Abstract
Purpose
To compare PET-CT to conventional imaging (CI) in staging pediatric rhabdomyosarcoma (RMS).
Subjects and Methods
Thirty subjects with RMS, median age 7.3 years, underwent PET-CT before therapy. PET-CTs and CI were independently reviewed by two radiologists and two nuclear medicine physician to determine the presence of nodal, pulmonary, bone, bone marrow and other sites of metastasis. Accuracy, sensitivity and specificity of PET-CT for detecting metastases was compared to CI using biopsy and clinical follow-up as reference standards. Maximum standardized uptake values (SUVmax) of primary tumors, lymph nodes and pulmonary nodules were measured.
Results
Primary tumors had an average SUVmax of 7.2 (range, 2.5-19.2). Accuracy rates for 17 subjects with nodal disease were 95% for PET-CT and 49% for CI. PET-CT had 94% sensitivity and 100% specificity for nodal disease. Of 7 pulmonary nodules detected by CI, 3 were not identified by PET-CT, 2 were indeterminate by PET-CT, and 1 was malignant with a SUVmax (3.4) > twice that of benign nodules. Two subjects had bone disease; both were identified by PET-CT but only 1 by CI. Four subjects had bone marrow disease, 2 had positive PET-CTs but none had positive CI. Two subjects had soft tissue metastases detected by PET-CT but not CI.
Conclusion
PET-CT performed better than CI in identifying nodal, bone, bone marrow, and soft tissue disease in children with RMS. CI remains essential for detection of pulmonary nodules. We recommend PET-CT for routine staging of children with RMS. CI with Tc99m bone scan can be eliminated.
Introduction
Rhabdomyosarcoma (RMS) is the most common pediatric sarcoma with approximately 350 new cases in the United States each year.1,2 At least 15% of patients present with distant metastases and the most common involved sites are lung (47%), bone marrow (38%), bone (34%) and distant lymph nodes (26%).3,4 It is imperative to identify all metastatic sites since cure depends on adequate local control of metastases.
The accepted workup of patients with RMS includes clinical examination, magnetic resonance imaging (MRI) or CT of the primary tumor and local-regional nodal basin, computed tomography (CT) of the lungs, 99mTechnetium methylene diphosphonate bone scintigraphy (99mTc MDP bone scan) and bilateral bone marrow aspirates and biopsies.5 Radiolabeled 18F-fluorodeoxyglucose positron emission tomography-computed tomography (FDG PET-CT) offers a method of assessing both structural information and metabolic activity, thus potentially offering advantages not afforded with conventional imaging (CI). Over the past decade, PET-CT has been used to evaluate RMS and other sarcomas in adults and children.6-16 However, the role of PET-CT in staging pediatric RMS has not been clearly established. The purpose of our study was to compare PET-CT to conventional evaluations for staging pediatric RMS in order to determine the relative value of PET-CT and to determine whether PET-CT could replace CI modalities.
Subjects and Methods
Subjects
This retrospective study was approved by our institutional review board. Eligible subjects were patients with newly diagnosed RMS between February 2003 and September 2009 who underwent CI (CT of the chest, CT or MRI of the primary site and local-regional nodal basin and 99m Tc MDP bone scan) and PET-CT prior to initiation of systemic therapy.
PET-CT scanning parameters and image review
All PET-CTs were performed at our institution on a Discovery LightSpeed PET-CT scanner (GE Healthcare, Milwaukee, WI). Our standard practice is to scan from the skull vertex to the toes. The CT was performed with milliamperes/second (mAs) adjusted for body weight (maximum 90 mAs), 120 kilovoltage peak (kVp), 5 mm slice thickness and without intravenous (IV) or oral contrast material. Subjects were instructed to fast for 4 hours before receiving an injection of 0.15 mCi/kg 18F-FDG (55 MBq/kg) approximately 60 minutes before imaging. Emission images were acquired in 2D mode for 5 minutes per bed position. Images were reconstructed in axial, coronal, and sagittal planes and reviewed at a Hermes workstation (Hermes Medical Solutions, Stockholm, Sweden). All PET-CTs were reviewed independently by one pediatric radiologist (GM) with 25 years nuclear medicine experience or two pediatric nuclear medicine physicians with 25 (BS) and 5 years experience, who were blinded to biopsy results and results of CI. Reviewers measured the maximum standardized uptake value (SUVmax) of the primary tumor, subjectively assessed the FDG avidity of the primary tumor and determined the presence of nodal, pulmonary, bone, bone marrow or other sites of metastatic disease. PET-CT findings were considered positive for malignancy if FDG avidity was greater than normal adjacent background tissue without a known physiologic explanation (such as brown fat or benign fibro-osseus defect).17 Lesions were considered indeterminate if the reviewer could not confidently classify them as benign or malignant. The SUVmax was obtained for all pulmonary nodules that could be identified on PET-CT and for the largest lymph node within nodal basins when nodes were identified as target lesions on CI.
Conventional imaging review
All CI was retrospectively reviewed by one pediatric radiologist (MBM) with 15 years of experience, who was blinded to results of PET-CT and biopsies. The original Response Evaluation Criteria in Solid Tumors (RECIST) were used for this study because they were used clinically during the study period.18 Lesions identified on CI, including lymph nodes measuring ≥ 1.0 cm in greatest diameter, were assessed and determined to be malignant, benign or indeterminate based on the reviewer's experience. The reviewer recorded the location and size of pulmonary nodules and enlarged lymph nodes, the sites of other suspected solid organ, soft-tissue and bone metastases and the presence or absence of bone marrow disease.
CT scanning parameters
Subjects were scanned with a GE LightSpeed Ultra helical eight-row detector CT scanner (GE Medical Systems, Milwaukee, WI) or a GE Lightspeed VCT 64-row detector scanner. Using a 1.35:1 pitch subjects weighing ≤ 14.4 kg were scanned with 3.75 mm slice thickness and those > 14.4 kg with 5 mm slice thickness. All patients were scanned using 120 kVp and the auto-mAs function, presetting the noise level to 5. Patients able to follow breath-holding instructions (generally ≥ 6 years old) were scanned during suspended inspiration. Iodinated oral and IV contrast materials were administered for abdominal and pelvic scans and IV contrast for chest scans as indicated.
