Abstract
Background: Biochemical evidence has linked the coordinate control of fatty acid (FA) synthesis with the activity of stearoyl-CoA desaturase-1 (SCD1). The ratio of 16:1n–7 to 16:0 [SCD1(16)] in plasma triacylglycerol FA has been used as an index to reflect liver SCD1(16) activity and has been proposed as a biomarker of FA synthesis, although this use has not been validated by comparison with isotopically measured de novo lipogenesis (DNLMeas).
Objective: We investigated plasma lipid 16:1n–7 and FA indexes of elongation and desaturation in relation to lipogenesis.
Design: In this cross-sectional investigation of metabolism, 24 overweight adults, who were likely to have elevated DNL, consumed D2O for 10 d and had liver fat (LF) measured by magnetic resonance spectroscopy. Very-low-density lipoprotein (VLDL)-triacylglycerols and plasma free FA [nonesterified fatty acids (NEFAs)] were analyzed by using gas chromatography for the FA composition (molar percentage) and gas chromatography–mass spectrometry and gas chromatography–combustion isotope ratio mass spectrometry for deuterium enrichment.
Results: In all subjects, VLDL-triacylglycerol 16:1n–7 was significantly (P < 0.01) related to DNLMeas (r = 0.56), liver fat (r = 0.53), and adipose insulin resistance (r = 0.56); similar positive relations were shown with the SCD1(16) index, and the pattern in NEFAs echoed that of VLDL-triacylglycerols. Compared with subjects with low LF (3.1 ± 2.7%; n = 11), subjects with high LF (18.4 ± 3.6%; n = 13) exhibited a 45% higher VLDL-triacylglycerol 16:1n–7 molar percentage (P < 0.01), 16% of subjects had lower 18:2n–6 (P = 0.01), and 27% of subjects had higher DNL as assessed by using a published DNL index (ratio of 16:0 to 18:2n–6; P = 0.03), which was isotopically confirmed by DNLMeas (increased 2.5-fold; P < 0.01). Compared with 16:0 in the diet, the low amount of dietary 16:1n–7 in VLDL-triacylglycerols corresponded to a stronger signal of elevated DNL.
Conclusion: The current data provide support for the use of the VLDL-triacylglycerol 16:1n–7 molar percentage as a biomarker for elevated liver fat when isotope use is not feasible; however, larger-scale confirmatory studies are needed. This trial was registered at clinicaltrials.gov as NCT01371396.
Keywords: palmitoleic fatty acid, VLDL triacylglycerol, biomarker, lipogenesis, stearoyl-CoA desaturase
INTRODUCTION
Elevated de novo lipogenesis (DNL)5 is increasingly recognized as an important contributor to hepatic triacylglycerol concentrations in nonalcoholic fatty liver disease (NAFLD) (1, 2) and other states of insulin resistance (3–6). Along with quantitative contributions to liver triacylglycerols (1), the DNL pathway has qualitative implications whereby the principal fatty acid (FA) products of DNL are saturated (7), which can have negative consequences on cellular functions such as insulin signaling (8). Isotopic labeling is the most objective method for measuring DNL but is technically challenging and costly to perform, making it unrealistic for large-scale studies. With the use of defined feeding conditions, Hudgins et al. developed the linoleic acid dilution method, which measures changes in the ratio of palmitate (16:0) to linoleate (18:2n–6), referred to as the lipogenic index (9, 10). This technique has been systematically validated against isotopically measured de novo lipogenesis (DNLMeas) (9, 10) and extended to create a standardized test of lipogenic sensitivity to acute fructose intake (11). However, the usefulness of this index to reflect DNL on its own (without fructose intervention) in larger studies may be limited because of the significant effect of recent dietary intake on the VLDL-triacylglycerol FA composition, particularly for 18:2n–6 (12, 13).
In plasma triacylglycerols, the saturated compared with unsaturated balance depends on the FA composition of dietary fat and adipose tissue as well as the activities of liver enzymes catalyzing FA synthesis, elongation, and desaturation. Stearoyl-CoA desaturase 1 (SCD1) desaturates stearate (18:0) and 16:0 into oleate (18:1n–9) and palmitoleate (16:1n–7), respectively (14). The plasma FA composition has been used to provide insight into cellular metabolic pathways; specifically, the ratios (molar percentage:molar percentage) of 18:1n–9 to 18:0 [SCD1(18)] and 16:1n–7 to 16:0 [SCD1(16)] are used to estimate SCD1 activity (15–20). The SCD1(16) index has also been proposed as an indirect biomarker of DNL. As reviewed by Jump (21), the activation of DNL upregulates SCD1, leading to the generation of 16:1n–7, vaccenic acid (18:1n–7), and 18:1n–9. In addition, the activation of FA synthesis and elongation through the elongation of the very-long-chain fatty acid protein 6 (ELOVL6) enzyme may occur simultaneously, with the ratio of 18:0 (the product of elongation) to 16:0 reflecting ELOVL6 activity (22). However, the importance of these processes to the development of excess liver fat is unknown.
The coordinate control of DNL, elongation, and desaturation was supported by observational studies in humans. Here, elevated DNL (measured directly via isotopic enrichment and indirectly by the 16:0 to 18:2n–6 ratio) was associated with greater proportions of MUFAs in VLDL-triacylglycerols, particularly 16:1n–7 (7, 18, 20). Furthermore, conditions associated with increased DNL, such as obesity (23–26), insulin resistance (27), and hypertriglyceridemia (28), also showed an increased proportion of 16:1n–7 in plasma lipids. However, to our knowledge, no study to date has compared a direct measure of DNL with the SCD1 product biomarker (16:1n–7); accordingly, the current study investigated DNL, FA composition, and indices of liver enzyme activity in VLDL-triacylglycerols and nonesterified fatty acids (NEFAs) from insulin–resistant adults who were likely to have elevated DNL. Our results suggest that the VLDL triacylglycerol 16:1n–7 molar percentage measured simply by using gas chromatography (GC) (with no isotopic labeling needed) correlates with both DNL measured isotopically and liver fat. Thus, measurements of the plasma FA composition should be considered to estimate lipogenesis in large population studies.
