMA-β, homeostasis model assessment of β cell function; U, units.
Mean ± SD (all such values) (continuous variables).
Dietary assessments based on the average of the responses on 2 FFQs administered in 1989–1990 and 1995–1996; data from one FFQ was used when the other was not available (27% of participants).
Multivariable-adjusted associations of demographic, lifestyle, and dietary factors with these phospholipid fatty acids are shown in Supplemental Tables 3 and 4. Consistent with experimental data and previous results in the study (17), alcohol intake and increased dietary carbohydrate as an isocaloric replacement for fat were significant, independent correlates of higher palmitic acid and oleic acid but lower stearic acid. Increased dietary protein as a replacement for fat was associated with higher palmitic acid and lower stearic acid but not oleic acid. In additional analyses that estimated the isocaloric substitution of carbohydrate for subtypes of fat (Figure 2), increased carbohydrate in place of SFAs was associated with lower palmitic acid and oleic acid; increased carbohydrate in place of MUFAs was associated with higher palmitic acid but lower oleic acid; and increased carbohydrate in place of polyunsaturated fat (PUFA) was associated with higher oleic acid.
FIGURE 2.
Standardized differences (95% CIs) in plasma phospholipid 16:0, 18:0, and 18:1n–9 concentrations for each additional unit of potential demographic or lifestyle determinants. Associations were assessed by using multivariable-adjusted linear regression with each fatty acid as the dependent variable with adjustment for all variables in the figure as well as education and enrollment site. The nutrient density method was used to model the effect of the isocaloric substitution of dietary macronutrients.
In multivariable-adjusted cross-sectional analyses (Table 2), palmitic acid and stearic acid showed both similarities and differences in relation to metabolic risk factors. Each acid was associated with higher BMI, waist circumference, triglycerides, and HOMA-IR (P-trend < 0.01 each). Conversely, palmitic acid was associated with lower LDL cholesterol, higher HDL cholesterol, higher CRP, and lower fibrinogen (P-trend < 0.05 each), but not with HOMA-β, whereas stearic acid was associated with higher LDL cholesterol, lower HDL cholesterol, higher fibrinogen, but also better β cell function assessed by HOMA-β (P-trend < 0.001 each). In contrast to both palmitic acid and stearic acid, oleic acid generally associated with more favorable concentrations of adiposity, LDL cholesterol, HDL cholesterol, and fibrinogen, but still with higher triglycerides (P-trend < 0.001 each) and without associations with HOMA-IR or HOMA-β. Likewise, other circulating fatty acids were each associated with metabolic risk factors in mixed directions (Supplemental Table 5).
TABLE 2.
Multivariable-adjusted associations of key plasma phospholipid saturated and monounsaturated fats in the de novo lipogenesis pathway with metabolic risk factors (n = 3004)1
| Quintiles of plasma phospholipid fatty acids |
||||||
| I | II | III | IV | V | P-trend | |
| 16:0 | ||||||
| Median, percentage of total fatty acids | 23.4 | 24.4 | 25.2 | 26.0 | 27.3 | — |
| Adiposity | ||||||
| BMI, kg/m2 | 25.3 ± 0.22 | 26.1 ± 0.2 | 26.7 ± 0.2 | 26.8 ± 0.2 | 26.8 ± 0.2 | <0.001 |
| Waist circumference, cm | 93.2 ± 0.5 | 95.3 ± 0.5 | 96.2 ± 0.5 | 97.1 ± 0.5 | 97.1 ± 0.5 | <0.001 |
| Blood lipids | ||||||
| LDL cholesterol, mg/dL | 133 ± 1 | 128 ± 1 | 130 ± 1 | 126 ± 1 | 119 ± 1 | <0.001 |
| HDL cholesterol, mg/dL | 54.5 ± 0.5 | 53.5 ± 0.5 | 53.4 ± 0.5 | 53.5 ± 0.5 | 57.3 ± 0.5 | 0.001 |
| Triglycerides, mg/dL | 120 ± 3 | 125 ± 3 | 138 ± 3 | 146 ± 3 | 157 ± 3 | <0.001 |
| Total:HDL cholesterol ratio | 4.1 ± 0.05 | 4.1 ± 0.05 | 4.2 ± 0.05 | 4.2 ± 0.05 | 3.9 ± 0.05 | 0.03 |
| Inflammation | ||||||
| CRP, mg/L | 3.5 ± 0.4 | 4.4 ± 0.4 | 4.3 ± 0.4 | 5.4 ± 0.4 | 6.2 ± 0.4 | <0.001 |
| Fibrinogen, mg/dL | 327 ± 3 | 325 ± 3 | 331 ± 3 | 325 ± 3 | 318 ± 3 | 0.04 |
| Glucose-insulin homeostasis | ||||||
| HOMA-IR, U | 1.3 ± 0.04 | 1.4 ± 0.04 | 1.4 ± 0.04 | 1.5 ± 0.04 | 1.4 ± 0.04 | 0.006 |
| HOMA-β, U | 100 ± 1 | 102 ± 1 | 101 ± 1 | 105 ± 1 | 102 ± 1 | 0.12 |
