Abstract
Homozygosity of loss-of-function mutations in ANGPTL3 (angiopoietin-like protein 3)-gene results in FHBL2 (familial combined hypolipidaemia, OMIM #605019) characterized by the reduction of all major plasma lipoprotein classes, which includes VLDL (very-low-density lipoprotein), LDL (low-density lipoprotein), HDL (high-density lipoprotein) and low circulating NEFAs (non-esterified fatty acids), glucose and insulin levels. Thus complete lack of ANGPTL3 in humans not only affects lipid metabolism, but also affects whole-body insulin and glucose balance. We used wild-type and ANGPTL3-silenced IHHs (human immortalized hepatocytes) to investigate the effect of ANGPTL3 silencing on hepatocyte-specific VLDL secretion and glucose uptake. We demonstrate that both insulin and PPARγ (peroxisome-proliferator-activated receptor γ) agonist rosiglitazone down-regulate the secretion of ANGPTL3 and TAG (triacylglycerol)-enriched VLDL1-type particles in a dose-dependent manner. Silencing of ANGPTL3 improved glucose uptake in hepatocytes by 20–50% and influenced down-regulation of gluconeogenic genes, suggesting that silencing of ANGPTL3 improves insulin sensitivity. We further show that ANGPTL3-silenced cells display a more pronounced shift from the secretion of TAG-enriched VLDL1-type particles to secretion of lipid poor VLDL2-type particles during insulin stimulation. These data suggest liver-specific mechanisms involved in the reported insulin-sensitive phenotype of ANGPTL3-deficient humans, featuring lower plasma insulin and glucose levels.
Keywords: ANGPTL3 silencing, hypolipidaemia, insulin signalling, liver, rosiglitazone, VLDL
Abbreviations: ANGPTL, angiopoietin-like protein; CCD, coiled coil domain; FHBL2, familial combined hypolipidaemia; GLUT2, glucose transporter 2; HDL, high-density lipoprotein; IHH, immortalized human hepatocyte; IR, insulin receptor; IRS, insulin receptor substrate; LDL, low-density lipoprotein; LPL, lipoprotein lipase; NEFA, non-esterified fatty acid; PEPCK, phosphoenolpyruvate carboxykinase; PGC1α, peroxisome proliferator-activated receptor γ co-activator 1-α; PI3K, phosphoinositide 3-kinase; PL, phospholipid; PPAR, peroxisome-proliferator-activated receptor; QPCR, quantitative PCR; shRNA, small hairpin RNA; TAG, triacylglycerol; TRB3, tribbles homologue 3; VLDL, very-low-density lipoprotein
Short abstract
We show that silencing of ANGPTL3 in human hepatocytes in addition to reducing secretion of TAG-enriched VLDL upon insulin stimulation enhances glucose uptake and improves insulin response. Thus, our data provide insight into the lower insulin and glucose levels observed in humans with ANGPTL3 loss-of-function mutation.
INTRODUCTION
The ANGPTLs (angiopoietin-like proteins) are a family of secreted proteins which share tertiary structural domains with angiopoietins [1], with N-terminal CCD (coiled coil domain) and C-terminal FLD (fibrinogen-like domain) of which the CCD is functionally associated with reduced plasma TAGs (triacylglycerols) [2]. ANGPTL3 is almost exclusively expressed in the liver with minor expression found in kidney [3,4]. ANGPTL3 has been shown to inhibit LPL (lipoprotein lipase) in vitro and in vivo [5–7], and mice lacking ANGPTL3 protein have increased LPL activity and reduced levels of plasma TAG [8,9].
GWAS (genome wide association studies) in humans have shown that SNPs (single nucleotide polymorphisms) at loci near ANGPTL3 are associated with plasma TAG levels [10–12]. Sequencing of the coding region of ANGPTL3 revealed additional loss-of-function mutations associated with low levels of plasma TAG [4]. Furthermore, Musunuru et al. [13] have identified E128X and S17X as two novel nonsense mutations in ANGPTL3. Loss of function in ANGPTL3 results in a condition referred to as FHBL2 (familial combined hypolipidaemia, OMIM #605019), featuring reduction in all major plasma lipoproteins VLDL (very-low-density lipoprotein), LDL (low-density lipoprotein) and HDL (high-density lipoprotein). In addition to FHBL2 phenotype also plasma NEFAs (non-esterified fatty acids), insulin, fasting plasma glucose and HOMA-IR (homoeostatic model assessment of insulin resistance) values are significantly lower in individuals homozygous for S17X mutation compared with carriers and non-carriers [14,15] linking the hypolipidaemic effect of ANGPTL3-deficiency with insulin sensitivity.
Insulin is the primary hormone controlling whole body glucose and lipid homoeostasis. Activation of the insulin-signalling pathway suppresses hepatic secretion of TAG-enriched VLDL1 particles without affecting the secretion of lipid-poor VLDL2 [16]. The number of TAG-enriched VLDL1 particles is elevated during insulin resistance, contributing to hypertriglyceridaemia [17]. Despite elevated plasma glucose and TAG levels liver persistently continues glucose production and VLDL secretion during IR [18,19]. Insulin inhibits VLDL secretion through PI3K (phosphoinositide 3-kinase)-mediated abruption in the second-step bulky TAG-assembly of VLDL1 particles and apoB-100 availability [20]. Unlike in the muscle and adipose tissue, insulin does not directly increase glucose uptake in the liver. Postic et al. [21] demonstrated down-regulation of GLUT2 (glucose transporter 2) by insulin in a dose-dependent manner in vivo and in vitro. Hepatic GLUT2 expression is induced by high glucose concentration which, during feeding, prevent the inhibitory effect of insulin on GLUT2 expression [21].
We utilized wild-type IHHs (immortalized human hepatocytes) and IHH cells with silenced ANGPTL3 to study how the secretion levels of ANGPTL3 are regulated in hepatocytes and how the silencing of ANGPTL3 affects VLDL secretion and hepatocyte-specific glucose uptake. The aim of the research is to seek further insight into the molecular mechanisms related to hypolipidaemia and insulin sensitivity reported in humans with ANGPTL3 deficiency [14].
