Summary
Background
Bence Jones proteinuria is a disorder that is defined by the excretion of monoclonal light-chain protein. About 15–20% of patients with multiple myeloma secrete monoclonal light chains only, without expression of the normal immunoglobulin heavy chain, which constitutes light-chain multiple myeloma. The definition, prevalence, and progression of these premalignant phases of light-chain multiple myeloma have not been fully characterised. We aimed to identify a subset of patients with idiopathic Bence Jones proteinuria who had a high risk of progression to light-chain multiple myeloma analogous to that seen in patients with smouldering multiple myeloma.
Methods
In this retrospective cohort study, we studied all patients seen at the Mayo Clinic (Rochester, MN, USA) within 30 days of diagnosis of idiopathic Bence Jones proteinuria between Jan 1, 1960, and June 30, 2004. Inclusion criteria were monoclonal light chain in the urine (≥0·2 g/24 h), absence of intact monoclonal immunoglobulin (M protein) in the serum, and no evidence of multiple myeloma, light-chain amyloidosis, or other related plasma-cell proliferative disorders. The primary endpoint was progression to symptomatic multiple myeloma or light-chain amyloidosis. We examined the cumulative probability of progression and the association of potential risk factors on progression rates to identify patients with a high risk of progression to multiple myeloma or light-chain amyloidosis.
Findings
We identified 101 patients with idiopathic Bence Jones proteinuria. During 901 total person-years of follow-up, 27 (27%) patients developed multiple myeloma and seven (7%) developed light-chain amyloidosis. The major risk factors for progression were amount of urinary excretion of M protein per 24 h, proportion of bone marrow plasma cells, presence of a markedly abnormal free-light-chain ratio (<0·01 or >100), and reduction of all three uninvolved immunoglobulins. Based on the risk of progression, monoclonal light-chain excretion of 0·5 g/24 h or greater or at least 10% bone marrow plasma cells, or both, in the absence of end-organ damage was used to define light-chain smouldering multiple myeloma. The cumulative probability of progression to active multiple myeloma or light-chain amyloidosis in patients with light-chain smouldering multiple myeloma was 27·8% (95% CI 14·2–39·2) at 5 years, 44·6% (27·9–57·4) at 10 years, and 56·5% (36·3–70·2) at 15 years.
Interpretation
Light-chain smouldering multiple myeloma as defined in this study is associated with a high risk of progression to symptomatic light-chain multiple myeloma, and this subset of patients needs careful observation and could benefit from clinical trials of early intervention.
Funding
Jabbs Foundation (Birmingham, UK), US National Cancer Institute, and Henry J Predolin Foundation (Madison, WI, USA).
Introduction
Multiple myeloma is a plasma-cell malignancy that is associated with monoclonal immunoglobulin (M protein) production, osteolytic bone lesions, hyper calcaemia, anaemia, and renal failure. 80–85% of patients with multiple myeloma secrete intact immunoglobulin. This subset of patients almost always have an asymptomatic premalignant phase for many years before diagnosis.1–8 This premalignant phase, termed monoclonal gammopathy of undetermined significance, is present in more than 3% of the general population older than 50 years.7 Monoclonal gammopathy of undetermined significance and multiple myeloma are linked by an intermediate stage, termed smouldering multiple myeloma, that is characterised by a higher amount of serum M protein or proportion of clonal plasma cells and has a greater risk of progression than in patients with monoclonal gammopathy of undetermined significance.2,5 Previously, we have developed the disease definitions and described the long-term outcome of patients with monoclonal gammopathy of undetermined significance9 and those with smouldering multiple myeloma;5 1% of patients with monoclonal gammopathy of undetermined significance progress to multiple myeloma or a related malignancy per year, whereas 10% of patients with smouldering multiple myeloma progress per year during the first 5 years after recognition.
About 15–20% of patients with multiple myeloma secrete monoclonal light chains only, without expression of the normal immunoglobulin heavy chain, which constitutes light-chain multiple myeloma.10 Monoclonal light-chain excretion, to our knowledge, was first described in 1847, and subsequently has been referred to as idiopathic Bence Jones proteinuria.11,12 The definition, prevalence, and progression of these premalignant phases of light-chain multiple myeloma have not been fully characterised. A few patients have been described with idiopathic or so-called benign Bence Jones proteinuria, but these reports are limited by inadequate follow-up.13–17 More than 30 years ago, we described a case series of seven patients with idiopathic Bence Jones proteinuria with an M-protein urinary excretion of greater than 1·0 g/24 h.12
In 2010, we defined light-chain monoclonal gammopathy of undetermined significance, which is present in 0·8% of the general population older than 50 years.18 The diagnosis of light-chain monoclonal gammopathy of undetermined significance requires an abnormal free-light-chain (FLC) ratio (ie, the ratio of κ FLCs to λ FLCs in the serum), an increase in the affected light-chain isotype, and absence of end-organ damage attributable to the plasma-cell disorder. Light-chain monoclonal gammopathy of undetermined significance represents the early premalignant phase of light-chain multiple myeloma; it is the equivalent of monoclonal gammopathy of undetermined significance, but is characterised by complete loss of the heavy chain of the immunoglobulin molecule. The stage between light-chain monoclonal gammopathy of undetermined significance and light-chain multiple myeloma has not been defined so far. This missing link is the light-chain equivalent of smouldering multiple myeloma, and it has been hard to define because the inability to recognise the disorder has precluded longitudinal studies that can accurately define and describe the clinical course and prognosis.