Magnetic resonance imaging parameters
MRI was performed on a Siemens Symphony or Avanto 1.5T scanner or a Siemens Trio 3T scanner (Siemens Medical Solutions, Malvern, PA) using an extremity or phased array body coil as appropriate. Our standard practice is to obtain T1W and short tau-inversion recovery coronal and T2W axial images of the primary tumor and local-regional nodal basin before administration of contrast media. Fat-suppressed T1W axial and coronal images of the primary tumor were obtained following an IV bolus injection of 2 mL/kg gadolinium contrast agent (maximum 20 mL).
Nuclear bone scan parameters
Two hours after IV injection of 12 mCi/m2 (maximum, 20 mCi) of 99mTc MDP, skeletal scintingraphy was performed with a dual headed Siemens Multispec 2 (Chicago, IL), GE Infinia Hawkeye (Milwaukee, WI), or Siemens Ecam Duet gamma camera (Hoffman Estates, IL). Whole body planar images were obtained in the anterior-posterior projection allowing one minute of signal acquisition per 12 cm. body length. Images of 500,000 counts were obtained of the ribs in the anterior-posterior and both oblique projections and the skull in anterior-posterior and lateral projections. Additional images of areas of interest were obtained at the discretion of the interpreting physician.
Bone marrow examination and metastatic site biopsy
All bone marrow aspirates and biopsies were obtained by an institutional pediatric oncologist and evaluated by an institutional pathologist. Other biopsies were performed by the interventional radiology or surgical services as clinically appropriate and reviewed by an institutional pathologist.
Clinical assessment
One study investigator (SS) retrospectively assessed whether metastatic disease was present at suspicious sites identified on CI that were not biopsied. This assessment was based on the primary physician's documented impression or, when documentation was lacking, the study oncologist's impression based on clinical experience and patient follow-up.
Statistical Analysis
For assessment of nodal and pulmonary disease, lymph nodes and pulmonary nodules, rather than subjects, were the units of analysis. For calculating sensitivity and specificity, biopsy results, or clinical assessment when biopsy was not performed, were the reference standards. Because many lymph nodes were considered indeterminate by CI we calculated “worst case scenario” sensitivity and specificity by including indeterminate cases as negative when calculating sensitivity and positive when calculating specificity. We determined the overall accuracy rates for PET-CT and CI.19 Exact Wilcoxon rank sum tests were used to examine associations between SUVmax and lymph node size and SUVmax and clinical assessment (benign vs. malignant). Spearman's correlation coefficient was also used to explore the association between lymph node size and SUVmax.
Results
Thirty eligible patients were studied. Patient demographics and tumor features are shown in Table 1. Eight subjects had undergone primary tumor excisional biopsy before PET-CT and one had an unknown primary site. All 21 remaining primary tumors were FDG avid. The SUVmax measurements of 3 of these 21 could not be obtained due to technical errors in 2 and because a bladder tumor could not be distinguished from surrounding urine in the third. The average SUVmax of the remaining 18 tumors was 7.2 (range, 2.5-19.2)
Table 1.
Demographic and tumor features of 30 study subjects.
| Characteristic | Number |
|---|---|
|
| |
| Gender | |
| Male | 17 (57%) |
| Female | 13 (43%) |
|
| |
| Race | |
| White | 19 (63%) |
| Black | 9 (30%) |
| Other | 1 (3%) |
| Race Unknown | 1 (3%) |
|
| |
| Age at diagnosis (years) | |
| Median | 7.3 |
| Range | 1.3 – 23.5 |
|
| |
| Histology | |
| Alveolar | 11 (37%) |
| Embryonal | 14 (47%) |
| Spindle Cell | 1 (3%) |
| Botryoid | 1 (3%) |
| Mixed | 2 (7%) |
| Not otherwise specified | 1 (3%) |
|
| |
| Primary Sites | |
| Extremity | 9 |
| Parameningeal | 8 |
| Head and neck | 4 |
| Trunk | 4 |
| Bladder/prostate | 3 |
| Paratesticular | 1 |
| Unknown | 1 |
Evaluation of nodal disease
On CI, 17 subjects (17/30; 57%) had 37 nodes measuring ≥ 1 cm. in greatest diameter. The median time between CI and PET-CT in these 17 subjects was 4 days (range, 0-21 days). The median length of follow-up of these subjects was 34 months (range, 9-91 months). Using biopsy and follow-up in 6 subjects and follow-up alone in 11, 5 had nodal involvement. Table 2 summarizes the comparison of CI and PET-CT with regard to nodal disease. Four of the 5 subjects with nodal involvement had positive PET-CTs; PET-CT was false negative in 1. The 12 subjects without nodal involvement were negative by PET-CT. By CI 49% (18/37) of nodes were classified as indeterminate while only 1 (1/37, 3%) was indeterminate by PET-CT. One node that was malignant by CI was benign by PET (Table 2; Fig 1). This node was determined to be benign by clinical assessment, no local therapy was administered to this nodal bed, and the subject had no nodal recurrence. PET-CT did not upstage nodal disease in any subject. The agreement rate between clinical assessment and PET-CT was 94% (16/17 subjects). Using nodes as the unit of analysis the overall accuracy rate was 95% for PET-CT and 49% for CI. Sensitivity and specificity for PET-CT were 94% and 100%, respectively. Worst-case sensitivity and specificity for PET-CT remained high at 94% and 95%. Worst-case sensitivity for CI was 94%, however, specificity was low at 14%.
Table 2.
Comparison of PET-CT and conventional imaging (CI) assessment of lymph nodes measuring ≥ 1.0 cm in greatest diameter.
| PET-CT Assessment | CI Total (n) | |||
|---|---|---|---|---|
| Conventional Imaging Assessment | Benign (n) | Malignant (n) | Indeterminate (n) | |
| Benign | 3 | 0 | 0 | 3 |
| Malignant | 1 | 15 | 0 | 16 |
| Indeterminate | 17 | 0 | 1 | 18 |
| PET-CT Total (n) | 21 | 15 | 1 | 37 |
Fig. 1.
