Abstract
DNA-dependent protein kinase catalytic subunit (DNA-PKcs) and Ataxia telangiectasia mutated (ATM) are the two major kinases involved in DNA double-strand break (DSB) repair, and are required for cellular resistance to ionizing radiation. While ATM is the key upstream kinase for DSB signaling, DNA-PKcs is primarily involved in DSB repair through the non-homologous end-joining (NHEJ) mechanism. In addition to DSB repair, ATM has been shown to be involved in oxidative stress response and could be activated directly in vitro upon hydrogen peroxide (H2O2) treatment. However, the role of DNA-PKcs in cellular response to oxidative stress is not clear. We hypothesize that DNA-PKcs may participate in the regulation of ATM activation in response to oxidative stress, and that this regulatory role is independent of its role in DNA double strand break repair. Our findings reveal that H2O2 induces hyperactivation of ATM signaling in DNA-PKcs deficient, but not Ligase 4 deficient cells, suggesting an NHEJ-independent role for DNA-PKcs. Furthermore, DNA-PKcs deficiency leads to the elevation of reactive oxygen species (ROS) production, and to a decrease in cellular survival against H2O2. For the first time, our results reveal that DNA-PKcs plays a non-canonical role in the cellular response to oxidative stress, which is independent from its role in NHEJ. In addition, DNA-PKcs is a critical regulator of the oxidative stress response and contributes to the maintenance of redox homeostasis. Our findings reveal that DNA-PKcs is required for cellular resistance to oxidative stress and suppression of ROS build-up independently to its function in DSB repair.
Introduction
Cells living in an oxygen-rich environment are constantly challenged by oxidative stress, whereby the production of oxidants, including reactive oxygen and nitrogen species (ROS and RNS, respectively) exceeds the cellular anti-oxidative capacity. ROS, including the superoxide anion radical (O2•−), hydrogen peroxide (H2O2), and the hydroxyl radical (•OH) are generated as byproducts during aerobic metabolism in mitochondria, and are the primary causes for oxidative stress [1]. Among these ROS, •OH is highly bioreactive, and can react directly with the 2-deoxyribose moiety as well as with purine and pyrimidine bases, leading to the formation of single strand breaks, abasic sites, and oxidatively damaged bases, whereas O2•− and its dismutation product H2O2 do not exhibit detectable reactivity towards DNA in aqueous solutions [2, 3]. Despite their decreased reactivity, H2O2 can be transformed into •OH in the presence of ferrous cuprous ions through Fenton reaction. Additionally, there are other biologically relevant radical or non-radical oxidants that could trigger oxidative stress, such as singlet oxygen, generated upon photosensitized reaction [3]. Oxidative stress influences many physiological and pathophysiological processes, and can be provoked by diverse exogenous and endogenous stimuli, such as inflammatory cytokines, hypoxia, ultraviolet light, ionizing radiation (IR), and chemotherapeutics [4]. Excessive or chronic oxidative stress will damage all cellular components including DNA and the accumulation of oxidatively damaged DNA is being considered as the key driving force of aging-related diseases and cancer [5, 6].
Ataxia telangiectasia mutated (ATM) and DNA-dependent protein kinase catalytic subunit (DNA-PKcs) are members of PI3K-like kinases, and are key regulators of the DNA double-strand break (DSB) repair response [7]. ATM is the major DSB-signaling kinase and plays a versatile role in the cellular response to DSBs, including regulating cell cycle arrest, apoptosis, senescence, and proliferation [8]; however, DNA-PKcs is primarily involved in DSB repair through the non-homologous end-joining (NHEJ) mechanism [9]. Besides their similarity in structure and function, ATM and DNA-PKcs share overlapping roles in DNA damage response and mutually regulate each other [10, 11]. For example, ATM-dependent DNA-PKcs phosphorylation at the T2609 cluster is critical for DSB repair [12]. On the other hand, DNA-PKcs is required to facilitate ATM expression as ATM protein levels decrease in the absence of DNA-PKcs [13]. Therefore, DNA-PKcs can backup ATM function in signaling regulation in ATM deficient cells [10, 11].
In addition to its critical function in DSB repair, it has been speculated that ATM plays a pivotal role in the cellular response to oxidative stress as seen by the increased sensitivity to oxidative stress in ataxia telangiectasia (A-T) patients [14, 15]. The direct evidence and molecular mechanism was shown by Guo et al. that H2O2 treatment induces ATM dimerization and kinase activation in vitro independent of the Mre11-Rad50-Nbs1 (MRN) complex[16]. These results suggest that ATM alone could be the direct sensor of ROS and trigger downstream signaling events. Despite the degree of overlap between ATM and DNA-PKcs, and the evidence linking ATM to oxidative stress, it is not clear whether DNA-PKcs is involved in the cellular response to oxidative stress.
In the present study, we report that the ATM signaling pathway is hyperactive in response to H2O2 treatment in the absence of DNA-PKcs. This hyperactivation of ATM correlates to an elevation in ROS production and/or oxidatively damaged DNA, but not in defective DSB repair. These results demonstrate for the first time that DNA-PKcs is critical to H2O2-induced oxidative stress response, and that it participates in redox homeostasis to prevent hyperactivation of ATM signaling pathway.
