Abstract
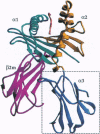
Class I major histocompatibility complex (MHC) molecules are ternary complexes of the soluble serum protein beta 2-microglobulin, MHC heavy chain, and bound peptide. The first two domains (alpha 1, alpha 2) of the heavy chain create the peptide binding cleft and the surface that contacts the T-cell receptor. The third domain (alpha 3) associates with the T-cell co-receptor, CD8, during T-cell recognition. Here we describe the x-ray crystal structure of a human class I MHC molecule, HLA-Aw68, from which the alpha 3 domain has been proteolytically removed. The resulting molecule shows no gross morphological changes compared to the intact protein. A decameric peptide complexed with the intact HLA-Aw68 is seen to bind to the proteolized molecule in the conventional manner, demonstrating that the alpha 3 domain is not required for the structural integrity of the molecule or for peptide binding.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blue M. L., Craig K. A., Anderson P., Branton K. R., Jr, Schlossman S. F. Evidence for specific association between class I major histocompatibility antigens and the CD8 molecules of human suppressor/cytotoxic cells. Cell. 1988 Jul 29;54(3):413–421. doi: 10.1016/0092-8674(88)90204-8. [DOI] [PubMed] [Google Scholar]
- Cerundolo V., Tse A. G., Salter R. D., Parham P., Townsend A. CD8 independence and specificity of cytotoxic T lymphocytes restricted by HLA-Aw68.1. Proc Biol Sci. 1991 May 22;244(1310):169–177. doi: 10.1098/rspb.1991.0066. [DOI] [PubMed] [Google Scholar]
- Colovos C., Yeates T. O. Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci. 1993 Sep;2(9):1511–1519. doi: 10.1002/pro.5560020916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T., Elvin J., Cerundolo V., Allen H., Townsend A. Structural requirements for the peptide-induced conformational change of free major histocompatibility complex class I heavy chains. Eur J Immunol. 1992 Aug;22(8):2085–2091. doi: 10.1002/eji.1830220819. [DOI] [PubMed] [Google Scholar]
- Ezquerra A., Bragado R., Vega M. A., Strominger J. L., Woody J., López de Castro J. A. Primary structure of papain-solubilized human histocompatibility antigen HLA-B27. Biochemistry. 1985 Mar 26;24(7):1733–1741. doi: 10.1021/bi00328a025. [DOI] [PubMed] [Google Scholar]
- Fremont D. H., Matsumura M., Stura E. A., Peterson P. A., Wilson I. A. Crystal structures of two viral peptides in complex with murine MHC class I H-2Kb. Science. 1992 Aug 14;257(5072):919–927. doi: 10.1126/science.1323877. [DOI] [PubMed] [Google Scholar]
- Garboczi D. N., Hung D. T., Wiley D. C. HLA-A2-peptide complexes: refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3429–3433. doi: 10.1073/pnas.89.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N., Margulies D. H. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- Giblin P. A., Leahy D. J., Mennone J., Kavathas P. B. The role of charge and multiple faces of the CD8 alpha/alpha homodimer in binding to major histocompatibility complex class I molecules: support for a bivalent model. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1716–1720. doi: 10.1073/pnas.91.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H. C., Jardetzky T. S., Garrett T. P., Lane W. S., Strominger J. L., Wiley D. C. Different length peptides bind to HLA-Aw68 similarly at their ends but bulge out in the middle. Nature. 1992 Nov 26;360(6402):364–366. doi: 10.1038/360364a0. [DOI] [PubMed] [Google Scholar]
- Guo H. C., Madden D. R., Silver M. L., Jardetzky T. S., Gorga J. C., Strominger J. L., Wiley D. C. Comparison of the P2 specificity pocket in three human histocompatibility antigens: HLA-A*6801, HLA-A*0201, and HLA-B*2705. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8053–8057. doi: 10.1073/pnas.90.17.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardetzky T. S., Lane W. S., Robinson R. A., Madden D. R., Wiley D. C. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Lehmann-Grube F., Dralle H., Utermöhlen O., Löhler J. MHC class I molecule-restricted presentation of viral antigen in beta 2-microglobulin-deficient mice. J Immunol. 1994 Jul 15;153(2):595–603. [PubMed] [Google Scholar]