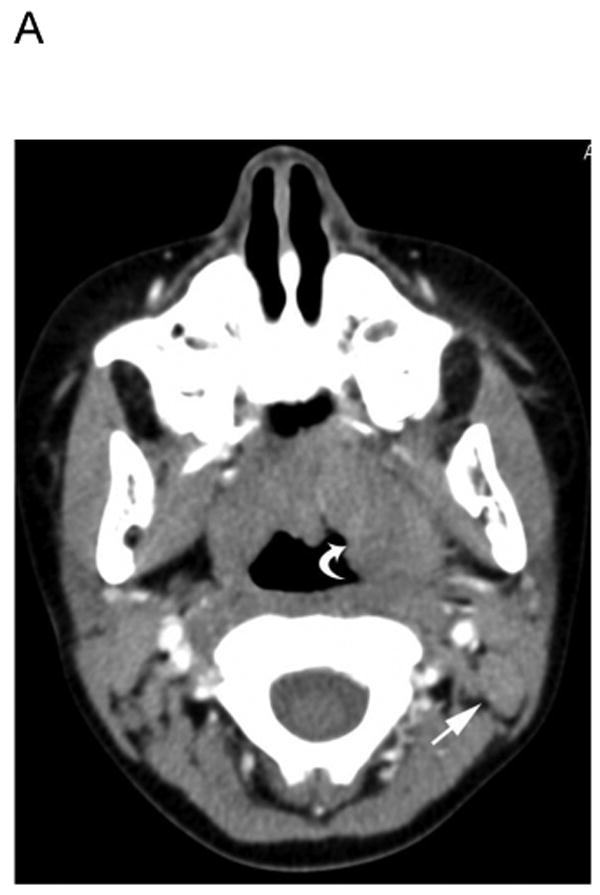
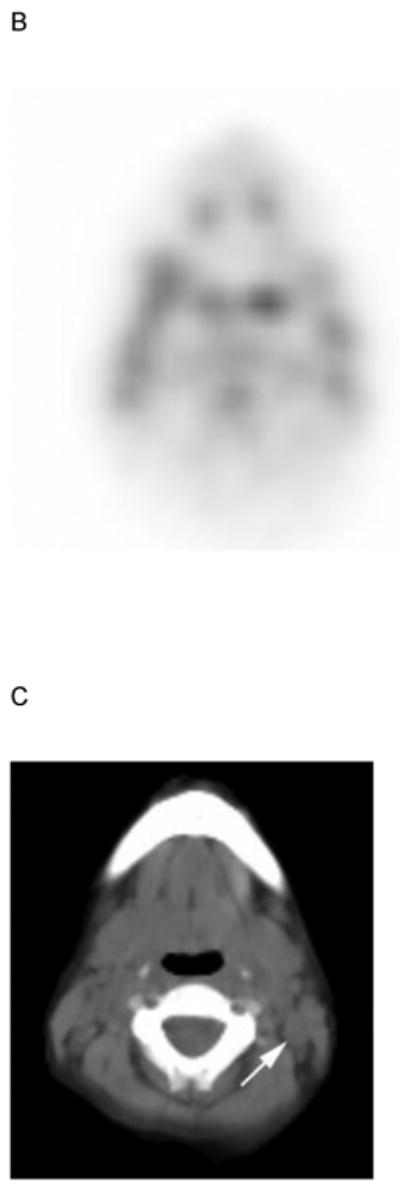
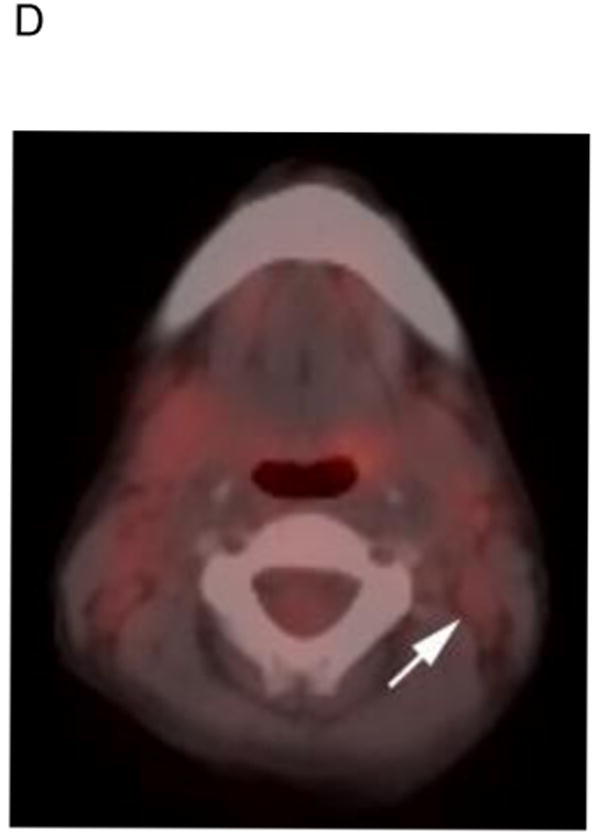
5 yo girl with left-sided nasopharyngeal rhabdomyosarcoma (not otherwise specified). A) Diagnostic computed tomography (CT) shows a 1.4 cm left posterior cervical lymph node (arrow) that was concerning for metastasic disease. Note primary tumor in left nasopharyngeal tonsil (curved arrow). B) Axial positron emission tomography (PET) image, C) co-registered non-diagnostic CT and D) fused PET-CT images show minimal uptake within the suspicious node (arrow) that was symmetric with the opposite side and interpreted as benign. The patient had no nodal recurrence or other evidence of nodal disease.