In this Article, we define light-chain smouldering multiple myeloma and outline the long-term outcome and risk factors for progression. To do this, we did a long-term study of a large cohort of patients with idiopathic Bence Jones proteinuria characterised by monoclonal free-light-chain production in the absence of signs and symptoms of overt multiple myeloma. Our goal was to identify the subset of patients with idiopathic Bence Jones proteinuria who had a high risk of progression to light-chain multiple myeloma analogous to that seen in patients with smouldering multiple myeloma.
Methods
Study design and participants
In this retrospective cohort study, we searched a computerised database and reviewed the records of all patients seen at the Mayo Clinic (Rochester, MN, USA) between Jan 1, 1960, and June 30, 2004, within 30 days of the recognition of a monoclonal light chain (ie, Bence Jones protein) in their urine. Inclusion criteria were measurable monoclonal light chain in the urine of 0·2 g/24 h or greater, absence of intact M protein in the serum, and no evidence of multiple myeloma, light-chain amyloidosis, or other related plasma-cell proliferative disorders. Patients with renal insufficiency, anaemia, hypercalcaemia, or bone lesions resulting from the plasma-cell proliferative disorder (including multiple myeloma or light-chain amyloidosis) or who had received chemotherapy or had a nephrotic syndrome were excluded. We gained approval to do the study from the Mayo Clinic institutional review board. We obtained a waiver of informed consent from the institutional review board (informed consent for retrospective chart review studies is not required under Minnesota law).
Procedures
We followed up these patients and established their cause of death (if applicable) by reviewing each patient’s medical record at the Mayo Clinic and medical records at other institutions. If they had died, we made telephone contact with next of kin and reviewed death certificates. We sent patients letters of inquiry every year if they had not visited the Mayo Clinic in the preceding year.
Serum and urine protein electrophoresis and immunofixation were done as previously described and we obtained the results.19 The serum free-light-chain assay (Freelite, Binding Site, Birmingham, UK) was done on frozen serum samples using a Dade Behring BNII automated nephelometer (Siemens, Newark, DE, USA). The assay reports free κ and λ light-chain concentrations and the κ-to-λ ratio (normal range 0·26–1·65).20 We obtained, from the medical record, data for all other risk factors for progression, measured within 30 days of diagnosis, including haemoglobin, serum calcium, creatinine, and quantitative immunoglobulin (IgA, IgG, and IgM) concentrations, serum and urine monoclonal protein concentrations, and the proportion of plasma cells in the bone marrow. These data were entered for analysis into an electronic database by one of the investigators (RAK) and trained study coordinators.
Outcomes
The primary endpoint was progression to symptomatic multiple myeloma (defined by hypercalcaemia, renal insufficiency, anaemia, or bone lesions attributable to the plasma-cell proliferative process) or light-chain amyloidosis. The study had no secondary endpoints.
Statistical analysis
We estimated prognosis in terms of the cumulative probability of progression, calculated by Kaplan-Meier analysis21 in which data from patients who had died were censored; we compared curves using the log-rank test.22 We examined association of potential risk factors (sex, age, age >65 years, haemoglobin, calcium, serum creatinine, serum albumin, reduction in concentration of serum immunoglobulins, urine light-chain type, proportion of bone marrow plasma cells, amount of urine M protein, and FLC ratio) on progression rates in univariate Cox proportional hazards models, expressed as hazard ratios (HRs) with 95% CIs.23
We assessed the relative risk of progression to active multiple myeloma or light-chain amyloidosis, compared with that in the general population, with standardised incidence ratios24 whereby observed cases were compared with the number of expected cases by applying age-specific and sex-specific incidence for multiple myeloma in a cohort of mainly white individuals (99 patients with Bence Jones proteinuria were white, one was black, and one was of unknown race) from the Iowa Surveillance Epidemiology and End Results Program (SEER)25,26 to the person-years of follow-up specific for age, sex, and calendar year in our study cohort. The expected age-specific and sex-specific incidence of light-chain amyloidosis was based on data from Olmsted County, MN, USA.27 We did statistical analyses using SAS version 9.2 and R version 3.0.2.
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author (RAK), last author (SVR), and statisticians (TMT and DRL) had full access to all the data in the study. All authors of this paper had final responsibility for the decision to submit for publication.
Results
101 patients fulfilled the criteria for diagnosis of idiopathic Bence Jones proteinuria during the 45-year period (1960–2004). Patient characteristics are listed in table 1. Haemoglobin concentration was lower than 100 g/L (10 g/dL) in eight (8%) patients (only one of whom progressed to multiple myeloma or light-chain amyloidosis), but was unrelated to their underlying plasma-cell disorder; causes of anaemia were gastrointestinal bleeding (in two [2%] patients), chronic renal insufficiency from biopsy-proven nephrosclerosis (one [<1%]), hypertension and side-effects of non-steroidal anti-inflammatory drugs (one [<1%]), connective tissue disorders (two [2%]), myelodysplasia (one [<1%]), and postoperative bleeding (one [<1%]). Serum calcium concentration was higher than 2·75 mmol/L (11·0 mg/dL) in three (3%) patients, but metastatic cancer was responsible for this result in two patients, and the high concentration was transient in the other patient. Serum creatinine concentration was higher than 176·8 µmol/L (2·0 mg/dL) in ten (10%) patients (only one [<1%] progressed), but none of the results was related to Bence Jones proteinuria; two patients had hypertension, two had renal vascular disease, two had prostate cancer, one had polycystic kidney disease, one had acquired Fanconi syndrome, one had chronic renal failure from severe cardiovascular disease, and in one patient the result was transient.
Table 1.