| 18:0 | ||||||
| Median, percentage of total fatty acids | 12.1 | 12.9 | 13.4 | 14.0 | 14.9 | — |
| Adiposity | ||||||
| BMI, kg/m2 | 25.1 ± 0.2 | 25.9 ± 0.2 | 26.5 ± 0.2 | 26.9 ± 0.2 | 27.1 ± 0.2 | <0.001 |
| Waist circumference, cm | 92.3 ± 0.5 | 94.2 ± 0.5 | 95.8 ± 0.5 | 97.6 ± 0.5 | 98.8 ± 0.5 | <0.001 |
| Blood lipids | ||||||
| LDL cholesterol, mg/dL | 117 ± 1 | 126 ± 1 | 129 ± 1 | 130 ± 1 | 133 ± 1 | <0.001 |
| HDL cholesterol, mg/dL | 59.9 ± 0.5 | 55.4 ± 0.5 | 54.4 ± 0.5 | 52.6 ± 0.5 | 49.9 ± 0.5 | <0.001 |
| Triglycerides, mg/dL | 122 ± 3 | 122 ± 3 | 130 ± 3 | 144 ± 3 | 167 ± 3 | <0.001 |
| Total:HDL cholesterol ratio | 3.6 ± 0.05 | 3.9 ± 0.04 | 4.0 ± 0.04 | 4.2 ± 0.04 | 4.5 ± 0.05 | <0.001 |
| Inflammation | ||||||
| CRP, mg/L | 5.2 ± 0.4 | 4.2 ± 0.4 | 4.6 ± 0.4 | 4.5 ± 0.4 | 5.3 ± 0.4 | 0.73 |
| Fibrinogen, mg/dL | 315 ± 3 | 319 ± 3 | 329 ± 3 | 330 ± 3 | 333 ± 3 | <0.001 |
| Glucose-insulin homeostasis | ||||||
| HOMA-IR, U | 1.2 ± 0.04 | 1.3 ± 0.04 | 1.4 ± 0.04 | 1.5 ± 0.04 | 1.7 ± 0.04 | <0.001 |
| HOMA-β, U | 98 ± 1 | 98 ± 1 | 101 ± 1 | 103 ± 1 | 110 ± 1 | <0.001 |
| 18:1n–9 | ||||||
| Median, percentage of total fatty acids | 6.3 | 7.0 | 7.5 | 8.0 | 9.0 | — |
| Adiposity | ||||||
| BMI, kg/m2 | 26.6 ± 0.2 | 26.7 ± 0.2 | 26.6 ± 0.2 | 26.1 ± 0.2 | 25.6 ± 0.2 | <0.001 |
| Waist circumference, cm | 96.4 ± 0.5 | 96.6 ± 0.5 | 96.6 ± 0.5 | 95.1 ± 0.5 | 94.0 ± 0.5 | <0.001 |
| Blood lipids | ||||||
| LDL cholesterol, mg/dL | 134 ± 1 | 133 ± 1 | 125 ± 1 | 123 ± 1 | 121 ± 1 | <0.001 |
| HDL cholesterol, mg/dL | 53.4 ± 0.5 | 53.4 ± 0.5 | 54.0 ± 0.5 | 55.4 ± 0.5 | 56.0 ± 0.5 | <0.001 |
| Triglycerides, mg/dL | 123 ± 3 | 134 ± 3 | 139 ± 3 | 138 ± 3 | 152 ± 3 | <0.001 |
| Total:HDL cholesterol ratio | 4.2 ± 0.05 | 4.2 ± 0.05 | 4.1 ± 0.05 | 3.9 ± 0.05 | 3.9 ± 0.05 | <0.001 |
| Inflammation | ||||||
| CRP, mg/L | 4.7 ± 0.4 | 4.7 ± 0.4 | 5.8 ± 0.4 | 4.5 ± 0.4 | 4.1 ± 0.4 | 0.18 |
| Fibrinogen, mg/dL | 331 ± 3 | 331 ± 3 | 329 ± 3 | 321 ± 3 | 314 ± 3 | <0.001 |
| Glucose-insulin homeostasis | ||||||
| HOMA-IR, U | 1.4 ± 0.04 | 1.4 ± 0.04 | 1.4 ± 0.04 | 1.5 ± 0.04 | 1.4 ± 0.04 | 0.55 |
| HOMA-β, U | 102 ± 1 | 102 ± 1 | 102 ± 1 | 103 ± 1 | 101 ± 1 | 0.91 |
P-trend vales were computed by assigning the median concentration in each quintile to participants and evaluating this as a continuous variable. CRP, C-reactive protein; HOMA-β, homeostasis model assessment of β cell function; U, units.
Adjusted means ± SEs from multivariable linear regression (all such values). Values were adjusted for age, sex, race, education, clinic, smoking status, alcohol consumption, leisure-time physical activity, prevalence of ischemic heart disease, hypertension at baseline, and consumption of protein (percentage of energy), carbohydrate (percentage of energy), and total energy. Results for measures of blood lipids, inflammation, and glucose-insulin homeostasis were also adjusted for BMI and waist circumference.
During 30,763 person-years of follow-up, 297 incident cases of diabetes occurred (9.7 cases/1000 person-years). In the 3 primary fatty acids of interest, palmitic acid and stearic acid were associated with higher diabetes risk (Table 3). Compared with the lowest quintile, the multivariable-adjusted HR (95% CI) in the highest quintile was 1.89 (1.27, 2.83; P-trend = 0.001) for palmitic acid and 1.62 (1.09, 2.41; P-trend = 0.006) for stearic acid. Additional adjustment for dietary factors or mutual adjustment for palmitic acid and stearic acid did not appreciably alter these associations. After additional adjustment for clinical factors that could be either confounders or mediators including the total:HDL cholesterol ratio, plasma triglycerides, CRP, fibrinogen, and HOMA-IR, the association of palmitic acid with diabetes was only slightly attenuated (extreme-quintile HR: 1.73; 95% CI: 1.14, 2.62; P-trend = 0.009), whereas the association of stearic acid was attenuated and no longer significant (extreme-quintile HR: 1.35; 95% CI: 0.89, 2.04; P-trend = 0.11); the largest changes in these associations were seen with additional adjustment for triglycerides, the total:HDL cholesterol ratio, and HOMA-IR.
TABLE 3.