- Lüthy R., Bowie J. U., Eisenberg D. Assessment of protein models with three-dimensional profiles. Nature. 1992 Mar 5;356(6364):83–85. doi: 10.1038/356083a0. [DOI] [PubMed] [Google Scholar]
- MacFerrin K. D., Chen L., Terranova M. P., Schreiber S. L., Verdine G. L. Overproduction of proteins using expression-cassette polymerase chain reaction. Methods Enzymol. 1993;217:79–102. doi: 10.1016/0076-6879(93)17057-c. [DOI] [PubMed] [Google Scholar]
- Madden D. R., Garboczi D. N., Wiley D. C. The antigenic identity of peptide-MHC complexes: a comparison of the conformations of five viral peptides presented by HLA-A2. Cell. 1993 Nov 19;75(4):693–708. doi: 10.1016/0092-8674(93)90490-h. [DOI] [PubMed] [Google Scholar]
- Madden D. R., Gorga J. C., Strominger J. L., Wiley D. C. The structure of HLA-B27 reveals nonamer self-peptides bound in an extended conformation. Nature. 1991 Sep 26;353(6342):321–325. doi: 10.1038/353321a0. [DOI] [PubMed] [Google Scholar]
- Monzingo A. F., Collins E. J., Ernst S. R., Irvin J. D., Robertus J. D. The 2.5 A structure of pokeweed antiviral protein. J Mol Biol. 1993 Oct 20;233(4):705–715. doi: 10.1006/jmbi.1993.1547. [DOI] [PubMed] [Google Scholar]
- Olsen A. C., Pedersen L. O., Hansen A. S., Nissen M. H., Olsen M., Hansen P. R., Holm A., Buus S. A quantitative assay to measure the interaction between immunogenic peptides and purified class I major histocompatibility complex molecules. Eur J Immunol. 1994 Feb;24(2):385–392. doi: 10.1002/eji.1830240218. [DOI] [PubMed] [Google Scholar]
- Parker K. C., Wiley D. C. Overexpression of native human beta 2-microglobulin in Escherichia coli and its purification. Gene. 1989 Nov 15;83(1):117–124. doi: 10.1016/0378-1119(89)90409-5. [DOI] [PubMed] [Google Scholar]
- Rosenstein Y., Ratnofsky S., Burakoff S. J., Herrmann S. H. Direct evidence for binding of CD8 to HLA class I antigens. J Exp Med. 1989 Jan 1;169(1):149–160. doi: 10.1084/jem.169.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert J., Sidney J., Celis E., Kubo R. T., Grey H. M., Sette A. Prominent role of secondary anchor residues in peptide binding to HLA-A2.1 molecules. Cell. 1993 Sep 10;74(5):929–937. doi: 10.1016/0092-8674(93)90472-3. [DOI] [PubMed] [Google Scholar]
- Salter R. D., Norment A. M., Chen B. P., Clayberger C., Krensky A. M., Littman D. R., Parham P. Polymorphism in the alpha 3 domain of HLA-A molecules affects binding to CD8. Nature. 1989 Mar 23;338(6213):345–347. doi: 10.1038/338345a0. [DOI] [PubMed] [Google Scholar]
- Saper M. A., Bjorkman P. J., Wiley D. C. Refined structure of the human histocompatibility antigen HLA-A2 at 2.6 A resolution. J Mol Biol. 1991 May 20;219(2):277–319. doi: 10.1016/0022-2836(91)90567-p. [DOI] [PubMed] [Google Scholar]
- Silver M. L., Guo H. C., Strominger J. L., Wiley D. C. Atomic structure of a human MHC molecule presenting an influenza virus peptide. Nature. 1992 Nov 26;360(6402):367–369. doi: 10.1038/360367a0. [DOI] [PubMed] [Google Scholar]
- Silver M. L., Parker K. C., Wiley D. C. Reconstitution by MHC-restricted peptides of HLA-A2 heavy chain with beta 2-microglobulin, in vitro. Nature. 1991 Apr 18;350(6319):619–622. doi: 10.1038/350619a0. [DOI] [PubMed] [Google Scholar]
- Stern L. J., Wiley D. C. Antigenic peptide binding by class I and class II histocompatibility proteins. Structure. 1994 Apr 15;2(4):245–251. doi: 10.1016/s0969-2126(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Townsend A., Elliott T., Cerundolo V., Foster L., Barber B., Tse A. Assembly of MHC class I molecules analyzed in vitro. Cell. 1990 Jul 27;62(2):285–295. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- Young A. C., Zhang W., Sacchettini J. C., Nathenson S. G. The three-dimensional structure of H-2Db at 2.4 A resolution: implications for antigen-determinant selection. Cell. 1994 Jan 14;76(1):39–50. doi: 10.1016/0092-8674(94)90171-6. [DOI] [PubMed] [Google Scholar]