MATERIALS AND METHODS
ANGPTL3-silenced cell-line and cell culture conditions
Liver IHHs immortalized by SV40 large T-Antigen (IHH, ATCC® PTA-5565™) were transduced with MISSION™ shRNA (small hairpin RNA) Lentiviral Vector particles (TRCN0000242782, Sigma Aldrich) targeting ANGPTL3 (NM_014495.2) or with non-target shRNA (SHCOO2, Sigma Aldrich) [MOI (multiplicity of infection) 1]. Positive cells were selected against 5 μg/ml puromycin for 12 days. The transduction was repeated once after the first selection. Cells were cultured in Williams medium E (Gibco by Life Technologies, 22551-022) with added 10% (v/v) FBS and glutamine 0.2 mg/ml and incubated +37 °C. FBS was removed during experiments and total protein from cell lysates was used to normalize the data. Cells were washed with PBS (pH 7.4) and lysed in RIPA buffer. Protein concentration was measured with Bradford protein assay (Bio-Rad). Following compounds were used: insulin (bovine, Sigma-Aldrich), Wortmannin (Sigma-Aldrich), Akt1/2 inhibitor (Sigma-Aldrich), rosiglitazone (Cayman Chemical) and GW9662 (Sigma-Aldrich).
Enzymatic measurements of TAG and PLs (phospholipids)
TAGs from media and cell lysates were measured with an enzymatic method (GPO-PAP 1488872 kit; Roche Diagnostics Gmbh). Phospholipids were measured from the cell culture media with an enzymatic method (FS 60080970; Diasys Diagnostics).
ANGPTL3 and apoB-100 ELISA assays
Concentration of ANGPTL3 was measured utilizing ELISA assay developed by Robciuc et al. [22]. ApoB-100 was measured with Human apolipoprotein B ELISA kit (Mabtech) according to instructions provided by the manufacturer.
Labelled oleic acid and TAG extraction
Cells were incubated in the presence of 5.5 mM glucose and 0.5% (w/v) BSA-complexed with 0.375 mM oleic acid and 0.1 μCi [14C]-oleic acid (PerkinElmer) for 24 h. Cells were collected in 1 ml 2% NaCl–PBS buffer. Lipids were extracted by adding 2 ml of methanol and 1 ml chloroform and centrifuged (2500 rev/min for 10 min at room temperature). H2O (1 ml) and chloroform (1 ml) were added and again centrifuged (2500 rev/min, for 10 min at room temperature). The upper phase was removed and the lower phase dried under nitrogen and solubilized in 100 μl of chloroform and applied on a TLC-plate. The TLC-plates were run in a chamber containing (N-hexane, diethylether, acetic acid and H2O (65/15/1/0.25, v/v). Iodine vapour stained areas containing the TAGs were scraped out from the TLC-plate and radioactivity was measured by liquid scintillation counting (Wallac LS-Beta-Counter).
Measurement of glucose uptake
Cells were incubated in the presence of 5.5 or 20 mM glucose and 1 μCi of deoxy-D-glucose, 2-[1,2-3H (N)] (1 mCi/ml, lot 638084, Perkin Elmer). Radioactivity from cell lysates and media was measured by liquid scintillation counter (Wallac LS-Beta-Counter).
QPCR (quantitative PCR) analysis
RNA was extracted with Qiagen RNA purification kit and synthesized with SuperScript® VILO™ cDNA Synthesis Kit (Life Technologies). Each QPCR contained primers (see Table 1) at 300 nM concentration and 10 ng of cDNA. The total volume of each reaction was 10 μl containing SYBR® Green PCR Master Mix (Life Technologies). The QPCR program: 95 °C for 10 min followed by 45 cycles 95 °C 15 s, 60 °C 1 min.
Table 1. QPCR-primers.
| Name, gene name | Forward | Reverse |
|---|---|---|
| ACTIN, ACTB | GATGTGGATCAGCAAGCAGGA | AGCATTTGCGGTGGACGAT |
| ANGPTL3 | CCAGAACACCCAGAAGTAACT | TCTGTGGGTTCTTGAATACTAGTC |
| ANGPTL4 | GCCTATAGCCTGCAGCTCAC | CAAGTGGAGAAGGGTACGGA |
| ANGPTL8, C19ORF80 | AGGTCTTAAAGGCTCACG | TTCCATCCAGGCAGATTC |
| DGAT2 | CATCCTCATGTACATATTCTGC | TGGGAAAGTAGTCTCGAAAG |
| HNF4α, HNF4A | AGTACATCCCAGCTTTCTG | AATGTAGTCATTGCCTAGGAG |
| IL-6 | GGTACATCCTCGACGGCATCT | GTGCCTCTTTGCTGCTTTCAC |
| IRS-1 | CAGAATGAAGACCTAAATGACC | ATGCATCGTACCATCTACTG |
| IRS-2 | AAGAGTGAAGATCTGTCTGG | ATCTAACAGAGTCCACAGATG |
| PEPCK, PCK1 | GGGCATCCTCAGGCGGCT | GATAACCGTCTTGCTTTCGAT |
| PGC1A, PPARGC1A | GCAGACCTAGATTCAAACTC | CATCCCTCTGTCATCCTC |
| PI3K 85, PIK3R1 | AAGAAGACTTGAAGAAGCA | TCAACCACATCAAGTATTGG |
| PPARALPHA, PPARA | CCTAAAAAGCCTAAGGAAACC | GATCTCCACAGCAAATGATAG |
| PPARγ, PPARG | GATCCAGTGGTTGCAGATTACAA | GAGGGAGTTGGAAGGCTCTTC |
| PTEN | GGCTAAGTGAAGATGACAATC | GTTACTCCCTTTTTGTCTCTG |
| TGH, TGH/CES1 | GTCTTTCTGGGCATTCCATT | CTCTCCACGTCTTGTAGGCA |
| TNFα, TNFA | CCCAGGCAGTCAGATCATCTT | AGCTGCCCCTGAGCTTGA |
| TRB3, TRIB3 | GACCGTGAGAGGAAGAAG | GAGTATCTCAGGTCCCAC |
Statistics
The experiments were conducted in triplicate (n=3) and performed using 2–3 separately transduced IHH cell cultures. Results are expressed as means±S.D. One-way or two-way ANOVA was used to compare the significance between groups followed by Games–Howell post hoc test. The difference between groups was considered statistically significant if P<0.05 using Games–Howell post hoc test (*<0.05, **<0.01, ***<0.001).
RESULTS
We first tested insulin response in wild-type IHH cells by incubating the cells in the presence of insulin and measured the secretion rate of VLDL by analysing the cell culture media for TAG, apoB-100 and PLs after 24 h. Insulin treatment resulted in a dose dependent down-regulation of the secreted TAG, PL (Figure 1a) and apoB-100 (Figure 1b). Insulin in a dose-dependent manner reduced also the secretion of ANGPTL3 (Figure 1c). We calculated a TAG/apoB-100 ratio and demonstrated that insulin stimulation results in a significant reduction of hepatocyte TAG secreted per apoB-100 protein (Figure 1d). These data are in line with reports on insulin mediated down-regulation of TAG-enriched VLDL1 particles without affecting the (lipid-poor) VLDL2 secretion [16,23].