Lymph nodes that were subjectively positive on PET were significantly larger than PET negative nodes (median size, 3.4 cm vs. 1.4 cm; p < 0.0001). The SUVmax in 27 of the 37 enlarged nodes (10 had missing clinical data or technical error) was significantly positively correlated with size (Fig. 2; correlation coefficient 0.61, p < 0.001). The median SUVmax of 21 benign nodes was 1.6 (range, 0.66-2.8) and was significantly lower than 6 malignant nodes (median 7.9, range, 2.6-10.3; p < 0.0001).
Figure 2.
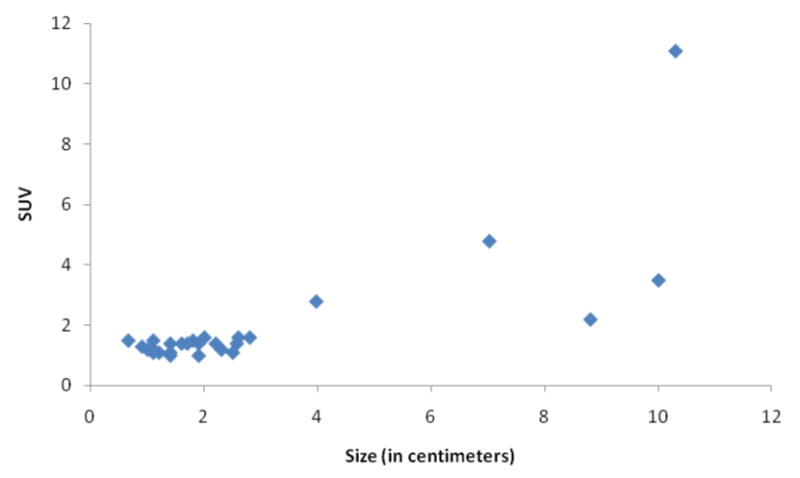
Scatter plot of 27 lymph nodes showing association between node size and maximum SUV. Publish on-line only.
Evaluation of pulmonary disease
Six subjects had 7 pulmonary nodules identified on chest CT. The median time from chest CT to PET-CT for these subjects was 5 days (range, 0-19 days). Their median length of follow-up was 41 months (range, 8-60 months). Table 3 summarizes the CT, PET-CT and clinical assessment of these nodules. None of the pulmonary nodules were biopsied although one subject had suspected pulmonary metastatic disease on both CT and PET-CT that was confirmed at autopsy. Of note, the SUVmax of this nodule (3.4) was more than twice that of 2 benign nodules that were measured.
Table 3. Comparison of clinical assessment and reviewers interpretations of PET-CT and chest CT for classification of pulmonary disease in 6 subjects with pulmonary nodules.
| Subject | Nodule Size | CT Interpretation | PET-CT Interpretation | SUV | Clinical Assessment |
|---|---|---|---|---|---|
| 1 | 0.8 cm | M | M | • | B |
| 2 | 1.8 cm | I | I | 1.36 | B |
| 3 | 0.2 cm | M | NV | • | B |
| 0.3 cm | M | NV | • | ||
| 4 | 0.3 cm | I | NV | • | B |
| 5 | 1.7 cm | M | M | 3.4 | M |
| 6 | 0.3 cm | I | I | 0.66 | B |
M = metastatic, B = benign, I = indeterminate, NV = not visualized, • = missing data
Evaluation of bone and bone marrow disease
The median time from bone scan to PET-CT in 8 subjects with bone or bone marrow imaging abnormalities or positive biopsies was 1 day (maximum interval, 5 days). Table 4 summarizes the findings on bone scan, PET-CT, clinical assessment and bone marrow biopsy of these subjects. Four of the 30 subjects (13%) had bone marrow involvement by biopsy. All 4 had negative bone scans while PET-CT identified marrow disease in 2 (Fig. 3). Notably, the 2 with positive marrow biopsies but PET-CTs negative for diffuse marrow involvement had focal bone disease on PET imaging. Only 1 subject had evidence of focal bone disease on bone scan, whereas PET-CT detected focal bone disease in 3. Therefore, PET-CT was more sensitive than bone scan for both bone and bone marrow disease.
Table 4.
Comparison of interpretations of 99mTc MDP bone scan, PET-CT, bone marrow biopsy and clinical assessment of bone and bone marrow disease.
| Bone Disease | Bone Marrow Disease | Bone or Bone Marrow Recurrence | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Subject | Bone Scan Interpretation | PET-CT Interpretation | Clinical Impression | Bone Scan Interpretation | PET-CT Interpretation | Clinical Impression | BM Bx Results | |
|
|
||||||||
| 1 | − | − | − | − | + | + | + | Unknown |
| 2 | − | − | − | − | − | − | − | N |
| 3 | + | + | + | − | − | + | + | N |
| 4 | − | + | − | − | − | − | + | Y (bone) |
| 5 | I | − | − | − | − | − | − | N |
| 6 | I | − | − | − | − | − | − | N |
| 7 | − | − | − | − | − | − | − | N |
| 8 | − | + | + | − | + | + | + | Y (bone marrow) |
I = indeterminate, + = positive for metastasis, − = negative for metastasis, N = no, Y = yes
Figure 3.