Initial laboratory data
| Study participants (N=101) | |
|---|---|
| Age, years (n=101) | |
| Median (range) | 67 (18–90) |
| <40 | 2 (2%) |
| Male sex (n=101) | 74 (73%) |
| Haemoglobin, g/L (n=100) | |
| Median (range) | 134 (67–181) |
| <100 | 8 (8%) |
| Serum calcium >2·75 mmol/L (n=96) | 3 (3%) |
| Serum creatinine >176·8 µmol/L (n=100) | 10 (10%) |
| Urinary light chain (n=101) | |
| κ | 50 (50%) |
| λ | 51 (50%) |
| Urinary M-protein excretion, g/24 h (n=101) | |
| Median (range) | 0·5 (0·2–4·7) |
| 0·2–0·49 | 50 (50%) |
| 0·5–0·99 | 22 (22%) |
| 1·0–1·99 | 19 (19%) |
| ≥2 | 10 (10%) |
| Reduction of uninvolved immunoglobulins (n=77) | |
| IgG | 41 (53%) |
| IgA | 26 (34%) |
| IgM | 27 (35%) |
| 1 immunoglobulin | 14 (18%) |
| 2 immunoglobulins | 22 (29%) |
| 3 immunoglobulins | 12 (16%) |
| Proportion of plasma cells in bone marrow (n=61) | |
| Median (range) | 9% (0–35) |
| <10% | 31 (51%) |
| 10–20% | 18 (30%) |
| >20% | 12 (20%) |
Data are number (%) unless otherwise specified. M protein=monoclonal immunoglobulin.
Immunofixation of serum revealed a monoclonal light chain in 56 (62%) of 91 patients, and was normal in the remaining 35 (38%) patients. Bone marrow examination was done in 61 (60%) of 101 patients within 30 days of diagnosis. Risk of progression was nearly the same in patients who had a bone marrow examination compared with those who did not (HR 1·34 [95% CI 0·61–2·98]; p=0·47). 31 (51%) of 61 patients had less than 10% of plasma cells in the bone marrow.
During 901 person-years of follow-up (median 7·1 years [IQR 2·2–14·1]), 89 (88%) of the 101 patients with idiopathic Bence Jones proteinuria died. Causes of death were cardiovascular events (22 [25%] of 89), infections (14 [16%]), renal failure (eight [9%]), cerebrovascular events (three [3%]), myeloma or light-chain amyloidosis (14 [16%]), other malignancies (nine [10%]), and unknown causes (19 [21%]). Four of the patients for whom cause of death was unknown had multiple myeloma or light-chain amyloidosis, so this disorder was probably the cause of death. Five of the 12 living patients have developed multiple myeloma, leaving only seven living patients at risk for progression. The median follow-up of these seven patients so far is 13·4 years (IQR 9·1–18·6).
During follow-up, active multiple myeloma developed in 27 (27%) of 101 patients. Documentation of CRAB (hyperCalcaemia, Renal failure, Anaemia, and Bone lesions) criteria was available for 13 of these patients; six others had progressed to greater than 30% plasma cells in their bone marrow, requiring therapy, but we did not have access to data to verify CRAB features. The other eight patients did not return to the Mayo Clinic at the time of treatment for their multiple myeloma. Since only 0·2 cases of multiple myeloma were expected from the SEER data for a population size of 101, the relative risk of multiple myeloma in this cohort of patients was 130 (95% CI 86–189). Seven (7%) of 101 patients developed light-chain amyloidosis, when only 0·09 were expected to on the basis of the Olmsted County data (relative risk 81 [95% CI 33–167]). Four patients had both multiple myeloma and light-chain amyloidosis and were assigned to multiple myeloma for the purposes of our analyses (two patients) or light-chain amyloidosis (two patients) according to their major clinical features. Median duration of survival after diagnosis of multiple myeloma was 46 months (IQR 17·8–88·7), whereas survival of the seven patients with light-chain amyloidosis ranged from 0 months to 37·6 months.
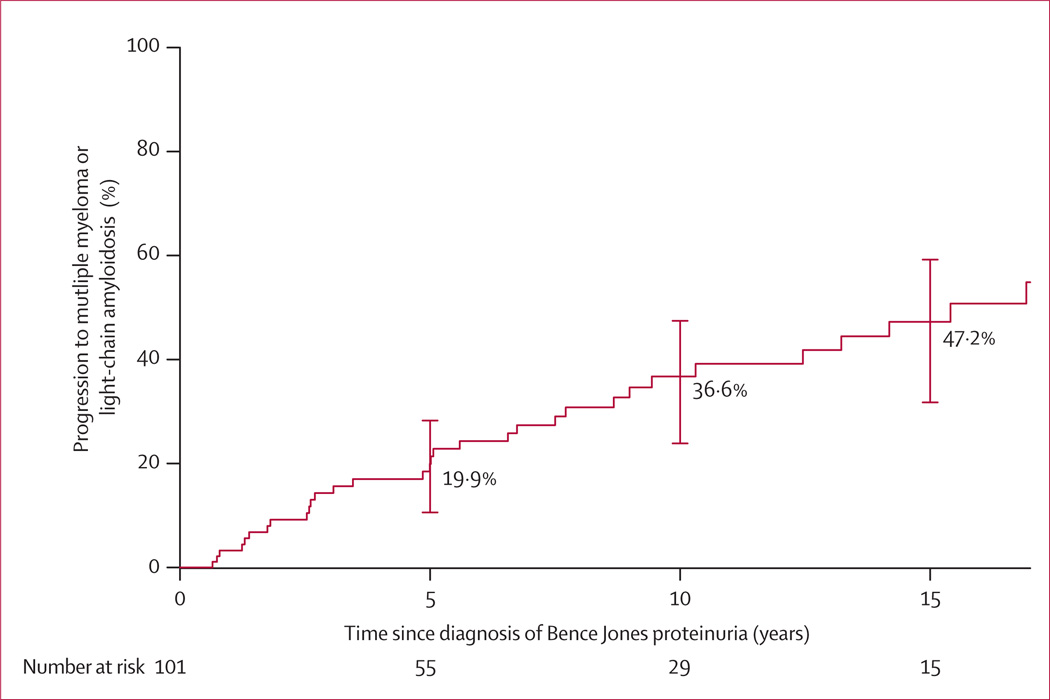
The cumulative probability of progressing to active multiple myeloma or light-chain amyloidosis in this cohort of 101 patients with idiopathic Bence Jones proteinuria was 19·9% (95% CI 10·6–28·3) at 5 years, 36·6% (23·8–47·3) at 10 years, and 47·2% (31·7–59·1) at 15 years (figure 1; table 2). Progression occurred at a steady rate of 3–4% yearly throughout the period of observation, and showed no signs of slowing after the first 5 years. The median time to progression was 15·4 years (IQR 6·6 to >35).
