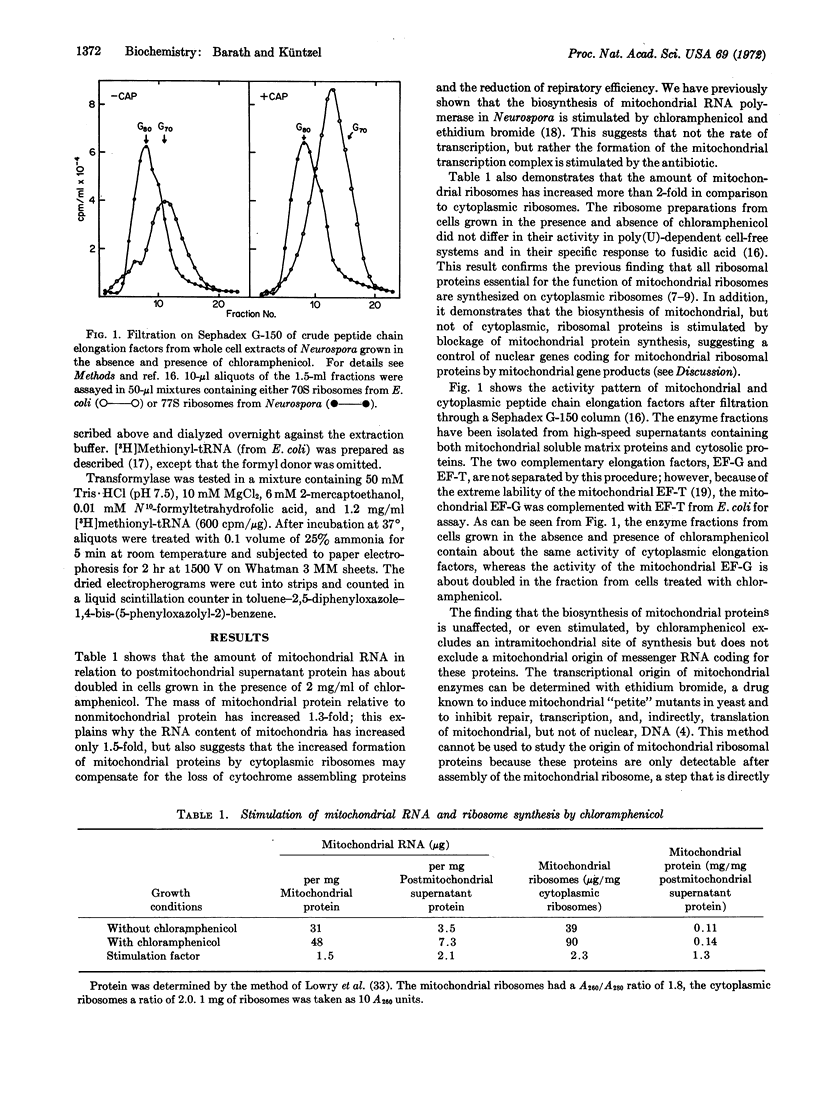
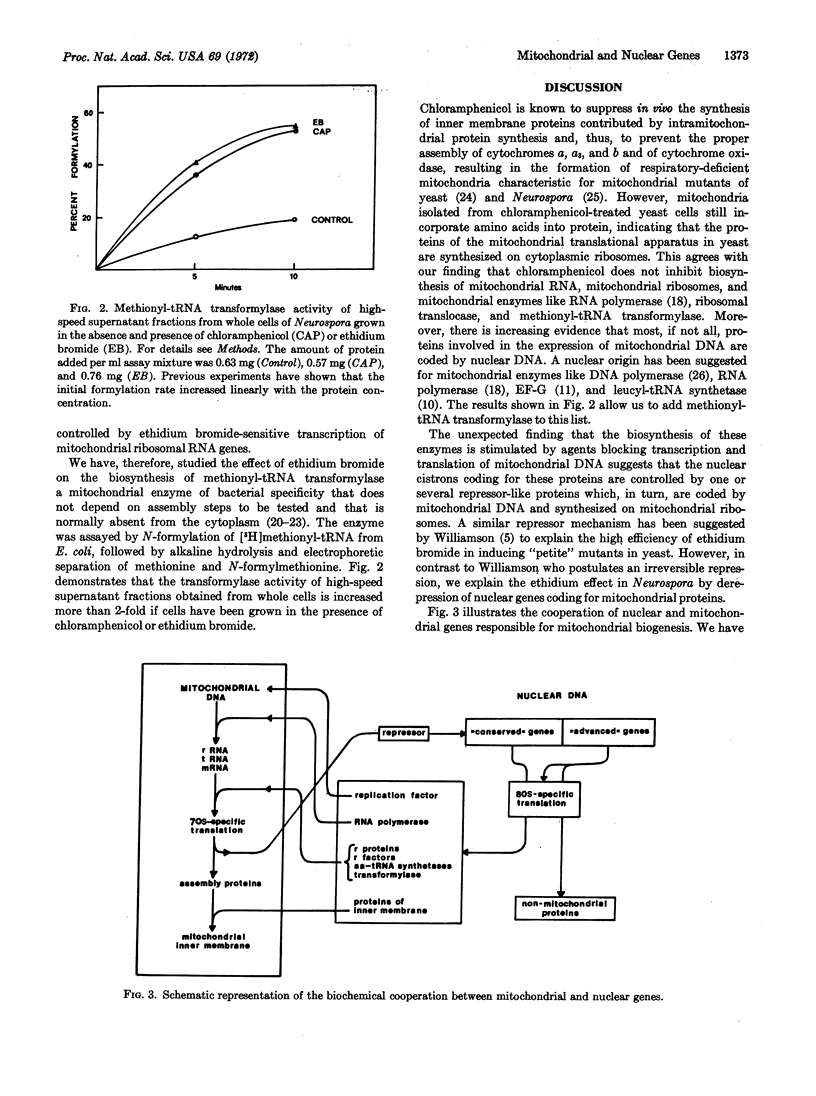
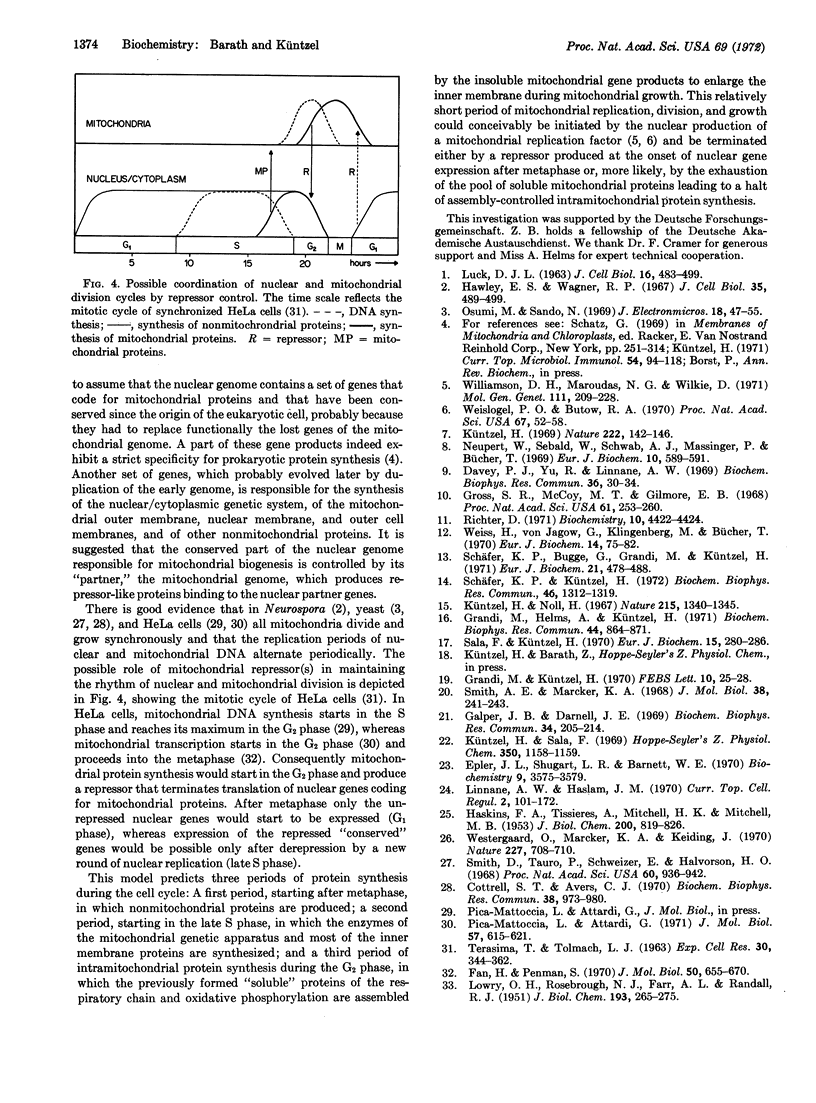
Abstract
Enzymes involved in the expression of the mitochondrial genome in Neurospora crassa are induced by chloramphenicol and ethidium bromide, which block transcription and translation of mitochondrial DNA. It is concluded that most, if not all, proteins of the mitochondrial genetic apparatus are coded by nuclear genes, synthesized on cytoplasmic ribosomes, and controlled by a repressor-like mitochondrial gene product. A model explaining the coordination of nuclear and mitochondrial division cycles by repressor control is discussed.