SUBJECTS AND METHODS
Subjects
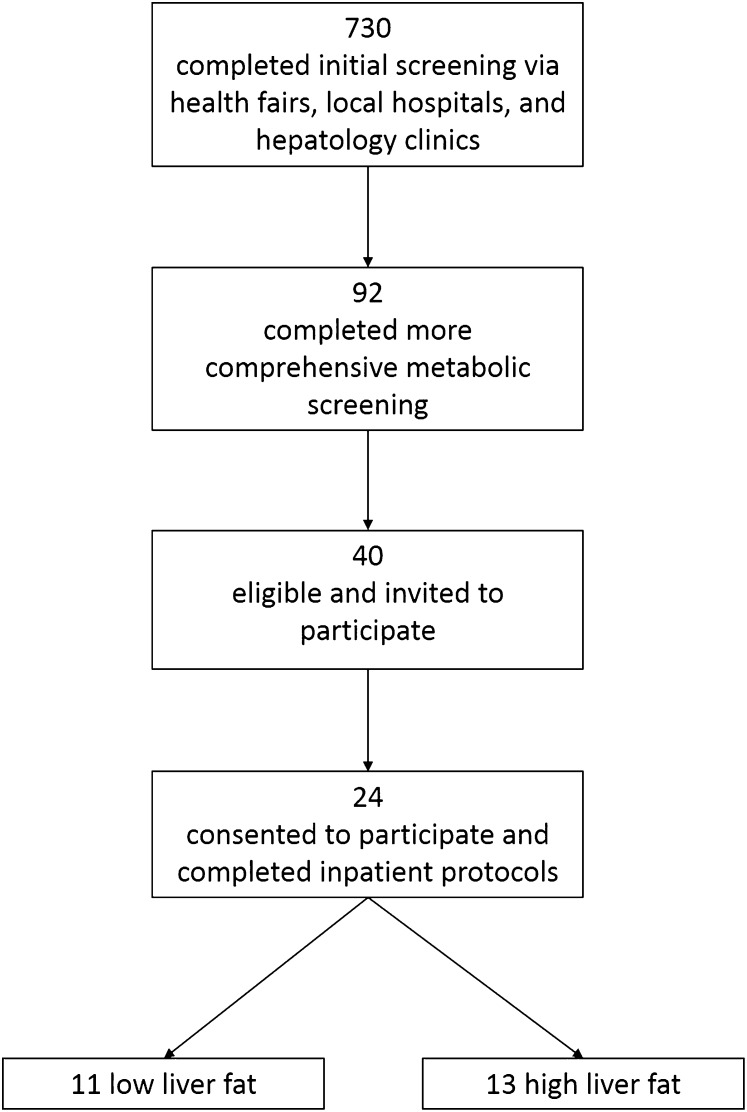
The current data represent a comprehensive analysis of the FA composition and labeling from a larger investigation of insulin resistance and liver metabolism (29). Overweight and obese adults were recruited from the local Dallas metropolitan area through health fairs, local hospitals, and hepatology clinics (see flowchart shown in Figure 1). Through these channels, 730 individuals completed an initial screening for elevated liver enzymes (alanine aminotransferase concentration >30 U/L and aspartate aminotransferase concentration > 30 U/L), fasting plasma glucose concentrations (>90 mg/dL), and BMI (in kg/m2) >25. On the basis of the results of this initial screening, subjects who were eligible were invited to attend a more comprehensive second screening visit to explain the study to them, assess characteristics of the metabolic syndrome (including elevated plasma triacylglycerols, waist circumference, glucose concentration >90 but <125 mg/dL, blood pressure, or low HDL cholesterol) (30), obtain a medical and weight history (to ensure stable body weight), and rule out diabetes and pre-existing liver disease (including hepatitis, cirrhosis, cholestasis, and biliary disorders). Ninety-two subjects agreed to come in for this screening visit, which was designed to identify overweight and obese adults who were nondiabetic with and without NAFLD and well matched for age, adiposity, dietary intake, activity levels, lipids, and other metabolic variables. Subjects were excluded if they smoked, had known metabolic abnormalities including elevated thyroid hormone concentrations, or elevated alcohol consumption (>140 g/wk for men and >70 g/wk for women). After this second screening, 40 subjects were eligible and invited to participate in the research protocol, which had high subject burden related to controlled food intake and physical activity as well as multiple inpatient study visits with intravenous line placement and overnight hospital stays. Twenty-four subjects consented to participate and completed the full study protocol. Each individual set of inpatient studies took 6 wk to accomplish; recruitment and data collection began in 2008 and ended in 2011. The sample size of the original study was determined on the basis of the ability to detect a significant difference in lipogenesis of ≥10 U (percentage of liver fat) between groups (P < 0.05) with 95% power (29). This study was approved by the Institutional Review Board at the University of Texas Southwestern Medical Center (062007–025), and all subjects provided written informed consent. This trial was registered at clinicaltrials.gov as NCT01371396.
FIGURE 1 .
Flowchart of study participants throughout screening and recruitment.
Study design
The study design and baseline characteristics of the subjects have been described elsewhere (29). Briefly, subjects were admitted to the Clinical Translational Research Center (CTRC) at University of Texas Southwestern Medical Center to assess body composition, glucose tolerance, and blood lipids. Before admission, subjects completed a 24-h dietary recall and filled out a 3-d food diary. These items were reviewed by a registered dietitian who interviewed the subjects to achieve a comprehensive assessment of usual dietary intake. On the basis of the analysis of these records with the Nutrition Data System for Research (NDSR 2009; University of Minnesota), a weight-maintaining menu was designed by the dietitian, and all foods and beverages were prepared by the CTRC kitchen and provided to the subject to consume over a 10-d period. During this time, subjects also consumed multiple 50-g doses of 70% deuterated water (D2O; Cambridge Isotope Laboratories Inc.) (31). Body composition was measured by using dual-energy X-ray absorptiometry (Hologic Discovery W, QDR series; Hologic), and the liver fat content was measured by using 3.0-T 1H-magnetic resonance spectroscopy (32). Subjects were categorized as having high liver fat (HighLF) or low liver fat (LowLF) according to published criteria and with the use of ≤5.6% as the cutoff for LowLF (33). Subjects also wore an Actical accelerometer on the hip (Actical; V2.1; Philips Healthcare) for 4d to assess physical activity levels (34). After the accelerometer measurement was completed, the subject was admitted to the CTRC for an overnight stay before the assessment of fasting blood lipids. Briefly, at 1800, a standardized evening meal was administered. The subject was allowed to consume noncaffeinated, nonenergy–containing drinks (e.g., herbal tea), but no other food was consumed until the fasting blood draw the next day at 1030. Subjects were studied sequentially over a period of 3 y. For each subject, 3 wk were needed for inpatient and outpatient sample collections (screening in addition to initial and inpatient visits) followed by 5 wk of biochemical and analytic data generation per subject.
Analysis of blood metabolites
Blood was collected in chilled tubes containing EDTA for the measurement of plasma glucose, insulin, NEFA, and triacylglycerols. Plasma was immediately separated and kept on ice while preservatives and antioxidants were added (phenylmethylsulfonyl fluoride, chloramphenicol, gentamicin sulfate, benzamidine, and Trolox) as previously described (35). Samples were immediately frozen and analyzed as soon as possible by using enzymatic assays, and fluorescence antibody kits were used to measure plasma concentrations of plasma NEFAs (991–34891; Wako Diagnostics; CV: 3.1%), glucose (439–90901; Wako Diagnostics; CV: 6.6%), insulin (EZHI-14L; Millipore Corp.; CV: 3.4%), and total plasma triacylglycerols and VLDL-triacylglycerols (461–08992 and −09092; Wako Diagnostics; CV: 1.3%). Enzymatic plates were read on a Power wave XS microtiter plate reader (Biotek Inc.). HOMA was also calculated as follows (36):
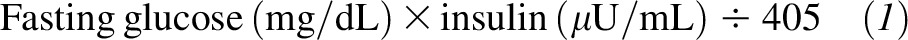 |
An index of adipose insulin resistance (AdipoIR) was calculated as the product of fasting insulin and NEFA concentrations (37).
Laboratory procedures and lipid isolation
For the analysis of blood lipids, plasma underwent ultracentrifugation at 40,000 × g for 20 h in a Beckman 50.3 Ti fixed-angle rotor (Beckman Instruments) at 15°C immediately after the collection period as described previously (35). VLDL particles were obtained by tube slicing to remove the top 2 mL (35), whereas the remaining bottom 4 mL solution were kept for isolation of plasma NEFAs. VLDL-triacylglycerols were isolated, and fatty acid methyl-esters (FAMEs) were prepared as described previously (35). To prepare NEFAs for FA analysis, the 4 mL infranatant that remained after VLDL isolation was extracted with heptane:isopropanol (30:70 mixture; 4 mL) and 1 mol H2SO4/L (100 μL). NEFAs were isolated by using thin-layer chromatography with a 80:20:1 hexane:ethyl ether:acetic acid solvent system and silica G plates (20 × 20 cm, 250 μm; Analtech Inc.) and prepared as FAMEs for GC analysis.
FA analysis
The FA composition of the diet was determined as part of the background dietary assessment with Nutrition Data System for Research software. The FAME composition of VLDL-triacylglycerols and NEFAs was analyzed by using an HP 6890 series GC gas chromatograph (Hewlett-Packard) equipped with a flame ionization detector. Samples were introduced via a splitless injector and separated on an SP-2560 column (100 m, 0.25-mm inside diameter, 0.20-μm film thickness, Supelco; Sigma-Aldrich) by using helium as the carrier gas. The initial oven temperature was kept at 140°C for 5 min and then ramped up to 240°C by increments of 4°C/min. The temperature was held at 240°C for 20 min before ramping back down to 140°C. Peak areas were quantified with HP Chemstation software (Hewlett-Packard). Individual FAs were identified by comparing their retention times with standard FAME mixtures (68A and NIHC; Nu-Chek Prep), and the molar percentage of each FA was calculated. The CV for these measurements was 0.1% for FAs in higher abundance (>6% of the total molar percentage) and 0.5% for FAs of lower abundance (≤6% of the total molar percentage). In the fasting state, the FA composition in plasma NEFAs primarily reflects FA arising from adipose tissue, although some FAs can arise from triacylglycerol hydrolysis in the plasma. The FA composition of plasma VLDL-triacylglycerols reflects liver-triacylglycerol FA sources (1) and composition (38). The current analysis focused on 7 FAs that represent 90–95% of total FAs current in VLDL-triacylglycerols and NEFAs. These 7 FAs were chosen on the basis of their major contribution to the plasma NEFA pool (16:0, 18:0, 18:1n–9, and 18:2n–6) and because they are direct products of lipogenesis (14:0 and 16:0) or elongation and desaturation (cis16:1n–7 and cis18:1n–7). For this data set, there were no undetected values for any of the 7 FAs investigated in either triacylglycerol or NEFA pools.