Prospective associations of key plasma phospholipid saturated and monounsaturated fats in the de novo lipogenesis pathway with incident diabetes (n = 3004)1
| Quintiles of plasma phospholipid fatty acids |
||||||
| 1 | 2 | 3 | 4 | 5 | P-trend | |
| 16:0 | ||||||
| Percentage of total fatty acids | 23.4 (19.5–24.0)2 | 24.4 (24.0–24.8) | 25.2 (24.8–25.5) | 26.0 (25.5–26.5) | 27.3 (26.5–32.4) | — |
| Cases, total person-years | 47 (6548) | 64 (6371) | 53 (6127) | 67 (5913) | 66 (5803) | — |
| Demographic adjusted3 | 1.00 (reference) | 1.42 (0.97, 2.07) | 1.31 (0.88, 1.95) | 1.84 (1.25, 2.69) | 1.88 (1.28, 2.76) | 0.001 |
| Multivariable adjusted4 | 1.00 (reference) | 1.35 (0.93, 1.98) | 1.23 (0.83, 1.83) | 1.69 (1.14, 2.49) | 1.89 (1.27, 2.83) | 0.001 |
| 18:0 | ||||||
| Percentage of total fatty acids | 12.1 (8.2–12.6) | 12.9 (12.6–13.2) | 13.4 (13.2–13.7) | 14.0 (13.7–14.4) | 14.9 (14.4–18.9) | — |
| Cases, total person-years | 38 (5816) | 49 (5977) | 50 (6269) | 72 (6392) | 88 (6310) | — |
| Demographic adjusted3 | 1.00 (reference) | 1.23 (0.80, 1.88) | 1.17 (0.77, 1.79) | 1.62 (1.09, 2.41) | 1.94 (1.31, 2.86) | <0.001 |
| Multivariable adjusted4 | 1.00 (reference) | 1.16 (0.76, 1.78) | 1.07 (0.70, 1.65) | 1.37 (0.91, 2.05) | 1.62 (1.09, 2.41) | 0.006 |
| 14:0 | ||||||
| Percentage of total fatty acids | 0.19 (0.08–0.22) | 0.24 (0.22–0.25) | 0.27 (0.25–0.29) | 0.31 (0.29–0.34) | 0.37 (0.34–1.6) | — |
| Cases, total person-years | 56 (5995) | 64 (5929) | 64 (6272) | 63 (6204) | 50 (6363) | — |
| Demographic adjusted3 | 1.00 (reference) | 1.31 (0.91, 1.89) | 1.27 (0.88, 1.83) | 1.32 (0.90, 1.92) | 1.04 (0.69, 1.55) | 0.97 |
| Multivariable adjusted4 | 1.00 (reference) | 1.31 (0.90, 1.90) | 1.18 (0.81, 1.71) | 1.22 (0.83, 1.79) | 0.98 (0.65, 1.47) | 0.70 |
| 16:1n–7 | ||||||
| Percentage of total fatty acids | 0.29 (0.11–0.33) | 0.38 (0.33–0.41) | 0.44 (0.41–0.49) | 0.54 (0.49–0.61) | 0.73 (0.61–1.9) | — |
| Cases, total person-years | 53 (6439) | 54 (6239) | 66 (6075) | 67 (6176) | 57 (5834) | — |
| Demographic adjusted3 | 1.00 (reference) | 1.15 (0.78, 1.68) | 1.43 (0.99, 2.08) | 1.56 (1.07, 2.28) | 1.38 (0.93, 2.05) | 0.09 |
| Multivariable adjusted4 | 1.00 (reference) | 1.09 (0.74, 1.61) | 1.42 (0.97, 2.07) | 1.48 (1.00, 2.19) | 1.27 (0.84, 1.92) | 0.24 |
| 16:1n–9 | ||||||
| Percentage of total fatty acids | 0.07 (0.04–0.07) | 0.08 (0.07–0.08) | 0.09 (0.08–0.09) | 0.10 (0.09–0.10) | 0.12 (0.10–0.78) | — |
| Cases, total person-years | 71 (6712) | 61 (6009) | 68 (6584) | 50 (5632) | 47 (5826) | — |
| Demographic adjusted3 | 1.00 (reference) | 0.97 (0.69, 1.37) | 1.03 (0.73, 1.44) | 0.89 (0.62, 1.29) | 0.82 (0.56, 1.19) | 0.26 |
| Multivariable adjusted4 | 1.00 (reference) | 0.97 (0.68, 1.37) | 1.00 (0.71, 1.40) | 0.92 (0.63, 1.33) | 0.83 (0.67, 1.22) | 0.33 |
| 18:1n–7 | ||||||
| Percentage of total fatty acids | 1.1 (0.79–1.1) | 1.2 (1.1–1.2) | 1.3 (1.2–1.3) | 1.4 (1.3–1.5) | 1.6 (1.5–2.4) | — |
| Cases, total person-years | 89 (6537) | 64 (6613) | 49 (6200) | 57 (5968) | 38 (5445) | — |
| Demographic adjusted3 | 1.00 (reference) | 0.72 (0.52, 0.99) | 0.59 (0.41, 0.83) | 0.68 (0.49, 0.95) | 0.52 (0.35, 0.76) | 0.001 |
| Multivariable adjusted4 | 1.00 (reference) | 0.72 (0.52, 1.00) | 0.63 (0.44, 0.90) | 0.72 (0.51, 1.01) | 0.56 (0.38, 0.83) | 0.005 |
18:1n–9 is not shown because it did not meet the proportional hazard assumption. P-trend values were computed by assigning the median concentration in each quintile to participants and evaluating this as a continuous variable.
Median; range in parentheses (all such values).
All values are HRs (95% CIs) from a Cox regression model adjusted for age, sex, race, education, and clinic.
All values are HRs (95% CIs) from a Cox regression model adjusted for age, sex, race, education, clinic, smoking status, alcohol consumption, leisure-time physical activity, prevalence of ischemic heart disease, hypertension at baseline, BMI, waist circumference, and consumption of carbohydrate (percentage of energy), protein (percentage of energy), and total energy. Additional adjustment for the consumption of whole-fat dairy foods, red meat, fruits, vegetables, coffee, fish, fiber, saturated fat (percentage of energy), and monounsaturated fat (percentage of energy) did not substantially alter results (data not shown). For dietary factors, we used the average of the measurements from 2 food-frequency questionnaires or used one measurement from one food-frequency questionnaire when the other was not available.