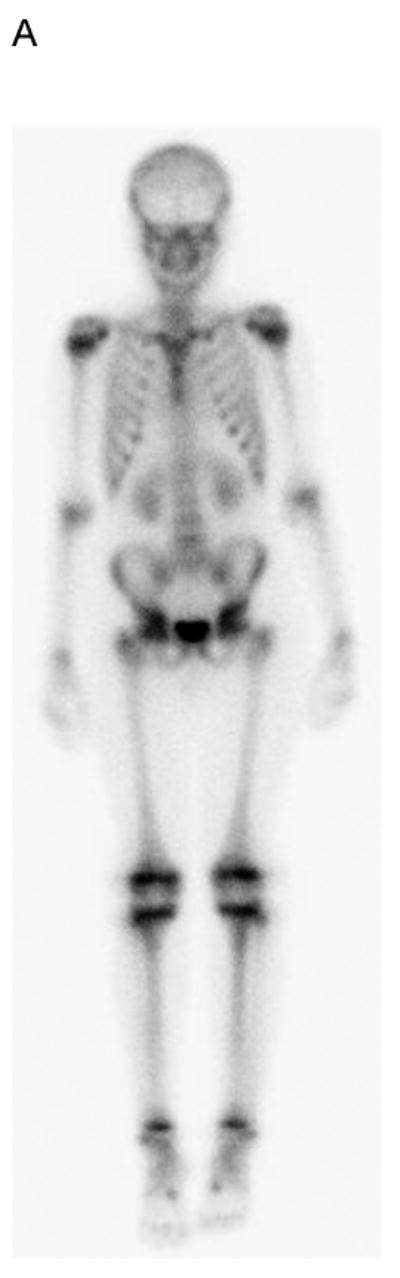
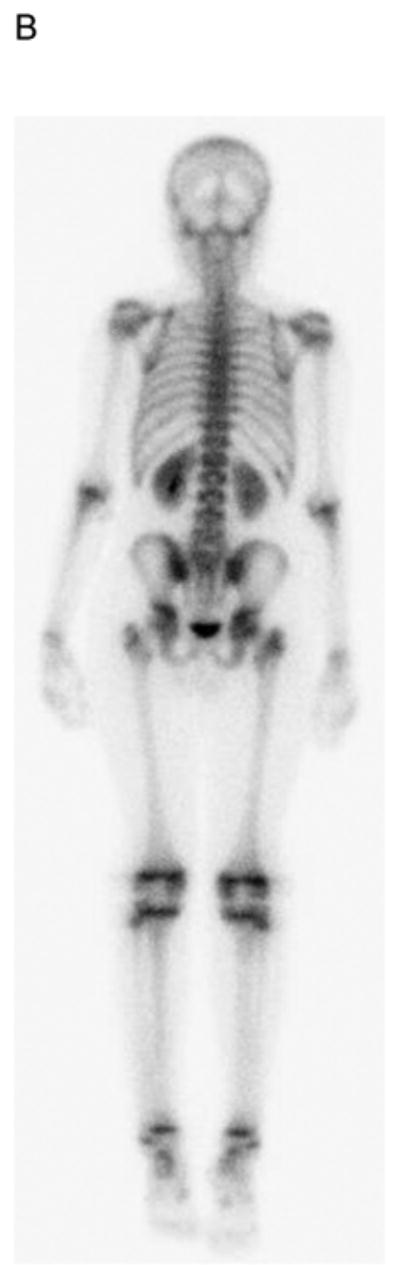
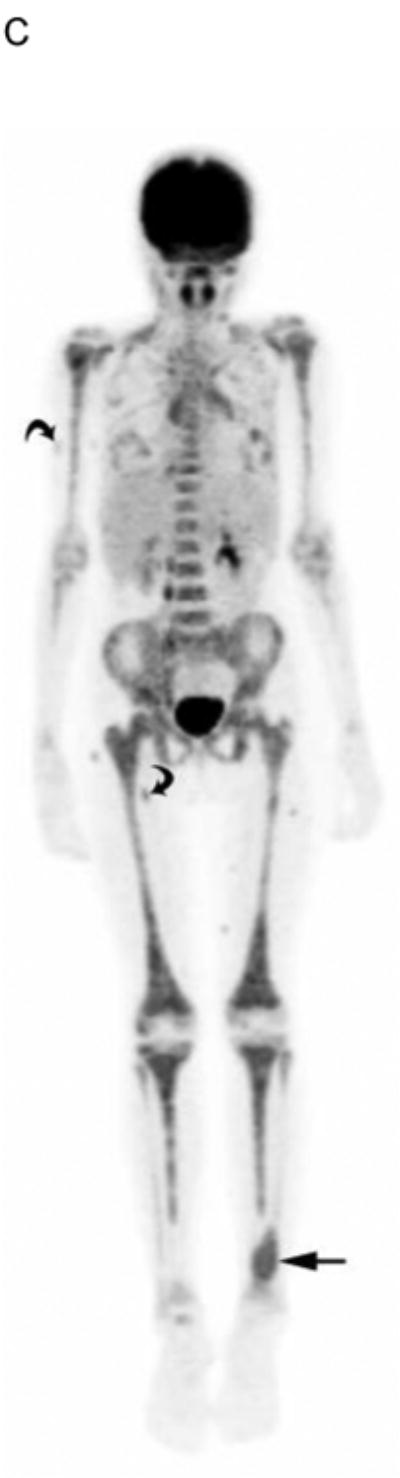
19 yo girl with embryonal RMS, primary site left lower leg. A) Anterior and B) posterior 99mTc bone scan images show no evidence of bone metastasis. C) Maximum intensity projection (MIP) PET-CT image showing diffusely abnormal and mildly asymmetric marrow FDG uptake throughout the upper and lower extremities, pelvis and spine, due to marrow disease proven by biopsy. The primary left lower leg tumor (straight arrow) and soft-tissue metastases (several indicated with curved arrows) are also evident. The soft tissue metastases were not detected by CI or physical examination.
Other sites of metastatic disease
Two subjects had metastatic soft tissue nodules in the extremities that were detected on PET-CT but not on CI or physical examination (Fig. 3). One of these 2 also had a deeply seated nodule in the anterior pelvis that was only detected with PET-CT (Fig. 4). The other had additional breast metastases that were evident on PET-CT and physical examination.
Figure 4.