Figure 1. The effect of insulin on VLDL and ANGPTL3 secretion.
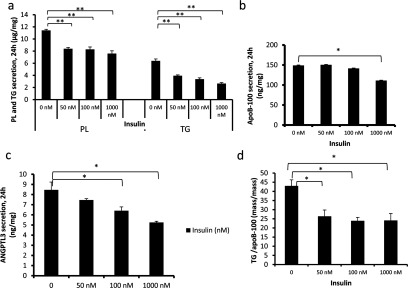
Wild-type IHH-cells were incubated in 5.5 mM glucose for 24 h at variable concentrations of insulin. (a) Secretion of TAG and PL during insulin stimulation (μg/mg). (b) Secretion of apoB-100 (ng/mg). (c) Secretion of ANGPTL3 (ng/mg). (d) The amount of TAG (ng) for each ng of apoB-100.
Insulin mediates its functions via IRS (insulin receptor substrate)–PI3K-dependent pathway [24]. To test whether VLDL secretion in IHH cells is regulated via PI3K and its downstream target Akt, we incubated cells with a PI3K inhibitor Wortmannin and Akt1/2 inhibitor. Treatment with Wortmannin partly abolished the inhibitory effect of insulin on TAG and PL secretion (Figure 2a). Akt1/2 inhibitor, much like Wortmannin, partially abolished the inhibitory effect of insulin on TAG secretion indicating that insulin regulates the secretion of VLDL via PI3K–Akt pathway in IHH cells (Figure 2b). Wortmannin or Akt1/2 did not abolish the inhibitory effect of insulin on ANGPTL3 secretion suggesting that secretion of ANGPTL3 is not down-regulated via PI3K or Akt (Figures 2c and 2d).
Figure 2. The effect of PI3K Wortmannin, Akt-specific inhibitor Akt1/2 and PPARγ antagonist GW9662 on VLDL and ANGPTL3 secretion.
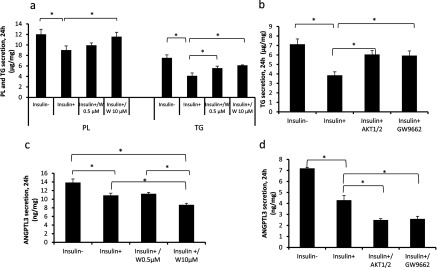
Wild-type IHH-cells were incubated in 5.5 mM glucose and 100 nM insulin (−/+), Wortmannin (0.5 μM,10 μM), Akt1/2 inhibitor (20 μM) or PPARγ antagonist GW9662 (10 μM) for 24 h. (a) Secretion of TAG and PL (μg/mg). (b) Secretion of TAG (μg/mg). (c) Secretion of ANGPTL3 (ng/mg). (d) Secretion of ANGPTL3 (ng/mg).
Since ANGPTL3 protein secretion is regulated by insulin, we investigated whether nuclear PPARs (peroxisome proliferator-activated receptors) α and γ and their agonists fenofibrate, used to treat high plasma TAG and cholesterol levels, and rosiglitazone, an insulin sensitizer, would affect the secretion of ANGPTL3. We demonstrate that PPARγ agonist rosiglitazone, in a dose-dependent manner, down-regulated mRNA expression (Figure 3a) and the secretion of ANGPTL3 (Figure 3b). PPARα agonist fenofibrate did not affect the secretion of ANGPTL3 (results not shown). Rosiglitazone, much like insulin, reduced the secretion of TAG in a dose-dependent manner (Figure 3c). PPARγ antagonist (GW9662) abolished the inhibitory effect of insulin on TAG secretion (Figure 2b). These data demonstrate that rosiglitazone mimics insulin action in cultured human hepatocytes by decreasing the secretion of TAG and ANGPTL3 in a dose-dependent manner. These data also support the hypothesis that insulin may down-regulate VLDL secretion via PPARγ. However, PPARγ-antagonist (GW9662) did not abolish the inhibitory effect of insulin on ANGPTL3 secretion (Figure 2d) indicating that insulin and PPARγ down-regulate the expression of ANGPTL3 via different pathways.
Figure 3. The effect of rosiglitazone on VLDL and ANGPTL3 secretion.
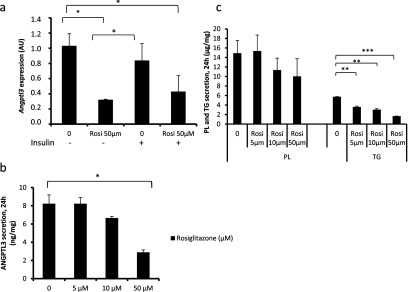
Wild-type IHH-cells were incubated in 5.5 mM glucose and 100 nM insulin (+/−) for 24 h following RNA extraction from cell lysates. Secretion of ANGPTL3 PL and TAG was measured in the presence of rosiglitazone. (a) Expression of ANGPTL3 (AU, arbitary unit). (b) Secretion of ANGPTL3 (ng/mg). (c) Secretion of TAG and PL (μg/mg).
To investigate ANGPTL3-specific functions in hepatocytes, we silenced ANGPTL3-gene by transducing wild-type IHH cells with a lentiviral vector carrying ANGPTL3-targeting shRNA-fragment. Control cells were transduced with non-targeting shRNA. We achieved 85% silencing in ANGPTL3 expression (Figure 4a) and a 90–95% decrease in ANGPTL3 protein secretion (Figure 4b). We then measured the concentration of TAG, PL and apoB-100 from the cell culture media after 24 h incubation. Surprisingly the secretion of apoB-100, PL and TAG were all increased in ANGPTL3-silenced cells compared with control cells under non-insulin conditions (−) (Figures 5a–5c). Also a short-term secretion rate of apoB-100 was higher in ANGPTL3-silenced cells under non-insulin conditions (Figure 5d). Insulin stimulation (+) reduced the secretion of TAG and PL in silenced cells (Figures 5a–5b) near to the level of TAG secreted by the control cells with a smaller decrease in secreted apoB-100 levels (Figure 5c). We calculated a TAG/apoB-100 ratio for both control and silenced cells and concluded that the ratio was more dramatically reduced in ANGPTL3-silenced cells [47%, from 60 to 32 (mass/mass)] compared with control cells [16%, from 45 to 38 (mass/mass)] during insulin stimulation (+) (Figure 5e). These findings suggest that the silencing of ANGPTL3 results in a more pronounced shift from the secretion of large TAG-enriched VLDL1-type particles into small lipid-poor VLDL2-type particles during insulin stimulation. These data are in line with plasma measures from ANGPTL3-deficient subjects who had a 4.3-fold reduction in the TAG/apoB-100 ratio in VLDL when compared with non-carriers [14] and suggests that ANGPTL3-silenced cells display enhanced insulin sensitivity as VLDL1 particles dominate the VLDL profile in insulin-resistant states.