DNL
Body D2O enrichments were measured by using isotope-ratio mass spectrometry (IRMS) (Metabolic Solutions); average enrichments were 0.90 ±1.00% of plasma water. Deuterium incorporation into VLDL-triacylglycerol 16:0 was determined by using gas chromatography–mass spectrometry (GC-MS) on an Agilent 6890N GC gas chromatograph coupled to a 5975 MS detector and 7683B injector (Agilent Technologies) as described previously (39). Enrichments were determined by selective ion monitoring for a m/z of 270, 271, and 272 under electron ionization, and the percentage of newly-made 16:0 in VLDL-triacylglycerols was calculated as described (31, 40). Fasting DNL (DNLMeas) was defined as the percentage contribution of newly synthesized 16:0 in the VLDL-triacylglycerol pool and, in this study, represented an average of 2 fasting blood samples taken in the morning.
To further investigate DNL, deuterium incorporation into 16:0 and 16:1n–7 were measured by Metabolic Solutions with a Delta V Isotope Ratio Mass Spectrometer coupled to a Thermo Trace GC Ultra with a GC combustion interface III and Conflow IV interface (Thermo Finnigan). Methyl esters of 16:0 and 16:1n–7 were analyzed by using a splitless injection (1 μL) at an inlet temperature of 300°C and a Zebron ZB-Semivolatiles column with a film thickness of 30 m × 0.25 mm × 0.50 μm (Phenomenex). The oven was programmed with an initial column temperature of 90°C with a 1-min hold followed by a ramp of 15°C/min to 230°C, a ramp at 5°C/min to 260°C, and 50°C to 345°C, and finally held for 2.5 min. Compounds eluting off the column were directed into the pyrolysis reactor, heated at 1420°C, and converted to hydrogen gas. The deuterium enrichment was first initially expressed in δ values, compared with a calibrated hydrogen gas, and converted to atom percent deuterium by using standard equations. Calibration standards of methyl palmitate were obtained from Arndt Schimmelmann, Biogeochemical Laboratories, Indiana University. The percentage of de novo 16:1n–7 was calculated as previously described (4). We chose gas chromatography–combustion isotope ratio mass spectrometry for the measurement of de novo 16:1n–7 rather than GC-MS because the double bond in 16:1n–7 makes the molecule highly labile when measured by GC-MS with electron ionization and even when analyzed by chemical ionization (the fragmentation pattern is highly variable depending on the inlet or source temperatures). Moreover, IRMS is more sensitive than GC-MS for detecting lower deuterium enrichment, with the former detecting tracer:tracee (mol:mol) ratios down to 10−5 (or 0.001 atom percent excess) compared with GC-MS, which can accurately detect the tracer:trace ratio down to 0.2 atom percent excess (41).
Calculation of FA ratios and statistical analysis
Past research studies have used ratios of FAs as representative of relative activities of SCD1 and ELOVL6 enzymes (15, 25, 38, 42). We calculated these ratios for both VLDL-triacylglycerol and plasma NEFA FA compositions (molar percentage). SCD1(16) and SCD1(18) were calculated as indices of SCD1, whereas the ratio of 18:0 to 16:0 was used as an index of elongase activity (15, 38, 42). Finally, the ratio of 16:0 to 18:2n–6 was tested in the current study as an index of de novo lipogenesis (DNLIndex) (9, 10, 18) and compared with the absolute lipogenesis measured by using the deuterium isotope (DNLMeas). Statistical calculations were performed with Microsoft Excel (version 2007; Microsoft) and GraphPad Prism (V6.01; GraphPad Software Inc.). LowLF and HighLF groups were compared by unpaired 2-tailed t test (data reported as means ± SDs), and associations between outcome variables were assessed with Pearson's correlation coefficient. P < 0.05 was considered statistically significant, with P < 0.10 reported as a trend.
RESULTS
Subject characteristics
With the use of predefined criteria (36), subjects were divided into 2 groups as having either HighLF (n = 13; 18.4 ± 3.6%) or LowLF (n = 11; 3.1 ± 2.7%), and groups were matched for anthropometric characteristics as described previously (29) and presented in Table 1. Briefly, the groups had similar fasting plasma glucose and lipid concentrations and glycated hemoglobin. Groups were also equally sedentary (79.8 ± 8.8% and 79.0 ± 10.5% of the day; P = 0.85). Fasting insulin concentration was slightly higher in the HighLF group than LowLF group, which is characteristic of subjects with NAFLD (43). As shown in Table 1, the groups were also similar in ad libitum (prestudy) dietary intakes of macronutrients as well as dietary FA compositions.
TABLE 1.
Anthropometric and fasting biochemical characteristics, macronutrient intake, and fatty acid composition of subjects’ habitual diets1
| LowLF (n = 11) | HighLF (n = 13) | P | |
| Anthropometric measures | |||
| Liver fat, % | 3.1 ± 2.7 | 18.4 ± 3.6 | <0.001 |
| BMI, kg/m2 | 35.3 ± 7.7 | 34.9 ± 5.2 | 0.89 |
| Body fat, % | 39.7 ± 10.5 | 39.2 ± 6.8 | 0.89 |
| Fasting plasma biochemistry | |||
| Glucose, mmol/L | 5.1 ± 0.5 | 5.5 ± 0.8 | 0.20 |
| Hb A1c, % | 5.8 ± 0.2 | 5.9 ± 0.3 | 0.12 |
| Insulin, mU/L | 7 ± 4 | 11 ± 5 | 0.05 |
| LDL cholesterol, mmol/L | 3.13 ± 0.29 | 3.27 ± 0.78 | 0.59 |
| Triglycerides, mmol/L | 1.24 ± 0.57 | 1.51 ± 0.61 | 0.28 |
| Dietary intake | |||
| Total energy intake, kcal · kg BW−1 · d−1 | 25.7 ± 6.6 | 26.2 ± 6.2 | 0.84 |
| Carbohydrate, percentage of total daily energy | 48 ± 10 | 47 ± 10 | 0.88 |
| Protein, percentage of total daily energy | 17 ± 4 | 17 ± 5 | 0.96 |
| Fat, percentage of total daily energy | 35 ± 8 | 36 ± 7 | 0.80 |
| SFA intake, g/d | |||
| 14:0 | 2.5 ± 1.6 | 2.7 ± 1.3 | 0.84 |
| 16:0 | 17.6 ± 7.1 | 18.3 ± 7.7 | 0.82 |
| 18:0 | 8.5 ± 4.0 | 8.0 ± 3.4 | 0.73 |
| Total SFA | 32.7 ± 14.6 | 31.8 ± 12.8 | 0.87 |
| MUFA intake, g/d | |||
| 16:1n–7 | 1.6 ± 0.7 | 1.8 ± 0.9 | 0.41 |
| 18:1n–9 | 39.6 ± 16.5 | 36.7 ± 16.0 | 0.68 |
| Total MUFAs | 41.9 ± 17.0 | 39.0 ± 16.3 | 0.68 |
| PUFA intake, g/d | |||
| 18:2n–6 | 23.5 ± 14.0 | 18.8 ± 9.0 | 0.35 |
| 18:3n–3 | 2.4 ± 1.4 | 2.4 ± 1.9 | 0.99 |
| 20:4n–6 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.41 |
| 20:5n–3 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.49 |
| 22:6n–3 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.51 |
| Total PUFAs | 26.5 ± 15.3 | 20.8 ± 10.0 | 0.29 |
| Total trans fatty acids | 4.6 ± 2.9 | 4.1 ± 2.9 | 0.68 |
All values are means ± SDs analyzed by using an unpaired 2-tailed t test. Dietary data were derived from 3-d food records compared with interview and food-frequency data in subjects with LowLF and HighLF. BW, body weight; Hb A1c, glycated hemoglobin; HighLF, high liver fat; LowLF, low liver fat.
