Abstract
Substantial treatment variation exists among patients with rectal cancer. A survey of surgeons demonstrated that fellowship trained colorectal surgeons and surgical oncologists were more likely than general surgeons to prefer guideline concordant staging procedures (transrectal ultrasound/magnetic resonance imaging) and pre-operative chemoradiation therapy for rectal cancer patients.
Keywords: rectal cancer, neo-adjuvant therapy, guideline adherence
Introduction
Treatment guidelines for rectal cancer have changed over the past several years due to advances in diagnostic/imaging procedures, improved surgical techniques, and adjuvant/neoadjuvant therapies.1 While post-operative (post-op) chemoradiation therapy (CRT) was the standard of care for locally advanced rectal cancer throughout most of the 1990’s, studies in the early 2000’s provided evidence in favor of pre-operative (pre-op) CRT due to studies demonstrating that pre-op CRT decreased loco-regional recurrence rates and reduced toxicity,2–4 and increased rates of sphincter preservation when compared to post-op CRT.3 For these reasons, the use of pre-op CRT for Stages II/III rectal adenocarcinoma based upon careful pretreatment staging has become incorporated into treatment guidelines such as those published by the National Comprehensive Cancer Network (NCCN).5 However, analyses of large databases such as Surveillance Epidemiology End Results (SEER) and Cancer Care Outcomes Research and Surveillance Consortium (CanCORS) have demonstrated that a substantial proportion of Stage II/III rectal cancer patients (24%) undergoing surgery and CRT are still receiving it post-operatively.6,7 While the proportion receiving pre-op CRT has been increasing and the proportion receiving post-op CRT has been decreasing, the most recent SEER data show that among those who received RT, the proportion receiving it pre-operatively is still below 80%; 21% of 2010 cases received RT post-operatively.8
The use of pre-op CRT has been shown to be associated with documented use of recommended loco-regional staging methods.7,9,10 NCCN guidelines recommend the use of either transrectal ultrasound (TRUS) or magnetic resonance imaging (MRI) of the abdomen/pelvis because they have demonstrated the capability to determine tumor depth and local lymph node metastases with high levels of sensitivity.5,11–15 However, studies have demonstrated consistent underutilization of TRUS and MRI in the loco-regional staging of rectal cancer.7,16,17
Few studies have elicited information directly from general surgeons, colorectal surgeons and surgical oncologists on their preferences for staging and treatment regimens for rectal cancer, or potential barriers to adhering to treatment guidelines. The objective of this study was to determine if surgeon preferences align with clinical guidelines, and if practices and perceived barriers to providing recommended care for rectal cancer vary by sub-specialty training and/or years in practice.
Materials and Methods
Surgeons practicing in the state of Florida were identified from a membership roster of The American College of Surgeons and The American Society of Colon and Rectal Surgeons, and their email addresses were obtained from the Florida Board of Medicine licensure verification site. From December 2006 to March 2007, surveys were sent via e-mail to 759 surgeons, and reminder e-mails were sent to non-responders at 2 and 4 weeks from the initial e-mail. The survey was not labeled with any identifiers, nor did it ask surgeons to document any personal identifiers; responses were therefore anonymous.