Figure 4. Silencing of ANGPTL3.
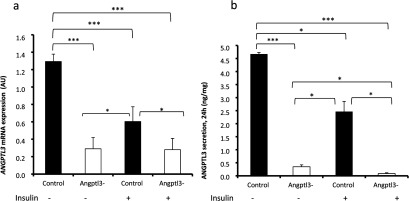
IHH-cells were transduced with ANGPTL3-targeting or non-targeting shRNA (control). Cells were incubated in 5.5 mM glucose with 100 nM insulin (+/−) for 24 h. RNA was extracted from cell lysates. (a) Expression of ANGPTL3 (AU, arbitrary unit). (b) Secretion of ANGPTL3 (ng/mg).
Figure 5. Secretion of VLDL from ANGPTL3-silenced and control IHH cells.
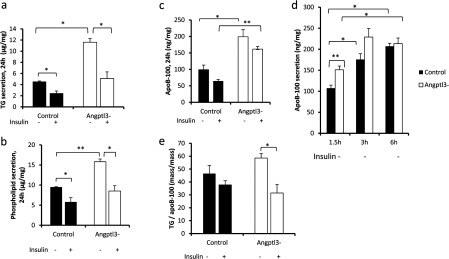
ANGPTL3-silenced and control cells were incubated in media containing 5.5 mM glucose and 100 nM insulin (+/−). (a–c) Secretion of TAG, PL (μg/mg) and apoB-100 (ng/mg). (d) Secretion of short-term apoB-100 (ng/mg). (e) The amount of TAG (ng) for each ng of apoB-100.
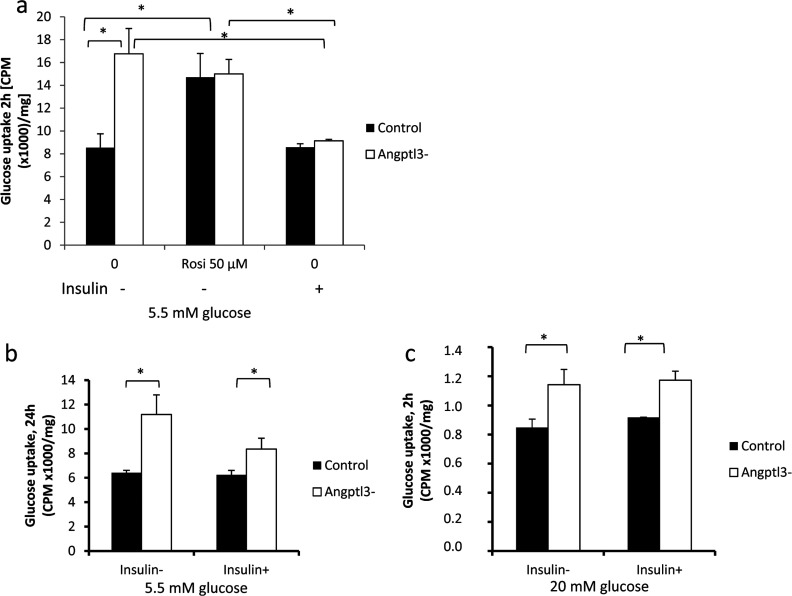
We then measured glucose uptake in control and silenced cells by utilizing a non-hydrolysable radiotracer, [3H]-labelled deoxy-D-glucose. Basal glucose uptake without insulin stimulus was increased by 50% in ANGPTL3 silenced cells compared with control cells after 2 h (Figure 6a) and after 24 h (Figure 6b). Treatment with rosiglitazone improved hepatocyte glucose uptake by 40% in control cells (Figure 6a). Acute insulin stimulus in the presence of 5.5 mM glucose did not increase hepatocyte glucose uptake in control cells and significantly decreased glucose uptake in ANGPTL3-silenced cells after 2 h (Figure 6a) and 24 h (Figure 6b). When the glucose concentration was increased up to 20 mM the inhibitory effect of insulin on glucose uptake was abolished (Figure 6c). We conclude that the silencing of ANGPTL3 results in increased glucose uptake comparable with rosiglitazone treatment in the absence of insulin stimulus and that insulin inhibits hepatocyte-specific glucose uptake in the presence of low glucose concentrations but not under high glucose concentration.
Figure 6. Hepatic glucose uptake in ANGPTL3-silenced and control cells.
ANGPTL3-silenced and control cells were incubated in the presence of [3H] labelled deoxy-D-glucose. Radioactivity (CPM) was measured from the cell lysates. (a) Hepatic glucose uptake measured after 2 h incubation period in media containing 5.5 mM glucose (CPM/mg). Cells were incubated with 50 μM rosiglitazone or 100 nM insulin (+/−). (b) Hepatic glucose uptake measured after 24 h incubation period in media containing 5.5 mM glucose and 100 nM insulin (+/−) (CPM/mg). (c) Hepatic glucose uptake measured after 2 h incubation period in media containing 20 mM glucose and 100 nM insulin (+/−) (CPM/mg).
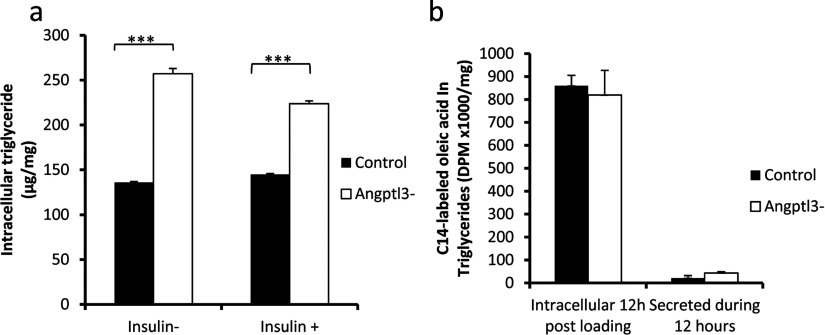
We reasoned that the increased glucose uptake in ANGPTL3-silenced hepatocytes would cause elevation in glycogen and intracellular TAG levels and measured the intracellular TAG levels after 24 h incubation period. We observed elevated intracellular TAG levels in ANGPTL3-silenced cells as compared with control cells (Figure 7a). To test whether silencing of ANGPTL3 affected hepatic fatty acid uptake we incubated control and ANGPTL3-silenced cells in media containing [14C]-labelled oleic acid bound to 0.5% BSA for 12 h and measured the uptake of fatty acids and its conversion into intracellular TAG. There were no differences in fatty acid incorporation in intracellular TAG and the secretion rates of TAG between control and ANGPTL3-silenced cells (Figure 7b).