VLDL-triacylglycerol and NEFA FA composition
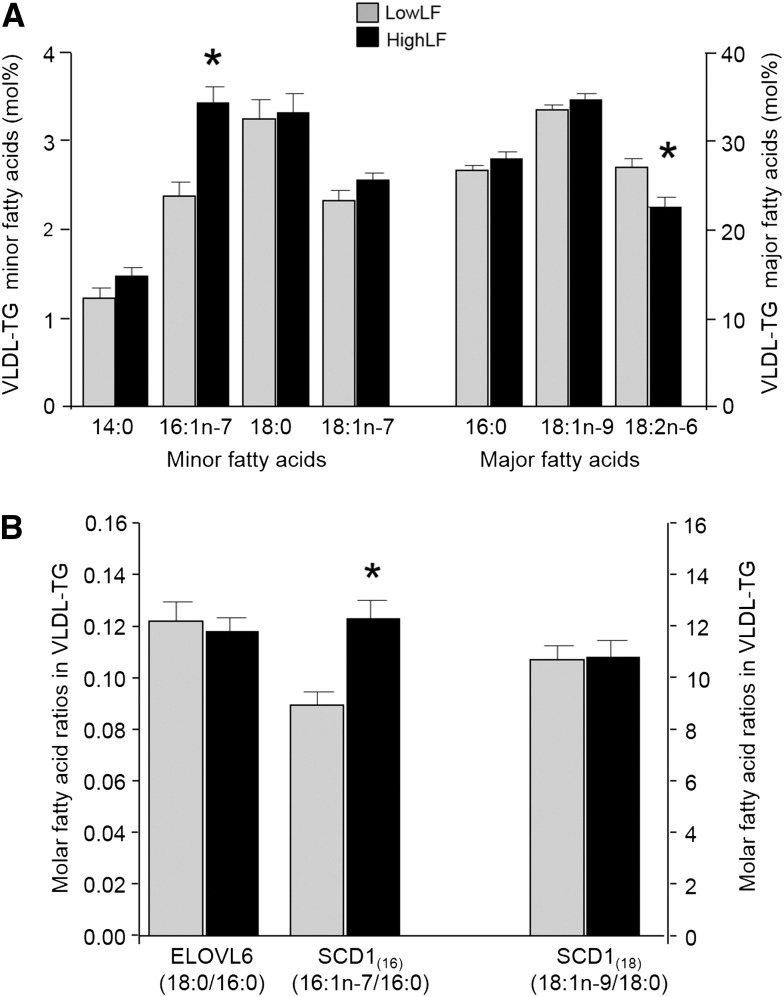
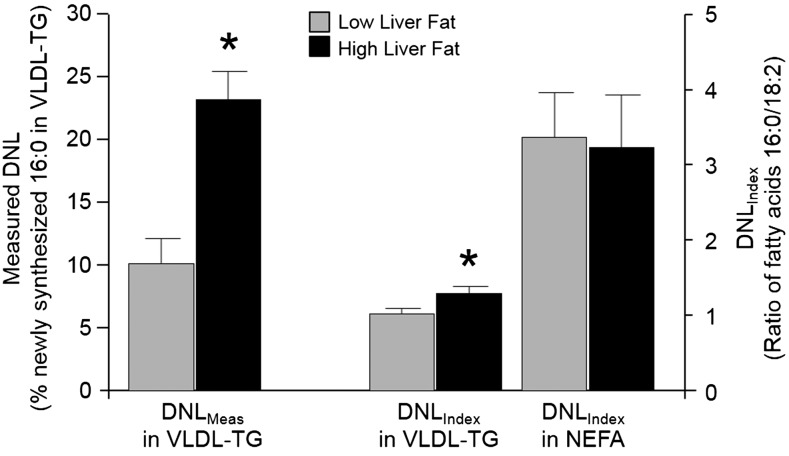
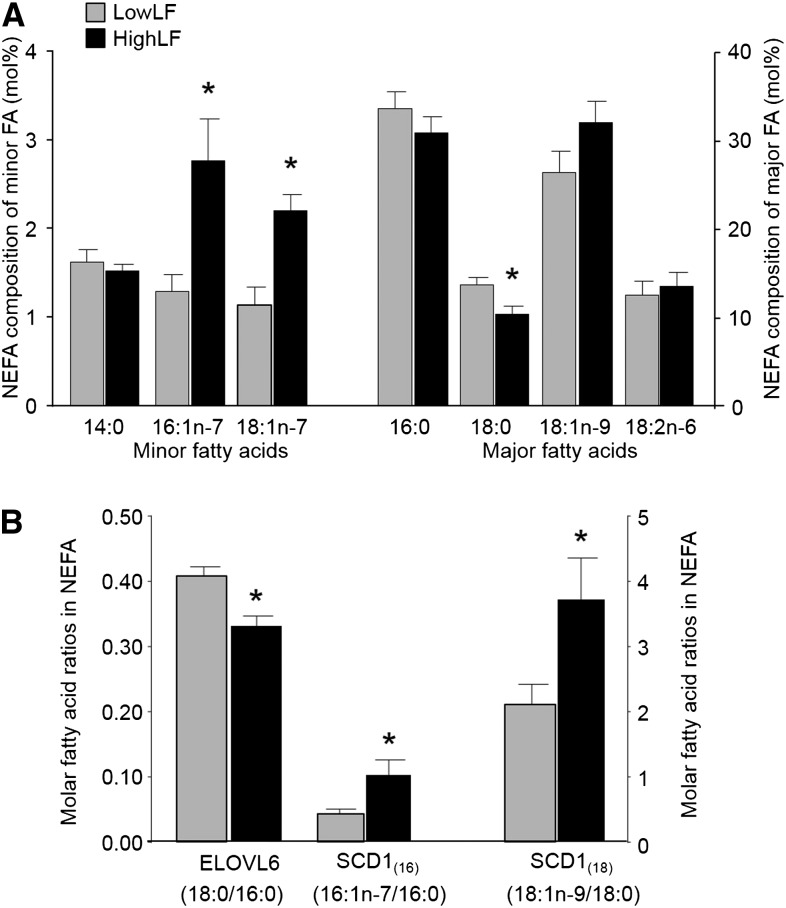
As shown in Figure 2A, the VLDL-triacylglycerol molar percentage of 14:0, 16:0, 18:0, 18:1n–7, and 18:1n–9 were similar between liver fat groups. By contrast, VLDL-triacylglycerol 16:1n–7 was 45% higher (P < 0.01), and 18:2n–6 was 16% lower (P = 0.01), in the HighLF group than LowLF group. The dietary intake of 18:2n–6 in the HighLF group was slightly, although not significantly, lower than that of the LowLF group (Table 1), whereas dietary intakes of other FAs, including 16:1n–7, were similar between groups. In addition, across both groups, dietary intakes of 16:1n–7 were much lower than of 16:0 and 18:2, as expected. Biomarkers of enzyme activity are presented in Figure 2B, and no difference was shown between groups for ELOVL6 activity. By contrast, the SCD1(16) index was 38% higher in the HighLF group, which was attributed to an isolated elevation of 16:1n–7 because 16:0 was not different between groups. Figure 3 presents the DNLIndex (ratio of 16:0 to 18:2n–6) in VLDL-triacylglycerols, which has been previously been validated as a measure of change in lipogenesis by Hudgins et al. (9, 10). The VLDL-triacylglycerol DNLIndex was 27% higher in the HighLF group. When VLDL-triacylglycerol DNL was measured directly by using deuterium-labeled water (DNLMeas), lipogenesis was 2.5-fold higher in the HighLF group (P < 0.01) than LowLF group (Figure 3). For the plasma NEFA pool, as shown in Figure 4A, proportions of 16:1n–7 and 18:1n–7 were greater, and 18:0 was lower, in the HighLF group. These findings resulted in the SCD1(16) index being 137% higher (P = 0.02; Figure 4B) and SCD1(18) index being 76% higher in the HighLF group (P < 0.05). By contrast, the elongase index (ELOVL6) was significantly lower in the HighLF group (Figure 4B). Similar proportions of NEFAs 16:0 and 18:2n–6 between groups (Figure 4A) resulted in a similar DNLIndex in the NEFA pool (Figure 3).
FIGURE 2 .