Results
Hydrogen peroxide induces ATM hyperactivation in DNA-PKcs deficient cells
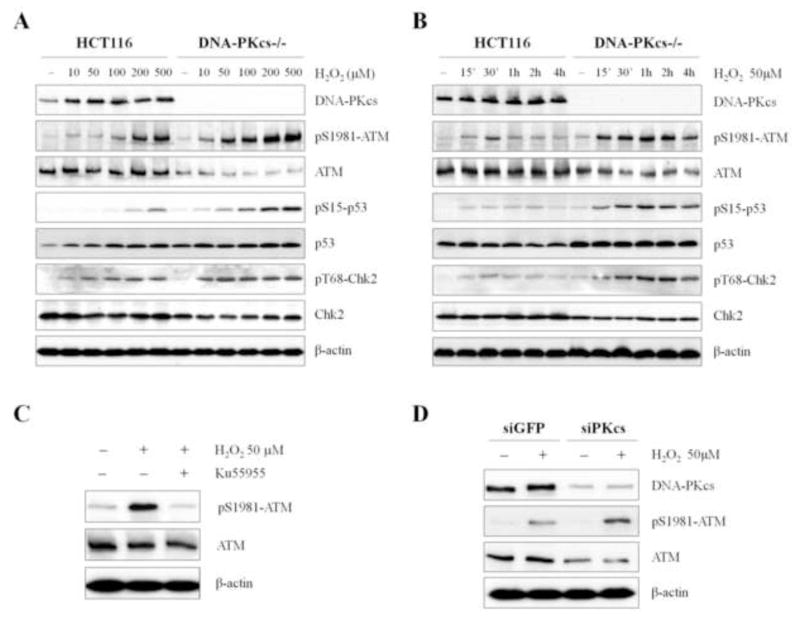
ATM and DNA-PKcs are the major kinases activated in response to DSB and they share some overlapping functions in DNA damage response. To examine whether DNA-PKcs plays a role in the cellular response to oxidative stress, human colon cancer HCT116 cells and its derivative DNA-PKcs knockout (DNA-PKcs−/−) cells were subjected to the increasing concentrations of H2O2 incubation for 30 min and were analyzed for the activation of ATM signaling pathway including ATM Ser1981 autophosphorylation as well as ATM dependent Chk2 and p53 phosphorylations. In DNA-PKcs−/− cells, there is a reduction of ATM protein levels as previously described [13]. Despite the reduction in total ATM protein levels, ATM Ser1981 phosphorylation was significantly induced in DNA-PKcs−/− cells, as compared to the parental HCT116 cells (Fig. 1A). Time course analysis further revealed that the levels of ATM Ser1981 phosphorylation were elevated and sustained in DNA-PKcs−/− cells in response to H2O2 (Fig. 1B). ATM Ser1981 phosphorylation was transiently induced by H2O2 treatment in the parental HCT116 cells with a peak induction at 30 min post treatment; in contrast, Ser1981 phosphorylation levels in DNA-PKcs−/− cells was significantly increased starting at 15 min and remained high for at least 4 h post H2O2 treatment. Both the dosage response and time course kinetics clearly demonstrates that ATM kinase is hyper-activated in response to H2O2 treatment in the absence of DNA-PKcs, despite the reduction of ATM protein expression levels. In agreement, siRNA-mediated DNA-PKcs depletion in HeLa cells also led to a reduction in ATM protein expression and an increase in Ser1981 autophosphorylation upon H2O2 treatment (Fig. 1D).
Figure 1. Hydrogen peroxide induces ATM hyperactivation in DNA-PKcs deficient cells.
(A) The parental human colon cancer HCT116 cells and derivative DNA-PKcs knockout (DNA-PKcs−/−) cells were subjected to increasing concentrations of H2O2 for 30 min. Whole cell lysates were probed against the specified antigens and analyzed by western blot. (B) Wild type and DNA-PKcs−/− HCT116 cells were treated with 50 μM H2O2 and harvested at the indicated time points prior to western blot analysis for the same proteins. (C) HCT116 cells were treated with 50 μM H2O2 in the presence or absence of ATM kinase inhibitor Ku55933 for 30 min and harvested for western blot analysis. (D) HeLa cells were transfected with control siRNA or DNA-PKcs siRNA at 0h and 24h. At 72h, transfected HeLa cells were treated with 50 μM H2O2 for 30 min followed by western blot analysis.
In addition to the hyperactivation of ATM, our data showed that ATM-mediated downstream signaling events, including Chk2 phosphorylation at Thr68 and p53 phosphorylation at Ser15, were both elevated in DNA-PKcs−/− cells in response to H2O2 treatment (Figs.1A and 1B). Taken together, our results suggest that DNA-PKcs deficiency leads to a hyperactivation of ATM and a sensitization of its signaling pathway in the wake of H2O2 treatment.
DNA-PKcs deficiency sensitizes ATM signaling to different oxidative stress agents
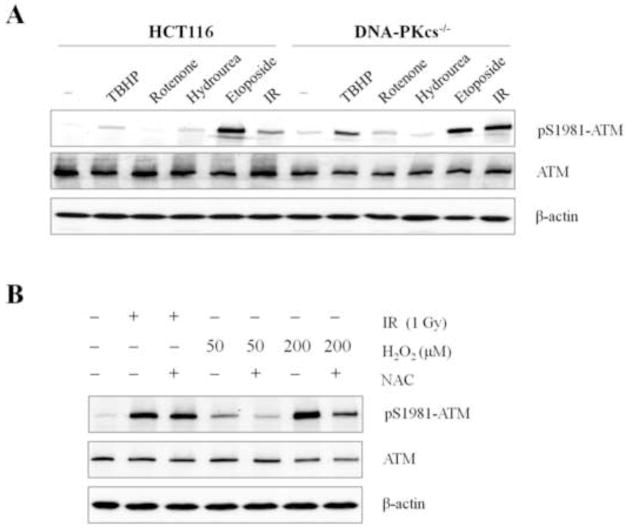
To examine whether ATM hyperactivation in the absence of DNA-PKcs is not limited to H2O2 treatment but reflects a general response to cellular oxidative stress, wild type and DNA-PKcs−/− HCT116 cells were subjected to various oxidative stress and genotoxic agents. As shown in Fig. 2A, hyperactivation of ATM Ser1981 phosphorylation was observed in DNA-PKcs−/− cells in response to tert-butyl hydroperoxide (TBHP, a short-chain organic hydroperoxide) and Rotenone (an inhibitor of mitochondrial electron transport), both of which are commonly used to induce the production of ROS and generate a state of oxidative stress. ATM hyperactivation was also observed following exposure to γ-rays, which produces both DSBs and an increase in •OH levels. Contrary to known oxidative stress inducers, etoposide and hydroxyurea (HU) causing DSBs and replication stress, respectively, did not further stimulate ATM Ser1981 phosphorylation in DNA-PKcs−/− cells. We observed similar results in HeLa cells after siRNA deletion of DNA-PKcs, whereby HU treatment did not further stimulate ATM Ser1981 hyper-phosphorylation, as compared to H2O2 treatment (Supplemental Fig. S1). Lastly, pretreatment with free radical scavenger N-acetyl-L-cysteine (NAC) significantly attenuates ATM Ser1981 phosphorylation in response to H2O2 but not after γ-rays irradiation (Fig. 2B).These results suggest that ATM hyperactivation is not due to an impaired DSB repair mechanism following the absence of DNA-PKcs, but point to a deficit in the cellular response to oxidative stress.
Figure 2. DNA-PKcs deficiency sensitizes ATM activation in response to oxidative stress.