Figure 1. Risk of progression to multiple myeloma or light-chain amyloidosis in patients with idiopathic Bence Jones proteinuria.
Error bars represent 95% CI.
Table 2.
Excretion of urinary monoclonal protein and risk of progression of idiopathic Bence Jones proteinuria
| Patients, n | 5-year progression (95% CI) |
10-year progression (95% CI) |
15-year progression (95% CI) |
|
|---|---|---|---|---|
| Overall | 101 | 19·9% (10·6–28·3) | 36·6% (23·8–47·3) | 47·2% (31·7–59·1) |
| <0·50 g/24 h | 50 | 7·8% (0·0–16·0) | 24·2% (5·8–39·0) | 29·7% (8·6–45·8) |
| 0·50–0·99 g/24 h | 22 | 18·8% (0·0–35·8) | 31·8% (4·3–51·4) | 48·9% (1·1–73·6) |
| ≥1·0 g/24 h | 29 | 39·1% (16·6–55·5) | 57·8% (31·7–73·9) | 69·9% (41·0–84·6) |
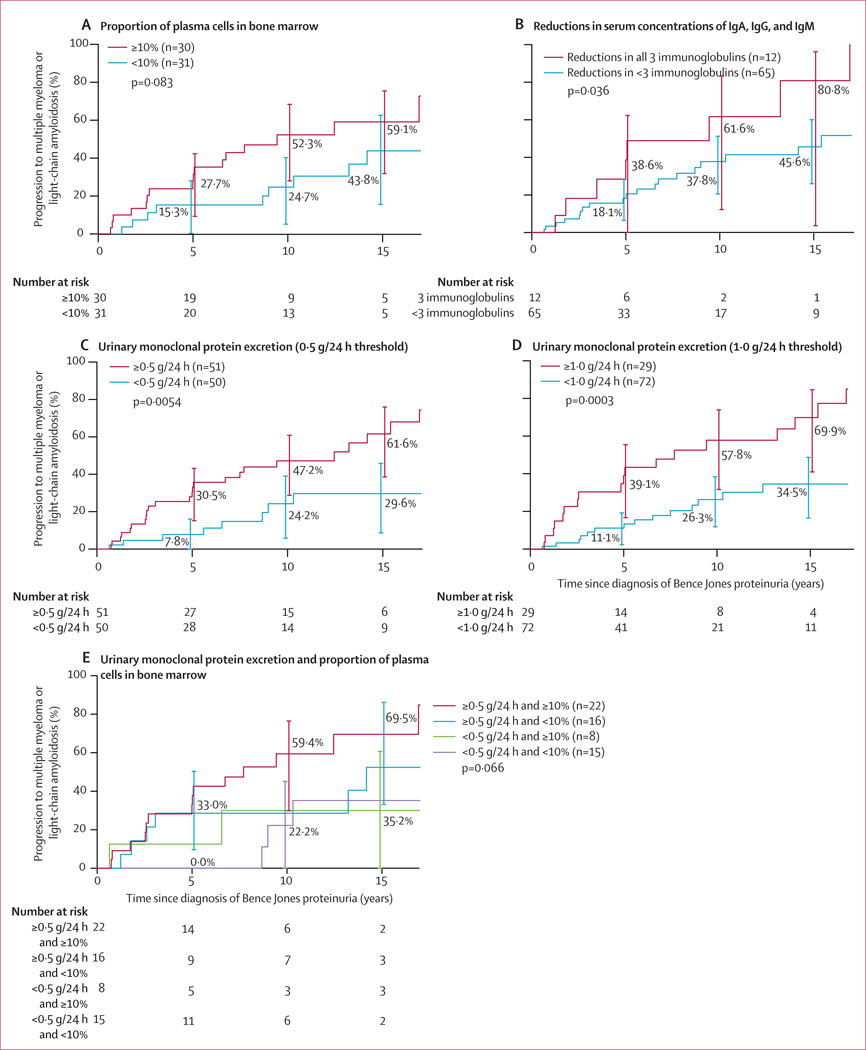
The amount of M protein in the urine was associated with a significantly increased relative risk of progression (HR 1·99 [95% CI 1·44–2·75]; p<0·0001; appendix). The risk of progression according to the varying amounts of urinary M-protein excretion is shown in table 2. Of the 61 patients for whom we had bone marrow data, the risk of progression at 10 years was greater in patients with more than 10% plasma cells in the bone marrow (52·3% [95% CI 28·0–68·4]) than in those with less than 10% plasma cells in the bone marrow (24·7% [5·1–41·6]), but was not statistically significant over the whole follow-up period (HR 2·00 [95% CI 0·90–4·44]; p=0·083; figure 2A). Of the 77 patients with quantitative immunoglobulins measured at diagnosis, the risk of progression was greater in the 12 patients with reduction in serum concentrations of all three immunoglobulins than in those with reduction in concentrations of fewer than three immunoglobulins (HR 2·36 [95% CI 1·03–5·42]; p=0·036; figure 2B). Progression did not significantly differ between patients who were tested for quantitative immunoglobulins and those who were not (HR 1·62 [95% CI 0·70–3·74]; p=0·26). An abnormal FLC ratio (<0·26 or >1·65) was detected in 64 (88%) of the 73 patients tested within 30 days of diagnosis, and these patients had a risk of progression of 49·4% (95% CI 32·1–62·3) at 10 years and 63·0% (43·3–77·6) at 15 years. The 73 patients whose FLC ratio was tested had increased progression compared with the 28 patients who were not tested (HR 3·89 [95% CI 1·37–11·06]; p=0·0060), but no difference in duration of survival was noted (data not shown; p=0·58). In the 27 patients with a markedly abnormal FLC ratio (<0·01 or >100), risk of progression at 10 years was 75·3% (95% CI 46·8–88·5) compared with 23·3% (5·6–37·7) for the 46 patients with an FLC ratio of 0·01–100 (HR 4·63 [95% CI 2·1–10·3]; p=0·0004).28 All nine patients with a normal FLC ratio (0·26–1·65) had urinary monoclonal protein excretion of less than 0·31 g/24 h. Only one of these nine patients progressed during observation.