Figure 7. Intracellular TAG and fatty acid uptake in ANGPTL3-silenced and control cells.
(a) Intracellular TAGs were measured in ANGPTL3-silenced and control cell lysates after 24 h incubation with 5.5 mM glucose and 100 nM insulin (+/−). (b) ANGPTL3-silenced and control cells were incubated in media containing 5.5 mM glucose, 0.375 mM oleic acid complexed with 0.5% BSA and C14− labelled oleic acid for 12 h. Radioactivity (DPM) in intracellular TAG and secreted TAG was measured (DPM/mg).
Finally we investigated whether silencing of ANGPTL3 changes the expression of insulin responsive genes. QPCR demonstrated that there were no significant changes in gene expression levels of ANGPTL8 or ANGPTL4 between control and silenced cells (Figure 8a). Insulin receptor IRS-2, GLUT2 and PI3K (regulatory subunit p85) show a moderate (but non-significant) up-regulation in ANGPTL3-silenced cells in non-insulin conditions without any differences in IRS-1 or PI3K inhibitor PTEN (phosphatase and tensin homologue deleted on chromosome 10) (Figure 8b). Expression of PGC1α (peroxisome proliferator-activated receptor γ co-activator 1-α) and its downstream targets PEPCK (phosphoenolpyruvate carboxy-kinase) and TRB-3 (tribbles homologue 3) were significantly down-regulated in ANGPTL3-silenced cells, whereas expression of PPARα was significantly up-regulated in ANGPTL3-silenced cells (Figure 8c). The mRNA levels of PPARγ displayed a moderate down-regulation in ANGPTL3-silenced cells upon insulin stimulation (Figure 8c). The expression of TGH (TAG hydrolase) and DGAT2 (diacylgylcerol acyltransferase 2) involved in VLDL assembly were up-regulated in ANGPTL3-silenced cells during insulin stimulus while the expression of inflammation marker TNFα (tumour necrosis factor α) was significantly down-regulated in the silenced cells (Figure 8d). There was no significant difference in IL-6 (interleukin-6) or HNF4α (hepatocyte nuclear factor α) expression (Figure 8d). These data suggest a down-regulation of gluconeogenic genes and an increase in genes promoting TAG synthesis in ANGPTL3-silenced cells as compared with control.
Figure 8. Gene expression patterns in ANGPTL3-silenced and control IHH cells during non-insulin condition (Ins-) and insulin stimulation (Ins+).
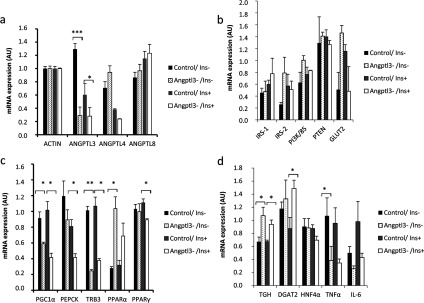
(a–d) ANGPTL3-silenced cells and control cells were incubated with 5.5 mM glucose and 100 nM insulin (+/−) for 24 h followed by total RNA extraction from cell lysates, cDNA synthesis and q-PCR (AU, arbitrary unit).
DISCUSSION
Since ANGPTL3 is primarily expressed in the liver [3,4] and affects TAG clearance [5–9] and possibly fatty acid release from adipose tissue [25] it is unclear how extracellular signals affect ANGPTL3 secretion rates and how silencing of ANGPTL3 would affect glucose and lipid metabolism in the liver. We show that activation of PPARγ by its agonist rosiglitazone, much like insulin, decreased the expression and secretion of ANGPTL3 accompanied by reduced secretion of TAG. The association of rosiglitazone and reduced VLDL–TAG secretion has been established in vivo [26,27]. Down-regulation of ANGPTL3 gene expression upon rosiglitazone treatment can also be found in microarray data of primary human hepatocytes [28] and is verified by our data including decreased dose-dependent protein secretion. The effect of insulin on ANGPTL3 expression and secretion was shown in HepG2 cells [29] and also verified by our data. These findings indicate that ANGPTL3 is a target gene for insulin and PPARγ action and demonstrate that stimulation of PPARγ by rosiglitazone, mediates or mimics insulin action with a concomitant reduction in ANGPTL3 and VLDL secretion.
Insulin stimulation reduces the secretion of TAG-enriched VLDL1-type particles in the liver without affecting the basal secretion of the lipid-poor VLDL2-type particles [16]. This balance is disrupted in IR leading to increased secretion of VLDL1 and increased plasma lipid levels [16,17]. Interestingly a loss of function mutation in the ANGPTL3-gene results in decreased levels of all lipoprotein types in plasma [14] but whether the hypolipidaemic phenotype is caused by decreased hepatic VLDL secretion or due to enhanced TAG clearance in the peripheral tissue (or both) has not been verified clearly. The work by Musunuru et al. [13] suggested lower apoB-100 production rate in homozygous human carriers of ANGPTL3 loss of function mutations. Shimizugawa et al. [9] and Ando et al. [30] suggest that the low plasma lipid levels (especially TAGs) observed in ANGPTL3-deficient mice were likely caused by increased VLDL–TAG clearance via induced LPL activity rather than decreased VLDL secretion.
We did not detect decreased apoB-secretion in ANGPTL3-silenced cells compared with control; however, silencing of ANGPTL3 caused a major decrease in secreted TAG during insulin stimulation with a more subtle effect on apoB-100 secretion. This observation is in line with the analysis on VLDL particles isolated from plasma of ANGPTL3-deficient subjects showing a dramatic reduction in VLDL particle size and a 4.3-fold reduction in the TAG/apoB-100 ratio [14]. We demonstrate that a shift from the secretion of TAG-enriched VLDL1-type particles into the secretion of TAG poor VLDL2-type particles under insulin stimulus was more pronounced in ANGPTL3 silenced cells compared with control cells suggesting elevated insulin sensitivity. Higher insulin sensitivity in homozygous ANGPTL3 loss-of-function carriers was demonstrated previously by Robciuc et al. [14] thus supporting our cell data. Additionally Inukai et al. [31] showed elevated ANGPTL3 expression in mice in both insulin-deficient and -resistant diabetic states. Therefore down-regulation of ANGPTL3 might have a protective role against IR.