(A) Wild type and DNA-PKcs−/− HCT116 cells were treated 30 min with following agents: tert-butyl hydroperoxide (TBHP), 50μM; Rotenone, 500nM; Hydroxyurea, 5mM); Etoposide, 100μM, and γ-ray (IR), 5Gy. Whole cell lysates were analyzed via western blot with the indicated antibodies. (B) Wild type HCT116 cells were pre-incubated with 10mM N-acetyl-L-cysteine (NAC) for 30 min and were subjected to γ-ray or H2O2 treatments. Cells were harvested at 30 min for western blot analysis.
In order to clarify the impact of DNA-PKcs downstream NHEJ activity in sensitizing the ATM signaling pathway, ATM hyperactivation following H2O2 treatment was further examined in HCT116 derivative Ligase4 knockout (Ligase4−/−) cells. If ATM hyperactivation in DNA-PKcs−/− cells is due to impaired DSB repair, we expect that ATM hyperactivation would also occur in Ligase4−/− cells. Contrary to DNA-PKcs−/− cells, ATM hyperactivation was only slightly enhanced in Ligase4−/− cells following H2O2, as seen by ATM Ser1981 phosphorylation levels that follow the trend of parental HCT116 cells (Figs. 3A and 3B). Similarly, our data showed that there was no significant induction of Chk2 Thr68 and p53 Ser15 phosphorylations in Ligase4−/− cells, whereas both events were intensified and maintained in DNA-PKcs−/− cells. Thus, H2O2-induced ATM hyperactivation in DNA-PKcs−/− cells cannot be explained simply by the impaired NHEJ or faulty DSB repair. Taken together, our results suggest that DNA-PKcs mediates a DSB repair-independent mechanism in the cellular response to oxidative stress.
Figure 3. Hyperactivation of ATM in DNA-PKcs deficient cells is independent of DSB repair through the NHEJ pathway.
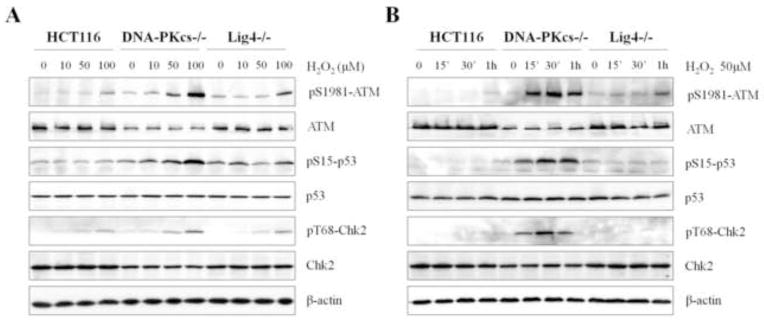
(A) Wild type, DNA-PKcs−/−, and Ligase4−/− HCT116 cells were subjected to the indicated concentrations of H2O2 for 30 min. Whole cell lysates were western blot were analyzed with the indicated antibodies. (B) The parental HCT116 cells were treated with 50 μM H2O2 for various durations. Protein levels from whole cell lysates were analyzed using western blot.
Involvement of DNA-PKcs in the oxidative stress response
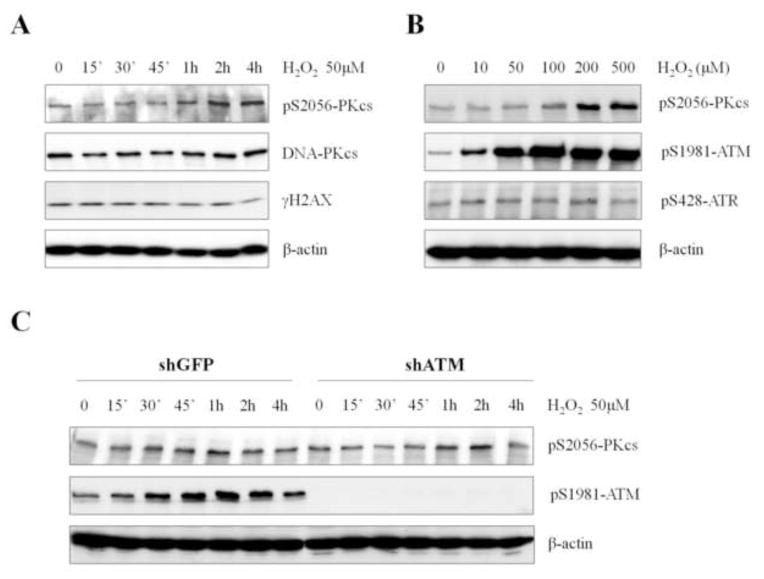
To determine whether DNA-PKcs kinase is activated in response to oxidative stress, DNA-PKcs autophosphorylation at Ser2056 (3) was analyzed in HCT116 cells following treatment with H2O2. Time course analysis revealed that H2O2 induced DNA-PKcs Ser2056 phosphorylation, but the kinetics was significantly slower than those of ATM Ser1981 phosphorylation. An increase in DNA-PKcs Ser2056 phosphorylation was apparent at 1 h and further increased at 2–4 h (Fig. 4A), whereas ATM Ser1981 phosphorylation was observed at 15 min and peaked at 30 min (Fig. 1B). It is unlikely that H2O2-induced DNA-PKcs Ser2056 phosphorylation is dependent on DSB production, since we did not detect a significant increase in γH2AX (Fig. 4A), a known marker of DSB [17]. Nonetheless, we cannot completely rule out this scenario, as higher concentrations of H2O2 could induce DSB formation and elicit DNA damage responses [18]. DNA-PKcs Ser2056 phosphorylation levels became visible in response to 30 min treatments with 100 μM or higher doses of H2O2 (Fig. 4B). In comparison, ATM Ser1981 phosphorylation could be detected robustly in response to H2O2 doses as low as 10 μM, whereas no induction was found in ATR phosphorylation at Ser428 (Fig. 4B).
Figure 4. H2O2 treatment induces DNA-PKcs activation independently of ATM status.
(A) Human HCT116 cells were treated with 50μM of H2O2 and were harvested at various time points. Whole cell lysates were analyzed via western blot with the indicated antibodies. (B) HeLa cells were treated with increasing concentrations of H2O2 for 30 min. Whole cell lysates were analyzed for autophosphorylations of DNA-PKcs, ATM, and ATR kinases. (C) HeLa cells expressing shRNA against GFP or ATM were treated with 50μM H2O2 for the indicated time points.