Figure 2. Risk of progression to multiple myeloma or light-chain amyloidosis in patients with idiopathic Bence Jones proteinuria according to various clinical characteristics measured at diagnosis.
Risk was compared according to measurements taken at diagnosis of the proportion of plasma cells in the bone marrow (A), reductions in serum concentrations of IgA, IgG, and IgM (B), monoclonal protein excretion (C, D), and a combination of the proportion of plasma cells in the bone marrow and monoclonal protein excretion (E). Error bars show 95% CIs.
We identified thresholds of urinary monoclonal protein excretion at which patients had a risk of progression sufficient to be regarded as having light-chain smouldering multiple myeloma. Patients who had monoclonal light-chain excretion of 0·5 g/24 h or higher at diagnosis had nearly twice the risk of progression at 10 years as those with an excretion rate of less than 0·5 g/24 h (47·2% [95% CI 28·7–60·8] vs 24·2% [5·8–39·9], respectively; p=0·0054; figure 2C). A higher threshold of 1·0 g/24 h did not increase the risk of progression meaningfully compared with the 0·5 g/24 h threshold; at 10 years, the risk of progression was 57·8% (95% CI 31·7–73·9) in patients with an excretion rate of 1·0 g/24 h versus 26·3% (11·8–38·4) in those with an excretion of less than 1·0 g/24 h (HR 3·23 [95% CI 1·64–6·36]; p=0·0003; figure 2D).
The risk of progression to multiple myeloma or light-chain amyloidosis at 10 years in patients with urinary monoclonal protein excretion of 0·5 g/24 h or higher and at least 10% plasma cells in the bone marrow was 59·4% (95% CI 29·9–76·5) compared with 22·2% (0·0–45·1) for those with urinary monoclonal protein excretion of less than 0·5 g/24 h and less than 10% plasma cells in the bone marrow (HR 4·03 [95% CI 1·15–14·07]; p=0·0183; figure 2E).
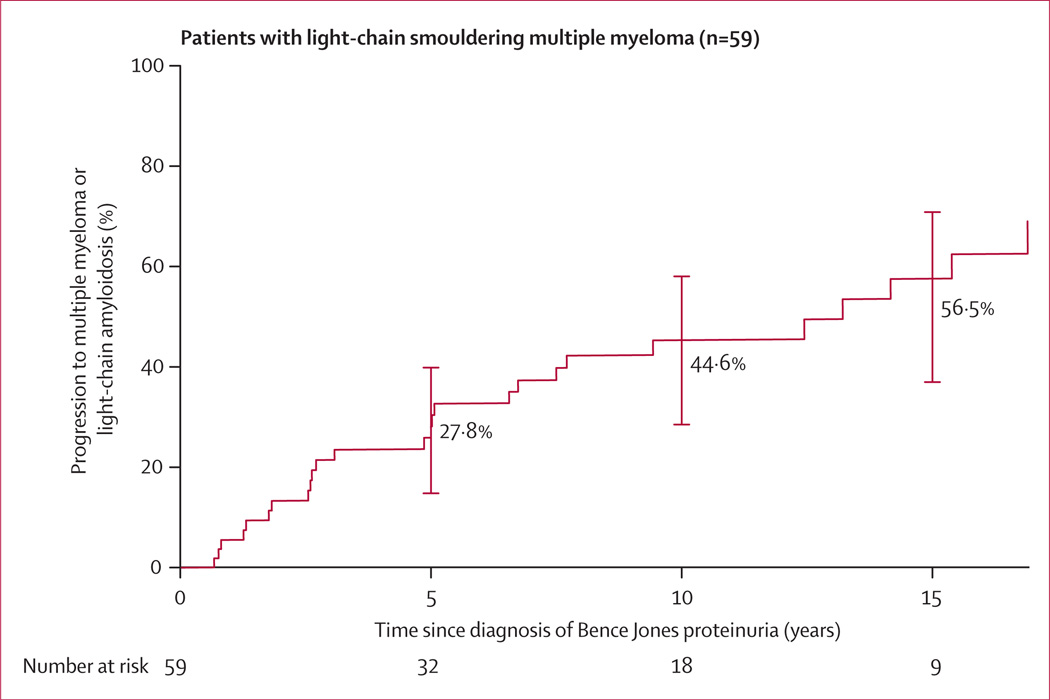
The risk of progression in the 59 patients with monoclonal protein excretion of 0·5 g/24 h or greater or 10% bone marrow plasma cells or higher, or both (which we have defined as light-chain smouldering multiple myeloma) was 27·8% (95% CI 14·2–39·2) at 5 years, 44·6% (27·9–57·4) at 10 years, and 56·5% (36·3–70·2) at 15 years (figure 3).