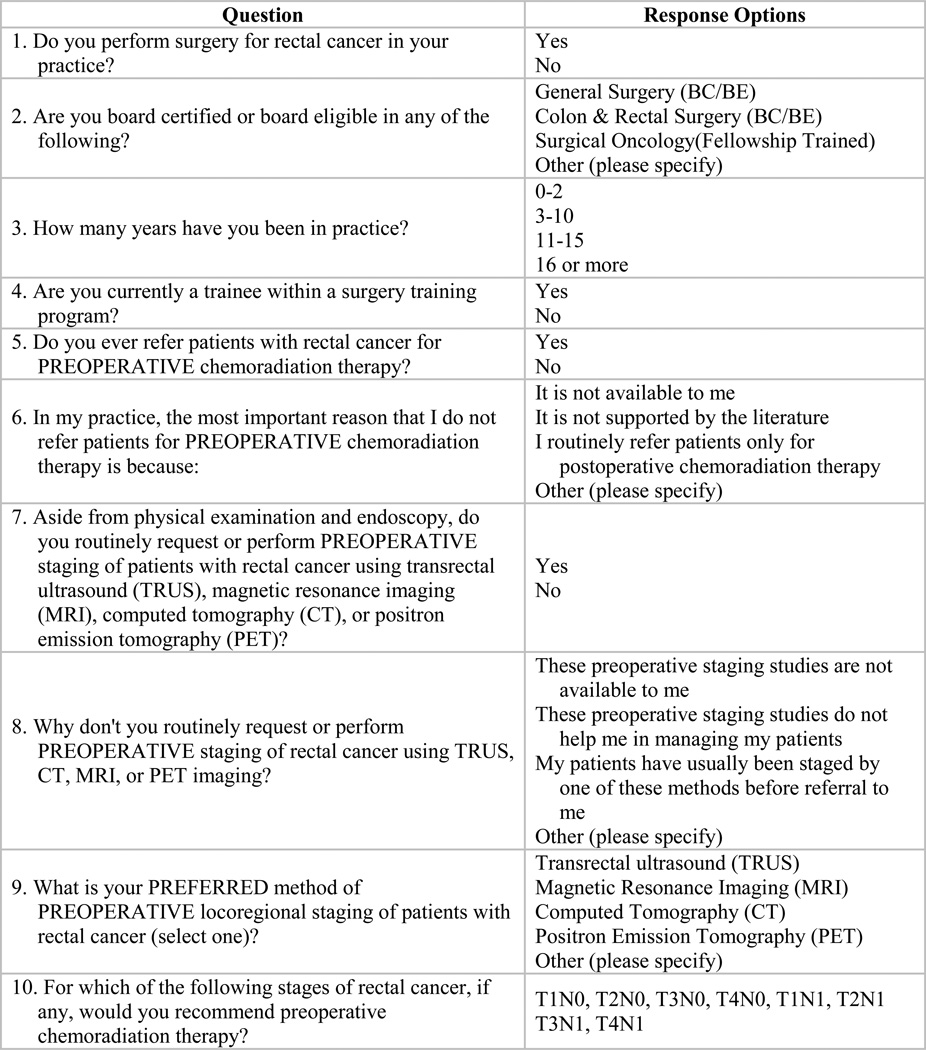
The survey instrument first ascertained whether or not the surgeon performed surgery for rectal cancer in his/her practice, if he/she was board-certified or board-eligible in general surgery or colon and rectal surgery or fellowship-trained in surgical oncology, and if he/she was currently a trainee within a surgery training program (Figure 1). Those who responded that they did not perform surgery on rectal cancer patients, were not board certified or board eligible in surgery or were a trainee were excluded from analysis. The remaining seven questions assessed years of practice, use of and preferred methods for loco-regional staging, use of pre-op CRT referral in practice and stages appropriate for pre-op CRT referral.
Figure 1.
Survey instrument questions and response options.
NCCN guidelines were used as a reference to evaluate the appropriateness of surgeon preferences. The guidelines recommend all patients with suspected rectal cancer undergo loco-regional staging involving a MRI of the abdomen/pelvis or a TRUS, so if the surgeons responded they routinely request or perform pre-op staging, and their preferred method of loco-regional staging was TRUS or MRI, then they were considered to have guideline concordant preferences. Likewise, if they responded that they recommend pre-op CRT for all Stage II/III rectal cancer patients (stages T3N0, T4N0 and TXN1) then they were considered to have guideline concordant recommendations for pre-op CRT.
Chi square tests were used to compare responses by physician specialty and years in practice. Logistic regression was used to model the multivariate relationship between predictor variables (physician specialty and years in practice) and guideline concordant staging preferences and pre-op CRT recommendations. All analyses were conducted using SAS® statistical software version 9.1 (Cary, NC).
Results
Of the 759 surgeons who were sent a survey instrument, 321 (42%) responded. Of those who responded, 117 (36%) were excluded because they did not treat rectal cancer patients, and 41 (13%) were excluded because they were trainees and/or not board certified or board eligible in surgery. The characteristics, practices and preferences by specialty are presented in Table 1.
Table 1.