It is possible that the rapid induction of the ATM signaling pathway upon oxidative stress is required for a subsequent activation of the DNA-PKcs kinase. To test this possibility, HeLa cells expressing small hairpin RNA against ATM (shATM) or green fluorescent protein (shGFP) were treated with H2O2, and DNA-PKcs Ser2056 phosphorylation levels were assessed. Immunoblotting results revealed that no further stimulation of DNA-PKcs Ser2056 phosphorylation was found in ATM deficient cells in response to H2O2 (Fig. 4C).
DNA-PKcs deficiency enhances ROS production in response to oxidative stress
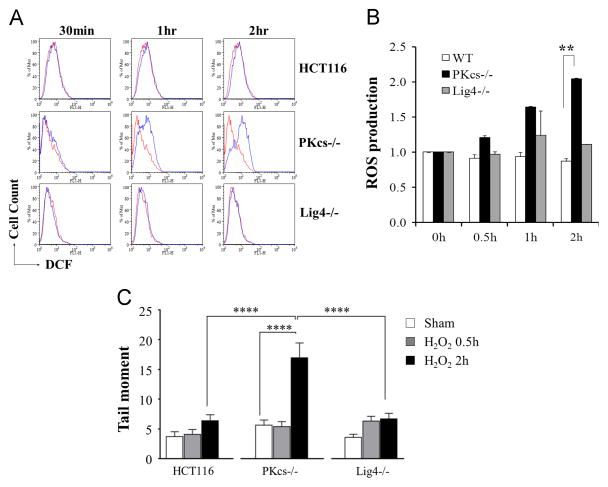
As ATM is a direct sensor of ROS, it is plausible that H2O2-induced ATM hyperactivation is due to a dysregulation in ROS production in DNA-PKcs deficient cells. To test this hypothesis, wild type, DNA-PKcs−/−, and Ligase4−/− HCT116 cells were exposed to H2O2, and were analyzed by flow cytometry using the commonly applied fluorescent indicator dichlorodihydrofluorescein diacetate (H2DCF-DA). While H2DCF-DA was initially used as a general ROS detection, it reacts primarily with •OH and is able to react with other types of organic radicals such as thiyl radicals [19–22]. Our analysis revealed that H2O2 treatment did not alter ROS production in HCT116 and Ligase4−/− cells; however, H2O2 caused significant ROS production in DNA-PKcs−/− cells (Fig. 5A). Increase in ROS production was detectable in DNA-PKcs−/− cells within 1 h. Quantification analysis revealed that there was a 2.05 ± 0.14 folds increase of ROS at 2 h in DNA-PKcs−/− cells compared to 0.87 ± 0.14 in wild type cells and 1.11 ± 0.15 in Ligase4−/− cells (Fig. 5B). In comparison, treatment with 50 mM H2O2 caused a 2.22±0.16 folds increase of ROS production in HeLa shATM cells and a 1.27±0.11 folds increase in HeLa shGFP cells (Supplemental Fig. S2). To further evaluate the DNA damage by elevated ROS production in DNA-PKcs−/− cells, relative to the parental HCT116 and Ligase4−/− cells, we performed the alkaline comet assay to measure the direct nicks and alkali-labile sites in DNA, presumably induced by oxidative stress [23–25]. Consistent with the elevation of ROS production, H2O2 treatment significantly increased comet tail moment in DNA-PKcs−/− cells (p<0.0001) at 2h (Fig. 5C). Furthermore, the production of oxidatively generated damage to DNA appears to be independent of DSB repair as Ligase4−/− cells and wild type HCT116 cells displayed similar and significantly less comet tail moment than DNA-PKcs−/− cells (p<0.0001).
Figure 5. DNA-PKcs deficiency enhances ROS production in response to H2O2 treatment.
(A) Wild type HCT116, DNA-PKcs−/−, and Ligase4−/− cells were incubated with 50 μM H2O2 for the indicated time points. ROS production in sham (red) and H2O2 treated cells was detected by H2DCF-DA incubation (20 μM at 37 °C for 45 min) by flow cytometry. (B) Normalized ROS production in HCT116, DNA-PKcs−/−, and Ligase4−/− cells. The result was generated from two independent experiments. Statistical analyses were performed by Student’s t-test, ** = P < 0.01. (C) HCT116 and derivative cells were incubated with 50 μM H2O2 for the indicated time points followed by alkaline comet assay to measure oxidatively damaged DNA production. **** = P < 0.0001.
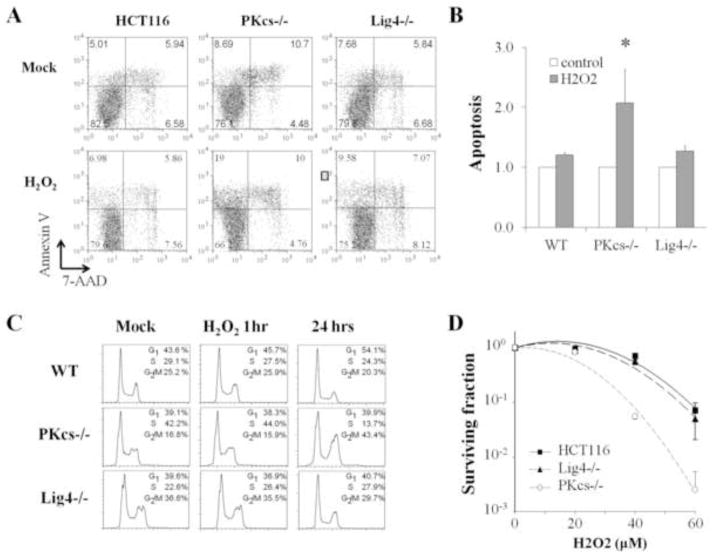
DNA-PKcs deficient cells are susceptible to oxidative stresses
It is possible that DNA-PKcs activity is required to counteract oxidative stress and prevent buildup of dangerous levels of ROS, which would otherwise interfere with normal cellular activities, leading to oxidatively damaged DNA and decreases in cell viability. To test this possibility, HCT116, DNA-PKcs−/−, and Ligase4−/− cells were subjected to a transient 50 μM H2O2 treatment for1 h and washed with normal culture medium. Flow cytometry analysis revealed that transient H2O2 treatment induces cell cycle checkpoint response and G2 arrest in DNA-PKcs−/− cells, but not in HCT116 or Ligase4−/− cells (Fig. 6C), which is consistent with sustained activation of ATM and Chk2 in DNA-PKcs−/− cells. At 24 h following transient H2O2 treatment, we also observed a specific induction of apoptotic cell population (Annexin-V positive and 7-AAD negative) in DNA-PKcs−/− cells (Fig. 6A). In untreated cell populations, similar fractions of apoptotic cells were found in all three cell lines. In response to 1 h H2O2 treatment, no significant induction of apoptosis occurred in HCT116 and Ligase4−/− cells, whereas there was a two-fold increase of apoptotic cells in DNA-PKcs−/− cells (Fig. 6B). Similar results were generated from clonogenic survival assay that DNA-PKcs−/− cells are susceptible to H2O2 treatments as compared to HCT116 and Ligase4−/− cells (Fig. 6D). Taken together, these results further confirm that there is a NHEJ-independent activity of DNA-PKcs required for the cellular response and resistance to oxidative stress.