VLDL-TG fatty acid composition and indicators of lipid enzyme activities. Data are means ± SDs for individual fatty acids in VLDL-TG from subjects with low liver fat (gray bars; n = 11) and high liver fat (black bars; n = 12) (A) and fatty acid ratios used as putative biomarkers in the literature for the activities of ELOVL6, which elongates palmitate (16:0) to stearate (18:0), and SCD1, which can desaturate palmitate (16:0) to palmitoleate (16:1n–7) or stearate (18:0) to oleate (18:1n–9) (B). *Difference between liver fat groups, P < 0.05 (unpaired 2-tailed t test). ELOVL6, elongation of very-long-chain fatty acid protein 6; HighLF, high liver fat; LowLF, low liver fat; mol%, molar percentage; SCD1, stearoyl-CoA desaturase-1; SCD1(16), ratio of 16:1n–7 to 16:0; SCD1(18), ratio of 18:1n–9 to 18:0; TG, triacylglycerol.
FIGURE 3 .
Comparison of DNLMeas with fatty acid indices of DNL. Data are means ± SDs from subjects with low liver fat (gray bars; n = 11) and high liver fat (black bars; n = 12 for VLDL-TG data and n = 13 for NEFA data). In the literature, the molar ratio of 16:0 to 18:2n–6 is used as a DNLIndex. In this figure, the index is compared with DNLMeas by using deuterated water administration and fatty acid analysis by GC-MS. * P value <0.05 for differences between liver fat groups, analyzed by unpaired 2-tailed t test. DNL, de novo lipogenesis; DNLIndex, index of de novo lipogenesis; DNLMeas, isotopically measured de novo lipogenesis; GC-MS, gas chromatography–mass spectrometry; NEFA, nonesterified fatty acid; TG, triacylglycerol.
FIGURE 4 .
NEFA fatty acid composition and indicators of lipid enzyme activities. Data are means ± SDs for individual fatty acids in plasma NEFAs from subjects with LowLF (gray bars; n = 11) and HighLF (black bars; n = 13) (A) and fatty acid ratios used as putative biomarkers in the literature for activities of ELOVL6, which elongates palmitate (16:0) to stearate (18:0), and SCD1, which can desaturate palmitate (16:0) to palmitoleate (16:1n–7) or stearate (18:0) to oleate (18:1n–9). *Difference between liver fat groups, P < 0.05 (unpaired 2-tailed t test). ELOVL6, elongation of very-long-chain fatty acid protein 6; HighLF, high liver fat; LowLF, low liver fat; mol%, molar percentage; NEFA, nonesterified fatty acid; SCD1, stearoyl-CoA desaturase-1; SCD1(16), ratio of 16:1n–7 to 16:0; SCD1(18), ratio of 18:1n–9 to 18:0; TG, triacylglycerol.
Correlations
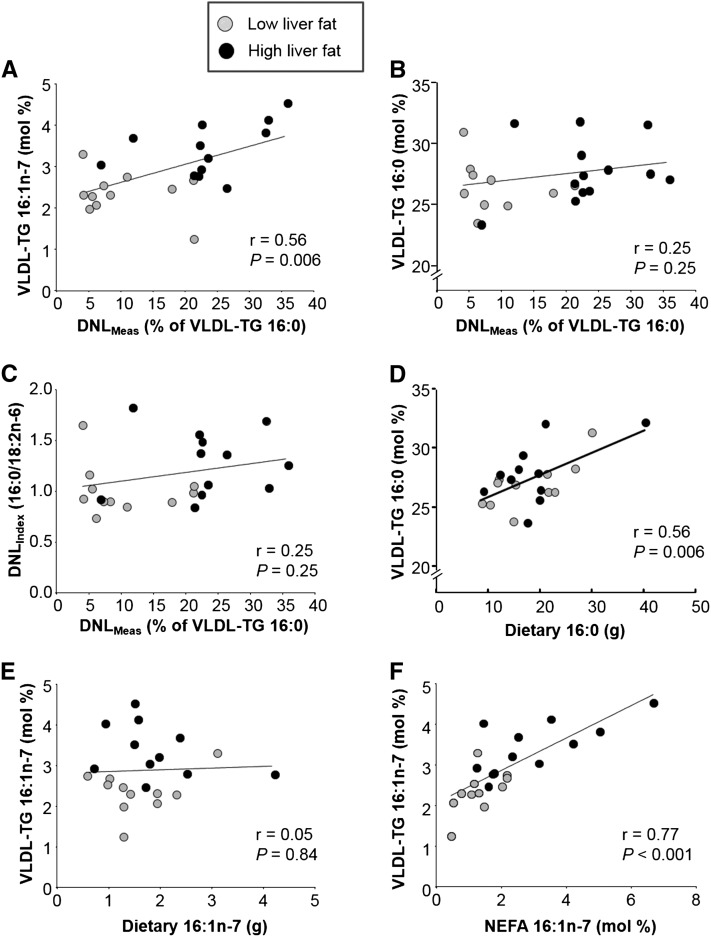
Relations were explored between FA compositions of VLDL-triacylglycerols, NEFAs, and the diet and characteristics of insulin resistance (Table 2, Figure 5). In VLDL-triacylglycerols, 16:1n–7 was significantly correlated with isotopically measured lipogenesis (Table 2, Figure 5A), liver fat, and AdipoIR. No other VLDL-triacylglycerol FA was associated with DNLMeas (Table 2), including 16:0 (Figure 5B). These relations with 16:1n–7 suggest that this FA could be derived directly from lipogenic pathways (i.e., channeling occurs between FA synthesis and desaturase enzymes). To test this hypothesis, we compared the deuterium labeling of 16:1n–7 and 16:0 and showed that 16:1n–7 (10 ± 7%) was significantly less (P = 0.004) labeled than 16:0 (23 ± 13%); this finding does not support the concept of channeling. Rather, unlabeled 16:1n–7 would be derived from desaturation of 16:0 originating from both endogenous (unlabeled) and labeled (D2 enriched during lipogenesis) sources. Calculations of indicators of liver enzyme activities revealed that the SCD1(16) was significantly associated with DNLMeas and liver fat, whereas the SCD1(18) was not (Table 2). Rather, the SCD1(18) was negatively correlated with HOMA and AdipoIR, and because of the mathematical relations between these variables, VLDL-triacylglycerols 18:0 and ELOVL6 were positively related to HOMA and AdipoIR. The DNLIndex (ratio of 16:0 to 18:2) did not correlate with DNLMeas (Figure 5C) but was associated with HOMA and AdipoIR (Table 2). With regard to dietary intake, VLDL-triacylglycerol 16:0 correlated with its presence in the diet (Figure 5D), but VLDL-triacylglycerol 16:1n–7 was not related to its presence in the diet (Figure 5E).
TABLE 2.
Pearson's correlations (r) between fatty acids in fasting VLDL-TGs and NEFAs and selected characteristics of insulin resistance1
| DNLMeas | Liver fat, % | HOMA | AdipoIR | |
| VLDL-TG fatty acid species (molar percentage) | ||||
| 16:1n–7 | 0.56§ | 0.53§ | NS | 0.56§ |
| 18:1n–7 | NS | NS | NS | NS |
| 18:1n–9 | NS | NS | NS | NS |
| 14:0 | NS | NS | NS | NS |
| 16:0 | NS | NS | NS | 0.42* |
| 18:0 | NS | NS | 0.50* | 0.52* |
| VLDL-TG fatty acid ratios as candidate enzyme indicators | ||||
| SCD1(16) | 0.50* | 0.52* | NS | 0.43* |
| SCD1(18) | NS | NS | −0.45* | −0.42* |
| DNLIndex | NS | NS | 0.52* | 0.59 § |
| ELOVL6 | NS | NS | 0.42* | 0.42* |
| Plasma NEFA species (molar percentage) | ||||
| 16:1n–7 | 0.60§ | NS | 0.58§ | 0.68¶ |
| 18:1n–7 | 0.43* | 0.57§ | 0.52§ | 0.53§ |
| 18:1n–9 | NS | NS | 0.46* | 0.45* |
| 14:0 | NS | NS | NS | NS |
| 16:0 | NS | NS | NS | NS |
| 18:0 | −0.48* | NS | −0.48* | −0.51* |
| Plasma NEFA ratios as candidate enzyme indicators (molar percentage) | ||||
| SCD1(16) | 0.58§ | NS | 0.55§ | 0.60§ |
| SCD1(18) | 0.54§ | NS | 0.55§ | 0.56§ |
| DNLIndex | NS | NS | NS | NS |
| ELOVL6 | −0.56§ | −0.50* | −0.44* | −0.57§ |
DNLMeas reflects VLDL-TG palmitate labeling using stable isotopes. AdipoIR is the product of fasting plasma insulin and NEFA concentrations. *P ≤ 0.05, §P ≤ 0.01, ¶P ≤ 0.001. AdipoIR, adipose insulin resistance; DNLIndex, index of de novo lipogenesis (ratio of 16:0 to 18:2n–6 molar percentages in VLDL and plasma nonesterified fatty acids); DNLMeas, isotopically measured de novo lipogenesis; ELOVL6, elongation of very-long-chain fatty acid protein 6 (ratio of 18:0 to 16:0); NEFA, nonesterified fatty acid; SCD1(16), ratio of 16:1n–7 to 16:0; SCD1(18), ratio of 18:1n–9 to 18:0; TG, triacylglycerol.