Figure 3. Risk of progression to multiple myeloma or light-chain amyloidosis in patients with light-chain smouldering multiple myeloma.
Light-chain smouldering multiple myeloma was defined as monoclonal light chain excretion of 0·5 g/24 h or higher, or at least 10% plasma cells in bone marrow, or both. Error bars show 95% CI.
Univariate analysis of other baseline parameters (age, sex, haemoglobin, serum calcium, and serum albumin) on the risk of progression to myeloma or light-chain amyloidosis in patients with idiopathic Bence Jones proteinuria is shown in the appendix.
Discussion
Results of this study show that patients with monoclonal light-chain excretion of 0·5 g/24 h or higher or at least 10% bone marrow plasma cells (or both), in the absence of end-organ damage, have a high risk of progression to multiple myeloma or light-chain amyloidosis. These patients are regarded as having light-chain smouldering multiple myeloma, and have a cumulative probability of progression to active multiple myeloma or light-chain amyloidosis of 27·8% at 5 years, 44·6% at 10 years, and 56·5% at 15 years.
Light-chain smouldering multiple myeloma defined in this Article represents the missing link between light-chain monoclonal gammopathy of undetermined significance and light-chain multiple myeloma (table 3; panel). It represents the light-chain equivalent of smouldering multiple myeloma and had not been defined thus far. Results of our study show that, above specific thresholds of M-protein excretion, idiopathic Bence Jones proteinuria represents light-chain smouldering multiple myeloma, with a clinical course analogous to that of smouldering multiple myeloma. It is a more advanced form of light-chain monoclonal gammopathy of undetermined significance and is the precursor of light-chain multiple myeloma. Light-chain monoclonal gammopathy of undetermined significance, light-chain smouldering multiple myeloma, and light-chain multiple myeloma are characterised by monoclonal plasma-cell proliferation with complete loss of expression of the heavy chain of the immunoglobulin molecule.18
Table 3.
Classification of multiple myeloma and its precursor disorders
| Monoclonal plasma-cell proliferative disorders with intact immunoglobulin |
Monoclonal plasma-cell proliferative disorders with complete loss of immunoglobulin heavy-chain expression (light chain only) |
|||||
|---|---|---|---|---|---|---|
| Monoclonal gammopathy of undetermined significance |
Smouldering multiple myeloma |
Multiple myeloma | Light-chain monoclonal gammopathy of undetermined significance |
Light-chain smouldering multiple myeloma |
Light-chain multiple myeloma |
|
| M-protein concentration* | <30 g/L in serum | ≥30 g/L in serum |
Presence of M protein in serum |
Abnormal free-light-chain ratio and increase of involved light chain with complete loss of heavy-chain expression in serum; urinary light chain M-protein excretion of <0·5 g/24 h |
Urinary light-chain M-protein excretion of ≥05 g/24 h |
Presence of light-chain only M protein (usually in urine, but can sometimes be seen in serum) |
| Proportion of plasma cells in bone marrow* |
<10% | ≥10% | ≥10% or biopsy-proven plasmacytoma |
<10% | ≥10% | ≥10% or biopsy-proven plasmacytoma |
| End-organ damage attributable to plasma-cell disorder |
Absent | Absent | Present | Absent | Absent | Present |
| Rate of progression | 1% per year | 10% per year for first 5 years and then decreases |
NA | 0·3% per year | 5% per year for 5 years, 3% per year for next 5 years, and 2% per year for next 5 years |
NA |
M-protein=monoclonal protein. NA=not applicable.
For the diagnosis of monoclonal gammopathy of undetermined significance and light-chain monoclonal gammopathy of undetermined significance, both M-protein and bone marrow plasma cell proportion requirements have to be fulfilled; conversely, for the diagnosis of smouldering multiple myeloma and light-chain smouldering multiple myeloma, M protein or bone marrow plasma cell proportion requirements have to be fulfilled.
Idiopathic Bence Jones proteinuria is infrequently recognised in clinical practice because the free-light-chain assay and urinary studies for M protein are not done on a routine basis. In fact, light-chain monoclonal gammopathy of undetermined significance was not recognised in Olmsted County residents7 when they were initially screened with serum protein electrophoresis and subsequent immunofixation of those with an abnormal electrophoretic pattern.7 When this population of Olmsted County residents7 was screened with the serum free-light-chain assay, we discovered that 0·8% of the general population older than 50 years had light-chain monoclonal gammopathy of undetermined significance.7,18 In this study, we examined all 101 patients with idiopathic Bence Jones proteinuria in detail to ensure that the initial diagnosis was accurate. Although anaemia was detected in some of these patients, a cause other than the plasma-cell proliferative process was identified; the same is true for those with hypercalcaemia or renal insufficiency. We then examined progression and the risk factors for progression to define the new entity of light-chain smouldering multiple myeloma. Although the study was done in patients followed up over a long period of time, the electrophoretic methods of estimating urinary M protein have undergone minimal changes, and any changes to the method were validated to ensure that the accuracy of the test remained unchanged.