In contrast to circulating concentrations, dietary intakes of SFAs and MUFAs in place of carbohydrate was not significantly associated with incident diabetes (Table 4). Associations remained null when we evaluated substitution effects of dietary intake of these fatty acids in place of PUFA (data not shown). Additional adjustment for dietary factors or potential confounders or mediators did not appreciably change these associations (data not shown).
TABLE 4.
Prospective associations of dietary SFAs and MUFAs with incident diabetes1
| Quintiles of dietary fatty acids |
||||||
| 1 | 2 | 3 | 4 | 5 | P-trend | |
| Dietary 14:0 (n = 4221) | ||||||
| Percentage of total energy | 0.53 (0.18, 0.60) | 0.66 (0.60, 0.73) | 0.79 (0.73, 0.85) | 0.92 (0.85, 1.0) | 1.1 (1.0, 2.0) | — |
| Cases, total person-years | 89 (10,713) | 91 (10,315) | 79 (10,066) | 92 (9609) | 95 (9471) | — |
| Demographic adjusted2 | 1.00 (reference) | 1.09 (0.81, 1.46) | 0.98 (0.72, 1.33) | 1.20 (0.89, 1.61) | 1.23 (0.92, 1.65) | 0.12 |
| Multivariable adjusted3 | 1.00 (reference) | 0.97 (0.72, 1.32) | 0.87 (0.63, 1.21) | 1.01 (0.71, 1.43) | 1.00 (0.66, 1.52) | 0.94 |
| Dietary 16:0 (n = 4221) | ||||||
| Percentage of total energy | 4.2 (1.8, 4.7) | 5.1 (4.7, 5.5) | 5.9 (5.5, 6.2) | 6.6 (6.2, 6.9) | 7.5 (6.9, 12.2) | — |
| Cases, total person-years | 92 (10,768) | 87 (10,434) | 91 (10,276) | 88 (9757) | 88 (8939) | — |
| Demographic adjusted2 | 1.00 (reference) | 1.01 (0.75, 1.36) | 1.05 (0.79, 1.41) | 1.07 (0.79, 1.43) | 1.12 (0.83, 1.51) | 0.55 |
| Multivariable adjusted3 | 1.00 (reference) | 0.99 (0.73, 1.36) | 1.01 (0.72, 1.43) | 1.02 (0.69, 1.51) | 1.14 (0.72, 1.79) | 0.55 |
| Dietary 18:0 (n = 4221) | ||||||
| Percentage of total energy | 1.7 (0.6, 2.0) | 2.2 (2.0, 2.4) | 2.6 (2.4, 2.7) | 2.9 (2.7, 3.1) | 3.4 (3.1, 5.9) | — |
| Cases, total person-years | 84 (10,642) | 88 (10,206) | 94 (10,226) | 86 (9617) | 94 (9484) | — |
| Demographic adjusted2 | 1.00 (reference) | 1.13 (0.83, 1.52) | 1.17 (0.87, 1.57) | 1.14 (0.84, 1.55) | 1.17 (0.87, 1.59) | 0.30 |
| Multivariable adjusted3 | 1.00 (reference) | 1.06 (0.77, 1.46) | 1.06 (0.74, 1.50) | 0.99 (0.66, 1.48) | 1.00 (0.62, 1.62) | 0.93 |
| Dietary 16:1 (n = 4221) | ||||||
| Percentage of total energy | 0.38 (0.07, 0.45) | 0.51 (0.45, 0.56) | 0.63 (0.56, 0.69) | 0.75 (0.69, 0.82) | 0.92 (0.82, 1.8) | — |
| Cases, total person-years | 76 (10,668) | 81 (10,485) | 113 (10,349) | 91 (9835) | 85 (8836) | — |
| Demographic adjusted2 | 1.00 (reference) | 1.11 (0.81, 1.53) | 1.55 (1.16, 2.08) | 1.27 (0.94, 1.73) | 1.28 (0.93, 1.75) | 0.20 |
| Multivariable adjusted3 | 1.00 (reference) | 1.05 (0.76, 1.45) | 1.37 (0.99, 1.89) | 1.08 (0.75, 1.56) | 1.13 (0.75, 1.71) | 0.63 |
| Dietary 18:1 (n = 3869) | ||||||
| Percentage of total energy | 7.3 (2.3, 8.4) | 9.2 (8.4, 9.8) | 10.4 (9.8, 11.0) | 11.7 (11.0, 12.4) | 13.3 (12.4, 20.7) | — |
| Cases, total person-years | 74 (9593) | 75 (9411) | 74 (9539) | 81 (9489) | 78 (8869) | — |
| Demographic adjusted2 | 1.00 (reference) | 1.02 (0.74, 1.41) | 0.97 (0.70, 1.35) | 1.04 (0.76, 1.43) | 1.00 (0.72, 1.39) | 0.95 |
| Multivariable adjusted3 | 1.00 (reference) | 1.01 (0.73, 1.41) | 0.93 (0.66, 1.32) | 1.02 (0.71, 1.47) | 0.99 (0.66, 1.49) | 0.99 |
Estimated fatty acid consumption expressed as the percentage of total energy intake was updated by cumulative updating (averaging first and second dietary estimates at the time of the second dietary assessment). Dietary 18:1 was estimated only for the first assessment. Covariates measured at the same visits as diet assessments were used. All participants had dietary intakes of fatty acids in the de novo lipogenesis pathway evaluated and without prevalent diabetes at the time of their first diet assessment (1989–1990 for the original cohort or 1995–1996 for the minority cohort). P-trend values were computed by assigning the median concentration in each quintile to participants and evaluating this as a continuous variable.
All values are HRs (95% CIs) from a Cox regression model adjusted for age, sex, race, education, and clinic.