Physician Characteristics, Practices and Preferences by Specialty
| Survey Responses | All Surgeons (N=163) |
Physician Specialty |
p-value | ||
|---|---|---|---|---|---|
| General Surgeon (N=115) |
Colorectal Surgeon (N=30) |
Surgical Oncologist (N=18) |
|||
| Years in Practice | p=0.46 | ||||
| 0–2 years | 12 (7%) | 8 (7%) | 2 (7%) | 2 (11%) | |
| 3–10 years | 42 (26%) | 25 (22%) | 9 (30%) | 8 (44%) | |
| 11–15 years | 21 (13%) | 15 (13%) | 4 (13%) | 2 (11%) | |
| 16+ years | 88 (54%) | 67 (58%) | 15 (50%) | 6 (33%) | |
| Refer patients for pre-op CRT | p=0.43 | ||||
| Yes | 159 (98%) | 111 (97%) | 30 (100%) | 18 (100%) | |
| No | 3 (2%) | 3 (3%) | 0 (0%) | 0 (0%) | |
| Missing response | 1 (1%) | 1 (1%) | 0 (0%) | 0 (0%) | |
| Routinely request/perform pre-op staging of patients using TRUS, MRI, CT or PET |
p=0.07 | ||||
| Yes | 151 (93%) | 103 (90%) | 30 (100%) | 18 (100%) | |
| No | 7 (4%) | 7 (6%) | 0 (0%) | 0 (0%) | |
| Missing response | 5 (3%) | 5 (4%) | 0 (0%) | 0 (0%) | |
| Preferred method of pre-op locoregional staging |
p=0.02 | ||||
| TRUS | 80 (49%) | 46 (40%) | 19 (63%) | 15 (83%) | |
| MRI | 12 (7%) | 9 (8%) | 3 (10%) | 0 (0%) | |
| CT | 55 (34%) | 46 (40%) | 6 (20%) | 3 (17%) | |
| PET | 7 (4%) | 7 (6%) | 0 (0%) | 0 (0%) | |
| Other | 2 (1%) | 1 (1%) | 1 (3%) | 0 (0%) | |
| Missing Response | 7 (4%) | 6 (5%) | 1 (3%) | 0 (0%) | |
| Recommend pre-op CRT for the following stages |
|||||
| T3N0 | 126 (77%) | 83 (72%) | 26 (87%) | 17 (94%) | p=0.04 |
| T4N0 | 128 (79%) | 83 (72%) | 28 (93%) | 17 (94%) | p<0.009 |
| T1N1 | 102 (63%) | 65 (57%) | 24 (80%) | 13 (72%) | p=0.04 |
| T2N1 | 109 (67%) | 71 (44%) | 25 (83%) | 13 (72%) | p=0.07 |
| T3N1 | 127 (78%) | 82 (71%) | 27 (90%) | 18 (100%) | p=0.005 |
| T4N1 | 118 (72%) | 74 (64%) | 27 (90%) | 17 (94%) | p=0.002 |
Among the 163 surgeons meeting eligibility criteria, 71% were general surgeons, 18% were colorectal surgeons, and 11% were surgical oncologists. There were no differences by specialty related to the number of years in practice or the percent reporting they have ever referred rectal cancer patients for pre-op CRT. Ninety-three percent of general surgeons reported that they routinely requested or performed pre-op staging with TRUS, MRI, CT or PET compared to 100% of colorectal surgeons and surgical oncologists. Of the seven general surgeons who did not request/perform pre-op staging, four indicated that their patients have usually been staged by one of these methods before referral to them, one reported these methods did not help him/her in managing patients, one said that he/she does it selectively and one did not specify a reason. There was a significant difference in preferred methods of pre-op loco-regional staging methods among the different specialties. The general surgeons were split between TRUS and CT, whereas a majority of colorectal surgeons and surgical oncologists preferred TRUS over CT.
There were also significant differences by specialty in terms of the specific stages for which they recommend pre-op CRT. Very high proportions (≥94%) of surgical oncologists recommended pre-op CRT for T3NX and T4NX stages, whereas lower proportions (72–79%) of general surgeons recommended pre-op CRT for these same stages, and colorectal surgeons were in the middle (87–93%). High proportions of colorectal surgeons reported that they recommended pre-op CRT for T1N1 and T2N1 stages (80% and 83%, respectively), whereas lower proportions of general surgeons recommended pre-op CRT for these stages (57% and 44%, respectively), with surgical oncologists in the middle (72%). Of three general surgeons who indicated that they do not refer rectal cancer patients for pre-op CRT, one reported that he/she routinely refers patients for post-op CRT, one said it is not supported by the literature and one did not specify a reason.