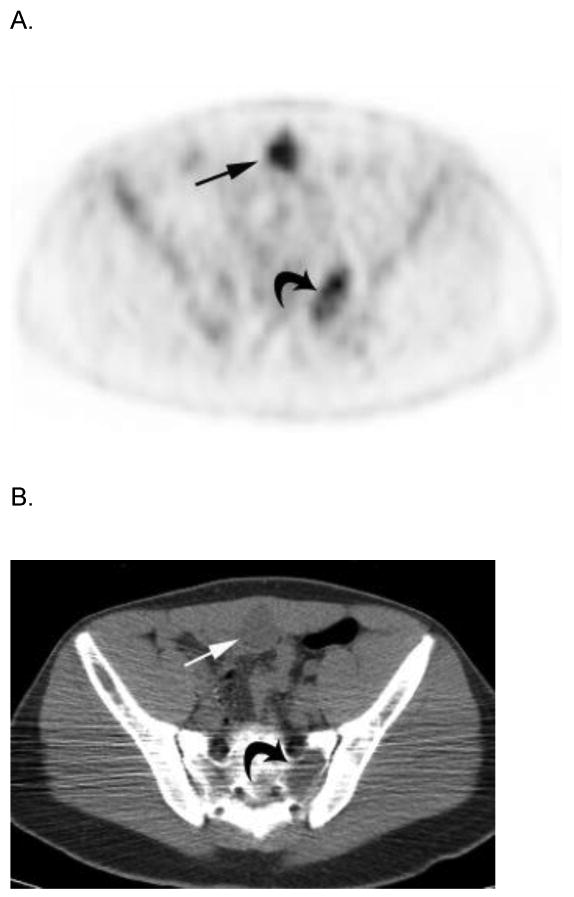
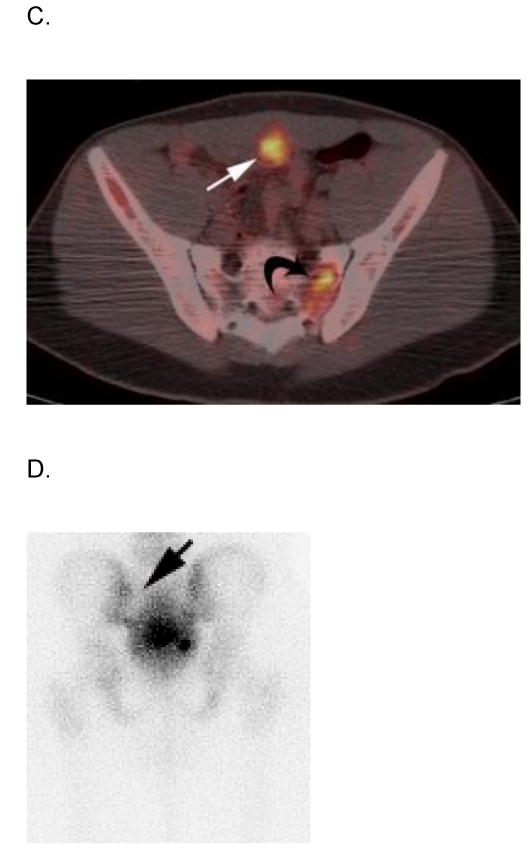
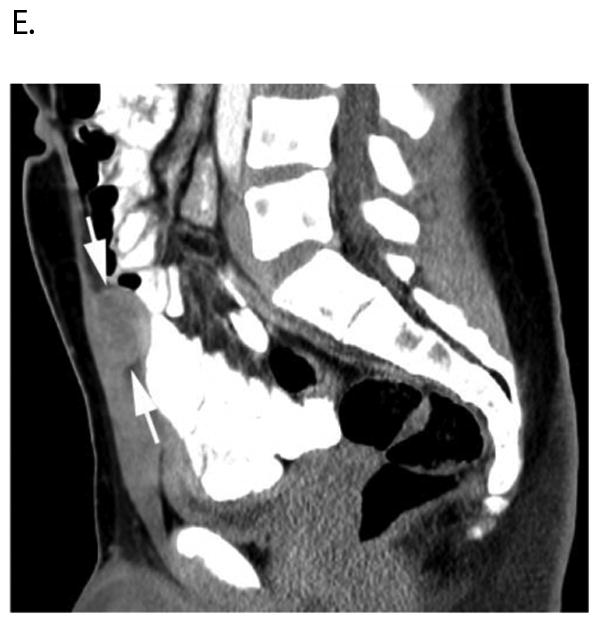
17 year-old boy with parameningeal alveolar RMS. Axial A) PET, B) co-registered non-diagnostic CT and C) fused PET-CT images show intense FDG avidity within a soft tissue metastasis on the inner surface of the anterior pelvic wall (straight arrows) that was not palpable on physical examination. Note lytic metastasis in left sacrum (curved arrows) that is also FDG avid but not seen on D) posterior bone scan image (straight arrow indicates corresponding area). This patient had bone marrow disease on pathologic inspection. E) Sagittal reconstructed diagnostic CT image better delineates the deeply seated, non-palpable, soft tissue metastasis (arrows). Publish on-line only.
Discussion
Several studies have shown that PET-CT has value in staging and re-staging patients with RMS, but they have several limitations not present in our study. 8,9,11,12 Tateishi and colleagues found that PET-CT was more accurate than CI in identifying sites of disease in patients with newly diagnosed or recurrent RMS but they used chest radiography rather than CT for detection of pulmonary metastases.11 Klem et al. compared PET-CT to CI at the time of RMS diagnosis in 24 subjects. Nine of their subjects had received 1 to 13 days of chemotherapy at the time of PET-CT and had primary tumor SUVs that were significantly lower than those who had not received chemotherapy. Therefore, they may have underestimated the true value of PET-CT at diagnosis.12 In contrast, we limited our study population to newly diagnosed subjects who underwent PET-CT before initiation of chemotherapy. Additionally, we compared PET-CT to current imaging modalities used in modern day practice in the United States.18
Perhaps our most significant finding was the substantially higher sensitivity and specificity of PET-CT for detection of nodal disease compared to CI. Our findings are superior to those reported by others, perhaps because we used the RECIST criteria to define adenopathy on CI which then guided our evaluation of nodal basins on PET-CT.11,12 Patients with RMS nodal disease have inferior outcomes and cure depends on radiotherapy of involved nodes.20,21 Therefore, judicious use of lymph node biopsy to clarify equivocal imaging findings is appropriate. Previous investigators have not assessed the potential value of the SUV in distinguishing benign from malignant lymph nodes in RMS. We found that malignant nodes had significantly higher SUVmax than benign nodes. Therefore, the SUVmax may provide an objective assessment of lymph nodes that could impact the decision to perform biopsy as well as direct biopsy to a specific lymph node.