All values are HRs (95% CIs) from a Cox regression model adjusted for age, sex, race, education, clinic, smoking status, alcohol consumption, physical activity, prevalence of ischemic heart disease, hypertension at baseline, BMI, and consumption of saturated fat (percentage of energy) and monounsaturated fat (percentage of energy) with each fatty acid excluded, polyunsaturated fat (percentage of energy), trans-fat (percentage of energy), protein (percentage of energy), and total energy. The nutrient density method modeled the effect of the isocaloric substitution of dietary fatty acids for carbohydrate. Additional adjustment for the consumption of fruit, vegetables, and fiber did not substantially alter results. For example, HRs (95% CIs) of diabetes in the highest quintile were 1.13 (0.72, 1.79; P-trend = 0.57) for dietary 16:0 and 1.00 (0.62, 1.62; P-trend = 0.92) for dietary 18:0.
For phospholipid oleic acid, the assumption of proportional hazards was not met (Supplemental Figure 1) perhaps related to the low 13-y reproducibility of oleic acid concentrations (within-individual correlation = 0.30 on the basis of a subset of 100 CHS participants). In analyses stratified by the midpoint of follow-up, oleic acid was not significantly associated with diabetes in the first 9 y (extreme-quintile HR: 1.06; 95% CI: 0.67, 1.66; P-trend = 0.59) but was associated with lower diabetes risk in the last 9 y of follow-up (extreme-quintile HR: 0.23; 95% CI: 0.07, 0.78; P-trend = 0.007).
In secondary analyses that investigated circulating concentrations of other fatty acids in the DNL pathway, only vaccenic acid was associated with diabetes with an extreme-quintile HR of 0.56 (95% CI: 0.38, 0.83; P-trend = 0.005) (Table 3). After additional adjustment for potential mediators, vaccenic acid became marginally associated with diabetes (extreme-quintile HR: 0.67; 95% CI: 0.45, 1.00; P-trend = 0.05).
The estimated SCD-1 activity based on the fatty acid ratio was not significantly associated with diabetes in the multivariable analysis (Supplemental Table 6), which was expected because the ratio was strongly correlated with the numerator (r = 0.89) (Supplemental Table 1). No independent additional association with diabetes was imparted by the estimated SCD-1 activity after adjustment for the component fatty acids of the ratio (Supplemental Table 6).
Age and sex did not significantly modify any of the associations (P-interaction > 0.05 each). In additional analyses, findings were similar when follow-up was limited to 9 y, excluding cases in the first 2 y, and excluding information on post-challenge glucose from the definition of diabetes (data not shown). Findings for palmitic acid and vaccenic acid were also similar when restricted to nondrinkers with higher diabetes risk for palmitic acid (extreme-quintile HR: 2.03; 95% CI: 1.22, 3.40; P-trend = 0.002) and lower diabetes risk for vaccenic acid (extreme-quintile HR: 0.51; 95% CI: 0.30, 0.88; P-trend = 0.03). The association of stearic acid with diabetes was no longer significant when restricted to nondrinkers (extreme-quintile HR: 1.34; 95% CI: 0.80, 2.26; P-trend = 0.14) although the interaction by alcohol use was not statistically significant (P-interaction = 0.33).
DISCUSSION
In this large, prospective cohort study, plasma phospholipid palmitic acid and stearic acid were associated with increased risk of incident diabetes, whereas vaccenic acid was associated with decreased risk. Oleic acid had divergent associations depending on the follow-up time, which was perhaps related to low long-term reproducibility or chance. Other circulating SFAs and MUFAs in the DNL pathway and their estimated dietary intake were not associated with diabetes risk.
Our findings support either direct harms of palmitic acid and stearic acid or harms of DNL which influence their concentrations, or both possibilities, on diabetes risk. Although the current findings could not distinguish between these interrelated possibilities, experimental evidence supported adverse effects of these fatty acids and the chronic upregulation of the DNL pathway on diabetes risk. In animal and in vitro studies, palmitic acid and stearic acid induced inflammation, endoplasmic reticulum stress, and insulin resistance (7–14), whereas increased DNL itself contributes to hepatic steatosis and insulin resistance (46). Our prospective results are consistent with these previous experimental studies and support the need to further examine the likely interrelated effects of DNL, its fatty acid products, and factors that activate or inhibit these pathways for their influence on glucose-insulin homeostasis.
Adverse associations of palmitic acid and stearic acid with diabetes have been observed in some (18, 24, 26–28, 30) but not all (23, 25, 29) previous prospective observational studies. Variations in the study population (e.g., age and ethnicity), compartment of fatty acid measurement (e.g., plasma, serum, or erythrocyte membranes), duration of follow-up, and statistical power (number of cases ranged from 30 to 12,403) may partly explain the inconsistent findings. Our results build on and expand our understanding of these fatty acids and diabetes. We focused on older adults, a growing population who has been underrepresented previously. The assessment of both circulating concentrations and estimated dietary intakes facilitated the inference on differences between metabolic compared with dietary exposure to these fatty acids (47). We also carefully assessed potential confounding from numerous dietary factors, which confirmed the independence of these associations.
In our investigation, higher palmitic acid and stearic acid concentrations were associated with generally worse lipid and inflammatory biomarker profiles, with mixed associations with glucose-insulin indices. On the basis of adverse effects of stearic acid on diabetes risk factors in experimental studies (12–14), its positive association with HOMA-β was an unexpected finding, which possibly reflected early insulin resistance leading to the hyperreactivity of β cell function; this possibility should be interpreted cautiously because of the cross-sectional nature of these results. The partial attenuation of the prospective association of palmitic acid and stearic acid with diabetes after adjustment for lipid, inflammation, and glucose-insulin homeostatic biomarkers suggested potential mediated effects of these biological pathways.