Keywords: chloramphenicol, ethidium bromide, repressor control, model
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cottrell S. F., Avers C. J. Evidence of mitochondrial synchrony in synchronous cell cultures of yeast. Biochem Biophys Res Commun. 1970 Mar 12;38(5):973–980. doi: 10.1016/0006-291x(70)90817-x. [DOI] [PubMed] [Google Scholar]
- Davey P. J., Yu R., Linnane A. W. The intracellular site of formation of the mitochondrial protein synthetic system. Biochem Biophys Res Commun. 1969 Jul 7;36(1):30–34. doi: 10.1016/0006-291x(69)90644-5. [DOI] [PubMed] [Google Scholar]
- Epler J. L., Shugart L. R., Barnett W. E. N-formylmethionyl transfer ribonucleic acid in mitochondria from Neurospora. Biochemistry. 1970 Sep 1;9(18):3575–3579. doi: 10.1021/bi00820a011. [DOI] [PubMed] [Google Scholar]
- Fan H., Penman S. Regulation of protein synthesis in mammalian cells. II. Inhibition of protein synthesis at the level of initiation during mitosis. J Mol Biol. 1970 Jun 28;50(3):655–670. doi: 10.1016/0022-2836(70)90091-4. [DOI] [PubMed] [Google Scholar]
- Galper J. B., Darnell J. E. The presence of N-formyl-methionyl-tRNA in HeLa cell mitochondria. Biochem Biophys Res Commun. 1969 Jan 27;34(2):205–214. doi: 10.1016/0006-291x(69)90633-0. [DOI] [PubMed] [Google Scholar]
- Grandi M., Helms A., Küntzel H. Fusidic acid resistance of mitochondrial G factor from Neurospora crassa. Biochem Biophys Res Commun. 1971 Aug 20;44(4):864–871. doi: 10.1016/0006-291x(71)90791-1. [DOI] [PubMed] [Google Scholar]
- HASKINS F. A., TISSIERES A., MITCHELL H. K., MITCHELL M. B. Cytochromes and the succinic acid oxidase system of poky strains of Neurospora. J Biol Chem. 1953 Feb;200(2):819–826. [PubMed] [Google Scholar]
- Hawley E. S., Wagner R. P. Synchronous mitochondrial division in Neurospora crassa. J Cell Biol. 1967 Dec;35(3):489–499. doi: 10.1083/jcb.35.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küntzel H. The genetic apparatus of mitochondria from Neurospora and yeast. Curr Top Microbiol Immunol. 1971;54:94–118. doi: 10.1007/978-3-642-65123-6_4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUCK D. J. Formation of mitochondria in Neurospora crassa. A quantitative radioautographic study. J Cell Biol. 1963 Mar;16:483–499. doi: 10.1083/jcb.16.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert W., Sebald W., Schwab A. J., Massinger P., Bücher T. Incorporation in vivo of 14C-labelled amino acids into the proteins of mitochondrial ribosomes from Neurospora crassa sensitive to cycloheximide and insensitive to Chloramphenicol. Eur J Biochem. 1969 Oct;10(3):589–591. doi: 10.1111/j.1432-1033.1969.tb00730.x. [DOI] [PubMed] [Google Scholar]
- Pica-Mattoccia L., Attardi G. Expression of the mitochondrial genome in HeLa cells. V. Transcription of mitochondrial DNA in relationship to the cell cycle. J Mol Biol. 1971 May 14;57(3):615–621. doi: 10.1016/0022-2836(71)90113-6. [DOI] [PubMed] [Google Scholar]
- Sala F., Küntzel H. Peptide chain initiation in homologous and heterologous systems from mitochondria and bacteria. Eur J Biochem. 1970 Aug;15(2):280–286. doi: 10.1111/j.1432-1033.1970.tb01005.x. [DOI] [PubMed] [Google Scholar]
- Schäfer K. P., Bugge G., Grandi M., Küntzel H. Transcription of mitochondrial DNA in vitro from Neurospora crassa. Eur J Biochem. 1971 Aug 25;21(4):478–488. doi: 10.1111/j.1432-1033.1971.tb01493.x. [DOI] [PubMed] [Google Scholar]
- Schäfer K. P., Küntzel H. Mitochondrial genes in neurospora: a single cistron for ribosomal RNA. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1312–1319. doi: 10.1016/s0006-291x(72)80118-9. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A. N-formylmethionyl transfer RNA in mitochondria from yeast and rat liver. J Mol Biol. 1968 Dec 14;38(2):241–243. doi: 10.1016/0022-2836(68)90409-9. [DOI] [PubMed] [Google Scholar]
- Smith D., Tauro P., Schweizer E., Halvorson H. O. The replication of mitochondrial DNA during the cell cycle in Saccharomyces lactis. Proc Natl Acad Sci U S A. 1968 Jul;60(3):936–942. doi: 10.1073/pnas.60.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss H., von Jagow G., Klingenberg M., Bücher T. Characterization of Neurospora crassa mitochondria prepared with a grind-mill. Eur J Biochem. 1970 May 1;14(1):75–82. doi: 10.1111/j.1432-1033.1970.tb00263.x. [DOI] [PubMed] [Google Scholar]
- Westergaard O., Marcker K. A., Keiding J. Induction of a mitochondrial DNA polymerase in Tetrahymena. Nature. 1970 Aug 15;227(5259):708–710. doi: 10.1038/227708a0. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Maroudas N. G., Wilkie D. Induction of the cytoplasmic petite mutation in Saccharomyces cerevisiae by the antibacterial antibiotics erythromycin and chloramphenicol. Mol Gen Genet. 1971;111(3):209–223. doi: 10.1007/BF00433106. [DOI] [PubMed] [Google Scholar]