The follow-up in this study is mature in that 93% of patients have died or progressed to multiple myeloma or light-chain amyloidosis; only 7% of the cohort is at risk for further progression. Patients with at least 10% bone marrow plasma cells had a risk of progression similar to smouldering multiple myeloma. Similarly, patients with urinary M-protein excretion of at least 0·5 g/24 h had a risk and pattern of progression that resembled smouldering multiple myeloma. A higher cutoff of 1·0 g/24 h did not improve discrimination. The risk of progression in the first 5 years is high, and hence is of particular importance when selecting a threshold for the definition of light-chain smouldering multiple myeloma. Furthermore, beyond that period the risk will also be affected by any change in urinary M-protein excretion that occurs over time, and we are less reliant on the baseline value for prognosis. The cumulative probability of progression to active multiple myeloma or light-chain amyloidosis in patients with monoclonal light-chain excretion of at least 0·5 g/24 h or at least 10% bone marrow plasma cells, or both, was high (28% at 5 years). Thus, we propose that the term light-chain smouldering multiple myeloma be adopted for patients with idiopathic Bence Jones proteinuria with urinary M-protein excretion of at least 0·5 g/24 h or at least 10% or more bone marrow plasma cells, or both. By contrast, the risk of progression in patients with monoclonal light chain excretion of less than 0·5 g/24 h and less than 10% bone marrow plasma cells was 0% in the first 5 years, and hence this population was not regarded as having light-chain smouldering multiple myeloma; these patients should be regarded as having light-chain monoclonal gammopathy of undetermined significance.
The proposed definition of light-chain smouldering multiple myeloma characterises a disease entity with a clinical course and progression that is slower than smouldering multiple myeloma; about 5·6% of patients per year with light-chain smouldering multiple myeloma progress compared with 10% per year with smouldering multiple myeloma.5 The reason for the slower progression needs to be established, but might be a result of a smaller relative clone size or a less clonally advanced neoplastic population in light-chain smouldering multiple myeloma. This study also clarifies the upper limit of light-chain excretion for light-chain monoclonal gammopathy of undetermined significance, which was not established when the entity was first described.18 Thus, the term light-chain monoclonal gammopathy of undetermined significance should be restricted to patients with a monoclonal light-chain excretion of less than 0·5 g/24 h (table 3), who did not progress in the first 5 years in this study; patients with this disorder should be followed up in 6 months with repeat testing and if stable every 2–3 years thereafter or at the time of symptoms.
One of the limitations of this study was that we were not able to measure serum FLC in all patients. As a result, we were unable to establish an FLC threshold for the definition of light-chain smouldering multiple myeloma. The FLC assay is serum-based and hence is easier to obtain than urinary M-protein measurement, which requires a 24 h collection.28 The FLC assay will also be useful in patients with existing renal impairment and low urinary output.
As with monoclonal gammopathy of undetermined significance and smouldering multiple myeloma, the rates of progression we provide are not adjusted for other competing causes of death; adjustment for competing risks would lower the risk estimates for progression in all premalignant plasma-cell disorders, and needs to be considered in clinical practice.
Patients with light-chain smouldering multiple myeloma should be reassessed after 3 months to exclude an evolving light-chain multiple myeloma, and if stable every 6 months thereafter. The FLC ratio is not proposed as part of the disease definition of light-chain smouldering multiple myeloma, but once the diagnosis is made should be considered during risk stratification. The risk of light-chain amyloidosis in light-chain smouldering multiple myeloma (seven of 101 patients) seems to be higher than that in smouldering multiple myeloma, and therefore monitoring of these patients should probably include a cardiac biomarker such as NT-proBNP. Translocation t(4;14), deletion 17p, and hyperdiploidy are known to increase risk of progression in smouldering multiple myeloma, and although their effect in light-chain smouldering multiple myeloma remains to be established, patients with such abnormalities need to be followed up more closely than patients without such abnormalities.29,30
Light-chain smouldering multiple myeloma represents the missing link between light-chain monoclonal gammopathy of undetermined significance and symptomatic light-chain multiple myeloma. Our definition identifies a subset of patients who do not need immediate therapy, but do need to be monitored more frequently than those with light-chain monoclonal gammopathy of undetermined significance (panel). With the availability of treatments that can delay progression in myeloma, we need to test new biomarkers to predict risk of progression, including cytogenetics and imaging in such patients, as well as clinical trials of early intervention. This study provides clarity to a disease entity that was recognised to exist, but proved elusive to define because of its rarity.
Supplementary Material
Panel: Research in context.
Systematic review
We searched PubMed with the search terms “idiopathic Bence Jones proteinuria”, “light chain smouldering myeloma”, and “light chain monoclonal gammopathy” for articles published in English only between Jan 1, 1980, and June 30, 2014. We found 57 articles and noted that the intermediate stage between light-chain monoclonal gammopathy of undetermined significance and light-chain multiple myeloma (ie, the equivalent of smouldering multiple myeloma) had not been defined.
Interpretation
To our knowledge, this study is the first to define the entity of light-chain smouldering multiple myeloma. Conventional smouldering multiple myeloma is defined using two markers: amount of serum monoclonal immunoglobulin (M protein) and proportion of plasma cells in bone marrow. In this study, light-chain smouldering multiple myeloma was also defined using similar parameters: concentration of urine M protein and bone marrow plasma cell proportion. On the basis of the risk of progression, urinary M-protein excretion of 0·5 g/24 h or higher or at least 10% bone marrow plasma cells, or both, in the absence of end-organ damage was defined as light-chain smouldering multiple myeloma. This study is important as it identifies the subset of patients with light-chain smouldering multiple myeloma, and estimates these patients’ risk of progression to multiple myeloma and light-chain amyloidosis. The study also provides the upper limit of M-protein excretion for the definition of light-chain monoclonal gammopathy of undetermined significance. It completes the systematic disease definition of the range of clonal plasma-cell disorders, and provides the link between light-chain monoclonal gammopathy of undetermined significance and light-chain multiple myeloma. Patients with light-chain smouldering multiple myeloma should be monitored more closely than patients without such abnormalities (every 3–4 months), similar to patients with smouldering multiple myeloma; by contrast, patients with monoclonal gammopathy of undetermined significance and light-chain monoclonal gammopathy of undetermined significance can be monitored yearly, since their risk of progression is much lower.