Although some experimental evidence suggested a protective role of oleic acid in metabolic health (10, 15, 16), we did not observe any significant overall association between circulating oleic acid and diabetes. Compared with the other fatty acids evaluated, oleic acid varies more substantially within individuals over time and causes increased misclassification and attenuation of true associations. However, such an effect would not have explained the inverse association with diabetes risk only during the latter half of follow-up; these findings could have been attributable to chance and should be interpreted with caution. Our results indicate the need for additional studies of oleic acid and incident diabetes, especially the use of serial fatty acid measurements over time.
Compared with palmitic acid and stearic acid, relatively little is known regarding metabolic effects of vaccenic acid. Emerging experimental evidence suggests that vaccenic acid may mediate the suppression of hepatic gluconeogenic gene expression and improve glucose metabolism by the enhanced activity of fatty acid elongase-5, which is the major enzyme for the endogenous synthesis of vaccenic acid from palmitoleic acid (48). Consistently, erythrocyte membrane vaccenic acid was prospectively associated with better insulin sensitivity and lower postprandial hyperglycemia in Finnish men (29), whereas phospholipid vaccenic acid was also associated with lower diabetes risk in middle-aged adults in the United Kingdom (26). To our knowledge, our results provide novel evidence that vaccenic acid or its metabolic determinants may also influence diabetes risk in older adults, suggesting that vaccenic acid should be a target for additional experimental, observational, and intervention studies.
We did not observe any significant association of dietary SFAs and MUFAs with diabetes risk. These findings are consistent with a meta-analysis of observational studies that showed SFA consumption was not related to incident diabetes (49). A potential explanation for the contrasting results of circulating compared with dietary palmitic acid and stearic acid is that circulating concentrations reflect endogenous pathways and metabolic processes that may influence the pathogenesis of diabetes. Consistent with previous reports (26, 28, 50, 51), we showed that circulating concentrations of palmitic acid and stearic acid were weakly correlated with their estimated dietary intakes. Dietary SFAs may be variably oxidized after ingestion for energy use (52), and habitual circulating concentrations appear to more-strongly reflect endogenous synthesis (e.g., driven by an excess of rapidly digested dietary carbohydrate) (20–22). In addition, phospholipid fatty acids are key components of cellular membranes where they can influence membrane fluidity and insulin signaling (7, 11).
Increased dietary carbohydrate replacing fat and alcohol intake, which are 2 factors known to stimulate DNL (3, 39), were positively associated with most of the fatty acids examined in our study, which suggested that usual habitual intakes of dietary carbohydrate and alcohol influence DNL in free-living populations. One exception was stearic acid, which was inversely associated with alcohol intake. In a previous investigation of the effect of alcohol withdrawal on circulating fatty acids, alcohol consumption increased absolute concentrations of circulating stearic acid but lowered stearic acid as percentage of total circulating fatty acids (53). This effect was attributable to a greater alcohol-induced increase in absolute concentrations of other DNL fatty acids, such as palmitic acid, relative to stearic acid (53). In light of these findings, the inverse association we observed between alcohol use and stearic acid may relate to a relatively greater increase in other DNL fatty acids.
Our findings, together with the likely harms of rapidly digested dietary carbohydrate on the development of diabetes (54, 55), provide support for reducing the consumption of rapidly digested carbohydrate to prevent diabetes. However, the observation that alcohol use contributes to DNL but is not associated with higher diabetes risk (56) suggests complex biological effects of alcohol or other confounding factors that require additional understanding.
Our study had several strengths. We used a well-established cohort with little loss of follow-up and a serial assessment of both glucose and medications, which minimized selection and ascertainment biases. The prospective design, exclusion of prevalent diabetes cases, and sensitivity analyses that excluded early cases reduced the potential for reverse causation. A large sample size provided the statistical power to detect relevant associations. Both circulating and dietary fatty acids were evaluated, which provided complementary evaluations of potential effects of the fatty acids. Information on demographics, lifestyle habits, and diet was collected by using standardized methods, which minimized the residual confounding.
Potential limitations should be considered. The cohort included older, mostly Caucasians, and results might not be generalizable to young adults or other races. However, our findings for palmitic acid and stearic acid are consistent with previous studies in younger cohorts. Although in vitro studies generally used triglyceride fatty acids or fatty acids salts, we evaluated phospholipid fatty acids because circulating triglyceride fatty acids vary much more over time. Circulating fatty acids were assessed at baseline, and changes over time would have caused misclassification and potentially attenuated true associations. To our knowledge, the reproducibility and validity for the assessment of some dietary fatty acids (e.g., myristic acid and stearic acid) have not been reported, and the estimation of dietary intake may have been limited by measurement error, which is prone to be random and attenuate associations with diabetes. Cardiometabolic risk factors were measured later in life on a single occasion, which limited cross-sectional assessments of relations with fatty acids. Although we adjusted for multiple important risk factors for diabetes, residual confounding because of unmeasured or imprecisely measured confounders could not be excluded.
In conclusion, we showed that, later in life, circulating palmitic acid and stearic acid were associated with higher risk of incident diabetes; and vaccenic acid was associated with lower risk. Dietary intakes of SFAs and MUFAs were not associated with diabetes risk. These results highlight the potentially varying metabolic effects of different fatty acids in the DNL pathway and the need to further investigate the biological mechanisms that may link these specific fatty acids to the pathogenesis of diabetes.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—WM and DM: designed the research; DSS, DM, RNL, KJM, LD, JRK, MLB, JAD, IBK, and XS: collected data; WM: analyzed data; QW: provided statistical advise; WM, JHYW, and DM: drafted the manuscript; and all authors: were involved in interpretation of data, critical revision of the manuscript for important intellectual content, and approval of the final manuscript for submission. DM reported ad hoc travel reimbursement or honoraria from Bunge, the Pollock Institute, Quaker Oats, and the Life Sciences Research Organization; ad hoc consulting fees from the McKinsey Health Systems Institute, Foodminds, Nutrition Impact, Amarin, Omthera, and Winston and Strawn LLP; membership with the Unilever North America Scientific Advisory Board; and royalties for a chapter on fish oil from UpToDate. LD reported ad hoc travel reimbursement from the International Nut & Dried Fruit Inc., investigator-initiated grants from GlaxoSmithKline and the California Walnut Commission, and being an ad hoc consultant for Merck. WM, JHYW, QW, RNL, KJM, IBK, XS, MLB, JAD, JRK, and DSS declared no conflicts of interest.