FIGURE 5 .
Relations between DNL and the fatty acid compositions of VLDL-TG, NEFAs, and the diet. Pearson's correlations in subjects with low liver fat (gray circles; n = 11) and high liver fat (black circles; n = 12 for VLDL-TG data and n = 13 for NEFA data). Data reflect the positive association between DNLMeas and VLDL-TG 16:1n–7 (A) but a lack of association with VLDL-TG 16:0 (B) as well as the DNLIndex (C). Instead, VLDL-TG 16:0 was associated with the dietary content of 16:0 (D), whereas this relation was not consistent for 16:1n–7 (E). Finally, the proportions of 16:1n–7 in VLDL-TGs and NEFAs were highly correlated with each other (F). Note interruption in y-axes in panels B and D. DNLIndex, index of de novo lipogenesis; DNLMeas, isotopically measured de novo lipogenesis; mol%, molar percentage; NEFA, nonesterified fatty acid; TG, triacylglycerol.
NEFA 16:1n–7 and VLDL-triacylglycerol 16:1n–7 were strongly correlated with each other (r = 0.77, P < 0.001; Figure 5F), whereas the other FAs in these lipid species were not intercorrelated (data not shown). NEFAs 16:1n–7 and 18:1n–7 were related to DNLMeas, HOMA, and AdipoIR (Table 2), and 18:1n–9 correlated with the latter two. Again, desaturase biomarkers SCD1(16) and SCD1(18) mirrored relations of their individual FA species. Similarly, 18:0 in NEFAs was negatively correlated with insulin resistance (i.e., the higher the NEFA 18:0, the lower the DNLMeas, HOMA, and AdipoIR), and the NEFA ELOVL6 was also inversely correlated with these variables.
DISCUSSION
Cellular FA composition, arising from the coordinated processes of lipogenesis, elongation, and desaturation, influences metabolic processes such as insulin sensitivity and lipid accrual. Because of the difficulty of obtaining biopsy tissue samples, many investigators measure the plasma lipid FA composition and use relative molar ratios to represent changes in intracellular desaturation and elongation (15, 25, 38, 42). In a series of elegant studies by Peter et al. (38, 42, 44), VLDL-triacylglycerol 16:1n–7 was strongly associated with the amount of both liver tissue triacylglycerol 16:1n–7 and liver SCD1 protein and expression. In addition, Peter and colleagues (45) recently associated an increase in the SCD1(16) index with less accrual of liver triacylglycerols during carbohydrate feeding in healthy men, although the range of liver fat was extremely narrow (<4% in all subjects). We showed the opposite to be true; in the current study, 16:1n–7 was positively related to liver fat across a wide range (0–27%). Our second key finding was a significant relation between proportions of VLDL-triacylglycerol 16:0 synthesized de novo and the molar percentage of 16:1n–7 in VLDL-triacylglycerols. This observed relation, on the basis of unequivocal measurements, strengthens indirect data from previous human studies and rodent models of fatty liver (7, 18, 20) and provides strong evidence that 16:1n–7 may be used as a marker of hepatic DNL and metabolic dysfunction.
Palmitate is the primary product of FA synthesis and, therefore, might be considered a candidate biomarker for DNL in humans. However, 16:0 is also a major dietary FA, and therefore, the amount of 16:0 in the body can represent either habitual dietary fat intake or the process of DNL (11). The VLDL-triacylglycerol DNLIndex is based on the principle that an increase in the synthesis of 16:0 dilutes the proportion of 18:2n–6. In the current study, this index suggested that lipogenesis was ∼30% higher in HighLF group, whereas the isotopically measured rate (DNLMeas) was 2.5-fold higher in the HighLF group than LowLF group. In addition, VLDL-triacylglycerol 16:0 did not correlate with DNLMeas but strongly correlated with dietary intake, suggesting that VLDL-triacylglycerol 16:0 better reflects dietary intake. These data suggest that an increase in 16:0 or the DNLIndex within an individual during a targeted intervention can be a marker for the stimulation of lipogenesis but may not accurately reflect DNL in an observational context. By contrast, 16:1n–7 can arise from the diet or via the SCD1-mediated desaturation of 16:0. However, 16:1n7 is present in food at very low amounts (1–4% of dietary FAs) (46), and thus, when 16:1n–7 is elevated in the blood, it reflects endogenous (SCD1) activity (38, 42, 44). These data and results from other authors (4, 47) raise the question of whether there is coordination between DNL and the generation of MUFAs through the modification of SFA species. Previously, the relation between 16:1n–7 and DNL has been indirectly suggested through observations of changes in the ratios of 16:0 to 18:2 and 16:1n–7 to 16:0 after dietary interventions (18), the conversion of labeled 16:0 to labeled 16:1n–7 (18), and associations of DNL-related enzymes with ELOVL6 and SCD (48). In the current study, we directly correlated the plasma lipid 16:1n–7 molar percentage with DNLMeas as well as liver fat.
The question of whether elevated 16:1n–7 in plasma lipids is driven by FA metabolism occurring in liver or adipose tissue raises the following 2 relevant issues: 1) DNL in adipose tissue is assumed to be low (49), disassociating SCD1 activity from the process of lipogenesis, and 2) NEFA flux from adipose tissue is the primary source of FAs for VLDL-triacylglycerols in lean individuals, suggesting that 16:1n–7 arising from adipose stores could contribute to 16:1n–7 in VLDL-triacylglycerols more than that derived from intrahepatic processes such as DNL. First, adequate accounting of adipose lipogenesis has been difficult because of the much larger amount of fat in this depot than in the liver (50, 51). Particularly in overweight and insulin resistant individuals, adipose lipogenesis may be greater than thought, as has been shown in the liver under these metabolic conditions (3). In the liver, the tight coupling of liver SCD activity, protein, and messenger RNA with the VLDL-triacylglycerol 16:1n–7 content has been shown repeatedly by Peter et al. (38, 42, 44) and suggests that VLDL-triacylglycerol 16:1n–7 reflects liver SCD1 activity, which we showed was elevated at the same time that DNL was elevated. Second, VLDL-triacylglycerol secretion is not always closely tied to NEFA flux (29, 52), especially for overweight and insulin resistant individuals in whom DNL makes a more substantial contribution to hepatic lipids. An emerging hypothesis is that the SCD1 pathway is stimulated during lipid overload resulting from both systemic NEFA delivery and hepatic DNL; this activity promotes desaturation of SFAs in the liver and adipose tissue, which may protect against lipotoxicity (53–56). The elevation of 16:1n–7 in the current subjects with HighLF was similar to previous observations (57) and was shown in both the plasma VLDL-triacylglycerol and NEFA pools, consistent with its elevation across a variety of lipid species in obesity (phospholipids, NEFAs, and triacylglycerols from plasma, VLDL, and the liver) (17, 26, 58, 59).