Liver uptake accounts for about 30% disposal of plasma glucose [32]. Silencing of ANGPTL3 resulted in 20–50% increase in glucose uptake compared with control cells and probably explaining glucose conversion into intracellular TAGs and their increased secretion in ANGPTL3-silenced cells under insulin-depleted conditions. Human data do not support an increased prevalence of hepatic steatosis in ANGPTL3-deficient subjects [15,33] suggesting that fluctuations in the availability of substrates, plasma glucose and insulin concentrations in vivo as well as uptake of glucose and fatty acids by other tissues regulate hepatic glucose uptake and VLDL secretion and thereby preventing hepatic TAG accumulation in ANGPTL3-deficient individuals.
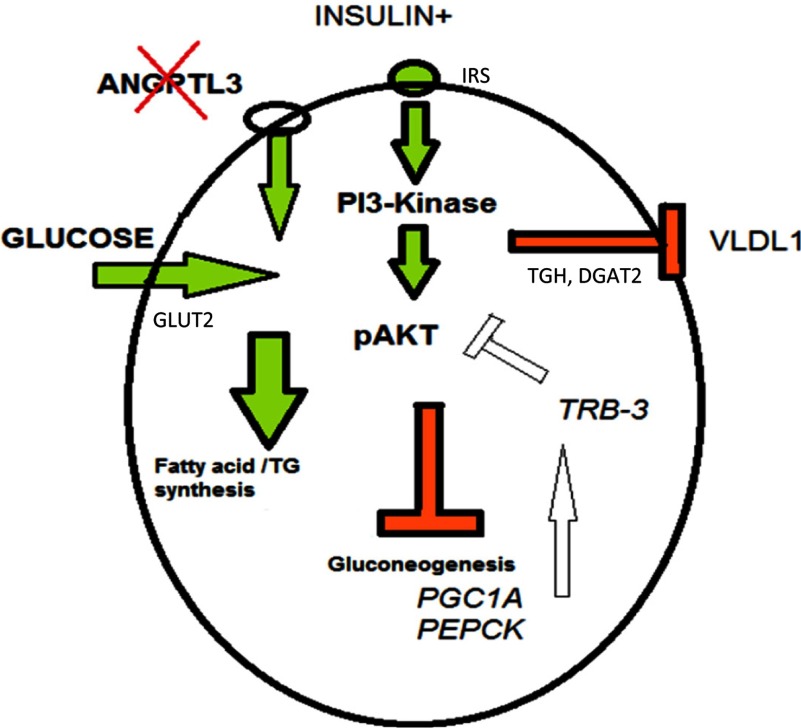
Our data on VLDL secretion and glucose uptake suggests that both insulin and PPARγ might mediate some of their functions via affecting the expression of ANGPTL3. Activation of PPARγ decreases the transcription of insulin-responsive genes involved in the control of glucose production (gluconeogenesis) [34] and PPARγ can directly activate GLUT2 transporter thus influencing hepatic glucose uptake [35] which can explain the increased short-term glucose uptake levels upon rosiglitazone treatment in the present study. We show that silencing of ANGPTL3 resulted in significant down-regulation of transcription factor PGC1α expression and its downstream targets PEPCK and TRB3 (tribbles homologue 3), a protein kinase acting as a negative inhibitor for Akt phosphorylation [36,37]. Elevation of PGC1α expression in liver has been linked to IR in several mouse and human studies [37–39] thus the down-regulation of PGC1α and its downstream targets as observed in ANGPTL3-silenced cells suggest decreased levels of gluconeogenesis and fatty acid oxidation, processes which display aberrant activation in insulin resistant states (See Figure 9 as a summary for the pathways).
Figure 9. Hypothetic model for ANGPTL3 function in a hepatocyte.
Silencing of ANGPTL3 enhances GLUT2-mediated glucose uptake, increases TAG synthesis and induces down-regulation of PGC1α and its downstream targets, including TRB3, hypothetically leading to enhanced Akt phosphorylation levels. Secretion of TAG-enriched VLDL-1-type particles is decreased upon insulin stimulus.
Insulin induces glucose uptake in insulin-responsive tissues (muscle, white adipose tissue) via GLUT4 but is not required for GLUT2-mediated glucose uptake by the liver [39]. In fact, insulin dose dependently decreases the expression of hepatocyte GLUT2 during the first 6–12 h of insulin stimulation in vitro and in vivo. The inhibitory effect of insulin on GLUT2 expression is abolished after 24 h despite continuous insulin stimulation and can also be reversed by high (10–20 mM) glucose concentration [21]. Therefore hepatic glucose uptake is driven by extracellular glucose concentration and not directly due to the elevated insulin levels (in other words GLUT2 is not insulin dependent). Increased glucose uptake in ANGPTL3-silenced cells might be due to higher production rate of GLUT2 and/or increased plasma membrane translocation and activation of GLUT2. In conclusion, our results give insights into the mechanisms of ANGPTL3-silencing in hepatic lipid and glucose metabolism. Since humans with ANGPTL3-deficiency display hypolipidaemia and insulin sensitivity it might be beneficial to target ANGPTL3 in the liver, the major site of ANGPTL3 expression, to balance lipid and glucose homoeostasis and lower the risk of cardiometabolic disorders.
ACKNOWLEDGEMENTS
We thank Sari Nuutinen from the National Institute for Health and Welfare (Helsinki, Finland) for her technical assistance and Marius Robciuc from the National Institute for Health and Welfare for his previous work with lentiviral shRNA particles and ANGPTL3-protein.
AUTHOR CONTRIBUTION
Matti Jauhiainen and Christian Ehnholm supervised the study. Jarkko Soronen participated in study design and scientific discussion of the data. Pirkka-Pekka Laurila contributed to the scientific discussion of the data. Jari Metso contributed to the biochemical analysis of the experiments.
FUNDING
The study was financially supported by the Academy of Finland [grant number 257545], Finnish Foundation for Cardiovascular Research, Jenny and Antti Wihuri Foundation, Finska Läkaresällskapet, Magnus Ehrnrooth foundation and Novo Nordisk Fonden.