Table 2 illustrates how guideline concordant preferences for staging methods and recommendations for pre-op CRT vary between general surgeons and fellowship-trained colorectal surgeons/surgical oncologists. A significantly higher proportion of colorectal surgeons/surgical oncologists reported preferences for MRI or TRUS (79%) and recommended pre-op CRT for all Stage II/III rectal cancers (non-metastatic T3N0, T4N0 and TXN1 stages) (71%) compared to general surgeons (50% and 45%, respectively).
Table 2.
NCCN guideline concordant preferences for staging methods and recommendations for pre-op CRT by physician specialty and years in practice
| NCCN guidelines Surgeon Characteristics |
Preferred method of pre-op loco-regional staging is MRI or TRUS* |
Recommend pre-op CRT for all Stage II/III |
|---|---|---|
| All Surgeons | 92 (59%) | 86 (53%) |
| Physician Specialty | ||
| General Surgeon | 55 (50%) | 52 (45%) |
| Colorectal Surgeon/Surgical Oncologist | 37 (79%) | 34 (71%) |
| p=0.001 | p=0.003 | |
| Years in Practice | ||
| 0 – 2 Years | 8 (67%) | 2 (17%) |
| 3 – 10 Years | 26 (63%) | 24 (57%) |
| 11 – 15 Years | 15 (71%) | 15 (71%) |
| 16+ Years | 43 (52%) | 45 (51%) |
| p=0.33 | p=0.02 |
N=156 due to missing responses.
Table 2 also displays the relationship between the number of years in practice and loco-regional staging method preferences and pre-op CRT recommendations. While there were no significant differences among the categories of years in practice (0–2 years, 3–10 years, 11–15 years or 16+ years) in relation to preferring TRUS/MRI for loco-regional staging (p=0.33), there were significant differences among the categories of years in practice and recommendations for pre-op CRT for all Stage II/III rectal cancers (p=0.02). The highest proportion of surgeons reporting guideline concordant recommendations was among those practicing 11–15 years (71%), and the lowest proportion was among those practicing 0–2 years (17%). Just over half of those practicing 3–10 years or 16+ years reported guideline-concordant recommendations for pre-op CRT.
Consistent with the univariate analyses, results of logistic regression models showed that general surgeons were less likely to report NCCN guideline concordant staging preferences compared to fellowship trained colorectal surgeons and surgical oncologists (OR=0.279, 95% CI: 0.125, 0.622) after controlling for years of practice, which was not significant in the model (Table 3). Both provider specialty and years in practice remained significant in the model predicting NCCN guideline concordant pre-op CRT recommendations, with general surgeons less likely to recommend pre-op CRT for all Stage II/III rectal cancer patients compared to colorectal surgeons/surgical oncologists (OR=0.306, 95% CI: 0.142, 0.661) and those in practice less than 2 years less likely than those practicing 16 or more years (OR=0.151, 95% CI: 0.030, 0.775). Interaction terms were tested but were not significant.
Table 3.
Multivariate models predicting NCCN guideline concordant staging method preferences and recommendations for pre-op CRT
| NCCN guidelines | Odds ratio and 95% Confidence Interval |
|---|---|
|
Model 1 Predictors of guideline concordant staging method preferences(N=156) |
|
| Physician Specialty | |
| General Surgeon | 0.28 (0.13, 0.62)* |
| Colorectal Surgeon/Surgical Oncologist | REF |
| Years in Practice | |
| 0 – 2 Years | 1.72 (0.05, 6.43) |
| 3 – 10 Years | 1.37 (0.61, 3.06) |
| 11 – 15 Years | 2.30 (0.79, 6.71) |
| 16+ Years | REF |
|
Model 2 Predictors of guideline concordant recommendations for pre- op CRT(N=163) |
|
| Specialty | |
| General Surgeon | 0.31 (0.14, 0.66)* |
| Colorectal Surgeon/Surgical Oncologist | REF |
| Years in Practice | |
| 0 – 2 Years | 0.15 (0.03, 0.78)* |
| 3 – 10 Years | 1.06 (0.49, 2.31) |
| 11 – 15 Years | 2.37 (0.82, 6.85) |
| 16+ Years | REF |
Significantly different from the reference at the p <0.05 level.