Figure 6. DNA-PKcs deficient cells are susceptible to H2O2 treatment.
(A) Wild type, DNA-PKcs−/−, and Ligase4−/− HCT116 cells were subjected to 50 μM H2O2 for 1h and were recovered in normal culture medium for 24h. H2O2-induced apoptotic responses were determined by Annexin-V and 7-Amino-Actinomycin (7-AAD) staining in flow cytometry. (B) Induction of apoptosis (Annexin-V positive and 7-AAD negative) was determined by xx independent experiments. (C) HCT116 cells subjected to the same H2O2 treatment (1h incubation and 24h recovery) were analyzed by PI staining for their cell cycle profiles. (D) HCT116 cell lines were subjected to the indicated concentrations of H2O2 and were analyzed for their clonogenic ability afterward.
Discussion
ATM and DNA-PKcs are the major DNA repair kinases in the cellular response to DSBs. ATM plays a central role in DSB signaling required for cell-cycle checkpoint and can also directly participate in DNA damage repair, whereas DNA-PKcs is involved primarily in NHEJ-mediated DSB repair. Despite their distinctive functions, ATM and DNA-PKcs share overlapping duties in substrate phosphorylation and could mutually regulate each other [10, 11, 26, 27]. Here we further demonstrate that DNA-PKcs coordinates with ATM in the cellular response to oxidative stress. In the absence of DNA-PKcs, ATM is hyper-reactive to H2O2 treatment, as illustrated by the elevation of ATM dependent phosphorylations at ATM itself, Chk2, and p53. Hyperactivation of ATM signaling pathway in DNA-PKcs−/− cells also correlates to cell cycle arrest at G2 checkpoint, increased apoptosis, and decrease of survival against H2O2 treatment. ATM hyperactivation is probably due to an imbalance of ROS production in DNA-PKcs−/− cells but not to defects in DSB repair as ATM signaling was normal and not elevated in Ligase4−/− cells. Furthermore, there were distinctions in ROS production and sensitivity toward H2O2 treatment between DNA-PKcs−/− and Ligase4−/− cells. Taken together, our results demonstrate an NHEJ-independent mechanism of DNA-PKcs in oxidative stress response, and that DNA-PKcs could modulate the production of ROS.
ATM deficiency in human has been well characterized in the A-T disorder including impaired DNA damage responses, increased cancer risk, and progressive cerebellar ataxia and degeneration [28]. In addition, A-T cells display high levels of oxidative stress indicating that ATM is an important regulator of oxidative stress response [15]. The molecular mechanism was demonstrated by a recent finding that H2O2 facilitates ATM homo-dimerization and its kinase activation even in the absence of the MRN complex [16]. Conversely, ATM activation upon DSBs requires MRN dependent recruitment of ATM and unwinding of the DNA ends to activate ATM [29]. H2O2-mediated ATM activation can be seen by ATM autophosphorylation at Ser1981 and its downstream target phosphorylations including p53 and Chk2 but not H2AX [16], which further demonstrates that H2O2-induced ATM activation is independent of DSB production. To generate DSBs in cellular DNA, two independent radical hits are required on each of the opposite DNA strands in close proximity, which typically occurs after IR due to the high density of radicals generated at the site of energy deposit. It is unlikely that H2O2 treatment and subsequent •OH production through Fenton type reactions will cause such DSB events. This is in agreement with our results that moderate dose of H2O2 activates ATM signaling without causing immediate induction of γH2AX (Fig 4A,B), and that DSB repair defective Ligase4−/− cells did not cause ATM hyperactivation (Fig 3) and were resistant to H2O2 treatment (Fig 6). Another interesting observation was that the antioxidant NAC significantly decreased ATM activation induced by H2O2 treatment but not after IR (Fig 2). NAC alone is able to scavenge the bioreactive •OH and it can also serve as a precursor of glutathione to reduce the levels of low reactive O2•− and H2O2 [30]. Thus, the protective effect of NAC against H2O2 but not against IR further indicates that DSB formation is neglectable in response to H2O2 treatment.
Our results indicate that DNA-PKcs kinase activity (as measured by Ser2056 autophosphorylation) is elicited upon H2O2 treatment (Fig 4). While H2O2 induces rapid activation of ATM signaling pathway, DNA-PKcs activation was detected 1 h after H2O2 treatment regardless the presence or absence of ATM protein and was not correlated to γH2AX (Fig 4). These results suggest that H2O2-induced DNA-PKcs activation (and/or Ser2056 autophosphorylation) is neither a downstream event of ATM signaling nor a DSB response, although it would be difficult to completely rule out the DSB connection with our current analyses. Nonetheless, multiple lines of recent evidence have implicated a role of DNA-PKcs in oxidative stress response. DNA-PKcs has been found to associate with base excision repair (BER) protein complex essential for removing oxidative base damage [31]. Among the core BER components, XRCC1 was shown to directly interact with and be phosphorylated by DNA-PKcs [32]. In addition, Peddi et al. reported that inhibition of DNA-PKcs attenuates the repair of oxidatively induced clustered DNA lesions upon IR or H2O2 treatment [33]. It is plausible that DNA-PKcs is activated and participates in the repair of oxidatively induced non-DSB lesions. Coincidentally, it was reported that Ku possesses a 5′-deoxyribose-5-phosphate (5′-dRP)/AP lyase activity resembling that of DNA polymerase beta (Polβ) and could excise abasic sites near DSBs in vitro (40). Thus, further investigation the role of DNA-PK complex in BER would clarify the mechanism of H2O2- induced DNA-PKcs activation.