Although we had a small number of subjects with bone or bone marrow involvement, our findings are consistent with prior reports and suggest that PET-CT is superior to bone scan for detection of such disease in patients with RMS. 8,11,12,14,22,23 Interestingly, no subject in our study had focal bone disease without also having bone marrow involvement. Larger studies are needed to determine whether, in RMS, “focal bone metastases” result from focal cortical destruction secondary to advanced marrow disease (which may have prognostic implications) or occur hematogenously.
We lacked a sufficient number of subjects with pulmonary nodules to draw firm conclusions regarding the value of PET-CT in this setting. However, consistent with prior reports, we found that PET-CT has limited value when nodules are small because they may be missed on non-diagnostic quality CT performed during PET-CT and have minimal FDG avidity.6,24 The potential role of the SUV, when nodules are FDG avid, for distinguishing benign from malignant histology has not been rigorously evaluated in children but warrants further investigation. Such information could guide decisions regarding biopsy and might prevent unneccessary invasive procedures to confirm benign nodule histology.
Our study again demonstrates the superiority of PET-CT in detecting soft-tissue metastases that may be missed by CI and physical examination.6,9,11 The breast (one of the most common soft tissue metastatic sites in RMS) is involved in about 4% of children with metastatic disease at diagnosis and is strongly associated with alveolar histology.25 Since CI and physical examination may miss small or deep breast metastases, the routine use of PET-CT in children with metastatic alveolar RMS may facilitate detection of subclinical breast involvement. The incidence of other sites of soft tissue metastasis in RMS is low, but may be underestimated since these sites are often not imaged. PET-CT could play an important role in the management and outcome of patients with RMS soft tissue metastases, particularly if there are no other sites of metastatic disease and when local control of these sites is feasible.
All primary tumors in our cohort were subjectively FDG avid and the average SUVmax was high at 7.2. Our findings provide further evidence that RMS is a malignancy that is generally FDG avid before initiation of therapy. Importantly, the primary tumor FDG avidity may have prognostic significance. Baum and colleagues recently showed that both subjective assessment of primary tumor FDG avidity and the SUVmax/SUVliver ratio at the time of diagnosis were predictive of overall and event free survival in children with RMS.7 Additional studies are needed to confirm these findings.
Our study has several limitations. The retrospective nature meant there was variability in image and data acquisition and several patients had missing data points. Additionally, because of the rarity of RMS, the cohort size and number of metastatic disease sites were small. The use of the original RECIST criteria for assessment of nodal disease is noteworthy. The revised RECIST criteria stipulate that a lymph node must have a short-axis diameter of ≥ 1.5 cm to be considered a target lesion.26 These criteria may improve the ability of CI to correctly predict malignant nodal disease but CI will remain limited by its inability to assess nodal metabolic activity. Finally, the oncology experience and expertise of reviewers in this study may have impacted our results. It is unclear whether our findings could be reproduced by others who may see few cases of RMS. This issue is especially important considering the learning curve encountered when implementing new imaging modalities.
In conclusion, our study provides further evidence that PET-CT is a reliable modality for assessment of children with untreated RMS. Compared to CI, PET-CT has higher accuracy and specificity for assessment of lymph node involvement. The role of the SUVmax in assessing lymph nodes should be investigated to determine if there is a threshold value above which biopsy should be performed or below which biopsy is not indicated. Our findings, coupled with previous reports, suggest that PET-CT is likely superior to bone scan for detection of bone marrow and focal bone involvement. A bone scan is probably not warranted when PET-CT is being performed unless the PET-CT is negative and the patient has symptoms related to bone. However, large prospective studies are needed to adequately assess the value of PET-CT in detecting focal bone and bone marrow disease in RMS. Such studies may also provide valuable insight into the biological nature of focal bone disease in this patient population, which might occur secondary to advanced bone marrow involvement rather than hematogenously. Diagnostic chest CT remains the reference standard for detection of pulmonary nodules, although when nodule SUVmax can be measured it may provide useful information regarding nodule histology. An important attribute of PET-CT relative to CI is its ability to assess of the entire body. Therefore, PET-CT can reveal unsuspected soft-tissue metastases and such information could have important implications for patient prognosis and management.
Acknowledgments
The authors thank Kim Johnson for data management for this project and Dr. Moinul Hossain for image review.