Discussion
Our analyses indicated that fellowship trained colorectal surgeons and surgical oncologists are more likely to prefer guideline concordant TRUS/MRI for loco-regional staging for all rectal cancer patients, and pre-op CRT for all Stage II/III rectal cancer patients compared to general surgeons. In addition, those practicing more than two years were more likely to report recommending pre-op CRT to all Stage II/III rectal cancer patients. These results suggest that both training and experience are important factors associated with surgeon preferences in the treatment of rectal cancer.
When asked about specific TN stages, a significantly smaller proportion of general surgeons reported referring patients for pre-op CRT in almost every stage compared to colorectal surgeons or surgical oncologists. The difference was greatest in node positive T1 and T2 cancers. Results from the multivariate model indicate that both sub-specialty training and years of experience were associated with referring Stage II/III rectal cancer patients for pre-op CRT, so it may be that greater cumulative experience with patients across TN stages makes surgeons of all types more apt to refer patients for pre-op CRT. This could, in part, be due to the formation of relationships with medical and radiation oncologists for the treatment of rectal cancer. Without well-developed relationships with these provider types, it is possible there may be market pressures that favor a surgery-first approach. For example, if the surgeon refers the patient to an oncologist for pre-op CRT, there may be concerns that the oncologist could send the patient to a different surgeon after CRT. Furthermore, in a previous study we demonstrated that interdisciplinary approach to rectal cancer treatment is associated with receipt of pre-op CRT.7
When asked about specific loco-regional staging methods, a significantly greater proportion of colorectal surgeons and surgical oncologists preferred TRUS or MRI, whereas more general surgeons preferred CT. Results from the multivariate model suggest that sub-specialty training is the most important predictor of preferred staging methods. In the authors’ experience, trainees in colorectal surgery and surgical oncology have far more exposure to TRUS and rectal MRI than general surgery trainees. It may be that general surgeons have a greater comfort level with CT and thus prefer to rely on this method in their usual practice related to rectal cancer staging. It is also possible that colorectal surgeons and surgical oncologists are more likely to practice in large tertiary care settings where there may be more available expertise on TRUS/MRI methods and interpretation. Also, while MRI for pelvic staging is gaining acceptance in the United States and is emerging as the most accurate method for loco-regional staging, it is possible some surgeons with older machines/protocols may not agree that it is superior to CT. In our current (unpublished) analyses of beneficiaries over 65 diagnosed with stages II-III rectal cancer in 2009 using SEER-Medicare files, we found that approximately 30% received TRUS during the staging period, and only 7% received MRI of the pelvis which is fairly consistent with the survey responses from the general surgeons in Florida (unpublished data). Of note, CT is also recommended during staging, but should be done in addition to, and not in place of, TRUS or pelvic MRI because of its limitations in depicting various layers of the rectal wall, mesorectal fascia, microscopic invasion of the fat surrounding the rectum, and tumor invasion in surrounding pelvic structures.18,19
The finding that surgeons who have recently completed training were less likely to report the use guideline-concordant recommendations for neoadjuvant therapy was unexpected. One potential alternative interpretation could be that they are more likely to be knowledgeable about emerging data on reserving chemoradiation for T3 cancers with threatened margins on pretreatment imaging. If this were the case, however, we would expect increased use of appropriate locoregional staging in this group of surgeons, which was not the case. In addition, this data was not available at the time this study was performed. A study examining trends in centralization in cancer surgery found that approximately one-third of rectal cancer cases in the Nationwide Inpatient Sample (NIS) were treated in hospitals that performed fewer than 16 rectal cancer surgeries per year, and there appears to be no shift to high-volume centers occurring over time.20 If these surgeries are spread between stages and among multiple surgeons and residents at these low volume facilities, it is possible that exposure to stage II/III rectal cancer patients can be quite low on a per surgeon basis and that newer surgeons have treated relatively few rectal cancer patients overall.