The time and technically demanding nature of tracer studies is a natural limitation to the sample size achievable with these protocols. However, strengths of the current study were the comprehensive phenotyping of FA metabolism and glucose tolerance as well as the high degree of matching between groups for adiposity, age, food intake, and physical activity. In addition, although the FA composition of adipose tissue was not measured, FA compositions of both VLDL-triacylglycerols and NEFAs were assessed in relation to the DNLMeas of both 16:0 and 16:1n–7; note that the synthesis of 16:1n–7 was reported in few studies (4). The 3-d prestudy diet was supplied by the CTRC kitchen and could have resulted in a similarity in the composition of food intake of all subjects, which would have tended to reduce the variability in the FA composition of blood lipids. However, the prestudy diet was based on each subject's ad libitum intake, and thus, blood lipids would have reflected each subject's unique diet. FA compositional analyses were not blinded to group assignment, although standardized protocols were used (GC-MS results were calculated from known standards, which made bias unlikely), but the IRMS determination of isotope enrichment in 16:1n–7 was performed in a blinded fashion. The current data were derived from the fasting state, and we have shown previously that subjects with high lipogenesis have elevated concentrations well into a fast (1, 29), whereas in lean healthy subjects, lipogenesis concentrations are very low in the fasting state and stimulated with feeding. Thus, in lean subjects, 16:1n–7 may not be an efficient marker of lipogenesis or liver fat, and generalizing these findings to larger data sets would require that the population have a wide range of body fat and insulin sensitivity.
In conclusion, to our knowledge, this is the first study to comprehensively compare isotopically labeled FA patterns in subjects with documented NAFLD. In both NEFA and VLDL-triacylglycerol pools, the following 3 outcomes were confirmed as markers of reduced insulin sensitivity and liver fat: the molar proportion of 16:1n–7 as a percentage of all VLDL-triacylglycerol FAs and the proportions of 16:0 and 16:1n–7 that were de novo synthesized (isotopically labeled). When combined with data from other studies, 16:1n–7 is identified in the current study as an objective lipid biomarker that has potential for use as an indicator of lipogenesis in studies in which the administration of isotopes may be more complicated for financial or analytic considerations or the population under study may be more challenging (e.g., studies in children or community or field work).
Acknowledgments
We thank Dora Bradford and Maressa Valdez for their excellent care of research subjects. We thank Vidya Vaidyanathan and Kim Borke for data generation.
The authors’ responsibilities were as follows—EJP: designed the overall research project, provided study oversight, and had primary responsibility for the final content of the manuscript; JJL, JEL, and EJP: wrote the manuscript; and all authors: conducted research and generated and analyzed data. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: AdipoIR, adipose insulin resistance; CTRC, Clinical Translational Research Center; DNL, de novo lipogenesis; DNLIndex, index of de novo lipogenesis; DNLMeas, isotopically measured de novo lipogenesis; ELOVL6, elongation of very-long-chain fatty acid protein 6; FA, fatty acid; FAME, fatty acid methyl-ester; GC, gas chromatography; GC-MS, gas chromatography–mass spectrometry; HighLF, high liver fat; IRMS, isotope-ratio mass spectrometry; LowLF, low liver fat; NAFLD, nonalcoholic fatty liver disease; NEFA, nonesterified fatty acid; SCD1, stearoyl-CoA desaturase-1; SCD1(16), ratio of 16:1n–7 to 16:0; SCD1(18), ratio of 18:1n–9 to 18:0.
REFERENCES
- 1.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non–alcoholic fatty liver disease. Diabetes Metab 2003;29:478–85. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz J-M, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr 2003;77:43–50. [DOI] [PubMed] [Google Scholar]
- 4.Wilke MS, French MA, Goh YK, Ryan EA, Jones PJ, Clandinin MT. Synthesis of specific fatty acids contributes to VLDL-triacylglycerol composition in humans with and without type 2 diabetes. Diabetologia 2009;52:1628–37. [DOI] [PubMed] [Google Scholar]
- 5.Vedala A, Wang W, Neese RA, Christiansen MP, Hellerstein MK. Delayed secretory pathway contributions to VLDL-triglycerides from plasma NEFA, diet, and de novo lipogenesis in humans. J Lipid Res 2006;47:2562–74. [DOI] [PubMed] [Google Scholar]
- 6.Marques-Lopes I, Ansorena D, Astiasaran I, Forga L, Martínez JA. Postprandial de novo lipogenesis and metabolic changes induced by a high-carbohydrate, low-fat meal in lean and overweight men. Am J Clin Nutr 2001;73:253–61. [DOI] [PubMed] [Google Scholar]
- 7.Aarsland A, Wolfe RR. Hepatic secretion of VLDL fatty acids during stimulated lipogenesis in men. J Lipid Res 1998;39:1280–6. [PubMed] [Google Scholar]
- 8.Field CJ, Ryan EA, Thomson AB, Clandinin MT. Diet fat composition alters membrane phospholipid composition, insulin binding, and glucose metabolism in adipocytes from control and diabetic animals. J Biol Chem 1990;265:11143–50. [PubMed] [Google Scholar]
- 9.Hudgins LC, Hellerstein M, Seidman C, Neese R, Diakun J, Hirsch J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J Clin Invest 1996;97:2081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hudgins LC, Hellerstein MK, Seidman CE, Neese RA, Tremaroli JD, Hirsch J. Relationship between carbohydrate-induced hypertriglyceridemia and fatty acid synthesis in lean and obese subjects. J Lipid Res 2000;41:595–604. [PubMed] [Google Scholar]
- 11.Hudgins LC, Parker TS, Levine DM, Hellerstein MK. A dual sugar challenge test for lipogenic sensitivity to dietary fructose. J Clin Endocrinol Metab 2011;96:861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008;47:348–80. [DOI] [PubMed] [Google Scholar]
- 13.Durrington PN, Bolton CH, Hartog M, Angelinetta R, Emmett P, Furniss S. The effect of a low-cholesterol, high-polyunsaturate diet on serum lipid levels, apolipoprotein B levels and triglyceride fatty acid composition. Atherosclerosis 1977;27:465–75. [DOI] [PubMed] [Google Scholar]
- 14.Emken EA. Metabolism of dietary stearic acid relative to other fatty acids in human subjects. Am J Clin Nutr 1994;60:1023S–8S. [DOI] [PubMed] [Google Scholar]
- 15.Sjögren P, Sierra-Johnson J, Gertow K, Rosell M, Vessby B, de Faire U, Hamsten A, Hellenius M-L, Fisher RM. Fatty acid desaturases in human adipose tissue: relationships between gene expression, desaturation indexes and insulin resistance. Diabetologia 2008;51:328–35. [DOI] [PubMed] [Google Scholar]
- 16.Gong J, Campos H, McGarvey S, Wu Z, Goldberg R, Baylin A. Adipose tissue palmitoleic acid and obesity in humans: does it behave as a lipokine? Am J Clin Nutr 2011;93:186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Almeida IT, Cortez-Pinto H, Fidalgo G, Rodrigues D, Camilo ME. Plasma total and free fatty acids composition in human non–alcoholic steatohepatitis. Clin Nutr 2002;21:219–23. [DOI] [PubMed] [Google Scholar]
- 18.Chong MF-F, Hodson L, Bickerton AS, Roberts R, Neville M, Karpe F, Frayn KN, Fielding BA. Parallel activation of de novo lipogenesis and stearoyl-CoA desaturase activity after 3 d of high-carbohydrate feeding. Am J Clin Nutr 2008;87:817–23. [DOI] [PubMed] [Google Scholar]
- 19.Fabbrini E, Magkos F, Su X, Abumrad NA, Nejedly N, Coughlin CC, Okunade AL, Patterson BW, Klein S. Insulin sensitivity is not associated with palmitoleate availability in obese humans. J Lipid Res 2011;52:808–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forsythe CE, Phinney SD, Feinman RD, Volk BM, Freidenreich D, Quann E, Ballard K, Puglisi MJ, Maresh CM, Kraemer WJ, et al. Limited effect of dietary saturated fat on plasma saturated fat in the context of a low carbohydrate diet. Lipids 2010;45:947–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jump DB. Fatty acid regulation of gene transcription. Crit Rev Clin Lab Sci 2004;41:41–78. [DOI] [PubMed] [Google Scholar]