Footnotes
Abbreviations used: CHS, Cardiovascular Health Study; CRP, C-reactive protein; DNL, de novo lipogenesis; FFQ, food-frequency questionnaire; HOMA-β, homeostasis model assessment of β cell function; SCD-1, stearoyl-CoA desaturase-1.
REFERENCES
- 1.Hellerstein MK, Schwarz JM, Neese RA. Regulation of hepatic de novo lipogenesis in humans. Annu Rev Nutr 1996;16:523–57. [DOI] [PubMed] [Google Scholar]
- 2.Schwarz JM, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr 2003;77:43–50. [DOI] [PubMed] [Google Scholar]
- 3.Chong MF, Hodson L, Bickerton AS, Roberts R, Neville M, Karpe F, Frayn KN, Fielding BA. Parallel activation of de novo lipogenesis and stearoyl-CoA desaturase activity after 3 d of high-carbohydrate feeding. Am J Clin Nutr 2008;87:817–23. [DOI] [PubMed] [Google Scholar]
- 4.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014;146:726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browning JD, Baker JA, Rogers T, Davis J, Satapati S, Burgess SC. Short-term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. Am J Clin Nutr 2011;93:1048–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res 2006;47:2726–37. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz EA, Zhang WY, Karnik SK, Borwege S, Anand VR, Laine PS, Su Y, Reaven PD. Nutrient modification of the innate immune response: a novel mechanism by which saturated fatty acids greatly amplify monocyte inflammation. Arterioscler Thromb Vasc Biol 2010;30:802–8. [DOI] [PubMed] [Google Scholar]
- 9.Weigert C, Brodbeck K, Staiger H, Kausch C, Machicao F, Haring HU, Schleicher ED. Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factor-kappaB. J Biol Chem 2004;279:23942–52. [DOI] [PubMed] [Google Scholar]
- 10.Sieber J, Lindenmeyer MT, Kampe K, Campbell KN, Cohen CD, Hopfer H, Mundel P, Jehle AW. Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am J Physiol Renal Physiol 2010;299:F821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynoso R, Salgado LM, Calderon V. High levels of palmitic acid lead to insulin resistance due to changes in the level of phosphorylation of the insulin receptor and insulin receptor substrate-1. Mol Cell Biochem 2003;246:155–62. [PubMed] [Google Scholar]
- 12.van den Berg SA, Guigas B, Bijland S, Ouwens M, Voshol PJ, Frants RR, Havekes LM, Romijn JA, van Dijk KW. High levels of dietary stearate promote adiposity and deteriorate hepatic insulin sensitivity. Nutr Metab (Lond) 2010;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu X, Liu L, Na L, Lu H, Li S, Li Y, Sun C. Sterol regulatory element-binding protein-1c mediates increase of postprandial stearic Acid, a potential target for improving insulin resistance, in hyperlipidemia. Diabetes 2013;62:561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson EK, Hill AA, Hasty AH. Stearic acid accumulation in macrophages induces toll-like receptor 4/2-independent inflammation leading to endoplasmic reticulum stress-mediated apoptosis. Arterioscler Thromb Vasc Biol 2012;32:1687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coll T, Eyre E, Rodriguez-Calvo R, Palomer X, Sanchez RM, Merlos M, Laguna JC, Vazquez-Carrera M. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem 2008;283:11107–16. [DOI] [PubMed] [Google Scholar]
- 16.Salvadó L, Coll T, Gomez-Foix AM, Salmeron E, Barroso E, Palomer X, Vazquez-Carrera M. Oleate prevents saturated-fatty-acid-induced ER stress, inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia 2013;56:1372–82. [DOI] [PubMed] [Google Scholar]
- 17.Wu JH, Lemaitre RN, Imamura F, King IB, Song X, Spiegelman D, Siscovick DS, Mozaffarian D. Fatty acids in the de novo lipogenesis pathway and risk of coronary heart disease: the Cardiovascular Health Study. Am J Clin Nutr 2011;94:431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zong G, Zhu J, Sun L, Ye X, Lu L, Jin Q, Zheng H, Yu Z, Zhu Z, Li H, et al. Associations of erythrocyte fatty acids in the de novo lipogenesis pathway with risk of metabolic syndrome in a cohort study of middle-aged and older Chinese. Am J Clin Nutr 2013;98:319–26. [DOI] [PubMed] [Google Scholar]
- 19.Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GS. Circulating palmitoleic acid and risk of metabolic abnormalities and new-onset diabetes. Am J Clin Nutr 2010;92:1350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knopp RH, Retzlaff B, Walden C, Fish B, Buck B, McCann B. One-year effects of increasingly fat-restricted, carbohydrate-enriched diets on lipoprotein levels in free-living subjects. Proc Soc Exp Biol Med 2000;225:191–9. [DOI] [PubMed] [Google Scholar]
- 21.Raatz SK, Bibus D, Thomas W, Kris-Etherton P. Total fat intake modifies plasma fatty acid composition in humans. J Nutr 2001;131:231–4. [DOI] [PubMed] [Google Scholar]
- 22.King IB, Lemaitre RN, Kestin M. Effect of a low-fat diet on fatty acid composition in red cells, plasma phospholipids, and cholesterol esters: investigation of a biomarker of total fat intake. Am J Clin Nutr 2006;83:227–36. [DOI] [PubMed] [Google Scholar]
- 23.Laaksonen DE, Lakka TA, Lakka HM, Nyyssonen K, Rissanen T, Niskanen LK, Salonen JT. Serum fatty acid composition predicts development of impaired fasting glycaemia and diabetes in middle-aged men. Diabet Med 2002;19:456–64. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Folsom AR, Zheng ZJ, Pankow JS, Eckfeldt JH, Investigators AS. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2003;78:91–8. [DOI] [PubMed] [Google Scholar]
- 25.Krachler B, Norberg M, Eriksson JW, Hallmans G, Johansson I, Vessby B, Weinehall L, Lindahl B. Fatty acid profile of the erythrocyte membrane preceding development of type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis 2008;18:503–10. [DOI] [PubMed] [Google Scholar]