This study has limitations that should be considered when interpreting our results. The survey was administered in only one state, and we achieved a modest response rate of 42%, so responses may not be representative of and generalizable to all surgeons throughout the United States. It is noteworthy, however, that Florida contains over 200 hospitals that include a variety of types (academic medical centers, non-profit community hospitals and for-profit hospitals), sizes and geography (from rural critical access to suburban to urban). In the interest of keeping the survey as brief and simple as possible to maximize response rates, surgeons were not queried on their actual volumes of rectal cancer patients, which may influence preferences and practices. However, the focus of this study was to quantify the preferences themselves rather than numbers of patients treated. Of note, the survey was administered in 2007, but aforementioned SEER data illustrate that non-adherence to treatment guidelines is still a relevant issue warranting examination, as 21% of patients still received post-op CRT in 2010, just 6% less than in 2007. The survey questions did not distinguish upper tumors from mid- or low-rectal tumors, which may have an impact on responses if some surgeons happen to only see mostly high (vs. low) tumors in their practice. Finally, surgeons were not asked about specific circumstances in which it may be warranted to take a surgery-first approach, such as in the case of a large obstruction causing acute problems. Rather, the focus was on surgeons’ general preferences and practices.
Conclusions
Approximately half of general surgeons surveyed reported preferences for staging and treatment that did not align with long-standing clinical guidelines. Prior studies have demonstrated improved outcomes for rectal cancer patients treated by subspecialists.21,22 Results of this study potentially suggest that closer adherence to treatment guidelines may be an explanation. An increased focus on the appropriate use of TRUS/MRI in the loco-regional staging of all patients with rectal cancer and of pre-op CRT in the treatment of patients with Stage II/III rectal cancer within general surgery training and educational programs would be warranted based upon this study.
Footnotes
Disclaimers: This manuscript is not under review elsewhere and there is no prior publication of manuscript contents. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The authors report no conflict of interest in regards to this study.
No funding was provided for this project.
Contributor Information
Mary E. Charlton, Assistant Professor, Department of Epidemiology, University of Iowa College of Public Health, Iowa City, IA & Core Investigator, VA Center for Comprehensive Access and Delivery Research and Evaluation (CADRE), Iowa City VA Health Care System, Iowa City, IA
Lorren R. Mattingly-Wells, Nurse Manager, VA Medical Center, Memphis, TN
Jorge E. Marcet, Professor of Surgery, Division Director Colon and Rectal Surgery, Department of Surgery, University of South Florida, Tampa, FL
Brenna C. McMahon Waldschmidt, Graduate Student, Department of Epidemiology, University of Iowa College of Public Health, Iowa City, IA
John W. Cromwell, Associate Professor and Director, Division of Gastrointestinal Surgery, Minimally Invasive and Bariatric Surgery, Department of Surgery, University of Iowa College of Medicine, Iowa City, IA
References
- 1.Wu JS, Fazio VW. Management of rectal cancer. J Gastrointest Surg. 2004 Feb;8(2):139–149. doi: 10.1016/j.gassur.2003.10.011. 2004. [DOI] [PubMed] [Google Scholar]
- 2.Roh MS, Colangelo LH, O'Connell MJ, et al. Preoperative Multimodality Therapy Improves Disease-Free Survival in Patients With Carcinoma of the Rectum: NSABP R-03. J Clin Oncol. 2009 Nov;27(31):5124–5130. doi: 10.1200/JCO.2009.22.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 4.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006 Sep 14;355(11):1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines™) Rectal Cancer. Fort Washington, PA: National Comprehensive Cancer Network; 2011. V 4.2011. [Google Scholar]