References
- 1.Davis S., Papadopoulos N., Aldrich T. H., Maisonpierre P. C., Huang T., Kovac L., Xu A., Leidich R., Radziejewska E., Rafique A., et al. Angiopoietins have distinct modular domains essential for receptor binding, dimerization and superclustering. Nat. Struct. Biol. 2003;10:38–44. doi: 10.1038/nsb880. [DOI] [PubMed] [Google Scholar]
- 2.Ono M., Shimizugawa T., Shimamura M., Yoshida K., Noji-Sakikawa C., Ando Y., Koishi R., Furukawa H. Protein region important for regulation of lipid metabolism in angiopoietin-like 3 (ANGPTL3): ANGPTL3 is cleaved and activated in vivo. J. Biol. Chem. 2003;278:41804–41809. doi: 10.1074/jbc.M302861200. [DOI] [PubMed] [Google Scholar]
- 3.Conklin D., Gilbertson D., Taft D. W., Maurer M. F., Whitmore T. E., Smith D. L., Walker K. M., Chen L. H., Wattler S., Nehls M., Lewis K. B. Identification of a mammalian angiopoietin-related protein expressed specifically in liver. Genomics. 1999;62:477–482. doi: 10.1006/geno.1999.6041. [DOI] [PubMed] [Google Scholar]
- 4.Romeo S., Yin W., Kozlitina J., Pennacchio L. A., Boerwinkle E., Hobbs H. H., Cohen J. C. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J. Clin. Invest. 2009;119:70–79. doi: 10.1172/JCI37118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J., Afroza H., Rader D. J., Jin W. Angiopoietin-like protein 3 inhibits lipoprotein lipase activity through enhancing its cleavage by proprotein convertases. J. Biol. Chem. 2010;285:27561–27570. doi: 10.1074/jbc.M110.144279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee E. C., Desai U., Gololobov G., Hong S., Feng X., Yu X. C., Gay J., Wilganowski N., Gao C., Du L. L., et al. Identification of a new functional domain in angiopoietin-like 3 (ANGPTL3) and angiopoietin-like 4 (ANGPTL4) involved in binding and inhibition of lipoprotein lipase (LPL) J. Biol. Chem. 2009;284:13735–13745. doi: 10.1074/jbc.M807899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shan L., Yu X. C., Liu Z., Hu Y., Sturgis L. T., Miranda M. L., Liu Q. The angiopoietin-like proteins ANGPTL3 and ANGPTL4 inhibit lipoprotein lipase activity through distinct mechanisms. J. Biol. Chem. 2009;284:1419–1424. doi: 10.1074/jbc.M808477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koishi R., Ando Y., Ono M., Shimamura M., Yasumo H., Fujiwara T., Horikoshi H., Furukawa H. Angptl3 regulates lipid metabolism in mice. Nat. Genet. 2002;30:151–157. doi: 10.1038/ng814. [DOI] [PubMed] [Google Scholar]
- 9.Shimizugawa T., Ono M., Shimamura M., Yoshida K., Ando Y., Koishi R., Ueda K., Inaba T., Minekura H., Kohama T., Furukawa H. ANGPTL3 decreases very low density lipoprotein triglyceride clearance by inhibition of lipoprotein lipase. J. Biol. Chem. 2002;277:33742–33748. doi: 10.1074/jbc.M203215200. [DOI] [PubMed] [Google Scholar]
- 10.Kathiresan S., Melander O., Guiducci C., Surti A., Burtt N. P., Rieder M. J., Cooper G. M., Roos C., Voight B. F., Havulinna A. S., et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat. Genet. 2008;40:189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teslovich T. M., Musunuru K., Smith A. V., Edmondson A. C., Stylianou I. M., Koseki M., Pirruccello J. P., Ripatti S., Chasman D. I., Willer C. J., et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willer C. J., Sanna S., Jackson A. U., Scuteri A., Bonnycastle L. L., Clarke R., Heath S. C., Timpson N. J., Najjar S. S., Stringham H. M., et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat. Genet. 2008;40:161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musunuru K., Pirruccello J. P., Do R., Peloso G. M., Guiducci C., Sougnez C., Garimella K. V., Fisher S., Abreu J., Barry A. J., et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N. Engl. J. Med. 2010;363:2220–2227. doi: 10.1056/NEJMoa1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robciuc M. R., Maranghi M., Lahikainen A., Rader D., Bensadoun A., Öörni K., Metso J., Minicocci I., Ciociola E., Ceci F., et al. ANGPTL3 deficiency is associated with increased insulin sensitivity, lipoprotein lipase activity, and decreased serum free fatty acids. Arterioscler. Thromb. Vasc. Biol. 2013;33:1706–1713. doi: 10.1161/ATVBAHA.113.301397. [DOI] [PubMed] [Google Scholar]
- 15.Minicocci I., Montali A., Robciuc M. R., Quagliarini F., Censi V., Labbadia G. Mutations in the ANGPTL3 gene and familial combined hypolipidemia: a clinical and biochemical characterization. J. Clin. Endocrinol. Metab. 2012;97:E1266–E1275. doi: 10.1210/jc.2012-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malmström R., Packard C. J., Caslake M., Bedford D., Stewart P., Yki-Järvinen H., Shepherd J., Taskinen M. R. Defective regulation of triglyceride metabolism by insulin in the liver in NIDDM. Diabetologia. 1997;40:454–462. doi: 10.1007/s001250050700. [DOI] [PubMed] [Google Scholar]
- 17.Garvey W. T., Kwon S., Zheng D., Shaughnessy S., Wallace P., Hutto A. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52:453–462. doi: 10.2337/diabetes.52.2.453. [DOI] [PubMed] [Google Scholar]
- 18.Wu Z., Jiao P., Huang X., Feng B., Feng Y., Yang S., Hwang P., Du J., Nie Y., Xiao G., Xu H. MAPK phosphatase-3 promotes hepatic gluconeogenesis through dephosphorylation of forkhead box O1 in mice. J. Clin. Invest. 2010;120:3901–3911. doi: 10.1172/JCI43250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu C., Okar D. A., Kang J., Lange A. J. Reduction of hepatic glucose production as a therapeutic target in the treatment of diabetes. Curr. Drug Targets Immune Endocr. Metabol Disord. 2005;5:51–59. doi: 10.2174/1568008053174769. [DOI] [PubMed] [Google Scholar]