In addition to cellular and biochemical analyses, studies from genetically modified mouse models also correlate ATM and DNA-PKcs in oxidative stress response. It was reported that there is an age-dependent loss of hematopoietic stem cells (HSCs) in older ATM−/− mice due to an elevation of ROS, and that treatment with free radical scavenger NAC was able to alleviate the oxidative stress response in ATM−/− mice [34]. Similarly, we reported that DNA-PKcs-3A knock-in mice harboring mutation at the human equivalent Thr2609 cluster (targeted by ATM and ATR kinases) let to HSCs loss and congenital bone marrow failure characters [35]. Overproduction of ROS was also detected in HSCs isolated from DNA-PKcs-3A mice [36]. However, NAC treatment was unable to rescue HSC defect in DNA-PKcs-3A mice, which probably reflects the differential severities of HSC impairment in these mouse models. It is likely that NAC treatment alone is unable to overcome the multiple DNA repair defects caused by the DNA-PKcs-3A mutant protein [35].
DNA-PKcs deficiency has been identified in mice and several mammals with the classic severe combined immunodeficiency (SCID) character due to defect in V(D)J recombination and failed development of T-B-lymphocytes [37, 38]. DNA-PKcs deficient SCID mice have a relative normal life span and are widely used in tumor xenograft studies. However, little was known about germline mutations in DNA-PKcs encoding PRKDC gene in human until recently [39, 40]. A missense mutation (L3062R) conferred impaired DSB repair and radiosensitivity was first identified from a SCID patient, although L3062R mutation it did not affect DNA-PKcs protein expression or its kinase activation [39]. A true hypomorphic RPKDC patient with different mutations at both alleles, one Δexon16 mutation inactivated DNA-PKcs and one missense A3574V mutation led to a diminished DNA-PKcs expression, was recently reported by Woodbine et al reported [40]. The patient suffered impaired DSB repair and SCID character as predicted. In addition, the patient displayed severe growth failure, microcephaly, and global impairment in neurological function not found in patients defective in NHEJ or other DNA repair pathway [41]. We can speculate that the severity of hypomorphic germline mutations of DNA-PKcs in human will lead to dysregulation of ROS production and a hyperactive ATM signaling pathway based on our current studies. It is foreseeable that ATM hyperactivation would severely compromise normal development due to elevated checkpoint and apoptosis responses.
ATM has been known to be a key regulator of the oxidative stress response. However, it is not clear whether DNA-PKcs, a closely related PIKK family member with functional overlapping actions with ATM, is involved in oxidative stress regulation. In this study, we provide several lines of evidences that deficiency of DNA-PKcs in human cells leads to dysregulation of ROS production, which we hypothesize, consequently lead to ATM hyperactivation and increased cell cycle checkpoint and cell death. Our results reveal that DNA-PKcs plays a novel function as a mediator to oxidative stress, independent of its canonical role in DSB repair by NHEJ. Our results also point to a possible explanation for the severity of hypomorphic germline mutations in DNA-PKcs encoding gene in human including the progressive and global impairment in neurological function. Taken together, the evidence demonstrates that DNA-PKcs is a critical regulator of oxidative stress response and contributes to the maintenance of redox homeostasis.
Materials and Methods
Cell Culture and Treatments
Human colorectal carcinoma HCT116 cells and derived knockout cells [42, 43], HeLa cells expressing shRNA against GFP or ATM [12] were maintained in α–minimum essential medium (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum and penicillin/streptomycin (Invitrogen). H2O2 solution, TBHP solution, rotenone, etoposide, HU, NAC, H2DCF-DA were from Sigma-Aldrich.
siRNA transfection
Chemical synthesis siRNA specifically against DNA-PKcs was purchased from Dharmacon. siRNA against GFP gene were served as a negative control. At 12–24 hours after plating, cell with confluence of 30–50% were transfected for the first round with RNAi Lipofectamine™ RNAiMAX transfection reagent (Invitrogen) following the manufacturer’s instruction. The second transfection round was performed after 24h, using the same protocol. After a 24h incubation period, cells were treated as indicated.
Western blot and antibodies
Following treatment, cells were placed on ice immediately, then washed twice with ice cold PBS. Loading buffer (0.05 M Tris-HCl PH 6.8, 4% SDS, 2% 2-ME, 12% glycerol) was added directly to the dishes and lyses on for 5 min. The cell lysate was collected by cell scraper and sonicated for 5–10 s. After heating to 100°C for 5 min, protein concentrations were measured using Bradford method. Fifty micrograms of whole cell lysate from each sample was applied to 8–12% two layers SDS-polyacrylamide gels and electrophoresed to resolve proteins. The proteins were then transferred to Nitrocellulose membranes and blocked in TBST containing 5% (w/v) nonfat dry milk. The membrane was incubated with specific primary antibody diluted in TBST containing 5% nonfat dry milk (regular antibodies) or 2.5% BSA (phosphorylation antibodies) overnight at 4°C. The membrane was then washed three times in TBST and incubated with horseradish peroxidase-labeled secondary antibody for 1 h at room temperature. The membrane was reacted with ECL reagents (GE Healthcare) and revealed with X-ray films. The band intensities were analyzed using the Gel Doc 2000 apparatus and software (Bio-Rad Laboratory). Antibodies were from these suppliers listed below: phospho-p53 Ser15, phospho-Chk2 Thr68, Chk2, Caspase3 from (Cell Signaling Technology), phospho-ATM Ser1981 (Epitomics), ATM (Abcam), p53 (Santa Cruz Biotechnology), β-actin (Sigma-Aldrich).
ROS production measurement
Cells were grown in 12-well plates and treated as indicated. Cells were washed twice with PBS containing 0.2% BSA (w/v) and then incubated with 20 μM H2DCF-DA at 37 °C for 45 min before immediate analysis a FACSCalibur flow cytometer and CellQuest software (BD Biosciences). During the incubation, H2DCF-DA can be cleaved by endogenous esterases and transformed to a fluorescent form when oxidized by the hydroxyl radial. The mean fluorescent intensity (FL1 channel) of 10,000 analyzed cells of each treatment group were normalized by the mean fluorescent intensity of control group of each cell line, then the ratios were taken as a measure for the total ROS load.
Single-Cell Gel Electrophoresis Assay
Single-Cell Gel Electrophoresis Assay under alkaline conditions [23] was performed using the Trevigen Comet Assay kit (Trevigen, Gaithersburg, MD). Briefly, 5.0×104 cells were embedded in 37°C agarose at a ratio of 1:10 (v/v), plated onto specially coated microscope slides, treated with lysis solution 60 min at 4°C, followed by alkaline solution incubation 30 min for DNA unwinding. Slides were subjected to electrophoresis for 30 min at 21 volts, air dried and stained with Propidium Iodide and imaged under a fluorescent microscope with corresponding filters. Greater than 150 cells for each condition were imaged and analyzed using ImageJ [44] with the OpenComet plugin [45].