- 6.Lin C, Charlton ME, Meza JL, et al. Temporal and Regional Variations in the Use of Preoperative Radiation Therapy for Rectal Cancer. Am J Clin Oncol. 2010;33(5):443–447. doi: 10.1097/COC.0b013e3181b4b175. [DOI] [PubMed] [Google Scholar]
- 7.Charlton M, Lin C, Jiang D, et al. Factors associated with use of pre-operative chemoradiation therapy for rectal cancer in the Cancer Care Outcomes Research and Surveillance Consortium. Am J Clin Oncol. 2012 doi: 10.1097/COC.0b013e318261082b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) Research Data (1973–2010), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013, based on the November 2012 submission
- 9.Harewood GC, Wiersema MJ, Nelson H, et al. A prospective, blinded assessment of the impact of preoperative staging on the management of rectal cancer. Gastroenterology. 2002 Jul;123(1):24–32. doi: 10.1053/gast.2002.34163. [DOI] [PubMed] [Google Scholar]
- 10.Marusch F, Koch A, Schmidt U, et al. Routine use of transrectal ultrasound in rectal carcinoma: results of a prospective multicenter study. Endoscopy. 2002 May;34(5):385–390. doi: 10.1055/s-2002-25292. 2002. [DOI] [PubMed] [Google Scholar]
- 11.Tjandra JJ, Kilkenny JW, Buie WD, et al. Practice Parameters for the Management of Rectal Cancer (Revised) Dis Colon Rectum. 2005;48:411–423. doi: 10.1007/s10350-004-0937-9. 2005. [DOI] [PubMed] [Google Scholar]
- 12.Fleshman JW, Myerson RJ, Fry RD, IJ K. Accuracy of transrectal ultrasound in predicting pathologic stage of rectal cancer before and after preoperative radiation therapy. Dis Colon Rectum. 1992;35(9):823–829. doi: 10.1007/BF02047866. 1992/09/01. [DOI] [PubMed] [Google Scholar]
- 13.Beets-Tan RGH, Beets GL, Borstlap ACW, et al. Preoperative assessment of local tumor extent in advanced rectal cancer: CT or high-resolution MRI? Abdom Imaging. 2000;25(5):533–541. doi: 10.1007/s002610000086. 2000/09/01. [DOI] [PubMed] [Google Scholar]
- 14.Kim NK, Kim MJ, Park JK, Park SI, Min JS. Preoperative staging of rectal cancer with MRI: accuracy and clinical usefulness. Ann Surg Oncol. 2000 Dec;7(10):732–737. doi: 10.1007/s10434-000-0732-3. [DOI] [PubMed] [Google Scholar]
- 15.Gualdi G, Casciani E, Guadalaxara A, d'Orta C, Polettini E, Pappalardo G. Local staging of rectal cancer with transrectal ultrasound and endorectal magnetic resonance imaging. Dis Colon Rectum. 2000;43(3):338–345. doi: 10.1007/BF02258299. 2000/03/01. [DOI] [PubMed] [Google Scholar]
- 16.Sengupta S, Tjandra JJ. Local excision of rectal cancer: what is the evidence? Dis Colon Rectum. 2001;44(9):1345–1361. doi: 10.1007/BF02234796. [DOI] [PubMed] [Google Scholar]
- 17.McMullen TPW, Easson AM, Cohen Z, Swallow CJ. The investigation of primary rectal cancer by surgeons: current pattern of practice. Can J Surg. 2005 Feb;48(1):19–26. [PMC free article] [PubMed] [Google Scholar]
- 18.Heriot A, Grundy A, Kumar D. Preoperative staging of rectal carcinoma. Br J Surg. 1999;86(1):17–28. doi: 10.1046/j.1365-2168.1999.00996.x. [DOI] [PubMed] [Google Scholar]
- 19.Bipat S, Glas AS, Slors FJM, Zwinderman AH, Bossuyt PMM, Stoker J. Rectal cancer: Local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging - A meta-analysis. Radiology. 2004 Sep;232(3):773–783. doi: 10.1148/radiol.2323031368. [DOI] [PubMed] [Google Scholar]
- 20.Stitzenberg K, Meropol N. Trends in Centralization of Cancer Surgery. Ann Surg Oncol. 2010;17(11):2824–2831. doi: 10.1245/s10434-010-1159-0. [DOI] [PubMed] [Google Scholar]
- 21.Porter GA, Soskolne CL, Yakimets WW, Newman SC. Surgeon-related factors and outcome in rectal cancer. Ann Surg. 1998 Feb;227(2):157–167. doi: 10.1097/00000658-199802000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen L, Stasik JJ, Reed JF, Olenwine JA, Aronoff JS, Sherman D. Variations in colon and rectal surgical mortality. Comparison of specialties with a state legislated database. Dis Colon Rectum. 1996;39(2):129–135. doi: 10.1007/BF02068065. [DOI] [PubMed] [Google Scholar]