Supported in part by Cancer Center Support CORE Grant P30 CA 21765 from the National Cancer Institute and by the American, Lebanese and Syrian Associated Charities
Footnotes
The authors have no disclosures or conflicts of interest
References
- 1.Gurney JG, Davis S, Severson RK, et al. Trends in cancer incidence among children in the U.S. Cancer. 1996;78:532–541. doi: 10.1002/(SICI)1097-0142(19960801)78:3<532::AID-CNCR22>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari A, Sultan I, Huang TT, et al. Soft tissue sarcoma across the age spectrum: a population-based study from the Surveillance Epidemiology and End Results database. Pediatr Blood Cancer. 2011;57:943–949. doi: 10.1002/pbc.23252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wexler LH, Meyer WH, Helman LJ. Rhabdoyosarcoma. In: Pizzo PA, Poplack's DG, editors. Priniciples and Practice of Pediatric Oncology. Philadelphia, PA: Lippincott Williams and Wilkins; 2011. [Google Scholar]
- 4.Oberlin O, Rey A, Lyden E, et al. Prognostic factors in metastatic rhabdomyosarcomas: results of a pooled analysis from United States and European cooperative groups. J Clin Oncol. 2008;26:2384–2389. doi: 10.1200/JCO.2007.14.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guillerman RP, McCarville MB, Kaste SC, et al. Imaging studies in the diagnosis and management of pediatric malignancies. In: Pizzo PA, Poplack's DG, editors. Principles and Practice of Pediatric Oncology. Philadelphia, PA: Lippincott Williams and Wilkins; 2011. [Google Scholar]
- 6.McCarville MB, Christie R, Daw NC, et al. PET/CT in the evaluation of childhood sarcomas. AJR Am J Roentgenol. 2005;184:1293–1304. doi: 10.2214/ajr.184.4.01841293. [DOI] [PubMed] [Google Scholar]
- 7.Baum SH, Frühwald M, Rahbar K, et al. Contribution of PET/CT to Prediction of Outcome in Children and Young Adults with Rhabdomyosarcoma. J Nucl Med. 2011;52:1535–1540. doi: 10.2967/jnumed.110.082511. [DOI] [PubMed] [Google Scholar]
- 8.Volker T, Denecke T, Steffen I, et al. Positron emission tomography for staging of pediatric sarcoma patients: results of a prospective multicenter trial. J Clin Oncol. 2007;25:5435–5441. doi: 10.1200/JCO.2007.12.2473. [DOI] [PubMed] [Google Scholar]
- 9.Arush MW, Israel O, Postovsky S, et al. Positron emission tomography/computed tomography with 18fluoro-deoxyglucose in the detection of local recurrence and distant metastases of pediatric sarcoma. Pediatr Blood Cancer. 2007;49:901–905. doi: 10.1002/pbc.21150. [DOI] [PubMed] [Google Scholar]
- 10.Arush MW, Bar Shalom R, Postovsky S, et al. Assessing the use of FDG-PET in the detection of regional and metastatic nodes in alveolar rhabdomyosarcoma of extremities. J Pediatr Hematol Oncol. 2006;28:440–445. doi: 10.1097/01.mph.0000212949.12856.02. [DOI] [PubMed] [Google Scholar]
- 11.Tateishi U, Hosono A, Makimoto A, et al. Comparative study of FDG PET/CT and conventional imaging in the staging of rhabdomyosarcoma. Ann Nucl Med. 2009;23:155–161. doi: 10.1007/s12149-008-0219-z. [DOI] [PubMed] [Google Scholar]
- 12.Klem ML, Grewal RK, Wexler LH, et al. PET for staging in rhabdomyosarcoma: an evaluation of PET as an adjunct to current staging tools. J Pediatr Hematol Oncol. 2007;29:9–14. doi: 10.1097/MPH.0b013e3180307693. [DOI] [PubMed] [Google Scholar]
- 13.Mody RJ, Bui C, Hutchinson RJ, et al. FDG PET imaging of childhood sarcomas. Pediatr Blood Cancer. 2010;54:222–227. doi: 10.1002/pbc.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricard F, Cimarelli S, Deshayes E, et al. Additional Benefit of F-18 FDG PET/CT in the staging and follow-up of pediatric rhabdomyosarcoma. Clin Nucl Med. 2011;36:672–677. doi: 10.1097/RLU.0b013e318217ae2e. [DOI] [PubMed] [Google Scholar]
- 15.Schuetze SM. Utility of positron emission tomography in sarcomas. Curr Opin Oncol. 2006;18:369–373. doi: 10.1097/01.cco.0000228744.49294.12. [DOI] [PubMed] [Google Scholar]
- 16.Lucas JD, O'Doherty MJ, Cronin BF, et al. Prospective evaluation of soft tissue masses and sarcomas using fluorodeoxyglucose positron emission tomography. Br J Surg. 1999;86:550–556. doi: 10.1046/j.1365-2168.1999.01090.x. [DOI] [PubMed] [Google Scholar]
- 17.Goodin GS, Shulkin BL, Kaufman RA, et al. PET/CT characterization of fibroosseous defects in children: 18F-FDG uptake can mimic metastatic disease. AJR Am J Roentgenol. 2006;187:1124–1128. doi: 10.2214/AJR.06.0171. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate The Response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Simel DL, Feussner JR, DeLong ER, et al. Intermediate, indeterminate, and uninterpretable diagnostic test results. Med Decis Making. 1987;7:107–114. doi: 10.1177/0272989X8700700208. [DOI] [PubMed] [Google Scholar]
- 20.Meza JL, Anderson J, Pappo AS, et al. Analysis of prognostic factors in patients with nonmetastatic rhabdomyosarcoma treated on intergroup rhabdomyosarcoma studies III and IV: the Children's Oncology Group. J Clin Oncol. 2006;24:3844–3851. doi: 10.1200/JCO.2005.05.3801. [DOI] [PubMed] [Google Scholar]
- 21.Rodeberg DA, Garcia-Henriquez N, Lyden ER, et al. Prognostic significance and tumor biology of regional lymph node disease in patients with rhabdomyosarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2011;29:1304–1311. doi: 10.1200/JCO.2010.29.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seshadri N, Wright P, Balan KK. Rhabdomyosarcoma with widespread bone marrow Infiltration. Clin Nuc Med. 2007;32:787–789. doi: 10.1097/RLU.0b013e318148b434. [DOI] [PubMed] [Google Scholar]
- 23.Iagaru A, Goris ML. Rhabdomyosarcoma diffusely metastatic to the bone marrow: suspicious findings on 99mTc-MDP bone scintigraphy confirmed by 18F-18 FDG PET/CT and bone marrow biopsy. Eur J Nucl Med Mol Imaging. 2008;35:1746. doi: 10.1007/s00259-008-0864-4. [DOI] [PubMed] [Google Scholar]
- 24.Coleman RE, Laymon CM, Turkington TG, et al. FDG imaging of lung nodules: a phantom study comparing SPECT, camera-based PET, and dedicated PET. Radiology. 1999;210:823–828. doi: 10.1148/radiology.210.3.r99mr18823. [DOI] [PubMed] [Google Scholar]
- 25.D'Angelo P, Carli M, Ferrari A, et al. Breast metastases in children and adolescents with rhabdomyosarcoma: Experience of the Italian Soft Tissue Sarcoma Committee. Pediatr Blood Cancer. 2010;55:1306–1309. doi: 10.1002/pbc.22729. [DOI] [PubMed] [Google Scholar]
- 26.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]