- 20.Chirieac D. V., Davidson N. O., Sparks C. E., Sparks J. D. PI3-kinase activity modulates apo B available for hepatic VLDL production in apobec-1−/− mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G382–G388. doi: 10.1152/ajpgi.00472.2005. [DOI] [PubMed] [Google Scholar]
- 21.Postic C., Burcelin R., Rencurel F., Pegorier J. P., Loizeau M., Girard J., Leturque A. Evidence for a transient inhibitory effect of insulin on GLUT2 expression in the liver: studies in vivo and in vitro. Biochem. J. 1993;293:119–124. doi: 10.1042/bj2930119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robciuc M. R., Tahvanainen E., Jauhiainen M., Ehnholm C. Quantitation of serum angiopoietin-like proteins 3 and 4 in a Finnish population sample. J. Lipid Res. 2010;51:824–831. doi: 10.1194/jlr.M002618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adiels M., Westerbacka J., Soro-Paavonen A., Häkkinen A. M., Vehkavaara S., Caslake M. J., Packard C., Olofsson S. O., Yki-Järvinen H., Taskinen M. R., Borén J. Acute suppression of VLDL1 secretion rate by insulin is associated with hepatic fat content and insulin resistance. Diabetologia. 2007;50:2356–2365. doi: 10.1007/s00125-007-0790-1. [DOI] [PubMed] [Google Scholar]
- 24.Rother K. I., Imai Y., Caruso M., Beguinot F., Formisano P., Accili D. Evidence that IRS-2 phosphorylation is required for insulin action in hepatocytes. J. Biol. Chem. 1998;273:17491–17497. doi: 10.1074/jbc.273.28.17491. [DOI] [PubMed] [Google Scholar]
- 25.Shimamura M, Matsuda M., Kobayashi S., Ando Y., Ono M., Koishi R., Furukawa H., Makishima M., Shimomura I. Angiopoietin-like protein 3, a hepatic secretory factor, activates lipolysis in adipocytes. Biochem. Biophys. Res. Commun. 2003;301:604–609. doi: 10.1016/S0006-291X(02)03058-9. [DOI] [PubMed] [Google Scholar]
- 26.Carpentier A., Taghibiglou C., Leung N., Szeto L., Van Iderstine S. C., Uffelman K. D., Buckingham R., Adeli K., Lewis G. F. Ameliorated hepatic insulin resistance is associated with normalization of microsomal triglyceride transfer protein expression and reduction in very low density lipoprotein assembly and secretion in the fructose-fed hamster. J. Biol. Chem. 2002;277:28795–28802. doi: 10.1074/jbc.M204568200. [DOI] [PubMed] [Google Scholar]
- 27.Brackenridge A. L., Jackson N., Jefferson W., Stolinski M., Shojaee-Moradie F., Hovorka R., Umpleby A. M., Russell-Jones D. Effects of rosiglitazone and pioglitazone on lipoprotein metabolism in patients with Type 2 diabetes and normal lipids. Diabet. Med. 2009;26:532–539. doi: 10.1111/j.1464-5491.2009.02729.x. [DOI] [PubMed] [Google Scholar]
- 28.Rogue A., Renaud M. P., Claude N., Guillouzo A., Spire C. Comparative gene expression profiles induced by PPARγ and PPARα/γ agonists in rat hepatocytes. Toxicol. Appl. Pharmacol. 2011;254:18–31. doi: 10.1016/j.taap.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Shimamura M., Matsuda M., Ando Y., Koishi R., Yasumo H., Furukawa H., Shimomura I. Leptin and insulin down-regulate angiopoietin-like protein 3,a plasma triglyceride-increasing factor. Biochem. Biophys. Res. Commun. 2004;322:1080–1085. doi: 10.1016/j.bbrc.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 30.Ando Y., Shimizugawa T., Takeshita S., Ono M., Shimamura M., Koishi R., Furukawa H. Decreased expression of angiopoietin-like 3 is protective against atherosclerosis in apoE-deficient mice. J. Lipid. Res. 2003;44:1216–1223. doi: 10.1194/jlr.M300031-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Inukai K., Nakashima Y., Watanabe M., Kurihara S., Awata T., Katagiri H., Oka Y., Katayama S. ANGPTL3 is increased in both insulin-deficient and -resistant diabetic states. Biochem. Biophys. Res. Commun. 2004;317:1075–1079. doi: 10.1016/j.bbrc.2004.03.151. [DOI] [PubMed] [Google Scholar]
- 32.Moore M. C., Coate K. C., Winnick J. J., An Z., Cherrington A. D. Regulation of hepatic glucose uptake and storage in vivo. Adv. Nutr. 2012;3:286–294. doi: 10.3945/an.112.002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minicocci I., Santini S., Cantisani V., Stitziel N., Kathiresan S., Arroyo J. A., Martí G., Pisciotta L., Noto D., Cefalù A. B., et al. Clinical characteristics and plasma lipids in subjects with familial combined hypolipidemia: a pooled analysis. J. Lipid Res. 2013;54:3481–3490. doi: 10.1194/jlr.P039875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Way J. M., Harrington W. W., Brown K. K., Gottschalk W. K., Sundseth S. S., Mansfield T. A., Ramachandran R. K., Willson T. M., Kliewer S. A. Comprehensive messenger ribonucleic acid profiling reveals that peroxisome proliferator-activated receptor gamma activation has coordinate effects on gene expression in multiple insulin-sensitive tissues. Endocrinology. 2001;142:1269–1277. doi: 10.1210/endo.142.3.8037. [DOI] [PubMed] [Google Scholar]
- 35.Kim H. I., Ahn Y. H. Role of peroxisome proliferator-activated receptor-gamma in the glucose-sensing apparatus of liver and beta-cells. Diabetes. 2004;53:S60–S65. doi: 10.2337/diabetes.53.2007.S60. [DOI] [PubMed] [Google Scholar]
- 36.Yoon J. C., Puigserver P., Chen G., Donovan J., Wu Z., Rhee J., Adelmant G., Stafford J., Kahn C. R., Granner D. K., et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 37.Koo S. H., Satoh H., Herzig S., Lee C. H., Hedrick S., Kulkarni R., Evans R. M., Olefsky J., Montminy M. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat. Med. 2004;10:530–534. doi: 10.1038/nm1044. [DOI] [PubMed] [Google Scholar]
- 38.Finck B. N., Kelly D. P. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J. Clin. Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olson A. L., Pessin J. E. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu. Rev. Nutr. 1996;16:235–256. doi: 10.1146/annurev.nu.16.070196.001315. [DOI] [PubMed] [Google Scholar]