Acknowledgements
This work was supported in part by the Jabbs Foundation (Birmingham, UK), the US National Cancer Institute (grants CA 168762, CA 107476), and the Henry J Predolin Foundation (Madison, WI, USA).
AD has received grants from Celgene, Millennium, Jannsen, and Pfizer. SK has received grants from Celgene, Millennium, Onyx, Sanofi/Genzyme, and Merck, and personal fees from Sharp Healthcare.
Footnotes
Contributors
RAK and SVR designed the research, analysed the data, and wrote and edited the report. DRL did the statistical analysis and reviewed the manuscript. SK, TMT, JTB, LJM, and AD participated in data interpretation, reviewed the manuscript, and provided critical comments. All authors reviewed and approved the final report.
Declaration of interests
All other authors declare no competing interests.
References
- 1.Kyle RA. Monoclonal gammopathy of undetermined significance. Natural history in 241 cases. Am J Med. 1978;64:814–826. doi: 10.1016/0002-9343(78)90522-3. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Greipp PR. Smoldering multiple myeloma. N Engl J Med. 1980;302:1347–1349. doi: 10.1056/NEJM198006123022405. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 4.International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–757. [PubMed] [Google Scholar]
- 5.Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356:2582–2590. doi: 10.1056/NEJMoa070389. [DOI] [PubMed] [Google Scholar]
- 6.Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–5417. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362–1369. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 8.Therneau TM, Kyle RA, Melton LJ3rd, et al. Incidence of monoclonal gammopathy of undetermined significance and estimation of duration before first clinical recognition. Mayo Clinic Proc. 2012;87:1071–1079. doi: 10.1016/j.mayocp.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–569. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 10.Rajkumar SV. Multiple myeloma: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol. 2013;88:225–235. doi: 10.1002/ajh.23390. [DOI] [PubMed] [Google Scholar]
- 11.Bence Jones H. Papers on chemical pathology: prefaced by the Gulstonian Lectures, read at the Royal College of Physicians, 1846. Lancet. 1847;50:88–92. [Google Scholar]
- 12.Kyle RA, Greipp PR. “Idiopathic” Bence Jones proteinuria: long-term follow-up in seven patients. N Engl J Med. 1982;306:564–567. doi: 10.1056/NEJM198203113061002. [DOI] [PubMed] [Google Scholar]
- 13.Hobbs JR. Paraproteins, benign or malignant? Br Med J. 1967;3:699–704. doi: 10.1136/bmj.3.5567.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldenström J. Diagnosis and treatment of multiple myeloma. New York: Grune & Stratton; 1970. [Google Scholar]
- 15.Cronstedt J, Carling L, Ostberg H. Idiopathic light chain dyscrasia—a new distinct entity? report of a case. Acta Med Scand. 1974;196:445–447. doi: 10.1111/j.0954-6820.1974.tb01037.x. [DOI] [PubMed] [Google Scholar]
- 16.Virella G, Lopes-Virella MF, Levine J, Ogawa M, Gonzalez J. “Idiopathic” Bence-Jones proteinuria. Acta Haematol. 1978;60:269–279. doi: 10.1159/000207723. [DOI] [PubMed] [Google Scholar]
- 17.Paladini G, Sala PG, Santini PA. Benign Bence Jones gammopathy. Acta Haematol. 1980;63:241–246. doi: 10.1159/000207409. [DOI] [PubMed] [Google Scholar]
- 18.Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375:1721–1728. doi: 10.1016/S0140-6736(10)60482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyle RA, Katzmann JA, Lust JA, Dispenzieri A. Immunochemical characterization of immunoglobulins. In: NR Rose, RG Hamilton, B Detrick., editors. Manual of Clinical Laboratory Immunology. 6th edn. Washington, DC: ASM Press; 2002. pp. 71–91. [Google Scholar]
- 20.Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48:1437–1444. [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc Ser A Stat Soc. 1972;135:185–207. [Google Scholar]
- 23.Cox DR. Regression models and life-tables. J R Stat Soc Ser B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 24.Berry G. The analysis of mortality by the subject-years method. Biometrics. 1983;39:173–184. [PubMed] [Google Scholar]
- 25.National Cancer Institute Surveillance, Epidemiology, and End Results Program. [accessed July 25, 2012]; www.seer.cancer.gov.
- 26.National Cancer Institute Surveillance, Epidemiology, and End Results Program. [accessed July 25, 2012]; SEER*Stat software version 7.1.0. www.seer.cancer.gov/seerstat.
- 27.Kyle RA, Linos A, Beard CM, et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood. 1992;79:1817–1822. [PubMed] [Google Scholar]
- 28.Larsen JT, Kumar SK, Dispenzieri A, Kyle RA, Katzmann JA, Rajkumar SV. Serum free light chain ratio as a biomarker for high-risk smoldering multiple myeloma. Leukemia. 2013;27:941–946. doi: 10.1038/leu.2012.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neben K, Jauch A, Hielscher T, et al. Progression in smoldering myeloma is independently determined by the chromosomal abnormalities del(17p), t(4;14), gain 1q, hyperdiploidy, and tumor load. J Clin Oncol. 2013;31:4325–4332. doi: 10.1200/JCO.2012.48.4923. [DOI] [PubMed] [Google Scholar]
- 30.Rajkumar SV, Gupta V, Fonseca R, et al. Impact of primary molecular cytogenetic abnormalities and risk of progression in smoldering multiple myeloma. Leukemia. 2013;27:1738–1744. doi: 10.1038/leu.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.