Apoptosis assay
Cells (5×105) were plated in 6-well plates and allowed to attach overnight. After being treated with 50 μM H2O2 for 1h at 37°C, cells were trypsinized and replated in 6-well plates. Cells were collected at 24h by trypsinzation and washed twice using ice cold PBS. Cells were stained using Annexin V-PE/7-Amino-Actinomycin (7-AAD) staining kit (BD Bioscience) following manufacturer’s instruction. The samples were analyzed immediately by flow cytometry as described.
Colony formation assay
Wild-type, DNA-PKcs−/−, and Ligase4−/− HCT116 cells were treated with increasing concentrations of H2O2 treatment for 1h then culture for 8 days.
Supplementary Material
Highlights.
DNA-PKcs deficiency causes hyperactivation of ATM signaling upon oxidative stress.
DNA-PKcs is required for cellular redox homeostasis independent of DSB repair.
DNA-PKcs participates in suppression of oxidative stress.
DNA-PKcs deficiency increases cellular susceptibility to oxidative stress.
Acknowledgments
This work is supported by National Institutes of Health (CA166677) and the Cancer Prevention Research Institute of Texas (RP110465) to BPC and National Natural Science Foundation of China (NSFC No. 81171904) to DW. We thank Dr. Eric Hendrickson for kindly providing HCT116 derivative DNA-PKcs−/− and Ligase4−/− cells.
List of Abbreviations
- APE1
apurinic/apyrimidinic endonuclease
- A-T
ataxia telangiectasia
- ATM
ataxia telangiectasia mutated
- BER
base excision repair
- DNA-PKcs
DNA-dependent protein kinase catalytic subunit
- DSB
DNA double-strand break
- ECL
enhanced chemiluminescence
- FA
Fanconi Anemia
- H2DCF-DA
dichlorodihydrofluorescein diacetate
- H2O2
hydrogen peroxide
- HDAC
histone deacetylase
- HIF-1α
hypoxia-inducible factor-1 α
- HSC
hematopoietic stem cell
- HU
hydroxyurea
- IR
ionizing radiation
- MRN
Mre11-Rad50-Nbs1
- NAC
N-acetyl-L-cysteine
- O2•−
superoxide anion radical
- •OH
hydroxyl radical
- NHEJ
non-homologous end-joining
- PIKK
PI3K like kinases
- Polβ
DNA polymerase beta
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SCID
severe combined immunodeficiency
- TBHP
tert-butyl hydroperoxide
- TBST
Tris-buffered saline with Tween 20
- XRCC1
X-ray repair cross-complementing protein 1
Footnotes
Author Disclosure Statement
The authors have declared that no competing interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 2.Dedon PC. The chemical toxicology of 2-deoxyribose oxidation in DNA. Chem Res Toxicol. 2008;21:206–219. doi: 10.1021/tx700283c. [DOI] [PubMed] [Google Scholar]
- 3.Cadet J, Loft S, Olinski R, Evans MD, Bialkowski K, Richard Wagner J, Dedon PC, Moller P, Greenberg MM, Cooke MS. Biologically relevant oxidants and terminology, classification and nomenclature of oxidatively generated damage to nucleobases and 2-deoxyribose in nucleic acids. Free Radic Res. 2012;46:367–381. doi: 10.3109/10715762.2012.659248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sedelnikova OA, Redon CE, Dickey JS, Nakamura AJ, Georgakilas AG, Bonner WM. Role of oxidatively induced DNA lesions in human pathogenesis. Mutat Res. 2010;704:152–159. doi: 10.1016/j.mrrev.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perry JJ, Fan L, Tainer JA. Developing master keys to brain pathology, cancer and aging from the structural biology of proteins controlling reactive oxygen species and DNA repair. Neuroscience. 2007;145:1280–1299. doi: 10.1016/j.neuroscience.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui H, Kong Y, Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct. 2012;2012:646354. doi: 10.1155/2012/646354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovejoy CA, Cortez D. Common mechanisms of PIKK regulation. DNA Repair (Amst) 2009;8:1004–1008. doi: 10.1016/j.dnarep.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol. 2013;14:197–210. [PubMed] [Google Scholar]
- 9.Davis AJ, Chen DJ. DNA double strand break repair via non-homologous end-joining. Transl Cancer Res. 2013;2:130–143. doi: 10.3978/j.issn.2218-676X.2013.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callen E, Jankovic M, Wong N, Zha S, Chen HT, Difilippantonio S, Di Virgilio M, Heidkamp G, Alt FW, Nussenzweig A, Nussenzweig M. Essential role for DNA-PKcs in DNA double-strand break repair and apoptosis in ATM-deficient lymphocytes. Mol Cell. 2009;34:285–297. doi: 10.1016/j.molcel.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomimatsu N, Mukherjee B, Burma S. Distinct roles of ATR and DNA-PKcs in triggering DNA damage responses in ATM-deficient cells. EMBO Rep. 2009;10:629–635. doi: 10.1038/embor.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen BP, Uematsu N, Kobayashi J, Lerenthal Y, Krempler A, Yajima H, Lobrich M, Shiloh Y, Chen DJ. Ataxia telangiectasia mutated (ATM) is essential for DNA-PKcs phosphorylations at the Thr-2609 cluster upon DNA double strand break. J Biol Chem. 2007;282:6582–6587. doi: 10.1074/jbc.M611605200. [DOI] [PubMed] [Google Scholar]
- 13.Peng Y, Woods RG, Beamish H, Ye R, Lees-Miller SP, Lavin MF, Bedford JS. Deficiency in the catalytic subunit of DNA-dependent protein kinase causes down-regulation of ATM. Cancer Res. 2005;65:1670–1677. doi: 10.1158/0008-5472.CAN-04-3451. [DOI] [PubMed] [Google Scholar]
- 14.Rotman G, Shiloh Y. The ATM gene and protein: possible roles in genome surveillance, checkpoint controls and cellular defence against oxidative stress. Cancer Surv. 1997;29:285–304. [PubMed] [Google Scholar]
- 15.Rotman G, Shiloh Y. Ataxia-telangiectasia: is ATM a sensor of oxidative damage and stress? Bioessays. 1997;19:911–917. doi: 10.1002/bies.950191011. [DOI] [PubMed] [Google Scholar]
- 16.Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 17.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 18.Driessens N, Versteyhe S, Ghaddhab C, Burniat A, De Deken X, Van Sande J, Dumont JE, Miot F, Corvilain B. Hydrogen peroxide induces DNA single- and double-strand breaks in thyroid cells and is therefore a potential mutagen for this organ. Endocr Relat Cancer. 2009;16:845–856. doi: 10.1677/ERC-09-0020. [DOI] [PubMed] [Google Scholar]