- 22.Kotronen A, Seppänen–Laakso T, Westerbacka J, Kiviluoto T, Arola J, Ruskeepää A-L, Oresic M, Yki-Järvinen H. Hepatic stearoyl-CoA desaturase (SCD)-1 activity and diacylglycerol but not ceramide concentrations are increased in the nonalcoholic human fatty liver. Diabetes 2009;58:203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein–Platat C, Drai J, Oujaa M, Schlienger J-L, Simon C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am J Clin Nutr 2005;82:1178–84. [DOI] [PubMed] [Google Scholar]
- 24.Kunesová M, Hainer V, Tvrzicka E, Phinney SD, Stich V, Parízková J, Zák A, Stunkard AJ. Assessment of dietary and genetic factors influencing serum and adipose fatty acid composition in obese female identical twins. Lipids 2002;37:27–32. [DOI] [PubMed] [Google Scholar]
- 25.Warensjö E, Rosell M, Hellenius M-L, Vessby B, De Faire U, Risérus U. Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: links to obesity and insulin resistance. Lipids Health Dis 2009;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paillard F, Catheline D, Le Duff F, Bouriel M, Deugnier Y, Pouchard M, Daubert J-C, Legrand P. Plasma palmitoleic acid, a product of stearoyl-coA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr Metab Cardiovasc Dis 2008;18:436–40. [DOI] [PubMed] [Google Scholar]
- 27.Vessby B, Ahrén B, Warensjö E, Lindgärde F. Plasma lipid fatty acid composition, desaturase activities and insulin sensitivity in Amerindian women. Nutr Metab Cardiovasc Dis 2012;22:176–81. [DOI] [PubMed] [Google Scholar]
- 28.Attie AD, Krauss RM, Gray-Keller MP, Brownlie A, Miyazaki M, Kastelein JJ, Lusis AJ, Stalenhoef AFH, Stoehr JP, Hayden MR, et al. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J Lipid Res 2002;43:1899–907. [DOI] [PubMed] [Google Scholar]
- 29.Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014;146:726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol 2006;21:1–6. [DOI] [PubMed] [Google Scholar]
- 31.Turner SM, Murphy EJ, Neese RA, Antelo F, Thomas T, Agarwal A, Go C, Hellerstein MK. Measurement of TG synthesis and turnover in vivo by 2H2O incorporation into the glycerol moiety and application of MIDA. Am J Physiol Endocrinol Metab 2003;285:E790–803. [DOI] [PubMed] [Google Scholar]
- 32.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 2005;288:E462–8. [DOI] [PubMed] [Google Scholar]
- 33.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–95. [DOI] [PubMed] [Google Scholar]
- 34.Crouter SE, Dellavalle DM, Horton M, Haas JD, Frongillo EA, Bassett DR. Validity of the Actical for estimating free-living physical activity. Eur J Appl Physiol 2011;111:1381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrows BR, Parks EJ. Contributions of different fatty acid sources to very low-density lipoprotein–triacylglycerol in the fasted and fed states. J Clin Endocrinol Metab 2006;91:1446–52. [DOI] [PubMed] [Google Scholar]
- 36.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 37.Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C, Finch J, Gastaldelli A, Harrison S, Tio F, et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 2012;55:1389–97. [DOI] [PubMed] [Google Scholar]
- 38.Peter A, Cegan A, Wagner S, Lehmann R, Stefan N, Königsrainer A, Königsrainer I, Häring H-U, Schleicher E. Hepatic lipid composition and stearoyl-coenzyme A desaturase 1 mRNA expression can be estimated from plasma VLDL fatty acid ratios. Clin Chem 2009;55:2113–20. [DOI] [PubMed] [Google Scholar]
- 39.Timlin MT, Barrows BR, Parks EJ. Increased dietary substrate delivery alters hepatic fatty acid recycling in healthy men. Diabetes 2005;54:2694–701. [DOI] [PubMed] [Google Scholar]
- 40.Murphy EJ. Stable isotope methods for the in vivo measurement of lipogenesis and triglyceride metabolism. J Anim Sci 2006;84:E94–104. [DOI] [PubMed] [Google Scholar]
- 41.Brenna JT. Use of stable isotopes to study fatty acid and lipoprotein metabolism in man. Prostaglandins Leukot Essent Fatty Acids 1997;57:467–72. [DOI] [PubMed] [Google Scholar]
- 42.Peter A, Cegan A, Wagner S, Elcnerova M, Königsrainer A, Königsrainer I, Häring H-U, Schleicher ED, Stefan N. Relationships between hepatic stearoyl-CoA desaturase-1 activity and mRNA expression with liver fat content in humans. Am J Physiol Endocrinol Metab 2011;300:E321–6. [DOI] [PubMed] [Google Scholar]
- 43.Gastaldelli A, Harrison SA, Belfort-Aguilar R, Hardies LJ, Balas B, Schenker S, Cusi K. Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology 2009;50:1087–93. [DOI] [PubMed] [Google Scholar]
- 44.Peter A, Weigert C, Staiger H, Machicao F, Schick F, Machann J, Stefan N, Thamer C, Häring H-U, Schleicher E. Individual stearoyl-coa desaturase 1 expression modulates endoplasmic reticulum stress and inflammation in human myotubes and is associated with skeletal muscle lipid storage and insulin sensitivity in vivo. Diabetes 2009;58:1757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silbernagel G, Kovarova M, Cegan A, Machann J, Schick F, Lehmann R, Häring H-U, Stefan N, Schleicher E, Fritsche A, et al. High hepatic SCD1 activity is associated with low liver fat content in healthy subjects under a lipogenic diet. J Clin Endocrinol Metab 2012;97:E2288–92. [DOI] [PubMed] [Google Scholar]
- 46.Hodson L, Karpe F. Is there something special about palmitoleate? Curr Opin Clin Nutr Metab Care 2013;16:225–31. [DOI] [PubMed] [Google Scholar]
- 47.Benhamed F, Denechaud P-D, Lemoine M, Robichon C, Moldes M, Bertrand-Michel J, Ratziu V, Serfaty L, Housset C, Capeau J, et al. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J Clin Invest 2012;122:2176–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts R, Hodson L, Dennis AL, Neville MJ, Humphreys SM, Harnden KE, Micklem KJ, Frayn KN. Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia 2009;52:882–90. [DOI] [PubMed] [Google Scholar]
- 49.Sjöström L. Fatty acid synthesis de novo in adipose tissue from obese subjects on a hypercaloric high-carbohydrate diet. Scand J Clin Lab Invest 1973;32:339–49. [DOI] [PubMed] [Google Scholar]
- 50.Hellerstein MK. De novo lipogenesis in humans: metabolic and regulatory aspects. Eur J Clin Nutr 1999;53:S53–65. [DOI] [PubMed] [Google Scholar]
- 51.Strawford A, Antelo F, Christiansen M, Hellerstein MK. Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. Am J Physiol Endocrinol Metab 2004;286:E577–88. [DOI] [PubMed] [Google Scholar]
- 52.Koutsari C, Mundi MS, Ali AH, Patterson BW, Jensen MD. Systemic free fatty acid disposal into very low-density lipoprotein triglycerides. Diabetes 2013;62:2386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem 2009;284:5637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oosterveer MH, van Dijk TH, Tietge UJF, Boer T, Havinga R, Stellaard F, Groen AK, Kuipers F, Reijngoud D-J. High fat feeding induces hepatic fatty acid elongation in mice. PLoS ONE 2009;4:e6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collins JM, Neville MJ, Hoppa MB, Frayn KN. De novo lipogenesis and stearoyl-CoA desaturase are coordinately regulated in the human adipocyte and protect against palmitate-induced cell injury. J Biol Chem 2010;285:6044–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA 2003;100:3077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Araya J, Rodrigo R, Videla LA, Thielemann L, Orellana M, Pettinelli P, Poniachik J. Increase in long-chain polyunsaturated fatty acid n - 6/n - 3 ratio in relation to hepatic steatosis in patients with non–alcoholic fatty liver disease. Clin Sci (Lond) 2004;106:635–43. [DOI] [PubMed] [Google Scholar]
- 58.Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GS. Circulating palmitoleic acid and risk of metabolic abnormalities and new-onset diabetes. Am J Clin Nutr 2010;92:1350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allard JP, Aghdassi E, Mohammed S, Raman M, Avand G, Arendt BM, Jalali P, Kandasamy T, Prayitno N, Sherman M, et al. Nutritional assessment and hepatic fatty acid composition in non–alcoholic fatty liver disease (NAFLD): a cross-sectional study. J Hepatol 2008;48:300–7. [DOI] [PubMed] [Google Scholar]