- 26.Patel PS, Sharp SJ, Jansen E, Luben RN, Khaw KT, Wareham NJ, Forouhi NG. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am J Clin Nutr 2010;92:1214–22. [DOI] [PubMed] [Google Scholar]
- 27.Hodge AM, English DR, O'Dea K, Sinclair AJ, Makrides M, Gibson RA, Giles GG. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr 2007;86:189–97. [DOI] [PubMed] [Google Scholar]
- 28.Kröger J, Zietemann V, Enzenbach C, Weikert C, Jansen EH, Doring F, Joost HG, Boeing H, Schulze MB. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr 2011;93:127–42. [DOI] [PubMed] [Google Scholar]
- 29.Mahendran Y, Agren J, Uusitupa M, Cederberg H, Vangipurapu J, Stancakova A, Schwab U, Kuusisto J, Laakso M. Association of erythrocyte membrane fatty acids with changes in glycemia and risk of type 2 diabetes. Am J Clin Nutr 2014;99:79–85. [DOI] [PubMed] [Google Scholar]
- 30.Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kroger J, Schulze MB, Crowe FL, Huerta JM, Guevara M, Beulens JW, et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol 2014;2:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–76. [DOI] [PubMed] [Google Scholar]
- 32.Matthan NR, Ip B, Resteghini N, Ausman LM, Lichtenstein AH. Long-term fatty acid stability in human serum cholesteryl ester, triglyceride, and phospholipid fractions. J Lipid Res 2010;51:2826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 34.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 1986;27:114–20. [PubMed] [Google Scholar]
- 35.Dobrzyn P, Dobrzyn A, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, Friedman JM, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc Natl Acad Sci USA 2004;101:6409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahman SM, Dobrzyn A, Dobrzyn P, Lee SH, Miyazaki M, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency elevates insulin-signaling components and down-regulates protein-tyrosine phosphatase 1B in muscle. Proc Natl Acad Sci USA 2003;100:11110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog Lipid Res 2004;43:91–104. [DOI] [PubMed] [Google Scholar]
- 38.Peter A, Cegan A, Wagner S, Lehmann R, Stefan N, Konigsrainer A, Konigsrainer I, Haring HU, Schleicher E. Hepatic lipid composition and stearoyl-coenzyme A desaturase 1 mRNA expression can be estimated from plasma VLDL fatty acid ratios. Clin Chem 2009;55:2113–20. [DOI] [PubMed] [Google Scholar]
- 39.Sozio M, Crabb DW. Alcohol and lipid metabolism. Am J Physiol Endocrinol Metab 2008;295:E10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumanyika S, Tell GS, Fried L, Martel JK, Chinchilli VM. Picture-sort method for administering a food frequency questionnaire to older adults. J Am Diet Assoc 1996;96:137–44. [DOI] [PubMed] [Google Scholar]
- 41.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6. [DOI] [PubMed] [Google Scholar]
- 42.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 1978;31:741–55. [DOI] [PubMed] [Google Scholar]
- 43.Hussein AA, Gottdiener JS, Bartz TM, Sotoodehnia N, Defilippi C, See V, Deo R, Siscovick D, Stein PK, Lloyd-Jones D. Inflammation and sudden cardiac death in a community-based population of older adults: the Cardiovascular Health Study.. Heart Rhythm 2013;10:1425–32. [DOI] [PubMed] [Google Scholar]
- 44.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–95. [DOI] [PubMed] [Google Scholar]
- 45.Willet W. Nutritional epidemiology. New York, NY: Oxford University Press; 2012. [Google Scholar]
- 46.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest 2008;118:829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mozaffarian D. Saturated fatty acids and type 2 diabetes: more evidence to re-invent dietary guidelines. Lancet Diabetes Endocrinol 2014;2:770–2. [DOI] [PubMed] [Google Scholar]
- 48.Tripathy S, Jump DB. Elovl5 regulates the mTORC2-Akt-FOXO1 pathway by controlling hepatic cis-vaccenic acid synthesis in diet-induced obese mice. J Lipid Res 2013;54:71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Micha R, Mozaffarian D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids 2010;45:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hodge AM, Simpson JA, Gibson RA, Sinclair AJ, Makrides M, O'Dea K, English DR, Giles GG. Plasma phospholipid fatty acid composition as a biomarker of habitual dietary fat intake in an ethnically diverse cohort. Nutr Metab Cardiovasc Dis 2007;17:415–26. [DOI] [PubMed] [Google Scholar]
- 51.Ma J, Folsom ARA, Shahar EE, Eckfeldt JHJ. Plasma fatty acid composition as an indicator of habitual dietary fat intake in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Am J Clin Nutr 1995;62:564–71. [DOI] [PubMed] [Google Scholar]
- 52.DeLany JP, Windhauser MM, Champagne CM, Bray GA. Differential oxidation of individual dietary fatty acids in humans. Am J Clin Nutr 2000;72:905–11. [DOI] [PubMed] [Google Scholar]
- 53.Teubert A, Thome J, Büttner A, Richter J, Irmisch G. Elevated oleic acid serum concentrations in patients suffering from alcohol dependence. Journal of Molecular Psychiatry 2013;1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhupathiraju SN, Tobias DK, Malik VS, Pan A, Hruby A, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr 2014;100:218–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Livesey G, Taylor R, Hulshof T, Howlett J. Glycemic response and health–a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr 2008;87:258S–68S. [DOI] [PubMed] [Google Scholar]
- 56.Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care 2005;28:719–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.