- 19.Chen XP, Zhong ZF, Xu ZT, Chen LD, Wang YT. 2′,7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radic Res. 2010;44:587–604. doi: 10.3109/10715761003709802. [DOI] [PubMed] [Google Scholar]
- 20.Karlsson M, Kurz T, Brunk UT, Nilsson SE, Frennesson CI. What does the commonly used DCF test for oxidative stress really show? Biochem J. 2010;428:183–190. doi: 10.1042/BJ20100208. [DOI] [PubMed] [Google Scholar]
- 21.Wrona M, Patel KB, Wardman P. The roles of thiol-derived radicals in the use of 2′,7′-dichlorodihydrofluorescein as a probe for oxidative stress. Free Radic Biol Med. 2008;44:56–62. doi: 10.1016/j.freeradbiomed.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Wrona M, Wardman P. Properties of the radical intermediate obtained on oxidation of 2′,7′-dichlorodihydrofluorescein, a probe for oxidative stress. Free Radic Biol Med. 2006;41:657–667. doi: 10.1016/j.freeradbiomed.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low-levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 24.Azqueta A, Collins AR. The essential comet assay: a comprehensive guide to measuring DNA damage and repair. Arch Toxicol. 2013;87:949–968. doi: 10.1007/s00204-013-1070-0. [DOI] [PubMed] [Google Scholar]
- 25.Collins AR. Measuring oxidative damage to DNA and its repair with the comet assay. Biochim Biophys Acta. 2014;1840:794–800. doi: 10.1016/j.bbagen.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 26.Stiff T, O’Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. doi: 10.1158/0008-5472.can-03-3207. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Guan J, Perrault AR, Wang Y, Iliakis G. Replication protein A2 phosphorylation after DNA damage by the coordinated action of ataxia telangiectasia-mutated and DNA-dependent protein kinase. Cancer Res. 2001;61:8554–8563. [PubMed] [Google Scholar]
- 28.Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9:759–769. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- 29.Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 30.Smeyne M, Smeyne RJ. Glutathione metabolism and Parkinson’s disease. Free Radic Biol Med. 2013;62:13–25. doi: 10.1016/j.freeradbiomed.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parlanti E, Locatelli G, Maga G, Dogliotti E. Human base excision repair complex is physically associated to DNA replication and cell cycle regulatory proteins. Nucleic Acids Res. 2007;35:1569–1577. doi: 10.1093/nar/gkl1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy N, Martz A, Bresson A, Spenlehauer C, de Murcia G, Menissier-de Murcia J. XRCC1 is phosphorylated by DNA-dependent protein kinase in response to DNA damage. Nucleic Acids Res. 2006;34:32–41. doi: 10.1093/nar/gkj409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peddi P, Loftin CW, Dickey JS, Hair JM, Burns KJ, Aziz K, Francisco DC, Panayiotidis MI, Sedelnikova OA, Bonner WM, Winters TA, Georgakilas AG. DNA-PKcs deficiency leads to persistence of oxidatively induced clustered DNA lesions in human tumor cells. Free Radic Biol Med. 2010;48:1435–1443. doi: 10.1016/j.freeradbiomed.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, Ikeda Y, Mak TW, Suda T. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 35.Zhang S, Yajima H, Huynh H, Zheng J, Callen E, Chen HT, Wong N, Bunting S, Lin YF, Li M, Lee KJ, Story M, Gapud E, Sleckman BP, Nussenzweig A, Zhang CC, Chen DJ, Chen BP. Congenital bone marrow failure in DNA-PKcs mutant mice associated with deficiencies in DNA repair. J Cell Biol. 2011;193:295–305. doi: 10.1083/jcb.201009074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang S, Chen BP. Disablement of DNA-PKcs phosphorylation induces hematopoietic failure in mice through the p53 signaling pathway. Treatment Strategies Hematol. 2012;1:36–40. [Google Scholar]
- 37.Kirchgessner CU, Patil CK, Evans JW, Cuomo CA, Fried LM, Carter T, Oettinger MA, Brown JM. DNA-dependent kinase (p350) as a candidate gene for the murine SCID defect. Science. 1995;267:1178–1183. doi: 10.1126/science.7855601. [DOI] [PubMed] [Google Scholar]
- 38.Fulop GM, Phillips RA. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990;347:479–482. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- 39.van der Burg M, Ijspeert H, Verkaik NS, Turul T, Wiegant WW, Morotomi-Yano K, Mari PO, Tezcan I, Chen DJ, Zdzienicka MZ, van Dongen JJ, van Gent DC. A DNA-PKcs mutation in a radiosensitive T-B-SCID patient inhibits Artemis activation and nonhomologous end-joining. J Clin Invest. 2009;119:91–98. doi: 10.1172/JCI37141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woodbine L, Neal JA, Sasi NK, Shimada M, Deem K, Coleman H, Dobyns WB, Ogi T, Meek K, Davies EG, Jeggo PA. PRKDC mutations in a SCID patient with profound neurological abnormalities. J Clin Invest. 2013;123:2969–2980. doi: 10.1172/JCI67349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Driscoll M, Jeggo PA. The role of double-strand break repair - insights from human genetics. Nat Rev Genet. 2006;7:45–54. doi: 10.1038/nrg1746. [DOI] [PubMed] [Google Scholar]
- 42.Ruis BL, Fattah KR, Hendrickson EA. DNA-PKcs regulates proliferation, telomere length and genomic stability in human somatic cells. Mol Cell Biol. 2008;28:6182–6195. doi: 10.1128/MCB.00355-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fattah F, Lee EH, Weisensel N, Wang Y, Lichter N, Hendrickson EA. Ku regulates the non-homologous end joining pathway choice of DNA double-strand break repair in human somatic cells. PLoS Genet. 2010;6:e1000855. doi: 10.1371/journal.pgen.1000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gyori BM, Venkatachalam G, Thiagarajan PS, Hsu D, Clement MV. OpenComet: An automated tool for comet assay image analysis. Redox Biol. 2014;2:457–465. doi: 10.1016/j.redox.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.